Seedling Survival Strategies of Zanthoxylum planispinum ‘Dintanensis’ and Zanthoxylum amatum ‘Novemfolius’, Based on Functional Traits in Karst Desertification Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plot Setting and Sample Collection
2.3. Sample Processing and Testing
2.4. Data Analysis
3. Results and Analysis
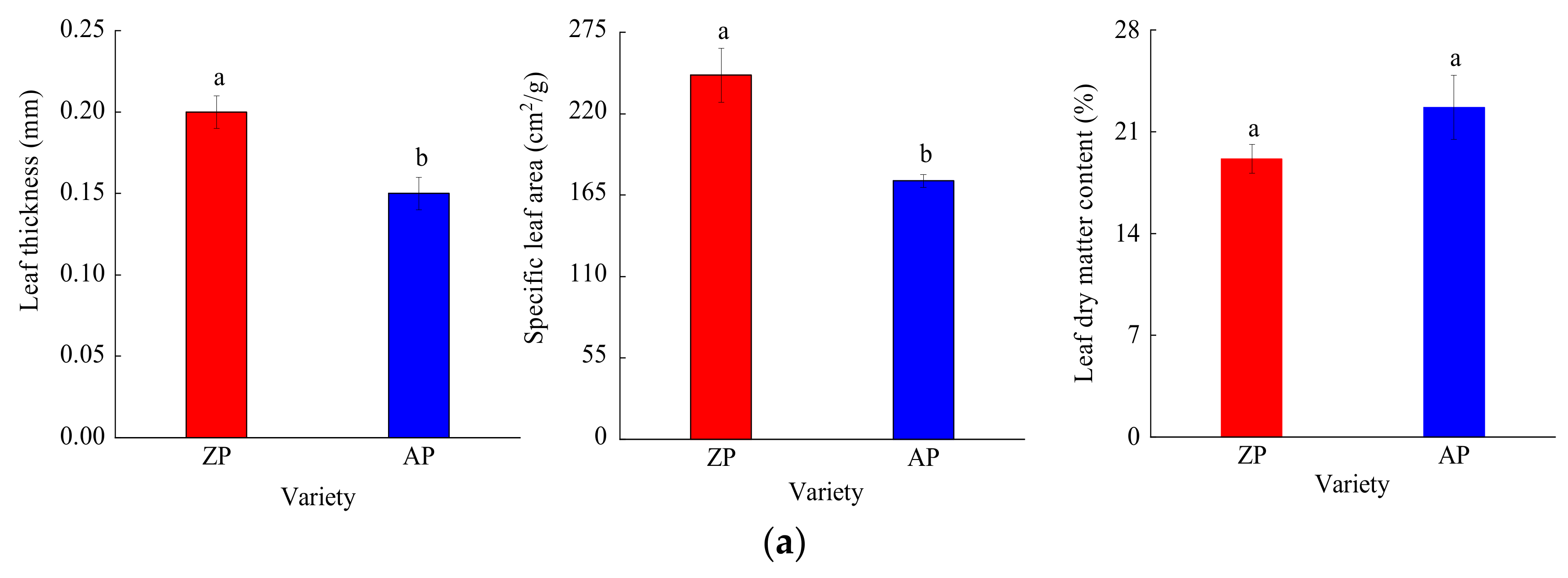
3.1. Leaf Functional Traits
3.1.1. Leaf Structural Traits
3.1.2. Leaf Nutrient Traits and Stoichiometry
3.1.3. Leaf Physiological Traits
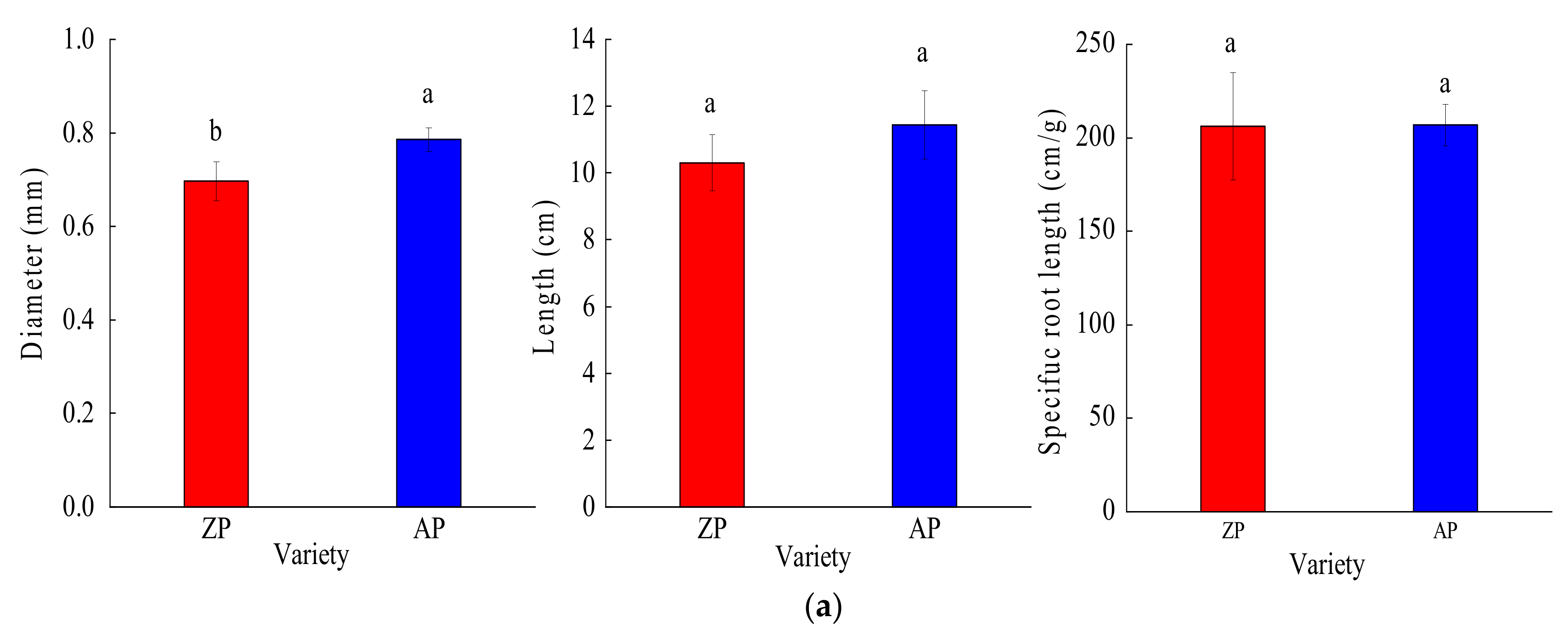
3.2. Root Functional Traits
3.2.1. Root Structural Traits
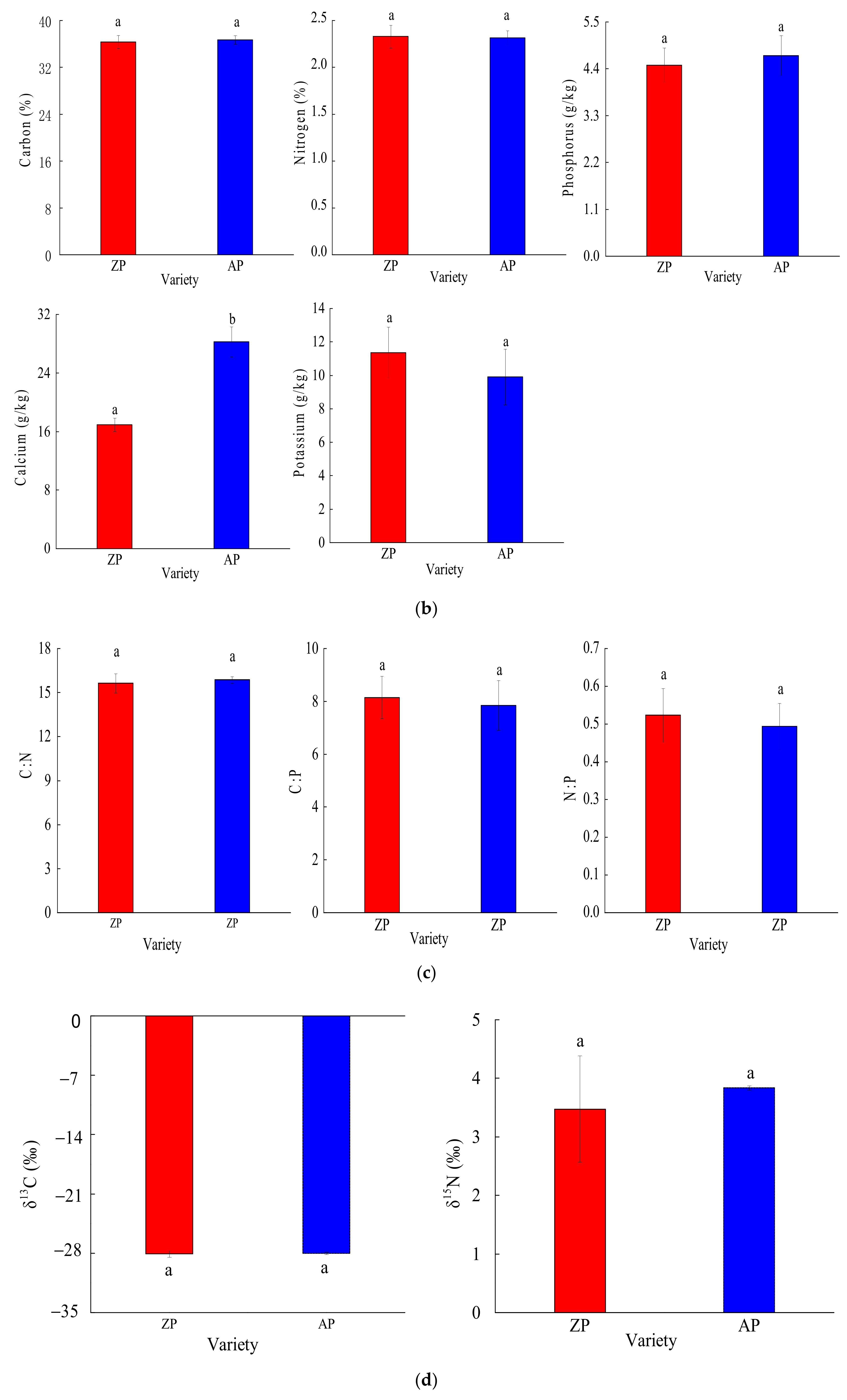
3.2.2. Root Nutrient and Chemical Traits
3.2.3. Root Physiological Traits
3.3. Screening for Major Functional Traits
3.4. Sensitivity to Changes in Major Functional Traits
3.5. Correlations between Major Functional Traits
4. Discussion
4.1. Characteristics of the Seedling Leaf Functional Traits of the Two Zanthoxylum Varieties
4.2. Characteristics of Root Functional Traits of the Two Zanthoxylum Varieties
4.3. Trade-Offs and Synergies among Major Functional Traits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; He, N.P.; Xu, L.; Li, S.G.; Li, M.X. Spatial variation in leaf potassium concentrations and its role in plant adaptation strategies. Ecol. Indic. 2021, 130, 108063. [Google Scholar] [CrossRef]
- Zhang, Y.; He, N.P.; Li, M.X.; Yan, P.; Yu, G.R. Community cholorphyll quantity determines the spatial variation of grassland productivity. Sci. Total Environ. 2021, 801, 149567. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.T.; Xiang, W.H.; Schafe, K.V.R.; Lei, P.F.; Deng, X.W.; Forrester, D.; Fang, X.; Zeng, Y.L.; Ouyang, S.; Chen, L.; et al. Photosynthetic and hydraulic traits influence forests resistance and resilience to drought stress across different biomes. Sci. Total Environ. 2022, 828, 154517. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Jin, G.Z.; Liu, Z.L. Effects of ontogenetic stage and leaf on leaf functional traits and the relationships between traits in Pinus koraiensis. J. For. Res. 2021, 32, 2459–2471. [Google Scholar] [CrossRef]
- Khan, A.; Yan, L.; Wang, W.; Xu, K.; Zou, G.W.; Liu, X.D.; Fang, X.W. Leaf traits and leaf nitrogen shift photosynthesis adaptive strategies among functional groups and diverse biomes. Ecol. Indic. 2022, 141, 109098. [Google Scholar] [CrossRef]
- Li, S.; Hamani, A.K.M.; Zhang, Y.Y.; Liang, Y.P.; Gao, Y.; Duan, A.W. Coordination of leaf hydraulic, anatomical, and economical traits in tomato seedlings acclimation to long-term drought. BMC Plant Biol. 2021, 21, 536. [Google Scholar] [CrossRef]
- Wliiiams, A.; Langride, H.; Straathof, A.L.; Muhamadali, H.; Hollywood, K.A.; Goodacre, R.; de Varie, F.T. Root functional traits explain root exudation rate and composition across a range of grassland species. J. Ecol. 2021, 110, 21–33. [Google Scholar] [CrossRef]
- Xu, H.D.; Zhu, B.; Wei, X.M.; Yu, M.K.; Cheng, X.R. Root functional traits mediate rhizosphere soil carbon stability in a subtropical forests. Soil Biol. Biochem. 2021, 162, 108431. [Google Scholar] [CrossRef]
- Zadmorny, M.; Mucha, J.; BBagniewska-Zadworna, A.; Zytkowiak, R.; Maderek, E.; Danusevicius, D.; Oleksyn, J.; Wyka, T.P.; McCormack, M.L. Higher biomass partitioning to absorptive roots improves needle nutrition but also does not alleviate stomatal limitation of northern Scots pine. Glob. Chang. Biol. 2021, 27, 3859–3869. [Google Scholar] [CrossRef]
- Wang, X.M.; Yan, B.G.; Shi, L.T.; Liu, G.C. Different responses of biomass allocation and leaf traits of Dodonaea viscous to concentrations of nitrogen and phosphorus. Chin. J. Plant Ecol. 2020, 44, 1247–1261. [Google Scholar] [CrossRef]
- Elberse, I.A.M.; Van Damme, J.M.M.; Van Tienderen, P.H. Plasticity of growth characteristics in wild barley (Hordeumspontaneum) in response to nutrient limitation. J. Ecol. 2003, 91, 371–382. [Google Scholar] [CrossRef]
- Qi, L.Y.; Chen, H.N.; Sairebieli, K.L.H.; Ji, T.Y.; Meng, G.D.; Qin, H.Y.; Wang, N.; Song, Y.X.; Liu, C.Y.; Du, N.; et al. Growth strategies of five shrub seedlings in warm temperate zone based on plant functional traits. Chin. J. Plant Ecol. 2022, 46, 1388–1399. [Google Scholar] [CrossRef]
- Ahmed, M.; Kheir, A.M.S.; Mehmood, M.Z.; Ahmad, S.; Hasanuzzaman, M. Changes in germination and seedling traits of sesame under simulated drought. Phyton-Int. J. Exp. Bot. 2022, 91, 713–726. [Google Scholar]
- Harrison, S.; Laforgia, M. Seedling traits predict drought-induced mortality linked to diversity loss. Proc. Natl. Acad. Sci. USA 2019, 116, 5576–5581. [Google Scholar] [CrossRef]
- Xu, R.; Liu, J.; Wang, L.Y.; Yan, Y.; Ma, X.Q.; Li, M. Analysis of root and leaf functional traits and C, N, P stoichiometry of Cunninghamia lanceolate from different provenances. Acta Ecol. 2022, 42, 6311–6319. [Google Scholar]
- Toledo-Aceves, T.; Lopez-Barrera, F.; Vasquez-Reyes, V. Preliminary analysis of functional traits in cloud forest tree seedlings. Trees-Struct. Funct. 2017, 31, 1253–1262. [Google Scholar] [CrossRef]
- Alan, M.; Ezen, T. Magnitude of genetic variation in seedling traits of Liquidambar orientalis populations. Fresenius Environ. Bull. 2018, 27, 1522–1531. [Google Scholar]
- Umana, M.N.; Zhang, C.C.; Cao, M.; Lin, L.; Swenson, N.G. Quantifying the role of intra-specific trait variation and organ-level traits in tropical seedling communities. J. Veg. Sci. 2018, 29, 276–284. [Google Scholar] [CrossRef]
- Hu, S.L.; Sanchez, D.L.; Wang, C.L.; Lipka, A.E.; Yin, Y.H.; Gardner, C.A.C.; Lubberstedt, T. Brassinosteroid and gibberellina control of seedling traits in maize (Zea mays L.). Plant Sci. 2017, 263, 132–141. [Google Scholar] [CrossRef]
- Ju, C.L.; Zhang, W.; Liu, Y.; Gao, Y.F.; Wang, X.F.; Yan, J.B.; Yang, X.H.; Li, J.S. Genetic analysis of seedling root traits reveals the association of root trait with other agronomic traits in maize. BMC Plant Biol. 2018, 18, 171. [Google Scholar] [CrossRef]
- Nahar, K.; Bovill, W.; McDonald, G. Assessing the contribution of seedling root traits to phosphorus responsiveness in wheat. J. Plant Nutr. 2022, 45, 2170–2188. [Google Scholar] [CrossRef]
- Ren, B.M. Research on the Driving Mechanism and Model Offarmers’ Cooperatives Ecological Industry in Karst Rocky Desertification Treatment. Ph.D. Thesis, Guizhou Normal University, Guiyang, China, 2022. (In Chinese). [Google Scholar]
- Bremer, E.; Janzen, H.H.; Johnston, A.M. Sensitivity of total, light fraction and mineralizable organic matter to management practices in a Lethbridge soil. Can. J. Soil Sci. 1994, 74, 131–138. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, U.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, W.C.; Kharouba, H.M.; Robinson, M.; Holyoak, M.; Karban, R. Variability in plant nutrients reduces insect herbivore performance. Nature 2016, 539, 425–427. [Google Scholar] [CrossRef]
- Luo, Q.Y.; Yu, M.S.; Yu, J.J.; Zheng, S.L.; Liu, J.J.; Yu, M.J. Effects of plant traits and the relative abundance of common woody species on seedling herbivory in the Thousand Island Lake region. Chin. J. Plant Ecol. 2017, 41, 1033–1040. [Google Scholar]
- Farquhar, G.D.; OLeary, M.H.; Berry, J.A. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Austrailan J. Plant Physiol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Z.H.; Sun, J.K.; Fu, Z.Y.; Zhao, Y.H.; Xu, W.J. Seasonal variation characteristics of C, N, and P stoichiometry and water use efficiency of Messerschmidia sibirica relationship with soil nutrients. Front. Ecol. Evol. 2022, 10, 948682. [Google Scholar] [CrossRef]
- Mukarram, M.; Khan, M.M.A.; Zehra, A.; Petrik, P.; Kurjak, D. Suffer or survive: Decoding salt- sensitivity of lemongrass and its implication on essential oil productivity. Front. Plant Sci. 2022, 13, 903954. [Google Scholar] [CrossRef]
- Shi, K.; Shao, H. Changes in the soil fungal community mediated by a Peganum harmala allelochemical. Front. Microbiol. 2022, 13, 911836. [Google Scholar] [CrossRef]
- Mungan, M.D.; Harbig, T.A.; Perez, N.H.; Edenhart, S.; Stegmann, E.; Nieselt, K.; Ziemert, N. Secondarymetabolite transcriptomic pipeline (SeMa-Trap), an expression-based exploration tool for increased secondary metabolite production in bacteria. Nucleic Acids Res. 2022, 50, W682–W689. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhang, Y.; Xia, X.L.; Yin, W.L.; Guo, H.W.; Li, Z.H. Resaerch advances in leaf senescence of woody plants. Sci. Sin. Vitae 2020, 50, 196–206. [Google Scholar]
- Keskitalo, J.; Bergquist, G.; Gardeström, P.; Jansson, S. A cellular timetable of autumn senescence. Plant Physiol. 2005, 139, 1635–1648. [Google Scholar] [CrossRef]
- Hu, H.C.; Fei, X.T.; He, B.B.; Chen, X.; Ma, L.; Han, P.; Luo, Y.L.; Liu, Y.H.; Wei, A.Z. UPLC-MS/MS profile combined with RNA-Seq reveals the amino acid metabolism in Zanthoxylum bungeanum leaves under drought stress. Front. Nutr. 2022, 9, 921742. [Google Scholar] [CrossRef]
- Carmona, C.P.; Bueno, C.G.; Toussaint, A.; Träger, S.; Díaz, S.; Moora, M.; Mouson, A.D.; Pärtel, M.; Zobel, M.; Tamme, R. Fine-root traits in the global spectrum of plant form and function. Nature 2021, 597, 683. [Google Scholar] [CrossRef]
- Ma, Z.Q.; Guo, D.L.; Xu, X.L.; Lu, M.H.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94. [Google Scholar] [CrossRef]
- Meng, W.P.; Ren, Q.Q.; Tu, N.; Leng, T.J.; Dai, Q.H. Characteristics of the adaptations of Epilithic Mosses to high-calcium habitats in the karst region of southwest China. Bot. Rev. 2021, 88, 204–219. [Google Scholar] [CrossRef]
- Poovaiah, H.W.; Reddy, A.S.N. Calcium and signal transduction in plants. Crit. Rev. Plant Sci. 1993, 12, 185–211. [Google Scholar] [CrossRef]
- Diao, H.Y.; Wang, A.Z.; Yuan, F.H.; Guan, D.X.; Yin, H.; Wu, J.B. Stable carbon isotopic characteristics of plant-ltter-soil continuum along a successional gradient of broadleaved Korean pine forests in Changbai Mountain, China. Chin. J. Appl. Ecol. 2019, 30, 1435–1444. [Google Scholar]
- Hobbic, E.A.; Werner, R.A. Intramolecular, compoundspecific, and bulk carbon isotope patterns in C3 and C4 plants: A review and synthesis. New Phytol. 2004, 161, 371–385. [Google Scholar] [CrossRef]
- Huang, M.J.; Wang, S.P.; Liu, X.; Nie, M.; Zhou, S.R.; Hautier, Y. Intra- and interspecific variability of specific leaf area mitigate the reduction of community stability in response to warming and nitrogen addition. Oikos 2022, 9, e09207. [Google Scholar] [CrossRef]
- Xu, X.N.; Sun, Y.X.; Liu, F.L. Modulating leaf thickness and calcium content impact on strawberry plant thermotolerance and water consumption. Plant Growth Regul. 2022, 98, 539–556. [Google Scholar] [CrossRef]
- Lin, W.Q.; Cai, J.H.; Xue, L. Responses of Cinnamomumcamphora seedling growth and leaf traits to additions of nitrogen and phosphorous under different planting densities. Acta Ecol. 2019, 39, 6738–6744. [Google Scholar]
- Zhang, J.H.; Tang, Y.Z.; Luo, Y.K.; Chi, X.L.; Chen, Y.H.; Fang, J.Y.; Shen, H.H. Resorption efficiency of leaf nutrients in woody plants on Mt. Dongling of Beijing, North China. J. Plant Ecol. 2014, 8, 530–538. [Google Scholar] [CrossRef]

| Month | Rainfall/mm | Land Surface Evaporation/mm | Available Rainfall/mm | Temperature/°C | Radiation /(W·m−2) | Relative Humidity /% |
|---|---|---|---|---|---|---|
| January | 17.00 | 16.49 | 0.51 | 12.17 | 227.26 | 80.02 |
| February | 10.40 | 10.32 | 0.08 | 14.79 | 320.71 | 70.44 |
| March | 12.60 | 12.51 | 0.09 | 19.15 | 373.30 | 67.19 |
| April | 33.50 | 32.84 | 0.66 | 26.40 | 572.70 | 64.11 |
| May | 31.20 | 30.65 | 0.55 | 26.20 | 465.80 | 67.24 |
| June | 253.00 | 125.07 | 127.93 | 27.41 | 582.78 | 78.13 |
| July | 156.60 | 112.47 | 44.13 | 27.40 | 585.91 | 78.62 |
| August | 75.60 | 70.09 | 5.51 | 28.70 | 761.22 | 73.05 |
| September | 296.40 | 105.78 | 190.62 | 25.30 | 587.69 | 73.80 |
| October | 101.00 | 80.77 | 20.23 | 23.80 | 332.78 | 70.10 |
| November | 57.60 | 45.43 | 12.17 | 14.30 | 282.56 | 68.47 |
| December | 43.50 | 36.05 | 7.45 | 12.00 | 247.72 | 72.34 |
| Variety | Altitude /m | Longitude | Latitude | Seedling Age/Month | Seedling Height/m | Insect Feed Rate |
|---|---|---|---|---|---|---|
| ZP | 620 | 105°41′30.17″ E | 35°39′49.35″ N | 8 | 0.3–0.4 | Less |
| AP | 620 | 105°41′30.17″ E | 35°39′49.35″ N | 8 | 0.3–0.4 | More |
| Factor | Principal Component (PC) | ||||
|---|---|---|---|---|---|
| PC 1 | PC 2 | PC 3 | PC 4 | PC 5 | |
| Specific leaf area | −0.963 | −0.018 | −0.214 | 0.058 | 0.154 |
| Leaf δ15N | −0.923 | 0.320 | −0.077 | −0.057 | 0.189 |
| Leaf dry matter content | 0.911 | 0.054 | −0.016 | 0.302 | 0.274 |
| Leaf thickness | −0.852 | 0.483 | −0.200 | 0.007 | 0.021 |
| Root diameter | 0.766 | −0.472 | 0.034 | −0.430 | 0.057 |
| Root length | 0.694 | 0.042 | 0.414 | 0.376 | 0.452 |
| Specific root length | 0.250 | 0.965 | 0.039 | 0.025 | −0.061 |
| Root δ15N | 0.151 | −0.891 | −0.388 | −0.176 | −0.048 |
| Leaf K content | −0.288 | 0.867 | −0.311 | 0.086 | −0.248 |
| Leaf N content | −0.463 | 0.860 | −0.108 | 0.159 | 0.095 |
| Leaf P content | −0.430 | 0.838 | 0.221 | −0.181 | −0.174 |
| Root P content | 0.243 | 0.182 | 0.941 | −0.148 | 0.026 |
| Leaf δ13C | 0.364 | −0.191 | 0.896 | 0.207 | −0.118 |
| Root Ca | 0.398 | −0.211 | 0.874 | 0.029 | −0.178 |
| Root K | −0.539 | 0.221 | 0.797 | 0.111 | 0.114 |
| Root K | 0.008 | −0.304 | −0.777 | −0.342 | 0.432 |
| Root C | 0.431 | 0.391 | −0.571 | −0.507 | 0.278 |
| Root δ13C | 0.200 | 0.118 | 0.166 | 0.955 | −0.085 |
| Leaf C | 0.141 | 0.307 | 0.188 | 0.194 | −0.902 |
| Leaf Ca | 0.221 | 0.340 | −0.506 | 0.477 | 0.594 |
| Eigenvalue | 5.909 | 5.086 | 4.910 | 2.149 | 1.946 |
| Cumulative contribution rate/% | 29.546 | 54.974 | 79.525 | 90.272 | 100 |
| No. | Functional Traits | ZP | AP | Interspecific |
|---|---|---|---|---|
| 1 | Leaf thickness | 0.11 | 0.14 | 0.50 |
| 2 | Specific leaf area | 0.15 | 0.05 | 0.56 |
| 3 | Leaf dry matter content | 0.11 | 0.21 | 0.33 |
| 4 | Leaf C content | 0.03 | 0.04 | 0.04 |
| 5 | Leaf N content | 0.03 | 0.02 | 0.04 |
| 6 | Leaf P content | 0.12 | 0.05 | 0.13 |
| 7 | Leaf K content | 0.44 | 0.53 | 0.70 |
| 8 | Leaf δ13C | 0.01 | 0.01 | 0.01 |
| 9 | Leaf δ15N | 0.13 | 0.12 | 1.78 |
| 10 | Specific root length | 0.32 | 0.11 | 0.32 |
| 11 | Root P | 0.19 | 0.21 | 0.25 |
| 12 | Root Ca | 0.08 | 0.11 | 0.82 |
| 13 | Root δ13C | 0.02 | 0.01 | 0.02 |
| 14 | Root δ15N | 0.50 | 0.02 | 0.50 |
| Index | Leaf Thickness | Specific Leaf Area | Leaf N | Leaf P | Leaf δ13C | Specific Root Length | Root P |
|---|---|---|---|---|---|---|---|
| Specific leaf area | 0.857 * | 1 | |||||
| Leaf dry matter content | −0.739 | −0.850 * | |||||
| Leaf N | 0.835 * | −0.332 | 1 | ||||
| Leaf P | 0.722 | 0.459 | 0.851 * | 1 | |||
| Leaf K | 0.721 | 0.335 | 0.903 * | 0.809 | |||
| leaf δ13C | −0.534 | 0.285 | −0.322 | −0.052 | 1 | ||
| Leaf δ15N | 0.961 ** | 0.932 ** | 0.720 | 0.626 | −0.469 | ||
| Specific root length | 0.244 | −0.278 | 0.708 | 0.716 | 0.051 | 1 | |
| Root P | −0.307 | −0.426 | −0.079 | 0.278 | 0.881 * | 0.268 | 1 |
| Root Ca | −0.619 | −0.596 | −0.472 | −0.129 | 0.974 ** | −0.058 | 0.872 * |
| Root δ15N | −0.484 | −0.044 | −0.827 * | −0.857 * | −0.242 | −0.838 * | −0.466 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Song, Y.; Li, Y. Seedling Survival Strategies of Zanthoxylum planispinum ‘Dintanensis’ and Zanthoxylum amatum ‘Novemfolius’, Based on Functional Traits in Karst Desertification Control. Forests 2023, 14, 386. https://doi.org/10.3390/f14020386
Yu Y, Song Y, Li Y. Seedling Survival Strategies of Zanthoxylum planispinum ‘Dintanensis’ and Zanthoxylum amatum ‘Novemfolius’, Based on Functional Traits in Karst Desertification Control. Forests. 2023; 14(2):386. https://doi.org/10.3390/f14020386
Chicago/Turabian StyleYu, Yanghua, Yanping Song, and Yitong Li. 2023. "Seedling Survival Strategies of Zanthoxylum planispinum ‘Dintanensis’ and Zanthoxylum amatum ‘Novemfolius’, Based on Functional Traits in Karst Desertification Control" Forests 14, no. 2: 386. https://doi.org/10.3390/f14020386
APA StyleYu, Y., Song, Y., & Li, Y. (2023). Seedling Survival Strategies of Zanthoxylum planispinum ‘Dintanensis’ and Zanthoxylum amatum ‘Novemfolius’, Based on Functional Traits in Karst Desertification Control. Forests, 14(2), 386. https://doi.org/10.3390/f14020386







