The Fine Root Distribution and Morphology of Mature White Poplar in Natural Temperate Riverside Forests under Periodically Flooded or Dry Hydrological Conditions
Abstract
1. Introduction
2. Material and Methods
2.1. Study Sites
2.2. Sampling Procedure
2.3. Fine Root Measurements and Physicochemical Properties of Soil
2.4. Statistical Analysis
3. Results
3.1. Soil Water Content
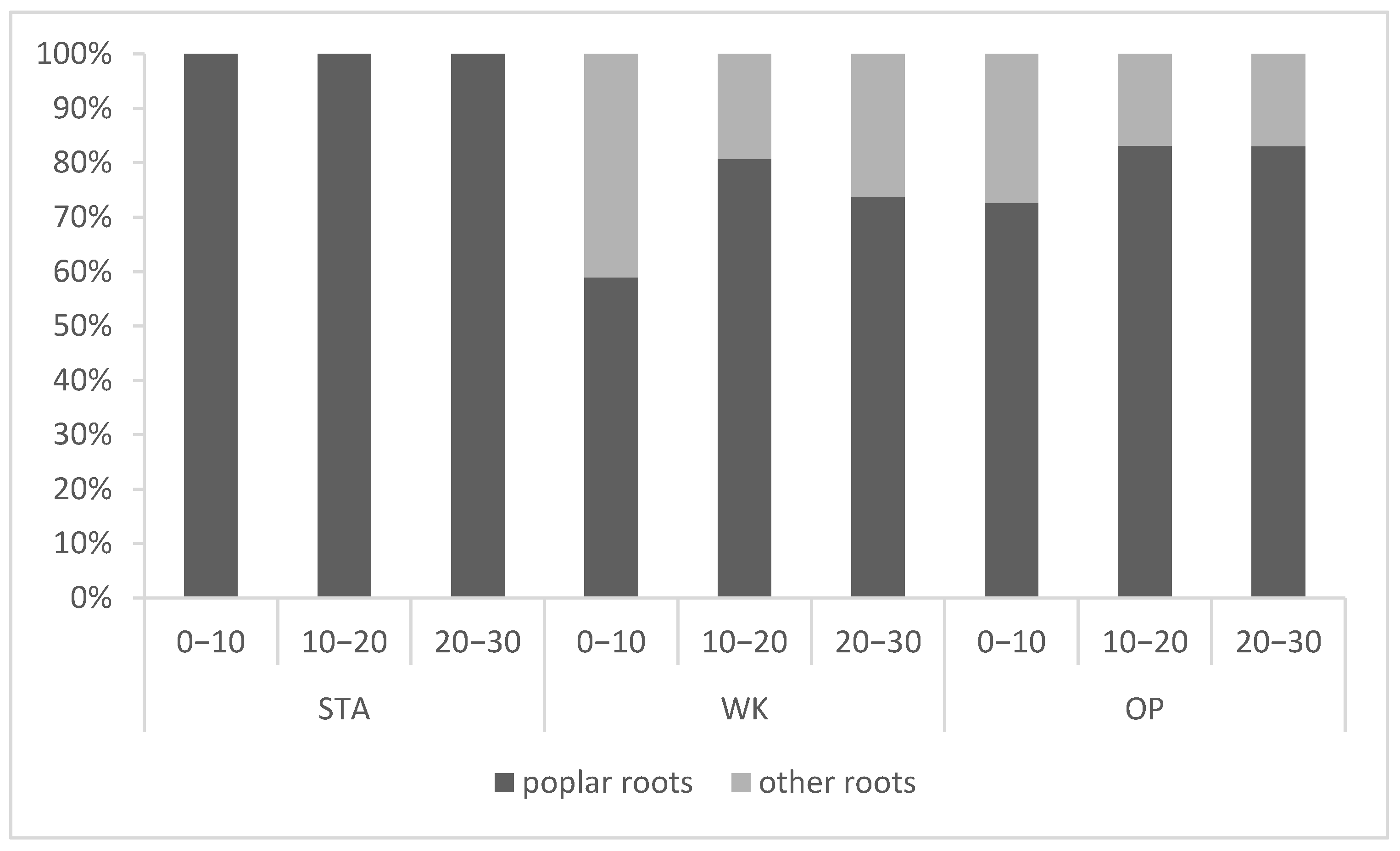
3.2. Share of Poplar Roots in the Pool of All Fine Roots
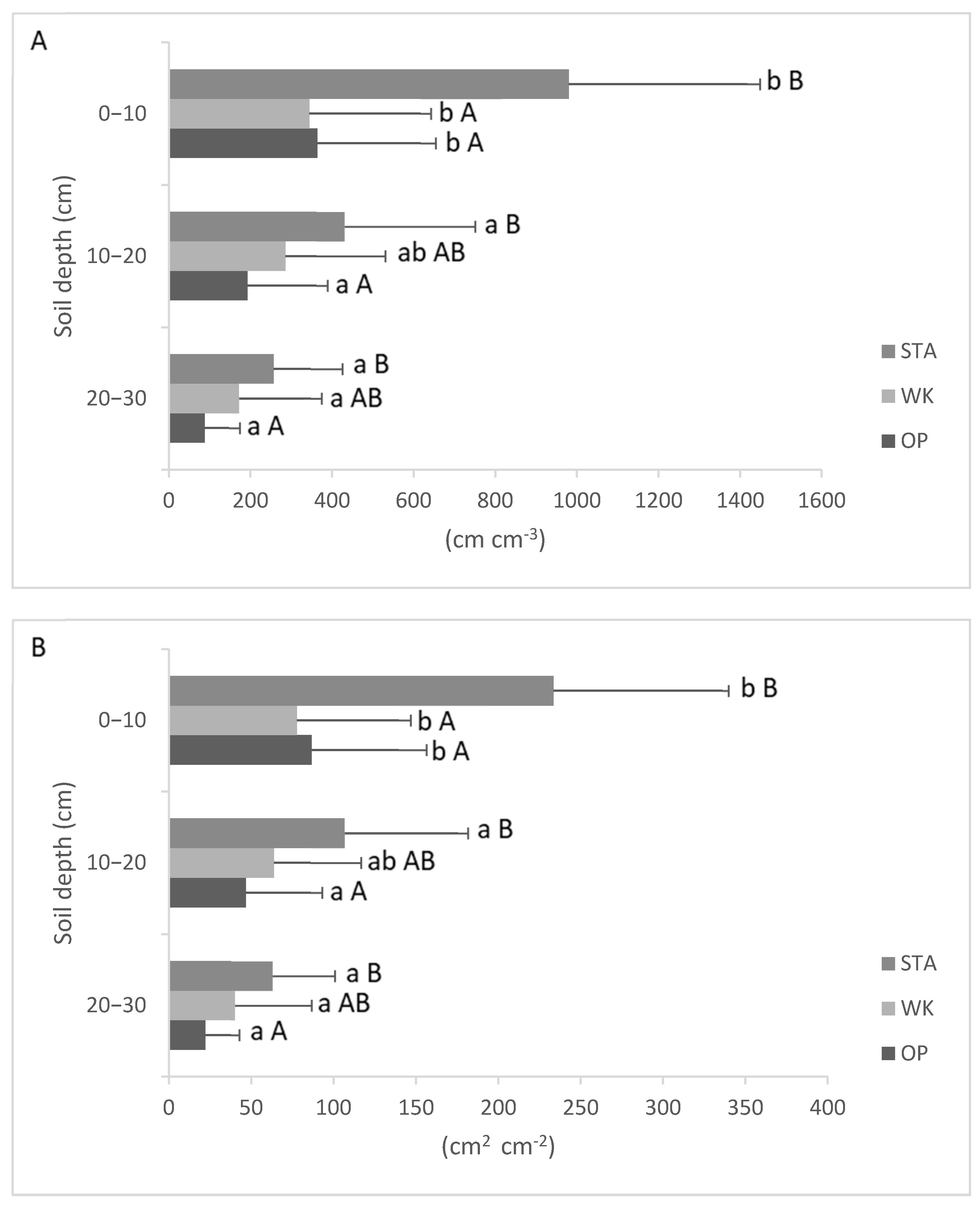
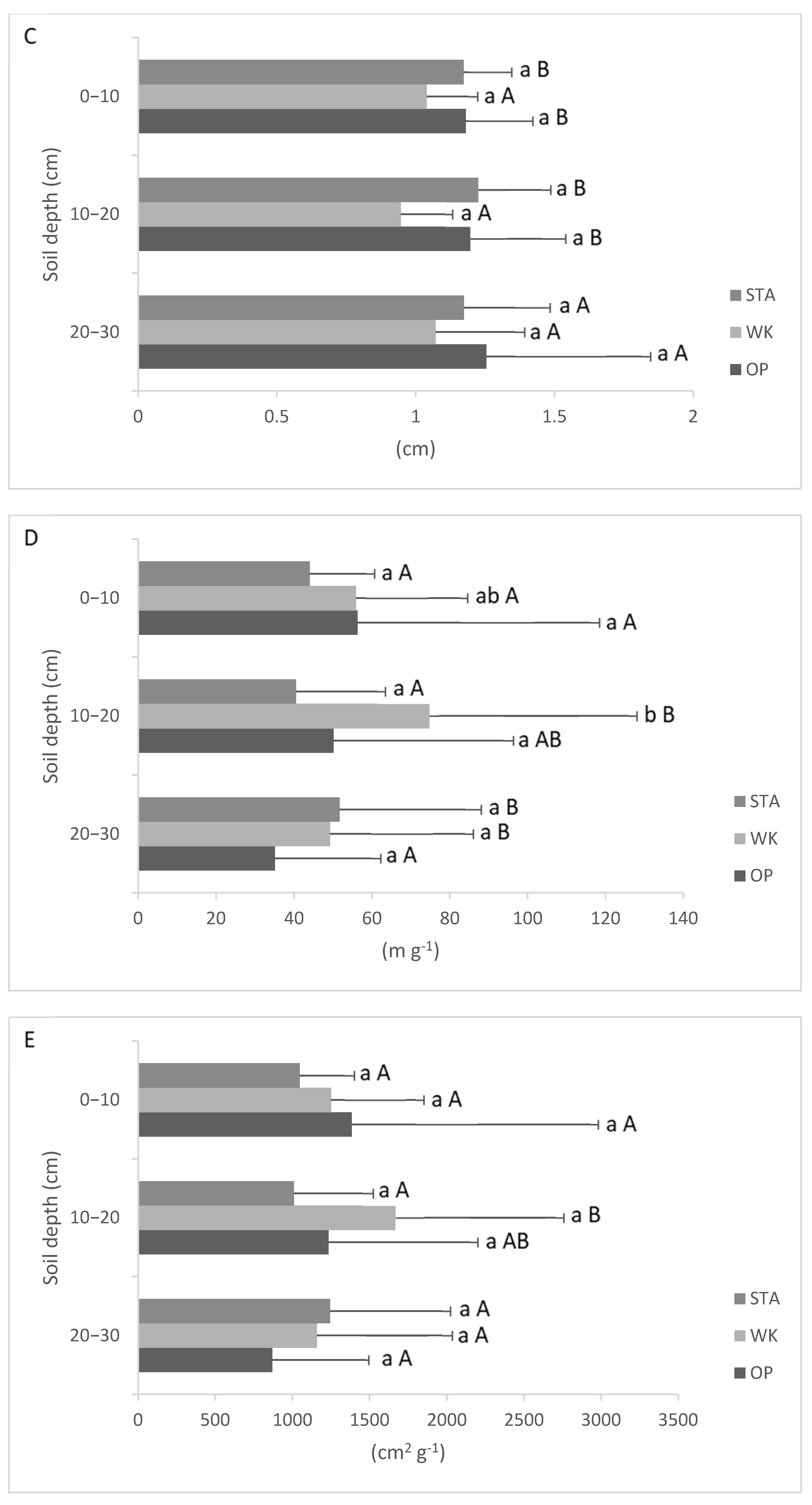
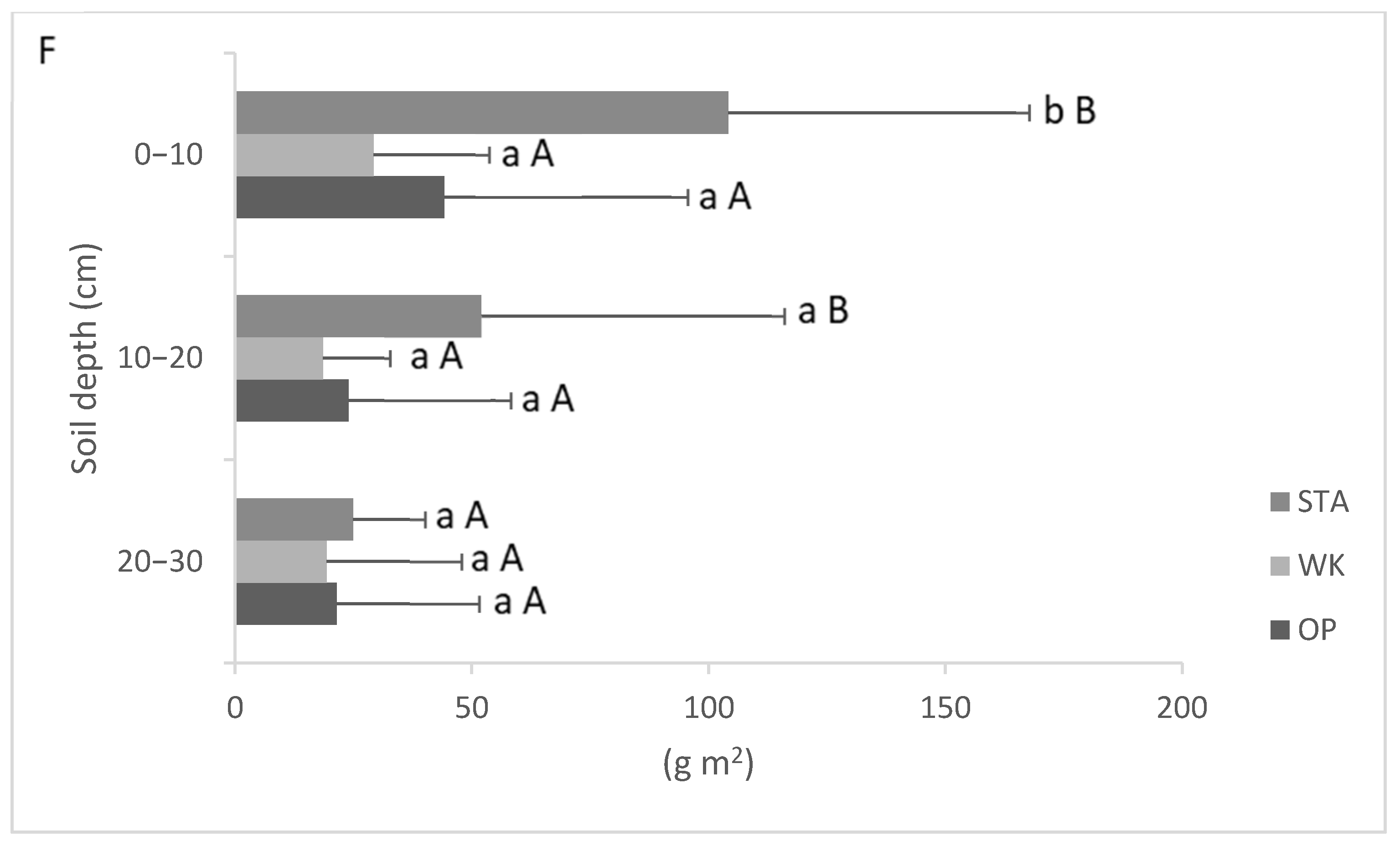
3.3. Fine Roots Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogt, K.A.; Vogt, D.J.; Palmiotto, P.A.; Boon, P.; O’Hara, J.; Asbjornsen, H. Review of root dynamics in forest ecosystems grouped by climate, climatic forest type and species. Plant Soil 1996, 187, 159–219. [Google Scholar] [CrossRef]
- Persson, H. Fine-root production, mortality and decomposition in forest eosystems. Plant Ecol. 1980, 41, 101–109. [Google Scholar] [CrossRef]
- Vogt, K.A.; Edmonds, R.L.; Grier, C.C. Seasonal-changes in biomass and vertical-distribution of mycorrhizal and fibrous-textured conifer fine roots in 23-year-old and 180-year-old subalpine Abies-amabilis stands. Can. J. For. Res. 1981, 11, 223–229. [Google Scholar] [CrossRef]
- Gill, R.A.; Jackson, R. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2000, 147, 13–31. [Google Scholar] [CrossRef]
- Helmisaari, H.-S.; Ostonen, I.; Lõhmus, K.; Dermore, J.; Lindroos, A.-J.; Merilä, P.; Nöjd, P. Ectomycorrhizal root tips in relation to site and stand characteristic in Norway spruce and Scots pine stands in boreal forest. Tree Physiol. 2009, 29, 445–456. [Google Scholar] [CrossRef]
- Makita, N.; Hirano, Y.; Dannoura, M.; Kominami, Y.; Mizoguchi, T.; Ishii, H.; Kanazawa, Y. Fine root morphological traits determine variation in root respiration of Quercus serrata. Tree Physiol. 2009, 29, 579–585. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Vogt, K.; Persson, H. Measuring Growth and Development of Roots. In Techniques, Approaches in Forest Tree Ecophysiology; Lassoie, J.P., Hinckley, T.M., Eds.; CRS Press: Boca Raton, FL, USA, 1991; pp. 477–501. [Google Scholar]
- Grayston, S.J.; Vaughan, D.; Jones, D. Rhizosphere carbon flow in trees, in comparison with annual plants: The importance of root exudation and its impact on microbial activity and nutrient availability. Appl. Soil Ecol. 1996, 5, 29–56. [Google Scholar] [CrossRef]
- Jackson, R.B.; Mooney, H.A.; Schulze, E.D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl. Acad. Sci. USA 1997, 94, 7363–7366. [Google Scholar] [CrossRef]
- Gaudinski, J.B.; Torn, M.S.; Riley, W.J.; Dawso, T.E.; Joslin, J.D.; Majdi, H. Measuring and modeling the spectrum of fine-root turnover times in three forests using isotopes, minirhizotrons, and the Radix model. Glob. Biogeochem. Cycles 2010, 24, GB3029. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; DeForest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hndrick, R.L. Fine root architecture of nine North American trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Guo, D.; Li, H.; Mitchell, R.J.; Han, W.; Hendricks, J.J.; Fahey, T.J.; Hendrick, R.L. Fine root heterogeneity by branch order: Exploring the discrepancy in root turnover esimates between minrhizotron and carbon isotopic methods. New Phytol. 2008, 177, 443–456. [Google Scholar] [CrossRef]
- Jia, S.; McLaughlin, N.B.; Gu, J.; Li, X.; Wang, Z. Relationships between root respiration rate and root morphology, chemistry and anatomy in Larix gmelinii and Fraxinus mandshurica. Tee Physiol. 2013, 33, 579–589. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.-S.; Obbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground conributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Withington, J.M.; Reich, P.B.; Oleksyn, J.; Eissenstat, D.M. Comparisons of structure and life span in roots and leaves among temperate trees. Ecol. Monogr. 2006, 76, 381–397. [Google Scholar] [CrossRef]
- Finér, L.; Helmisaari, H.-S.; Lõhmus, K.; Majdi, H.; Brunner, I.; Børja, I.; Eldhuset, T.; Godbold, D.; Grebenc, T.; Konôpka, B.; et al. Variation in fine root biomass of three European tree species: Beech (Fagus sylvatica L.), Norway spruce (Picea abies L. Karst.), and Scots pine (Pinus sylvestris L.). Plant Biosyst. 2007, 141, 394–405. [Google Scholar] [CrossRef]
- Du, H.; Liu, L.; Su, L.; Zeng, F.; Wang, K.; Peng, W.; Zhang, H.; Song, T. Seasonal changes and vertical distribution of fine root biomass during vegetation restoration in a Karst Area, Southwest China. Front. Plant Sci. 2019, 9, 2001. [Google Scholar] [CrossRef]
- Ostonen, I.; Lõhmus, K.; Lasn, R. The role of soil conditions in fine root ecomorphology in Norway spruce (Picea abies (L.) Karst.). Plant Soil 1999, 208, 283–292. [Google Scholar] [CrossRef]
- Jagodzinski, A.M.; Ziółkowski, J.; Warnkowska, A.; Prais, H. Tree Ae Effects on Fine Root Biomass and Morphology over Chronosequences of Fagus sylvatica, Quercus robur and Alnus glutinosa Stands. PLoS ONE 2016, 11, e0148668. [Google Scholar] [CrossRef]
- Vogt, K.A.; Publicover, D.A.; Bloomfield, J.; Perez, J.M.; Vogt, D.J.; Silver, W.L. Belowground responses as indicators of environ-mental change. Environ. Exp. Bot. 1993, 133, 189–205. [Google Scholar] [CrossRef]
- Cudlin, P.; Kieliszewska-Rokicka, B.; Rudawska, M.; Grebenc, T.; Alberton, O.; Lehto, T.; Bakker, M.R.; Børja, I.; Konôpka, B.; Leski, T.; et al. Fine roots and ectomycorrhizas as indicators of environmental change. Plant Biosyst. 2007, 141, 406–425. [Google Scholar] [CrossRef]
- Ostonen, I.; Püttsepp, Ü.; Biel, C.; Alberton, O.; Bakker, M.R.; Lõhmus, K.; Majdi, H.; Metcalfe, D.; Olsthoorn, A.F.M.; Pronk, A.; et al. Specific root length as an indicator of environmental change. Plant Biosyst. 2007, 141, 426–442. [Google Scholar] [CrossRef]
- Liu, Z.; Dickmann, D.I. Responses of two hybrid Populus clones to flooding, drought, and nitrogen availability. II. Gas exchange and water relations. Can. J. Bot. 1993, 71, 927–938. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, J.; Wang, W.; Zhang, T.; Li, J. Effects of root phenotypic changes on the deep rooting of Populus euphratica seedlings under drought stresses. PeerJ 2019, 7, e6513. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.E.; Mozo, I.; Cortizo, S.; Cappa, E.P.; Luquez, V.M.C. Early rooting and flooding tolerance in cottings from a Populus deltoides full-sib family under greenhouse conditions. Can. J. Bot. 2021, 51, 732–741. [Google Scholar]
- Dickmann, D.I.; Nguyen, P.V.; Pregitzer, K.S. Effects of irrigation and coppicing o above-ground growth, physiology, and fine-root dynamics of two field-grown hybrid poplar clones. For. Ecol. Manag. 1996, 80, 163–174. [Google Scholar] [CrossRef]
- Lukac, M.; Calfapietra, C.; Godbold, D.L. Root Production and Turnover in Populus Grown under Elevated CO2 using a Free Air Enrichment System POPFACE. In Plant Nutrition; Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 2001; Volume 92, pp. 688–689. [Google Scholar]
- Imada, S.; Yamanaka, N.; Tamai, S. Water table depth affects Populus alba fine root growth and whole plant biomass. Funct. Ecol. 2008, 22, 1018–1026. [Google Scholar] [CrossRef]
- Bo, H.; Wen, C.; Song, L.; Yue, Y.; Nie, L. Fine-root responses of Populus tomentosa forests to stand density. Forests 2018, 9, 562. [Google Scholar] [CrossRef]
- Di, N.; Liu, Y.; Mead, D.J.; Xie, Y.; Jia, L.; Xi, B. Root-system characteristic of plantation-grown Populus tomentosa adapted to seasonal fluctuation in the groundwater table. Trees 2018, 32, 137–149. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Zak, D.R.; Maziasz, J.; DeForest, J.; Curtis, P.S.; Lussenhop, J. Interactive effects of atmospheric CO2 and soil-N availability on fine roots of Populus tremuloides. Ecol. Appl. 2000, 10, 18–33. [Google Scholar]
- Singh, P.; Singh, B. Biomass and nitrogen dynamics of fine roots of poplar under differential N and P levels in an agroforestry system in Punjab. Trop. Ecol. 2016, 57, 143–152. [Google Scholar]
- Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Abiotic and biotic factors controlling fine root biomass, carbon and nutrients in closed-canopy hybrid poplar stands on post-agricultural land. Sci. Rep. 2019, 9, 6296. [Google Scholar] [CrossRef]
- Tufekcioglu, A.; Raich, J.W.; Isenhart, T.M.; Schultz, R.C. Fine root dynamics, coarse root biomass, root distribution, and soil respiration in a multi species riparian buffer in Central Iowa, USA. Agrofor. Syst. 1999, 44, 162–174. [Google Scholar]
- Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Root biomass and soil carbon distribution in hybrid poplar riparian buffers, herbaceous riparian buffers and natural riparian woodlots on farmland. Springerplus 2013, 2, 539. [Google Scholar] [CrossRef]
- Jones, A.; Fortier, J.; Gagnon, D.; Truax, B. Trading tree growth for soil degradation: Effects at 10 years of black plastic mulch on fine roots, earthworms, organic matter and nitrate in a multi-species riparian buffer. Trees For. People 2020, 2, 100032. [Google Scholar] [CrossRef]
- Holloway, J.V.; Rillig, M.C.; Gurnell, A.M. Physical environmental controls on riparian root profiles associated with black poplar (Populus nigra L.) along the Tagliamento River, Italy. Earth Surf. Process. Landf. 2016, 42, 1262–1273. [Google Scholar] [CrossRef]
- Si, J.; Feng, Q.; Li, J.; Zhao, J. Spatial distribution characteristics of fine roots of Populus euphratica in a desert riparian forest. Front. For. China 2008, 3, 429–433. [Google Scholar] [CrossRef]
- Rood, S.; Braatne, J.H.; Hughes, F.M.R. Ecophysiology of Riparian Cottonwoods: Stream Flow Dependency, Water Relations and Restoration. Tree Physiol. 2003, 23, 1113–1124. [Google Scholar] [CrossRef]
- Naiman, R.J.; Décamps, H.; Pollock, M. The role of riparian corridors in maintaining regional biodiversity. Ecol. Appl. 1993, 3, 209–212. [Google Scholar] [CrossRef]
- Tabacchi, E.; Lambs, L.; Guilloy, H.; Planty-Tabacchi, A.-M.; Muller, E.; Décamps, H. Impacts of riparian vegetation on hydrological processes. Hydrol. Process. 2000, 14, 2959–2976. [Google Scholar] [CrossRef]
- Deljouei, A.; Abdi, E.; Schwarz, M.; Majnounian, B.; Sohrabi, H.; Dumroese, R.K. Mechanical Characteristic of the Fine Roots of Two Broadleaved Tree Species form the Temperate Caspian Hyrcanian Ecoregion. Forests 2020, 11, 345. [Google Scholar] [CrossRef]
- Wójcik, G.; Marciniak, K. Climate. In Nature of the Kujawsko-Pomorskie Voivodeship; Przystalski, A., Ed.; Kujawsko-Pomorskie Voivodeship Office, Voivodeship Nature Conservator: Bydgoszcz, Poland, 2001; pp. 23–32. [Google Scholar]
- Gorączko, M. The Vistula River floods in the Bydgoszcz Region—Genesis, course and influence for hazard in the city. Probl. Landsc. Ecol. 2008, T. XXII, 7–17. [Google Scholar]
- Hausenbuiller, R. Soil Science Principles and Practice, 4th ed.; Wm. C. Brown Co.: Dubuque, IA, USA, 1975; p. 90. [Google Scholar]
- Frymark-Szymkowiak, A.; Karliński, L. Impacts of hydrological conditions on the activities of soil enzymes in temperate floodplain forest sites. Soil Res. 2022, 60, 637–647. [Google Scholar] [CrossRef]
- Persson, H. Adaptive Tactics and Characteristics of Tree Fine Roots. In The Supporting Roots of Trees and Woody Plans: Form, Function and Physiology; Stokes, A., Ed.; Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 2000; Volume 87. [Google Scholar]
- Bakker, M.R.; Augusto, L.; Achat, D.L. Fine root distribution of trees and understory in mature stands of maritime pine (Pinus pinaster) on dry and humid sites. Plant Soil 2006, 286, 37–51. [Google Scholar] [CrossRef]
- Børja, I.; De Wit, H.A.; Steffenrem, A.; Majdi, H. Stand age and fine root biomass, distribution and morphology in a Norway spruce chronosequence in southeast Norway. Tree Physiol. 2008, 28, 773–784. [Google Scholar] [CrossRef]
- Moresi, F.V.; Maesano, M.; Matteucci, G.; Romagnoli, M.; Sidle, R.C.; Mugnozza, G.S. Root Biomechanical Traits in a Montane Mediterranean Forest Watershed: Variations with Species Diversity and Soil Depth. Forests 2019, 10, 341. [Google Scholar] [CrossRef]
- Claus, A.; George, E. Effect of stand age on fine-root biomass and biomass distribution in three European forest chronosequences. Can. J. For. Res. 2005, 35, 1617–1625. [Google Scholar] [CrossRef]
- Steele, S.J.; Gower, S.T.; Vogel, J.G.; Norman, J.M. Root mass, net primary production and turnover in aspen, jack pine and black spruce forest in Saskatchewan and Manitoba, Canada. Tree Physiol. 1997, 17, 577–587. [Google Scholar] [CrossRef]
- Al Afas, N.; Marron, N.; Zavalloni, C.; Ceulemans, R. Growth and production of a short-rotation coppice culture of poplar-IV: Fine root characteristics of five poplar clones. Biomass Bioenergy 2008, 32, 494–502. [Google Scholar] [CrossRef]
- Karliński, L.; Rudawska, M.; Kieliszewska-Rokicka, B.; Leski, T. Relationship between genotype and soil environment during colonization of poplar roots by mycorrhizal and endophytic fungi. Mycorrhiza 2010, 20, 315–324. [Google Scholar] [CrossRef]
- Comas, L.; Bouma, T.; Eissenstat, D. Linking root traits to potential growth rate in six temperate tree species. Oecologia 2002, 132, 34–43. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Friend, A.L. The Structure and Function of Populus Root System. In Biology of Populus and Its Implications for Management and Conservation; Stettler, R.F., Ed.; NRC Research Press: Ottawa, ON, Canada, 1996; pp. 331–354. [Google Scholar]
- Berhongaray, G.; Janssens, I.A.; King, J.S.; Ceulemans, R. Fine root biomass and turnover of two fast-growing poplar genotypes in a short-rotation coppice culture. Plant Soil 2013, 373, 269–283. [Google Scholar] [CrossRef]
- Ostnen, I.; Lõhmus, K.; Pajuste, K. Fine root biomass, production and its proportion of NPP in a fertile middle-aged Norway spruce forest: Comarison of soil core and ingrowth core methods. For. Ecol. Manag. 2005, 212, 264–277. [Google Scholar] [CrossRef]
- Block, R.M.A.; Van Rees, K.C.J.; Knight, J.D. A Review of Fine Root Dynamics in Populus Plantations. Agrofor. Syst. 2006, 67, 73–84. [Google Scholar] [CrossRef]
- Montagnoli, A.; Terzaghi, M.; Di Iorio, A.; Scippa, G.S.; Chiatante, D. Fine-root morphological and growth traits in a Turkey-oak stand in relation to seasonal changes in soil moisture in the Southern Apennines, Italy. Ecol. Res. 2012, 27, 1015–1025. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; Liu, R.; Du, Z.; Zhou, L.; Li, S.; Liu, H.; Shao, J.; Wang, J.; Nie, Y.; et al. Soil fungi and fine root biomass mediate drought-induced reductions in soil respiration. Funct. Ecol. 2020, 34, 2634–2643. [Google Scholar] [CrossRef]
- Leuschner, C.; Hertel, D. Fine root biomass of temperate forest in relation to soil acidity and fertility, climate, age and species. Prog. Bot. 2002, 64, 405–438. [Google Scholar]
- Joslin, J.D.; Wolfe, M.H.; Hanson, P.J. Effects of altered water regimes on forest root systems. New Phytol. 2000, 147, 117–129. [Google Scholar] [CrossRef]
- Lauenroth, W.K.; Gill, R. Turnover of Root Systems. In Root Ecology; De Kroon, H., Visser, E.J.W., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2003; pp. 61–89. [Google Scholar]
- Pan, X.; Qin, Y.; Yuan, Z. Potential of a halophyte-associated endophytic fungus for sustaining Chinese white poplar growth under salinity. Symbiosis 2018, 76, 109–116. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Galiano, L.; Gessler, A. Plasticity of Fine-Root Traits Under Long-Term Irrigation of Water-Limited Scots Pine Forest. Front. Plant Sci. 2019, 10, 701. [Google Scholar] [CrossRef]
- Bogeat-Triboulot, M.-B.; Brosché, M.; Renaut, J.; Jouve, L.; Le Thiec, D.; Fayyaz, P.; Vinocur, B.; Witters, E.; Laukens, K.; Teichmann, T.; et al. Gradual Soil Water Depletion Results in Reversible Changes of Gene Expression, Protein Profiles, Ecophysiology, and Growth Performance in Populus euphratica, a Poplar Growing in Arid Regions. Plant Physiol. 2007, 143, 876–892. [Google Scholar] [CrossRef] [PubMed]
- Bűttner, V.; Leuschner, C. Spatial and temporal patterns of fine root abundance in a mixed oak-beech forest. For. Ecol. Manag. 1994, 70, 11–21. [Google Scholar] [CrossRef]
- Schmid, I.; Kazda, M. Vertical distribution and radial growth of coarse roots in pure and mixed stands of Fagus sylvatica and Picea abies. Can. J. For. Res. 2001, 31, 539–548. [Google Scholar] [CrossRef]
- Kalliokoski, T.; Pennanen, T.; Nygren, P.; Sievänen, R.; Helmisaari, H.-S. Belowground interspecific competition in mixed boreal forests: Fine root and ectomycorrhiza characteristics along stand development stage and soil fertility gradients. Plant Soil 2010, 330, 73–89. [Google Scholar] [CrossRef]
- Hishi, T.; Tashiro, N.; Maeda, Y.; Urakawa, R.; Shibata, H. Spatial patterns of fine root biomass and performances of understory dwarf bamboo and trees along with the gradient of soil N availability in broad-leaved natural forests and larch plantation. Plant Root 2015, 9, 85–94. [Google Scholar] [CrossRef]
- Beyer, F.; Hertel, D.; Leuschner, C. Fine root morphological and functional traits in Fagus sylvatica and Fraxinus excelsior saplings as dependent on species, root order and competition. Plant Soil 2013, 373, 143–156. [Google Scholar] [CrossRef]
- Kiley, D.K.; Schneider, R.L. Riparian roots through time, space and disturbance. Plant Soil 2005, 269, 259–272. [Google Scholar] [CrossRef]
| Depth (cm) | (%) | Texture | ||
|---|---|---|---|---|
| Sand | Silt | Clay | ||
| 0–10 | ||||
| STA | 33.33 (±2.89) | 53.67 (±2.08) | 13.00 (±1.00) | Silt loam |
| WK | 50.33 (±2.08) | 41.00 (±1.73) | 8.67 (±0.58) | Loam |
| OP | 42.02 (±9.51) | 53.93 (±8.41) | 4.05 (±1.13) | Silt loam |
| 10–20 | ||||
| STA | 29.00 (±12.12) | 55.00 (±7.00) | 16.00 (±5.29) | Silt loam |
| WK | 51.00 (±3.00) | 40.33 (±1.53) | 8.67 (0.58) | Loam |
| OP | 37.95 (±5.63) | 57.64 (±5.03) | 4.41 (±0.61) | Silt loam |
| 20–30 | ||||
| STA | 17.00 (±2.65) | 61.67 (±0.58) | 21.33 (±2.52) | Silt loam |
| WK | 54.33 (±5.86) | 37.33 (±4.73) | 8.33 (±1.15) | Sandy loam |
| OP | 38.76 (±6.66) | 56.00 (±5.29) | 5.24 (±1.37) | Silt loam |
| Depth (cm) | STA | WK | OP |
|---|---|---|---|
| 0–10 | 27.3 ± 6.3 bB | 30.6 ± 8.9 bB | 20.3 ± 6.4 aA |
| 10–20 | 24.6 ± 5.3 abA | 24.5 ± 7.2 aA | 20.5 ± 8.3 aA |
| 20–30 | 23.8 ± 4.8 aA | 20.1 ± 8.0 aA | 20.0 ± 8.6 aA |
| Mean values (0–30 cm) | 25.2 ± 5.5 B | 25.3 ± 9.1 B | 20.3 ± 7.7 A |
| Fine Root Length | Fine Root Surface Area | Fine Root Diameter | Specific Root Length | Specific Root Area | Fine Root Biomass | |
|---|---|---|---|---|---|---|
| Moisture at specific soil depth: | ||||||
| 0–10 cm | 0.023 | 0.125 | −0.298 ** | 0.072 | 0.027 | −0.028 |
| 10–20 cm | 0.302 ** | 0.317 ** | −0.059 | 0.161 | 0.132 | 0.207 * |
| 20–30 cm | 0.133 | 0.197 | −0.162 | 0.176 | 0.204 * | −0.162 |
| Moisture (mean values) | 0.201 *** | 0.251 ** | −0.164 ** | 0.141 * | 0.117 * | 0.115 * |
| Site | Depth | Season | |
|---|---|---|---|
| Fine root length | 44.38 (p < 0.001) | 46.57 (p < 0.001) | 0.87 (NS) |
| Fine root surface area | 51.04 (p < 0.001) | 52.54 (p < 0.001) | 3.56 (NS) |
| Fine root diameter | 7.86 (p < 0.001) | 0.47 (NS) | 1.37 (NS) |
| Specific root length | 2.57 (NS) | 1.48 (NS) | 4.93 (p < 0.05) |
| Specific root area | 1.10 (NS) | 1.13 (NS) | 4.46 (p < 0.05) |
| Fine root biomass | 19.68 (p < 0.001) | 18.75 (p < 0.001) | 0.41 (NS) |
| Study Site | Soil Layer | Root Diameter Class | |||||
|---|---|---|---|---|---|---|---|
| 0.0–0.5 mm | 0.5–1.0 mm | 1.0–2.0 mm | |||||
| cm cm−2 | % | cm cm−2 | % | cm cm−2 | % | ||
| STA | 0–10 cm | 467.56 ± 241.52 b | 60 | 196.85 ± 93.40 b | 58 | 316.12 ± 145.65 c | 58 |
| 10–20 cm | 194.84 ± 167.52 a | 25 | 90.34 ± 65.67 a | 26 | 144.93 ± 99.13 b | 27 | |
| 20–30 cm | 118.08 ± 90.63 a | 15 | 54.64 ± 36.25 a | 16 | 84.05 ± 47.24 a | 15 | |
| Mean values | 260.16 ± 230.72 | 46 | 113.94 ± 90.98 | 21 | 181.70 ± 142.71 | 33 | |
| WK | 0–10 cm | 181.24 ± 145.02 b | 43 | 69.46 ± 67.74 | 44 | 94.02 ± 90.85 | 43 |
| 10–20 cm | 156.41 ± 141.94 ab | 37 | 52.74 ± 43.29 | 34 | 76.62 ± 64.65 | 35 | |
| 20–30 cm | 87.14 ± 104.28 a | 20 | 34.30 ± 38.83 | 22 | 50.24 ± 62.73 | 22 | |
| Mean values | 141.60 ± 138.81 | 53 | 52.17 ± 54.19 | 19 | 73.63 ± 76.75 | 28 | |
| OP | 0–10 cm | 174.33 ± 139.82 b | 51 | 65.03 ± 59.81 b | 50 | 90.96 ± 95.75 b | 52 |
| 10–20 cm | 98.81 ± 97.66 a | 29 | 41.23 ± 42.21 a | 32 | 55.37 ± 61.69 a | 31 | |
| 20–30 cm | 66.78 ± 45.33 a | 20 | 22.94 ± 16.46 a | 18 | 28.92 ± 29.61 a | 17 | |
| Mean values | 113.31 ± 115.52 | 53 | 43.07 ± 48.82 | 20 | 58.42 ± 76.83 | 27 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frymark-Szymkowiak, A.; Kieliszewska-Rokicka, B. The Fine Root Distribution and Morphology of Mature White Poplar in Natural Temperate Riverside Forests under Periodically Flooded or Dry Hydrological Conditions. Forests 2023, 14, 223. https://doi.org/10.3390/f14020223
Frymark-Szymkowiak A, Kieliszewska-Rokicka B. The Fine Root Distribution and Morphology of Mature White Poplar in Natural Temperate Riverside Forests under Periodically Flooded or Dry Hydrological Conditions. Forests. 2023; 14(2):223. https://doi.org/10.3390/f14020223
Chicago/Turabian StyleFrymark-Szymkowiak, Anna, and Barbara Kieliszewska-Rokicka. 2023. "The Fine Root Distribution and Morphology of Mature White Poplar in Natural Temperate Riverside Forests under Periodically Flooded or Dry Hydrological Conditions" Forests 14, no. 2: 223. https://doi.org/10.3390/f14020223
APA StyleFrymark-Szymkowiak, A., & Kieliszewska-Rokicka, B. (2023). The Fine Root Distribution and Morphology of Mature White Poplar in Natural Temperate Riverside Forests under Periodically Flooded or Dry Hydrological Conditions. Forests, 14(2), 223. https://doi.org/10.3390/f14020223






