Bioaccumulation of Potentially Toxic Elements in Tilia tomentosa Moench Trees from Urban Parks and Potential Health Risks from Using Leaves and Flowers for Medicinal Purposes
Abstract
:1. Introduction
2. Materials and Methods
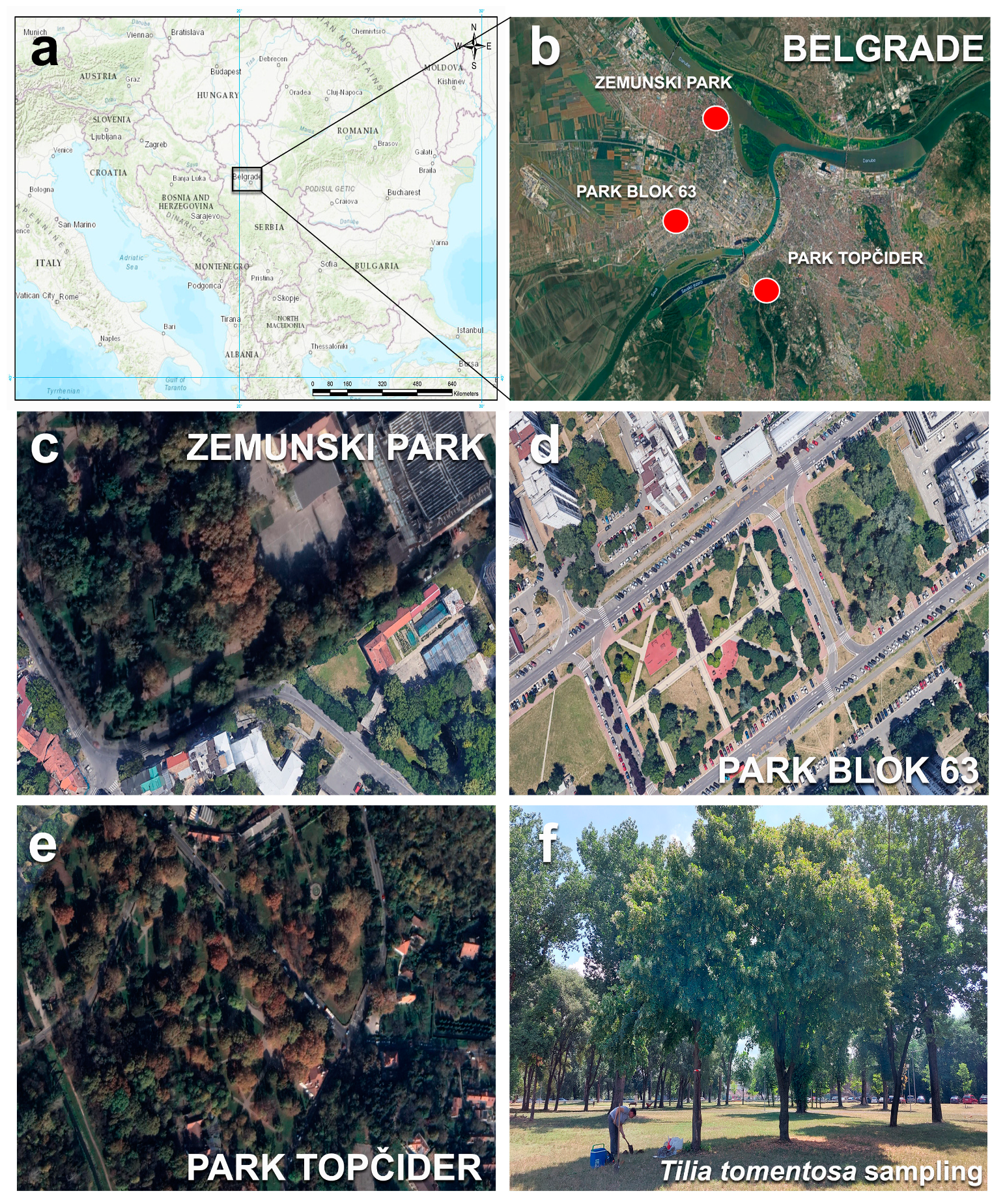
2.1. Study Areas and Sampling Procedure
2.2. Soil and Plant Analysis
2.3. Bioconcentration and Translocation Factors
2.4. Health Risk Indices
2.5. Data Analysis
3. Results and Discussion
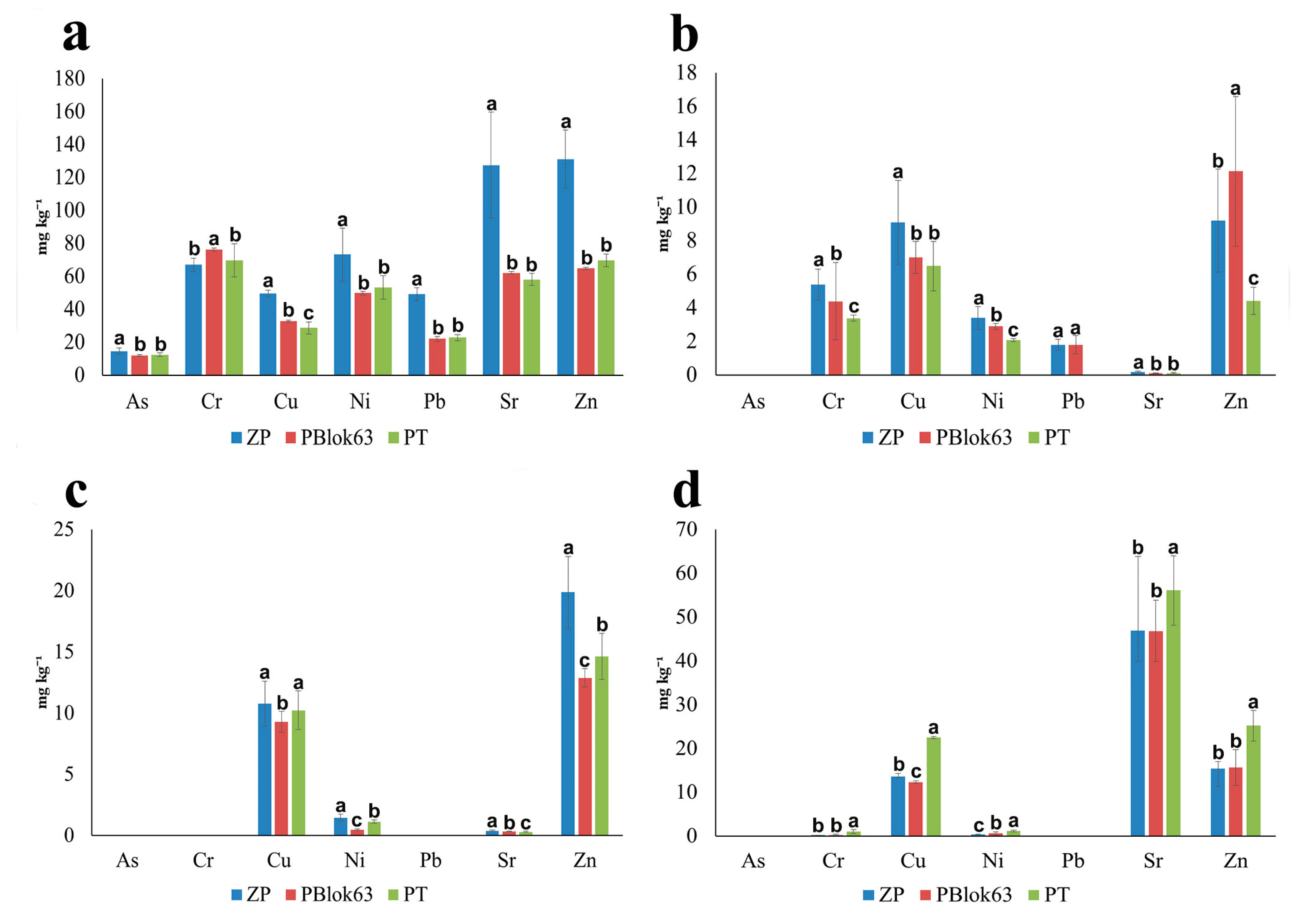
3.1. Potentially Toxic Element Concentrations, Reference Values, and Regulatory Reference Values for Soil
| As | Cr | Cu | Ni | Pb | Sr | Zn | Reference | |
|---|---|---|---|---|---|---|---|---|
| Results of this study | 11.11–16.49 | 60.75–72.43 | 46.81–53.21 | 52.37–92.76 | 45.17–55.70 | 91.27–170.33 | 104.74–147.03 | |
| Average for worldwide soils | 4.4–8.4 | 47–51 | 13–23 | 13–26 | 22–28 | 87–210 | 45–60 | [51] |
| Average for Serbian soils | 11 | 50 | 30 | 38 | 40 | / | 48 | [52] |
| Maximum permissible concentrations (MPC) | 25 | 100 | 100 | 50 | 100 | / | 300 | [54] |
| Critical range for plants | 20–50 | 75–100 | 60–125 | >100 | 100–400 | / | 70–400 | [53] |
| Belgrade | / | 83.5–118 | 30.4–98.4 | 62.4–73.6 | 54–237 | / | 122.7–215.6 | [26] |
| Belgrade | / | 22.6–34 | 20–23.3 | 27.9–62.1 | 9.2–27.6 | 9–31.1 | 41.2–51.5 | [28] |
| Reading (UK) | / | 5.1–8.7 | 6.7–38 | 2.2–15.4 | 18.4–141 | 4.2–47.2 | 24.5–137 | |
| Aviero (PT) | / | 6–16 | 7–61 | 6–28 | 7–41 | / | 18–134 | [55] |
| Glasgow (UK) | / | 17–131 | 24–678 | 16–53 | 41–894 | / | 64–377 | |
| Ljubljana (SI) | / | 11–33 | 20–101 | 15–43 | 39–255 | / | 81–301 | |
| Sevilla (ES) | / | 19–51 | 30–86 | 21–37 | 40–265 | / | 69–171 | |
| Torino (IT) | / | 105–310 | 44–308 | 154–335 | 53–257 | / | 108–317 | |
| Uppsala (SE) | / | 12–56 | 8–180 | 7–36 | 7–211 | / | 27–247 |
3.2. Accumulation of PTEs in T. tomentosa Roots, Leaves, and Flowers
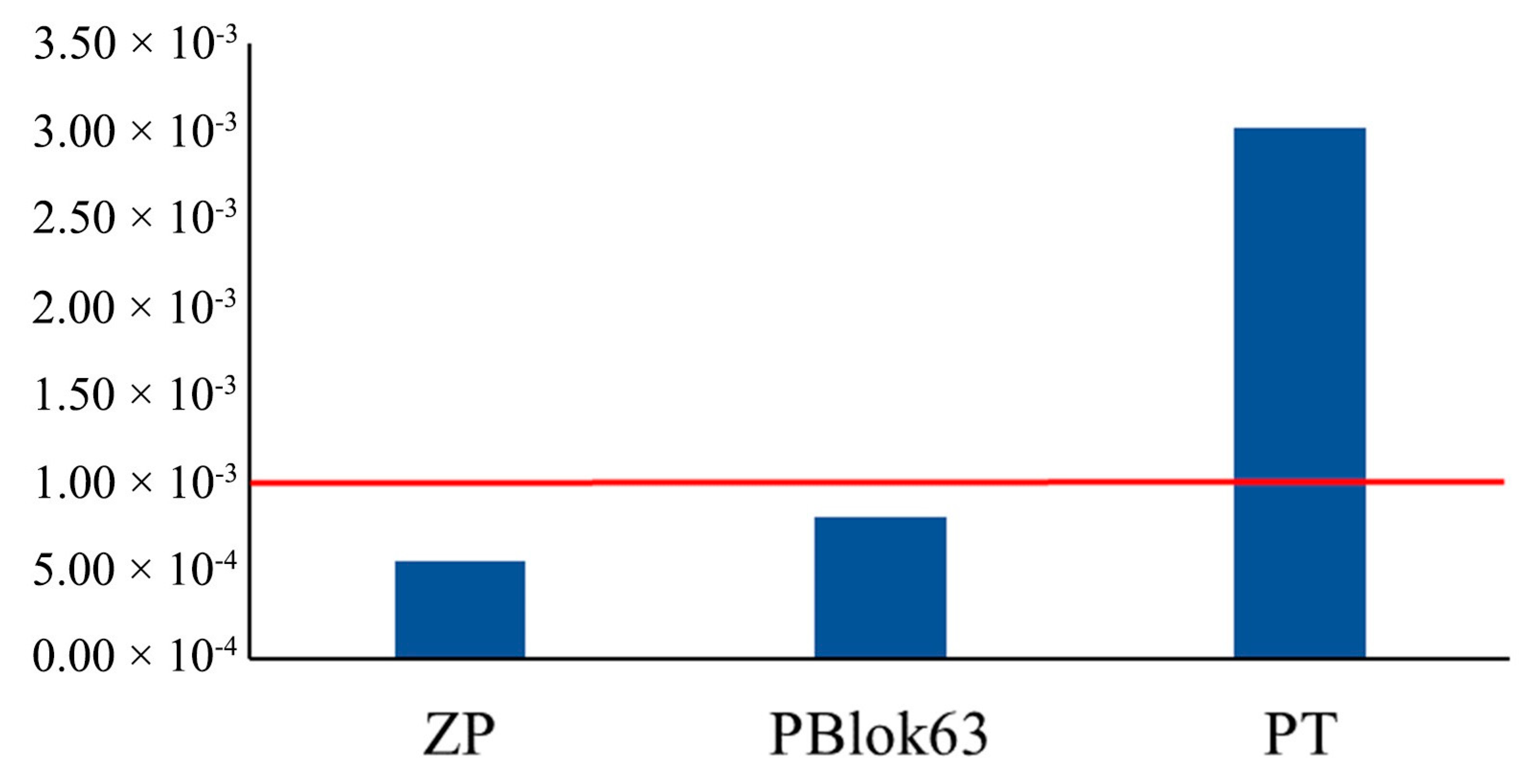
3.3. Potential Health Risks Arising from Using T. tomentosa Leaves and Flowers for Medicinal Purposes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Castells-Quintana, D.; Dienesch, E.; Krause, M. Air pollution in an urban world: A global view on density, cities and emissions. Ecol. Econ. 2021, 189, 107153. [Google Scholar] [CrossRef]
- Pavlović, D.; Pavlović, M.; Perović, V.; Mataruga, Z.; Čakmak, D.; Mitrović, M.; Pavlović, P. Chemical fractionation, environmental and human health risk assessment of potentially toxic elements in soil of industrialised urban areas in Serbia. Int. J. Environ. Res. Public Health 2021, 18, 9412. [Google Scholar] [CrossRef] [PubMed]
- Shaddick, G.; Thomas, M.L.; Mudu, P.; Ruggeri, G.; Gumy, S. Half the world’s population are exposed to increasing air pollution. npj Clim. Atmos. Sci. 2020, 3, 23. [Google Scholar] [CrossRef]
- Health Effects Institute. State of Global Air 2019; Special Report; Health Effects Institute: Boston, MA, USA, 2019. [Google Scholar]
- Liang, L.; Gong, P. Urban and air pollution: A multi-city study of long-term effects of urban landscape patterns on air quality trends. Sci. Rep. 2020, 10, 18618. [Google Scholar] [CrossRef]
- Li, G.; Sun, G.X.; Ren, Y.; Luo, X.S.; Zhu, Y.G. Urban soil and human health: A review. Eur. J. Soil Sci. 2018, 69, 196–215. [Google Scholar] [CrossRef]
- United Nations Environmental Programme (UNEP). Global Assessment of Soil Pollution—Summary for Policy Makers; FAO: Rome, Italy, 2021. [Google Scholar]
- Liu, Y.R.; van der Heijden, M.G.A.; Riedo, J.; Sanz-Lazaro, C.; Eldridge, D.J.; Bastida, F.; Moreno-Jiménez, E.; Zhou, X.-Q.; Hu, H.-Q.; He, J.-Z.; et al. Soil contamination in nearby natural areas mirrors that in urban greenspaces worldwide. Nat. Commun. 2023, 14, 1706. [Google Scholar] [CrossRef]
- Cameron, R.W.F.; Blanusa, T. Green infrastructure and ecosystem services—Is the devil in the detail? Ann. Bot. 2016, 118, 377–391. [Google Scholar] [CrossRef]
- Grote, R.; Samson, R.; Alonso, R.; Humberto Amorim, J.; Cariñanos, P.; Churkina, G.; Fares, S.; Le Thiec, D.; Niinemets, Ü.; Norgaard Mikkelsen, T.; et al. Functional traits of urban trees: Air pollution mitigation potential. Front. Ecol. Environ. 2016, 14, 543–550. [Google Scholar] [CrossRef]
- Roeland, S.; Moretti, M.; Amorim, J.H.; Branquinho, C.; Fares, S.; Morelli, F.; Niinemets, U.; Paoletti, E.; Pinho, P.; Sgrigna, G.; et al. Towards an integrative approach to evaluate the environmental ecosystem services provided by urban forest. J. For. Res. 2019, 30, 1981–1996. [Google Scholar] [CrossRef]
- Lee, A.C.K.; Maheswaran, R. The health benefits of urban green spaces: A review of the evidence. J. Public Health 2010, 33, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Cekstere, G.; Osvalde, A. A study of chemical characteristics of soil in relation to street trees in Riga, (Latvia). Urban For. Urban Green. 2013, 12, 69–78. [Google Scholar] [CrossRef]
- Manes, F.; Marando, F.; Capotorti, G.; Blasi, C.; Salvatori, E.; Fusaro, L.; Ciancarella, L.; Mircea, M.; Marchetti, M.; Chirici, G.; et al. Regulating Ecosystem Services of forests in ten Italian Metropolitan Cities: Air quality improvement by PM10 and O3 removal. Ecol. Indic. 2016, 67, 425–440. [Google Scholar] [CrossRef]
- Xing, Y.; Brimblecombe, P. Role of vegetation in deposition and dispersion of air pollution in urban parks. Atmos. Environ. 2019, 201, 73–83. [Google Scholar] [CrossRef]
- Pavlović, M.; Rakić, T.; Pavlović, D.; Kostić, O.; Jarić, S.; Mataruga, Z.; Pavlović, P.; Mitrović, M. Seasonal variations of trace element contents in leaves and bark of horse chestnut (Aesculus hippocastanum L.) in urban and industrial regions in Serbia. Arch. Biol. Sci. 2017, 69, 201–214. [Google Scholar] [CrossRef]
- Pavlović, D.; Pavlović, M.; Marković, M.; Karadžić, B.; Kostić, O.; Jarić, S.; Mitrović, M.; Gržetić, I.; Pavlović, P. Possibilities of assessing trace metal pollution using Betula pendula Roth. leaf and bark-experience in Serbia. J. Serb. Chem. Soc. 2017, 82, 723–737. [Google Scholar] [CrossRef]
- Tepanosyan, G.; Baldacchini, C.; Sahakyan, L. Revealing Soil and Tree Leaves Deposited Particulate Matter PTE Relationship and Potential Sources in Urban Environment. Int. J. Environ. Res. Public Health 2021, 18, 10412. [Google Scholar] [CrossRef]
- Bühler, O.; Kristoffersen, P.; Larsen, S.U. Growth of street trees in Copenhagen with emphasis on the effect of different establishment concepts. Arboric. Urban For. 2007, 33, 330–337. [Google Scholar] [CrossRef]
- Sjöman, H.; Nielsen, A.B. Selecting trees for urban paved cities in Scandinavia—A revive of information on stress tolerance and its relation to the requirements of tree planners. Urban For. Urban Green. 2010, 9, 281–293. [Google Scholar] [CrossRef]
- Sjöman, H.; Östberg, J.; Bühler, O. Diversity and distribution of the urban tree population in ten major Nordic cities. Urban For. Urban Green. 2012, 11, 31–39. [Google Scholar] [CrossRef]
- Massetti, L.; Petralli, M.; Orlandini, S. The effect of urban morphology on Tilia×europaea flowering. Urban For. Urban Green. 2015, 14, 187–193. [Google Scholar] [CrossRef]
- Weryszko-Chmielewska, E.; Piotrowska-Weryszko, K.; Dąbrowska, A. Response of Tilia sp. L. to climate warming in urban conditions—Phenological and aerobiological studies. Urban For. Urban Green. 2019, 43, 126369. [Google Scholar] [CrossRef]
- Helama, S.; Läänelaid, A.; Raisio, J.; Sohar, K.; Mäkelä, A. Growth patterns of roadside Tilia spp. affected by climate and street maintenance in Helsinki. Urban For. Urban Green. 2020, 53, 126707. [Google Scholar] [CrossRef]
- Tomašević, M.; Rajšić, S.; Đorđević, D.; Tasić, M.; Krstić, J.; Novaković, V. Heavy metals accumulation in tree leaves from urban areas. Environ. Chem. Lett. 2004, 2, 151–154. [Google Scholar] [CrossRef]
- Aničić, M.; Spasić, T.; Tomašević, M.; Rajšić, S.; Tasić, M. Trace elements accumulation and temporal trends in leaves of urban deciduous trees (Aesculus hippocastanum and Tilia spp.). Ecol. Indic. 2011, 11, 824–830. [Google Scholar] [CrossRef]
- Mitrović, M.; Blanusa, T.; Pavlović, M.; Pavlović, D.; Kostić, O.; Perović, V.; Jarić, S.; Pavlović, P. Using Fractionation Profile of Potentially Toxic Elements in Soils to Investigate Their Accumulation in Tilia sp. Leaves in Urban Areas with Different Pollution Levels. Sustainability 2021, 13, 9784. [Google Scholar] [CrossRef]
- Tomašević, M.; Aničić, M.; Jovanović, L.; Perić-Grujić, A.; Ristić, M. Deciduous tree leaves in trace elements biomonitoring: A contribution to methodology. Ecol. Indic. 2011, 11, 1689–1695. [Google Scholar] [CrossRef]
- Krzesłowska, M.; Timmers, A.C.J.; Mleczek, M.; Niedzielski, P.; Rabęda, I.; Woźny, A.; Goliński, P. Alterations of root architecture and cell wall modifications in Tilia cordata Miller (Linden) growing on mining sludge. Environ. Pollut. 2019, 248, 247–259. [Google Scholar] [CrossRef]
- Dadea, C.; Russo, A.; Tagliavini, M.; Mimmo, T.; Zerbe, S. Tree species as tools for biomonitoring and phytoremediation in urban environments: A review with special regard to heavy metals. Arboric. Urban For. 2017, 43, 155–167. [Google Scholar] [CrossRef]
- Mleczek, M.; Goliński, P.; Krzesłowska, M.; Gąsecka, M.; Magdziak, Z.; Rutkowski, P.; Budzyńska, S.; Waliszewska, B.; Kozubik, T.; Karolewski, Z.; et al. Phytoextraction of potentially toxic elements by six tree species growing on hazardous mining sludge. Environ. Sci. Pollut. Res. 2017, 24, 22183–22195. [Google Scholar] [CrossRef]
- Shchukin, V.M.; Kuzmina, N.E.; Blinkova, E.A.; Erina, A.A.; Zhigilei, E.S.; Luttseva, A.I. Accumulation Capacity of Linden Flowers and Leaves for Elemental Toxicants in Urban Areas Assessed by Inductively Coupled Plasma Mass Spectrometry. Pharm. Chem. J. 2022, 55, 1196–1200. [Google Scholar] [CrossRef]
- Viola, H.; Wolfman, C.; Levi de Stein, M.; Wasowski, C.; Peña, C.; Medina, J.H.; Paladini, A.C. Isolation of pharmacologically active benzodiazepine ligands from Tilia tomentosa (Tiliaceae). J. Ethnopharmacol. 1994, 44, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, M.; Goldberg, A.; Brinckmann, J. Herbal Medicine: Expanded Commission E Monographs; Integrative Medicine Communications: Newton, UK, 2000; pp. 78–83. [Google Scholar]
- Pérez-Ortega, G.; Guevara-Fefer, P.; Chávez, M.; Herrera, J.; Martínez, A.; Martínez, A.L.; Gonzalez-Trujano, M.E. Sedative and anxiolytic efficacy of Tilia americana var. mexicana inflorescences used traditionally by communities of State of Michoacan, Mexico. J. Ethnopharmacol. 2008, 116, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Allio, A.; Calorio, C.; Franchino, C.; Gavello, D.; Carbone, E.; Marcantoni, A. Bud extracts from Tilia tomentosa Moench inhibit hippocampal neuronal firing through GABAA and benzodiazepine receptors activation. J. Ethnopharmacol. 2015, 172, 288–296. [Google Scholar] [CrossRef]
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef]
- Ziaja, M.; Pawłowska, K.A.; Józefczyk, K.; Pruś, A.; Stefańska, J.; Granica, S. UHPLC-DAD-MS/MS analysis of extracts from linden flowers (Tiliae flos): Differences in the chemical composition between five Tilia species growing in Europe. Ind. Crops Prod. 2020, 154, 112691. [Google Scholar] [CrossRef]
- Petrova, S.; Velcheva, I.; Nikolov, B.; Vasileva, T.; Bivolarski, V. Antioxidant Responses and Adaptation Mechanisms of Tilia tomentosa Moench, Fraxinus excelsior L. and Pinus nigra J. F. Arnold towards Urban Air Pollution. Forests 2022, 13, 1689. [Google Scholar] [CrossRef]
- Republic Hydrometeorological Service of Serbia. Annual Bulletin for Serbia for the Year 2021; Department of Climate Monitoring and Forecasting: Belgrade, Serbia, 2022. Available online: https://www.hidmet.gov.rs/data/klimatologija/latin/2021.pdf (accessed on 26 August 2023).
- U.S. Environmental Protection Agency (US EPA). Method 3050B: Acid Digestion of Sediments, Sludges, and Soils; Revision 2; EPA: Washington, DC, USA, 1996. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA). Method 3052: Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices; EPA: Washington, DC, USA, 1996. [Google Scholar]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (US EPA). Regional Screening Levels (RSLs)-Equations. 2020. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-equations (accessed on 25 August 2023).
- Atique Ullah, A.K.M.; Maksud, M.A.; Khan, S.R.; Lutfa, L.N.; Quraishi, S.B. Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol. Rep. 2017, 4, 574–579. [Google Scholar] [CrossRef]
- Likuku, A.S.; Obuseng, G. Health Risk Assessment of Heavy Metals via Dietary Intake of Vegetables Irrigated with Treated Wastewater around Gaborone, Botswana. In Proceedings of the International Conference on Plant, Marine and Environmental Sciences (PMES-2015), Kuala Lumpur, Malaysia, 1–2 January 2015; Rahman, A., Ahmadi, R., Eds.; International Institute of Chemical, Biological & Environmental Engineering (IICBEE): Kuala Lumpur, Malaysia, 2015; ISBN 978-93-84422-38-7. [Google Scholar]
- SPSS Statistics for Windows, Version 21; SPSS Inc.: Chicago, IL, USA, 2016. Available online: http://www.spss.com.hk/corpinfo/ (accessed on 12 August 2023).
- Pavlović, P.; Sawidis, T.; Breuste, J.; Kostić, O.; Čakmak, D.; Đorđević, D.; Pavlović, D.; Pavlović, M.; Perović, V.; Mitrović, M. Fractionation of Potentially Toxic Elements (PTEs) in Urban Soils from Salzburg, Thessaloniki and Belgrade: An Insight into Source Identification and Human Health Risk Assessment. Int. J. Environ. Res. Public Health 2021, 18, 6014. [Google Scholar] [CrossRef]
- Oliver, M.A.; Gregory, P.J. Soil, food security and human health: A review. Eur. J. Soil Sci. 2015, 66, 257–276. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin, Germany, 2007. [Google Scholar] [CrossRef]
- Mrvić, V.; Zdravković, M.; Sikirić, B.; Čakmak, D.; Kostić-Kravljanac, L. Harmful and hazardous elements in soil. In the Fertility and Content of Hazardous and Harmful Substances in the Soils of Central Serbia; Mrvić, V., Antonović, G., Martinović, L., Eds.; Institute of Soil Science: Belgrade, Serbia, 2009. (In Serbian) [Google Scholar]
- Alloway, B. Heavy Metals in Soils. In Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2013. [Google Scholar]
- Official Gazette of the Republic of Serbia (SG RS 23/94). National Regulations on Permitted Quantities of Hazardous and Harmful Substances in Soil. Available online: http://demo.paragraf.rs/demo/combined/Old/t/t2008_12/t12_0236.htm (accessed on 25 January 2023).
- 53Madrid, L.; Diaz-Barrientos, E.; Ruiz-Cortés, E.; Reinoso, R.; Biasioli, M.; Davidson, C.M.; Duarte, A.C.; Grcman, H.; Hossack, I.; Hursthouse, A.S.; et al. Variability in concentrations of potentially toxic elements in urban parks from six European cities. J. Environ. Monit. 2006, 8, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Morel, J.L.; Schwartz, C.; Florentin, L.; de Kimpe, C. Urban Soils. In Encyclopedia of Soils in the Environment; Daniel, H., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 202–208, ISBN-13: 978-0123485304. [Google Scholar]
- Säumel, I.; Kotsyuk, I.; Hölscher, M.; Lenkereit, C.; Weber, F.; Kowarik, I. How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighborhoods in Berlin, Germany. Environ. Pollut. 2012, 165, 124–132. [Google Scholar] [CrossRef]
- Amato-Lourenco, L.F.; Moreira, T.C.L.; de Oliveira Souza, V.C.; Barbosa, F., Jr.; Saiki, M.; Saldiva, P.H.N.; Mauad, T. The influence of atmospheric particles on the elemental content of vegetables in urban gardens of Sao Paulo, Brazil. Environ. Pollut. 2016, 216, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Shacklette, H.T.; Erdman, J.A.; Harms, T.F. Trace elements in plants foodstuffs. In Toxicity of Heavy Metals in the Environment; Oehme, F.W., Ed.; Part I. Marcel Dekker Inc.: New York, NY, USA, 1978. [Google Scholar]
- Georgieva, S.K.; Georgieva, A.; Peteva, Z.; Dimova, D. Trace elements in commonly used medicinal plants from Varna region, Bulgaria. Environ. Sci. Pollut. Res. 2021, 28, 59277–59283. [Google Scholar] [CrossRef] [PubMed]
- Piechalak, A.; Tomaszewska, B.; Baralkiewicz, D.; Malecka, A. Accumulation and detoxification of lead ions in legumes. Phytochemistry 2002, 60, 153–162. [Google Scholar] [CrossRef]
- Fahr, M.; Laplaze, L.; Bendaou, N.; Hocher, V.; Mzibri, M.E.; Bogusz, D.; Smouni, A. Effect of lead on root growth. Front. Plant Sci. 2013, 6, 175. [Google Scholar] [CrossRef]
- World Health organization (WHO). WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; WHO Press: Geneva, Switzerland, 2007; ISBN 978 92 4 159444 8. [Google Scholar]
- Official Gazette RS (SG RS 23/94, 88/2010). National Regulations on the Quantities of Pesticides, Metals, Metalloids and Other Toxic Substances, Chemotherapy Drugs, Anabolics and Other Substances that Can Be Found in Foodstuffs: FRY 5/1992-67, 11/1992-151 (amend.), 32/2002-2, RS 25/2010-16 (Other Regulations), RS 28/2011-9 (Other Regulations). Available online: http://demo.paragraf.rs/demo/combined/Old/t/t2008_12/t12_0236.htm (accessed on 25 January 2023).
- Zehnder, H.J.; Kopp, P.; Eikenberg, J.; Feller, U.; Oertli, J.J. Uptake and transport of radioactive cesium and strontium into grapevines after leaf contamination. Radiat. Phys. Chem. 1995, 46, 61–69. [Google Scholar] [CrossRef]
- Brambilla, M.; Fortunati, P.; Carini, F. Foliar and root uptake of 134Cs, 85Sr and 65Zn in processing tomato plants (Lycopersicon esculentum Mill.). J. Environ. Radioactiv. 2002, 60, 351–363. [Google Scholar] [CrossRef]
- Tsukada, H.; Takeda, T.T.; Hasegawa, H.; Hisamatsu, S.; Inaba, J. Uptake and distribution of 90Sr and stable Sr in rice plants. J. Environ. Radioactiv. 2005, 81, 221–231. [Google Scholar] [CrossRef]
- Serbian Environmental Protection Agency. Annual Report on Air Quality in the Republic of Serbia in 2021; Serbian Environmental Protection Agency: Belgrade, Serbia, 2022. [Google Scholar]
- Başgel, S.; Erdemoğlu, S.B. Determination of mineral and trace elements in some medicinal herbs and their infusions consumed in Turkey. Sci. Total Environ. 2006, 359, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wang, B.; Jiang, J.; Fitzgerald, M.; Huang, Q.; Yu, Z.; Li, H.; Zhang, J.; Wei, J.; Yang, C.; et al. Heavy Metal Contaminations in Herbal Medicines: Determination, Comprehensive Risk Assessments, and Solutions. Front. Pharmacol. 2021, 11, 595335. [Google Scholar] [CrossRef] [PubMed]
- Alekseenko, V.A.; Shvydkaya, N.V.; Alekseenko, A.V.; Machevariani, M.M.; Bech, J.; Pashkevich, M.A.; Puzanov, A.V.; Nastavkin, A.V.; Roca, N. Element Accumulation Patterns of Native Plant Species under the Natural Geochemical Stress. Plants 2021, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Velciov, A.B.; Riviș, A.; Stoin, D.; Popescu, G.S.; Mitroi, C.L.; Birtea, A.I.; Rada, M. The chamomile and linden flowers—Sources of essential microelements—A review. J. Agroaliment. Process. Technol. 2021, 27 (Suppl. S4), 506–514. [Google Scholar]
- Barreiro Arcos, M.L.; Cremaschi, G.; Werner, S.; Coussio, J.; Ferraro, G.; Anesini, C. Tilia cordata Mill. Extracts and scopoletin (isolated compound): Differential cell growth effects on lymphocytes. Phytother. Res. 2006, 20, 34–40. [Google Scholar] [CrossRef]
- de Almeida Bezerra, L.; Callado, C.H.; Vasconcellos, T.J.; dos Santos Nogueira, T.O.C.; dos Santos, R.S.; de Lima Moreira, D.; de Mattos, J.C.P.; dos Anjos, M.J.; Murata, M.M.; Cunha, M.D. Chemical and cytotoxical changes in leaves of Eugenia uniflora L., a medicinal plant growing in the fourth largest urban centre of Latin America. Trees 2023, 37, 85–98. [Google Scholar] [CrossRef]
- Lüttge, U.; Buckeridge, M. Trees: Structure and function and the challenges of urbanization. Trees 2023, 37, 9–16. [Google Scholar] [CrossRef]
- Arjona-García, C.; Blancas, J.; Beltrán-Rodríguez, L.; López Binnqüist, C.; Colín Bahena, H.; Moreno-Calles, A.I.; Sierra-Huelsz, J.A.; López-Medellín, X. How does urbanization affect perceptions and traditional knowledge of medicinal plants? J. Ethnobiol. Ethnomed. 2021, 17, 48. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for copper. EFSA J. 2015, 13, 4253. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef]
- National Research Council (US). Recommended Dietary Allowance, 10th ed.; National Academic Press: Washington, DC, USA, 1989. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Summary Report of the Fifty-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); FAO: Rome, Italy, 1999. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA). Integrated Risk Information System; U.S. Environmental Protection Agency: Washington, DC, USA, 2016. Available online: https://www.epa.gov/iris/ (accessed on 7 September 2023).
- Wang, X.; Sato, T.; Xing, B.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (US EPA). Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites; OSWER 9355.4–24; US EPA: Washington, DC, USA, 2001. [Google Scholar]

| Locality | Transfer | Cr | Cu | Ni | Pb | Sr | Zn |
|---|---|---|---|---|---|---|---|
| Zemunski Park | BCF Root | 0.08 | 0.19 | 0.05 | 0.04 | 0.00 | 0.07 |
| BCF Leaf | 0.00 | 0.22 | 0.02 | 0.00 | 0.00 | 0.16 | |
| BCF Flower | 0.00 | 0.27 | 0.00 | 0.00 | 0.42 | 0.12 | |
| TF Leaf/Root | 0.00 | 1.23 | 0.44 | 0.00 | 2.21 | 2.32 | |
| TF Flower/Root | 0.04 | 1.62 | 0.10 | 0.00 | 260.00 | 1.83 | |
| Park Blok 63 | BCF Root | 0.06 | 0.21 | 0.06 | 0.08 | 0.00 | 0.19 |
| BCF Leaf | 0.00 | 0.29 | 0.01 | 0.00 | 0.01 | 0.20 | |
| BCF Flower | 0.00 | 0.38 | 0.01 | 0.00 | 0.76 | 0.24 | |
| TF Leaf/Root | 0.00 | 1.36 | 0.17 | 0.00 | 3.90 | 1.17 | |
| TF Flower/Root | 0.09 | 1.79 | 0.20 | 0.00 | 556.00 | 1.33 | |
| Park Topčider | BCF Root | 0.05 | 0.22 | 0.04 | 0.00 | 0.00 | 0.06 |
| BCF Leaf | 0.00 | 0.37 | 0.02 | 0.00 | 0.01 | 0.21 | |
| BCF Flower | 0.02 | 0.80 | 0.02 | 0.00 | 0.97 | 0.37 | |
| TF Leaf/Root | 0.00 | 1.73 | 0.54 | 0.00 | 2.98 | 3.52 | |
| TF Flower/Root | 0.30 | 3.68 | 0.55 | 0.00 | 573.00 | 6.00 |
| Flowers | Leaves | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Locality/Element | Cr | Cu | Ni | Sr | Zn | Cu | Ni | Sr | Zn |
| Zemunski park | 0.001 | 0.081 | 0.002 | 0.281 | 0.092 | 0.065 | 0.009 | 0.002 | 0.119 |
| Park Blok 63 | 0.001 | 0.074 | 0.003 | 0.281 | 0.094 | 0.056 | 0.003 | 0.002 | 0.077 |
| Park Topčider | 0.006 | 0.135 | 0.007 | 0.336 | 0.151 | 0.061 | 0.007 | 0.002 | 0.088 |
| Flowers | ||||||
|---|---|---|---|---|---|---|
| Adults | Children | |||||
| Locality/Element | Cu | Sr | Zn | Cu | Sr | Zn |
| Zemunski Park | 0.001 | 0.004 | 0.001 | 0.005 | 0.019 | 0.006 |
| Park Blok 63 | 0.001 | 0.004 | 0.001 | 0.005 | 0.019 | 0.006 |
| Park Topčider | 0.002 | 0.005 | 0.002 | 0.009 | 0.022 | 0.010 |
| Leaves | ||||||
| Adults | Children | |||||
| Locality/Element | Cu | Ni | Zn | Cu | Ni | Zn |
| Zemunski park | 0.001 | 0.000 | 0.002 | 0.004 | 0.001 | 0.008 |
| Park Blok 63 | 0.001 | 0.000 | 0.001 | 0.004 | 0.000 | 0.005 |
| Park Topčider | 0.001 | 0.000 | 0.001 | 0.004 | 0.000 | 0.006 |
| Flowers | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adults | Children | |||||||||||
| Locality/Element | Cr | Cu | Ni | Sr | Zn | TTHQ | Cr | Cu | Ni | Sr | Zn | TTHQ |
| Zemunski Park | 0.005 | 0.029 | 0.001 | 0.007 | 0.004 | 0.056 | 0.025 | 0.135 | 0.007 | 0.031 | 0.004 | 0.245 |
| Park Blok 63 | 0.008 | 0.026 | 0.002 | 0.007 | 0.004 | 0.062 | 0.036 | 0.123 | 0.011 | 0.031 | 0.004 | 0.274 |
| Park Topčider | 0.029 | 0.048 | 0.005 | 0.008 | 0.007 | 0.114 | 0.134 | 0.225 | 0.023 | 0.037 | 0.007 | 0.506 |
| Leaves | ||||||||||||
| Adults | Children | |||||||||||
| Locality/Element | Cr | Cu | Ni | Sr | Zn | TTHQ | Cr | Cu | Ni | Sr | Zn | TTHQ |
| Zemunski Park | 0.00 | 0.023 | 0.006 | 0.00 | 0.006 | 0.058 | 0.00 | 0.108 | 0.029 | 0.00 | 0.006 | 0.250 |
| Park Blok 63 | 0.00 | 0.020 | 0.002 | 0.00 | 0.004 | 0.046 | 0.00 | 0.093 | 0.010 | 0.00 | 0.004 | 0.202 |
| Park Topčider | 0.00 | 0.022 | 0.005 | 0.00 | 0.004 | 0.051 | 0.00 | 0.102 | 0.023 | 0.00 | 0.004 | 0.223 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitrović, M.; Kostić, O.; Miletić, Z.; Marković, M.; Radulović, N.; Sekulić, D.; Jarić, S.; Pavlović, P. Bioaccumulation of Potentially Toxic Elements in Tilia tomentosa Moench Trees from Urban Parks and Potential Health Risks from Using Leaves and Flowers for Medicinal Purposes. Forests 2023, 14, 2204. https://doi.org/10.3390/f14112204
Mitrović M, Kostić O, Miletić Z, Marković M, Radulović N, Sekulić D, Jarić S, Pavlović P. Bioaccumulation of Potentially Toxic Elements in Tilia tomentosa Moench Trees from Urban Parks and Potential Health Risks from Using Leaves and Flowers for Medicinal Purposes. Forests. 2023; 14(11):2204. https://doi.org/10.3390/f14112204
Chicago/Turabian StyleMitrović, Miroslava, Olga Kostić, Zorana Miletić, Milica Marković, Natalija Radulović, Dimitrije Sekulić, Snežana Jarić, and Pavle Pavlović. 2023. "Bioaccumulation of Potentially Toxic Elements in Tilia tomentosa Moench Trees from Urban Parks and Potential Health Risks from Using Leaves and Flowers for Medicinal Purposes" Forests 14, no. 11: 2204. https://doi.org/10.3390/f14112204
APA StyleMitrović, M., Kostić, O., Miletić, Z., Marković, M., Radulović, N., Sekulić, D., Jarić, S., & Pavlović, P. (2023). Bioaccumulation of Potentially Toxic Elements in Tilia tomentosa Moench Trees from Urban Parks and Potential Health Risks from Using Leaves and Flowers for Medicinal Purposes. Forests, 14(11), 2204. https://doi.org/10.3390/f14112204







