Resistance of Untreated and Torrefied Medium-Density Fiberboard (MDF) Residues to Xylophage Fungi
Abstract
1. Introduction
2. Material and Methods
2.1. Torrefaction Process
2.2. Chemical Characterization
2.3. Evaluation of Fungal Attack
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization, F. FAOSTAT: Forestry Production and Trade. Available online: https://www.fao.org/faostat/en/#data/FO (accessed on 12 November 2022).
- Instituto Brasileiro de Árvores. No Title. Relatório Anu. 2021, p93. Available online: https://iba.org/datafiles/publicacoes/relatorios/relatorioiba2021-compactado.pdf (accessed on 12 November 2022).
- Lubis, M.A.R.; Hong, M.K.; Park, B.D. Hydrolytic Removal of Cured Urea–Formaldehyde Resins in Medium-Density Fiberboard for Recycling. J. Wood Chem. Technol. 2018, 38, 1–14. [Google Scholar] [CrossRef]
- Lubis, M.A.R.; Park, B.D.; Kim, Y.S.; Yun, J.; Shin, H.C. Visual Inspection of Surface Mold Growth on Medium-Density Fiberboard Bonded with Oxidized Starch Adhesives. Wood Mater. Sci. Eng. 2022, 1–8. [Google Scholar] [CrossRef]
- Antov, P.; Mantanis, G.I.; Savov, V. Development of Wood Composites from Recycled Fibres Bonded with Magnesium Lignosulfonate. Forests 2020, 11, 613. [Google Scholar] [CrossRef]
- Bütün Buschalsky, F.Y.; Mai, C. Repeated Thermo-Hydrolytic Disintegration of Medium Density Fibreboards (MDF) for the Production of New MDF. Eur. J. Wood Wood Prod. 2021, 79, 1451–1459. [Google Scholar] [CrossRef]
- Hagel, S.; Joy, J.; Cicala, G.; Saake, B. Recycling of Waste MDF by Steam Refining: Evaluation of Fiber and Paper Strength Properties. Waste Biomass Valorization 2021, 12, 5701–5713. [Google Scholar] [CrossRef]
- Ferreira, S.D.; Altafini, C.R.; Perondi, D.; Godinho, M. Pyrolysis of Medium Density Fiberboard (MDF) Wastes in a Screw Reactor. Energy Convers. Manag. 2015, 92, 223–233. [Google Scholar] [CrossRef]
- Han, T.U.; Kim, Y.M.; Watanabe, C.; Teramae, N.; Park, Y.K.; Kim, S.; Lee, Y. Analytical Pyrolysis Properties of Waste Medium-Density Fiberboard and Particle Board. J. Ind. Eng. Chem. 2015, 32, 345–352. [Google Scholar] [CrossRef]
- Andrade, C.R.; Brito, J.O.; Dias Junor, A.F.; Lana, A.Q. Changes Caused by Torrefaction on Urban Wooden Waste Alterações Provocadas Pela Torrefação Sobre Resíduos Madeireiros de Origem Urbana. Sci. For. 2017, 45, 275–284. [Google Scholar] [CrossRef]
- He, C.; Tang, C.; Li, C.; Yuan, J.; Tran, K.Q.; Bach, Q.V.; Qiu, R.; Yang, Y. Wet Torrefaction of Biomass for High Quality Solid Fuel Production: A Review. Renew. Sustain. Energy Rev. 2018, 91, 259–271. [Google Scholar] [CrossRef]
- da Silva, C.M.S.; Carneiro, A.d.C.O.; Vital, B.R.; Figueiró, C.G.; Fialho, L.d.F.; de Magalhães, M.A.; Carvalho, A.G.; Cândido, W.L. Biomass Torrefaction for Energy Purposes–Definitions and an Overview of Challenges and Opportunities in Brazil. Renew. Sustain. Energy Rev. 2018, 82, 2426–2432. [Google Scholar] [CrossRef]
- Wang, L.; Barta-Rajnai, E.; Skreiberg, Ø.; Khalil, R.; Czégény, Z.; Jakab, E.; Barta, Z.; Grønli, M. Effect of Torrefaction on Physiochemical Characteristics and Grindability of Stem Wood, Stump and Bark. Appl. Energy 2018, 227, 137–148. [Google Scholar] [CrossRef]
- Gucho, E.M.; Shahzad, K.; Bramer, E.A.; Akhtar, N.A.; Brem, G. Experimental Study on Dry Torrefaction of Beech Wood and Miscanthus. Energies 2015, 8, 3903–3923. [Google Scholar] [CrossRef]
- Girods, P.; Dufour, A.; Rogaume, Y.; Rogaume, C.; Zoulalian, A. Thermal Removal of Nitrogen Species from Wood Waste Containing Urea Formaldehyde and Melamine Formaldehyde Resins. J. Hazard. Mater. 2008, 159, 210–221. [Google Scholar] [CrossRef]
- Debal, M.; Girods, P.; Lémonon, J.; Karama, J.P.; Donnot, A.; Rogaume, Y. TG-FTIR Kinetic Study of the Thermal Cleaning of Wood Laminated Flooring Waste. J. Therm. Anal. Calorim. 2014, 118, 141–151. [Google Scholar] [CrossRef]
- van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass Upgrading by Torrefaction for the Production of Biofuels: A Review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Shang, L.; Ahrenfeldt, J.; Holm, J.K.; Bach, L.S.; Stelte, W.; Henriksen, U.B. Kinetic Model for Torrefaction of Wood Chips in a Pilot-Scale Continuous Reactor. J. Anal. Appl. Pyrolysis 2014, 108, 109–116. [Google Scholar] [CrossRef]
- Xu, Z.; Zinchik, S.; Kolapkar, S.S.; Bar-Ziv, E.; Hansen, T.; Conn, D.; McDonald, A.G. Properties of Torrefied U.S. Waste Blends. Front. Energy Res. 2018, 6, 1–13. [Google Scholar] [CrossRef]
- de Castro, V.R.; de Castro Freitas, M.P.; Zanuncio, A.J.V.; Zanuncio, J.C.; Surdi, P.G.; Carneiro, A.d.C.O.; Vital, B.R. Resistance of in Natura and Torrefied Wood Chips to Xylophage Fungi. Sci. Rep. 2019, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stangerlin, D.M.; Costa, A.F.; Garlet, A.; Pastore, T.C.M. Resistência Natural Da Madeira de Três Espécies Amazônicas Submetidas Ao Ataque de Fungos Apodrecedores. Rev. Ciência Da Madeira-RCM 2013, 4, 15–32. [Google Scholar] [CrossRef]
- Hiscox, J.; O’Leary, J.; Boddy, L. Fungus Wars: Basidiomycete Battles in Wood Decay. Stud. Mycol. 2018, 89, 117–124. [Google Scholar] [CrossRef]
- Reinprecht, L.; Kmet’Ová, L. Fungal Resistance and Physical-Mechanical Properties of Beech Plywood Having Durable Veneers or Fungicides in Surfaces. Eur. J. Wood Wood Prod. 2014, 72, 433–443. [Google Scholar] [CrossRef]
- Barré, J.B.; Bourrier, F.; Brancheriau, L.; Bertrand, D.; Rey, F. Effects of Fungal Decay on Elasticity and Damping of Small-Diameter Silver Fir Logs Assessed by the Transverse Vibration Resonant Method. Wood Sci. Technol. 2018, 52, 403–420. [Google Scholar] [CrossRef]
- Stefanowski, B.K.; Curling, S.F.; Ormondroyd, G.A. Evaluating Mould Colonisation and Growth on MDF Panels Modified to Sequester Volatile Organic Compounds. Int. Wood Prod. J. 2016, 7, 188–194. [Google Scholar] [CrossRef]
- Kartal, S.N.; Green, F. Decay and Termite Resistance of Medium Density Fiberboard (MDF) Made from Different Wood Species. Int. Biodeterior. Biodegrad. 2003, 51, 29–35. [Google Scholar] [CrossRef]
- Köse, C.; Terzi, E.; Büyüksarı, Ü.; Avcı, E.; Ayrılmış, N.; Kartal, N.; Imamura, Y. Particleboard and Mdf Panels Made From a Mixture of Wood and Pinecones: Resistance To Decay Fungi. BioResources 2011, 6, 2045–2054. [Google Scholar]
- Kirker, G.; Zelinka, S.; Gleber, S.C.; Vine, D.; Finney, L.; Chen, S.; Hong, Y.P.; Uyarte, O.; Vogt, S.; Jellison, J.; et al. Synchrotron-Based X-Ray Fluorescence Microscopy Enables Multiscale Spatial Visualization of Ions Involved in Fungal Lignocellulose Deconstruction. Sci. Rep. 2017, 7, srep41798. [Google Scholar] [CrossRef]
- De Freitas Homem De Faria, B.; Lanvin, C.; Valette, J.; Rousset, P.; De Cássia Oliveira Carneiro, A.; Caldeira-Pires, A.; Candelier, K. Effect of Leaching and Fungal Attacks During Storage on Chemical Properties of Raw and Torrefied Biomasses. Waste Biomass Valorization 2021, 12, 1447–1463. [Google Scholar] [CrossRef]
- de Castro, P.G.S.; de Castro, V.R.; Zanuncio, A.J.V.; Zanuncio, J.C.; Carneiro, A.d.C.O.; Gominho, J.; Araújo, S.d.O. Energetic Characterization and Radiographic Analysis of Torrefied Coated MDF Residues. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- ASTM-D2017-05; ASTM D2017-Standard Test Method of Accelerated Laboratory Test of Natural Decay Resistance of Woods. ASTM International: West Conshohocken, PA, USA, 2005.
- Ates, S.; Kara, H.R.; Olgun, C.; Ozkan, O.E. Effects of Heat Treatment on Some Properties of MDF (Medium-Density Fiberboard). Wood Mater. Sci. Eng. 2017, 12, 158–164. [Google Scholar] [CrossRef]
- Wang, N.; Zhan, H.; Zhuang, X.; Xu, B.; Yin, X.; Wang, X.; Wu, C. Torrefaction of Waste Wood-Based Panels: More Understanding from the Combination of Upgrading and Denitrogenation Properties. Fuel Process. Technol. 2020, 206, 106462. [Google Scholar] [CrossRef]
- Moreno, A.I.; Font, R.; Conesa, J.A. Physical and Chemical Evaluation of Furniture Waste Briquettes. Waste Manag. 2016, 49, 245–252. [Google Scholar] [CrossRef]
- Sabino, T.P.F.; Surdi, P.G.; Vilela, A.P.; Metzker, S.L.O.; Coelho, N.P.F.; de Oliveira, T.J.P.; Mendes, R.F. Effect of the Post-Heat Treatment on the Properties of Medium Density Particleboard of Eucalyptus sp. Floresta e Ambient 2021, 28, 1–7. [Google Scholar] [CrossRef]
- Pereira, B.L.C.; Carneiro, A.d.C.O.; Carvalho, A.M.M.L.; Colodette, J.L.; Oliveira, A.C.; Fontes, M.P.F. Influence of Chemical Composition of Eucalyptus Wood on Gravimetric Yield and Charcoal Properties. BioResources 2013, 8, 4574–4592. [Google Scholar] [CrossRef]
- Barskov, S.; Zappi, M.; Buchireddy, P.; Dufreche, S.; Guillory, J.; Gang, D.; Hernandez, R.; Bajpai, R.; Baudier, J.; Cooper, R.; et al. Torrefaction of Biomass: A Review of Production Methods for Biocoal from Cultured and Waste Lignocellulosic Feedstocks. Renew. Energy 2019, 142, 624–642. [Google Scholar] [CrossRef]
- Fialho, L.d.F.; Carneiro, A.d.C.O.; Carvalho, A.M.M.L.; Figueiró, C.G.; da Silva, C.M.S.; Magalhães, M.A.; Peres, L.C. Bio-Coal Production with Agroforestry Biomasses in Brazil. Maderas Cienc. y Tecnol. 2019, 21, 357–366. [Google Scholar] [CrossRef]
- Esteves, B.M.; Pereira, H.M. Wood Modification by Heat Treatment: A Review. BioResource 2009, 4, 370–404. [Google Scholar] [CrossRef]
- de Castro, V.R.; Pereira, M.P.d.C.F.; Surdi, P.G.; Berger, M.d.S.; Zanuncio, J.C.; Carneiro, A.d.C. Resistance of the Eucalyptus Wood in Natura and Torrefied Exposed to the Attack of Cryptotermes Brevis. J. Agric. Sci. Technol. B 2018, 8, 512–516. [Google Scholar] [CrossRef]
- Brocco, V.F.; Paes, J.B.; da Costa, L.G.; Brazolin, S.; Arantes, M.D.C. Potential of Teak Heartwood Extracts as a Natural Wood Preservative. J. Clean. Prod. 2017, 142, 2093–2099. [Google Scholar] [CrossRef]
- Candelier, K.; Dumarçay, S.; Pétrissans, A.; Desharnais, L.; Gérardin, P.; Pétrissans, M. Comparison of Chemical Composition and Decay Durability of Heat Treated Wood Cured under Different Inert Atmospheres: Nitrogen or Vacuum. Polym. Degrad. Stab. 2013, 98, 677–681. [Google Scholar] [CrossRef]
- Candelier, K.; Thevenon, M.F.; Petrissans, A.; Dumarcay, S.; Gerardin, P.; Petrissans, M. Control of Wood Thermal Treatment and Its Effects on Decay Resistance: A Review. Ann. For. Sci. 2016, 73, 571–583. [Google Scholar] [CrossRef]
- Li, T.; Cheng, D.L.; Avramidis, S.; Wålinder, M.E.P.; Zhou, D. guo Response of Hygroscopicity to Heat Treatment and Its Relation to Durability of Thermally Modified Wood. Constr. Build. Mater. 2017, 144, 671–676. [Google Scholar] [CrossRef]
- Thybring, E.E. Water Relations in Untreated and Modified Wood under Brown-Rot and White-Rot Decay. Int. Biodeterior. Biodegrad. 2017, 118, 134–142. [Google Scholar] [CrossRef]
- de Freitas, F.P.; Carvalho, A.M.M.L.; Carneiro, A.d.C.O.; Vital, B.R.; Gonçalves, L.M.S.; de Jesus, M.S.; Xisto, M.F. Influence of Time and Heat Treatment Temperature on Permeability of Eucalyptus Grandis Wood. Rev. Arvore 2019, 43, e430301. [Google Scholar] [CrossRef]
- Thybring, E.E.; Kymäläinen, M.; Rautkari, L. Moisture in Modified Wood and Its Relevance for Fungal Decay. IForest 2018, 11, 418–422. [Google Scholar] [CrossRef]
- Yuan, J.; Zheng, X.; Cheng, F.; Zhu, X.; Hou, L.; Li, J.; Zhang, S. Fungal Community Structure of Fallen Pine and Oak Wood at Different Stages of Decomposition in the Qinling Mountains, China. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Park, B.D. Microscopic Observation of Wood-Based Composites Exposed to Fungal Deterioration. J. Wood Sci. 1999, 45, 64–68. [Google Scholar] [CrossRef]
- Stirling, R.; Sturrock, R.N.; Braybrooks, A. Fungal Decay of Western Redcedar Wood Products—A review. Int. Biodeterior. Biodegrad. 2017, 125, 105–115. [Google Scholar] [CrossRef]
- Alcalde, M. Engineering the Ligninolytic Enzyme Consortium. Trends Biotechnol. 2015, 33, 155–162. [Google Scholar] [CrossRef]
- Kong, W.; Chen, H.; Lyu, S.; Ma, F.; Yu, H.; Zhang, X. Characterization of a Novel Manganese Peroxidase from White-Rot Fungus Echinodontium Taxodii 2538, and Its Use for the Degradation of Lignin-Related Compounds. Process Biochem. 2016, 51, 1776–1783. [Google Scholar] [CrossRef]
- Liu, B.; Olson, Å.; Wu, M.; Broberg, A.; Sandgren, M. Biochemical Studies of Two Lytic Polysaccharide Monooxygenasesfrom the White-Rot Fungus Heterobasidion Irregulare and Their Roles in Lignocellulose Degradation. PLoS ONE 2017, 12, e0189479. [Google Scholar] [CrossRef] [PubMed]
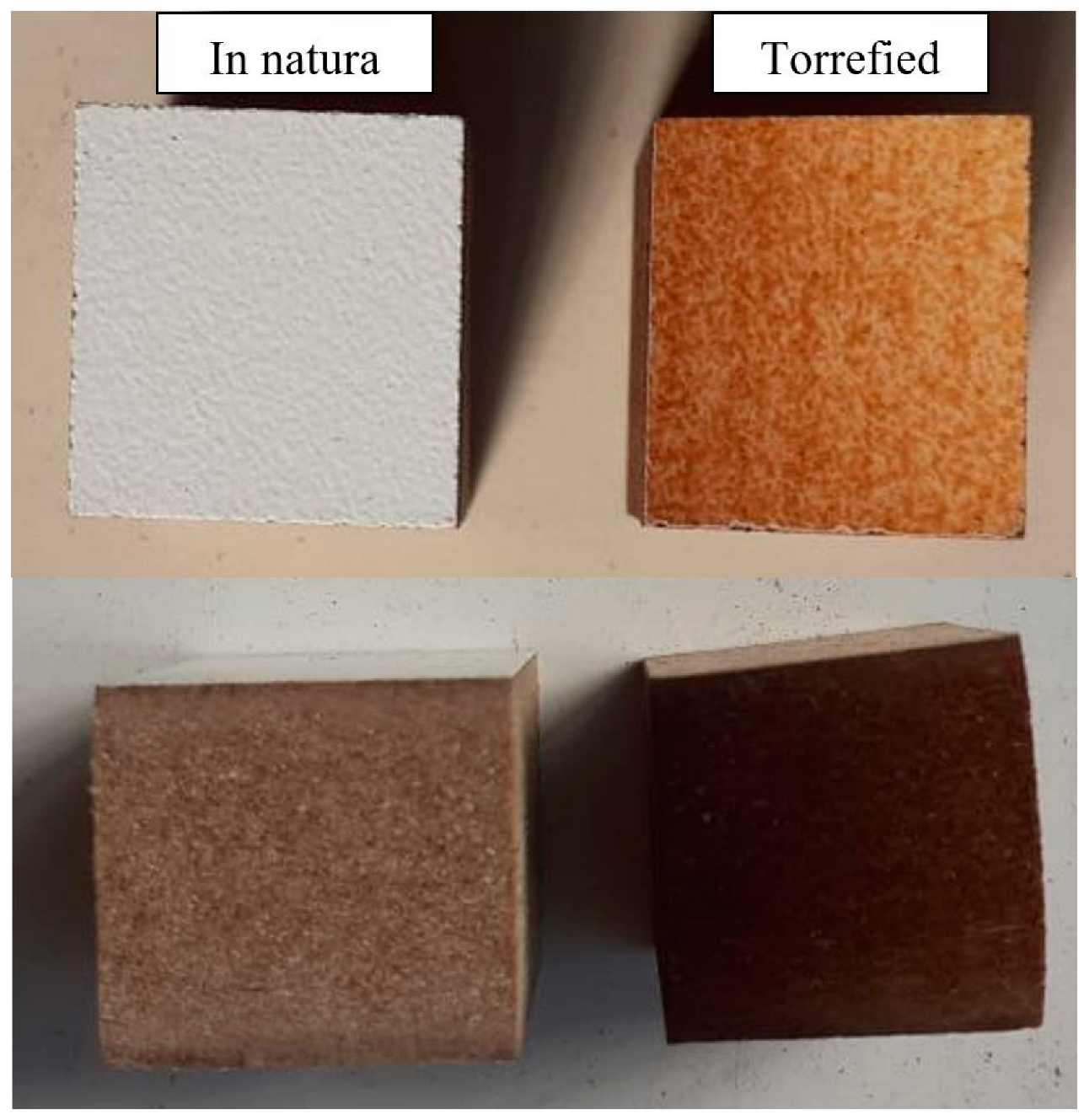
| Properties | Torrefaction Time | |||
|---|---|---|---|---|
| Untreated | 20 min | 30 min | 40 min | |
| EMC (%) | 9.66 | 7.69 | 8.79 | 8.49 |
| Holocelluloses (%) | 55.28 | 56.85 | 52.22 | 51.35 |
| Total lignin (%) | 32.06 | 32.83 | 40.23 | 42.36 |
| Extractives (%) | 12.66 | 10.32 | 7.54 | 6.27 |
| Ash (%) | 0.27 | 0.26 | 0.29 | 0.27 |
| GY (%) | 100 | 99.43 | 95.78 | 90.47 |
| ODD (g.cm−3) | 0.68 | 0.66 | 0.64 | 0.61 |
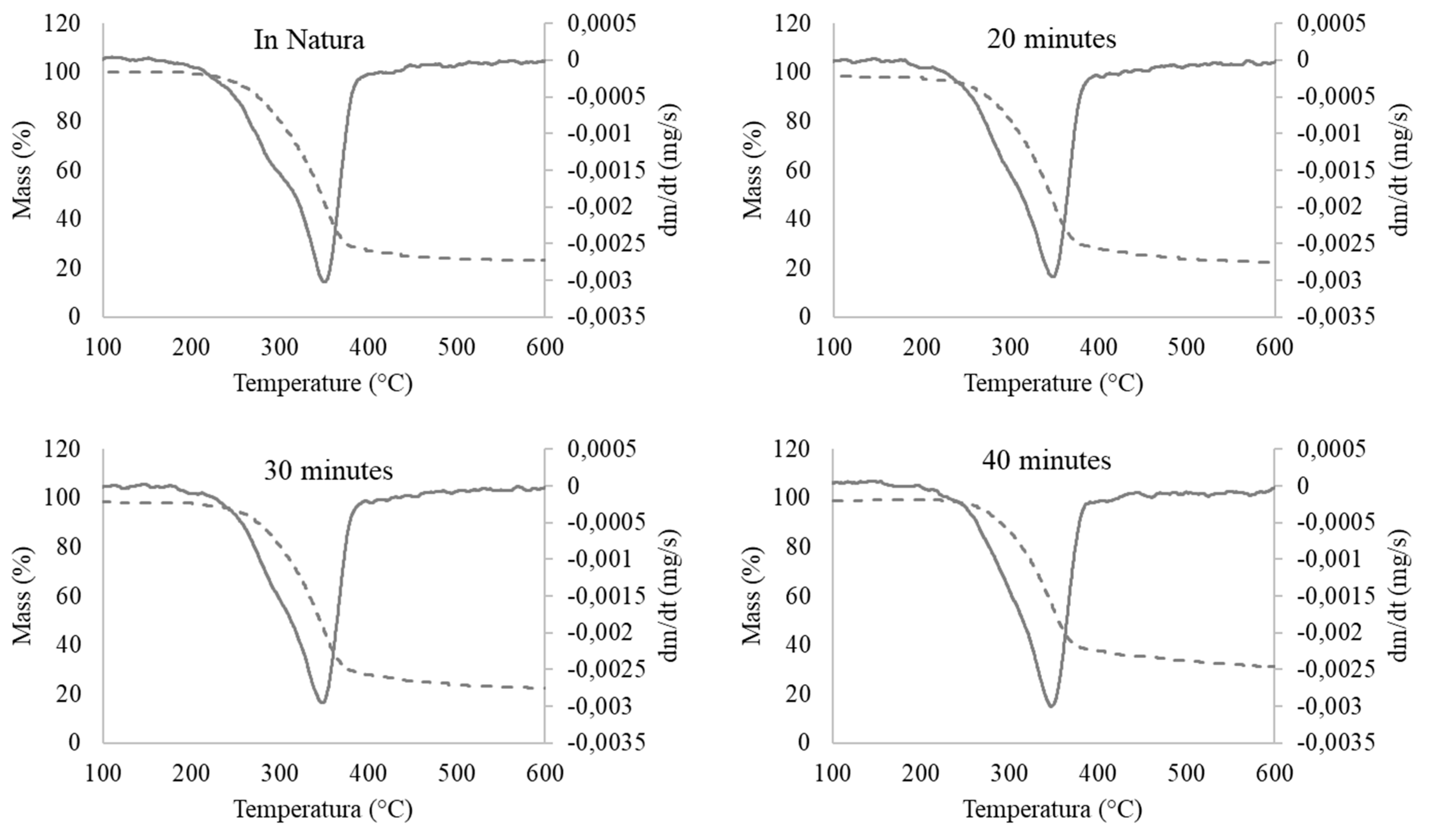
| Temperature (°C) | ||||||
| 100 to 150 | 150 to 250 | 250 to 350 | 350 to 400 | 400 to 450 | 450 to 500 | Residual Mass (%) |
| Mass loss (%) | ||||||
| untreated | ||||||
| 0 | 3.688 | 43.805 | 17.983 | 1.844 | 0.922 | 31.757 |
| 20 min | ||||||
| 2.073 | 3.109 | 49.222 | 17.617 | 2.591 | 1.554 | 23.834 |
| 30 min | ||||||
| 2.073 | 3.109 | 48.705 | 18.135 | 2.591 | 1.554 | 23.834 |
| 40 min | ||||||
| 0.485 | 1.942 | 42.718 | 17.476 | 1.942 | 1.942 | 33.495 |
| Fungus | Torrefaction Time | |||
|---|---|---|---|---|
| Untreated | 20 min | 30 min | 40 min | |
| Irpex lacteus | 47.54 ± 5.09aA | 47.54 ± 10.72aA | 6.10 ± 1.55bA | 4.32 ± 0.94bA |
| Trametes versicolor | 14.14 ± 4.26aB | 6.04 ± 1.01bB | 4.19 ± 0.95bA | 2.62 ± 0.80bA |
| Postia placenta | 6.21 ± 2.22aC | 5.01 ± 0.93aB | 4.75 ± 1.25aA | 3.13 ± 0.65aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surdi, P.G.; Castro, V.R.d.; Lima, N.N.; Portilho, G.R.; Lopes, N.F.; Andrade, F.A.; Zanuncio, A.J.V.; Zanuncio, J.C.; Carneiro, A.d.C.O.; Araújo, S.d.O. Resistance of Untreated and Torrefied Medium-Density Fiberboard (MDF) Residues to Xylophage Fungi. Forests 2023, 14, 307. https://doi.org/10.3390/f14020307
Surdi PG, Castro VRd, Lima NN, Portilho GR, Lopes NF, Andrade FA, Zanuncio AJV, Zanuncio JC, Carneiro AdCO, Araújo SdO. Resistance of Untreated and Torrefied Medium-Density Fiberboard (MDF) Residues to Xylophage Fungi. Forests. 2023; 14(2):307. https://doi.org/10.3390/f14020307
Chicago/Turabian StyleSurdi, Paula Gabriella, Vinicius Resende de Castro, Nidia Niela Lima, Gabriel Reis Portilho, Nayara Franzini Lopes, Frances Alves Andrade, Antônio José Vinha Zanuncio, José Cola Zanuncio, Angélica de Cássia Oliveira Carneiro, and Solange de Oliveira Araújo. 2023. "Resistance of Untreated and Torrefied Medium-Density Fiberboard (MDF) Residues to Xylophage Fungi" Forests 14, no. 2: 307. https://doi.org/10.3390/f14020307
APA StyleSurdi, P. G., Castro, V. R. d., Lima, N. N., Portilho, G. R., Lopes, N. F., Andrade, F. A., Zanuncio, A. J. V., Zanuncio, J. C., Carneiro, A. d. C. O., & Araújo, S. d. O. (2023). Resistance of Untreated and Torrefied Medium-Density Fiberboard (MDF) Residues to Xylophage Fungi. Forests, 14(2), 307. https://doi.org/10.3390/f14020307











