Effects of Enclosure Succession on the Morphological Characteristics and Nutrient Content of a Bamboo Whip System in a Moso Bamboo (Phyllostachys edulis) Forest on Wuyi Mountain, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of Experimental Conditions
2.2. Experimental Materials
2.3. Assessing Indicators of the Characteristics and Nutrient Content of Moso Bamboo Whips
2.4. Statistical Analysis
3. Results
3.1. Morphological Characteristics of Moso Bamboo Whips
3.2. Nutrient Content of Moso Bamboo Whip
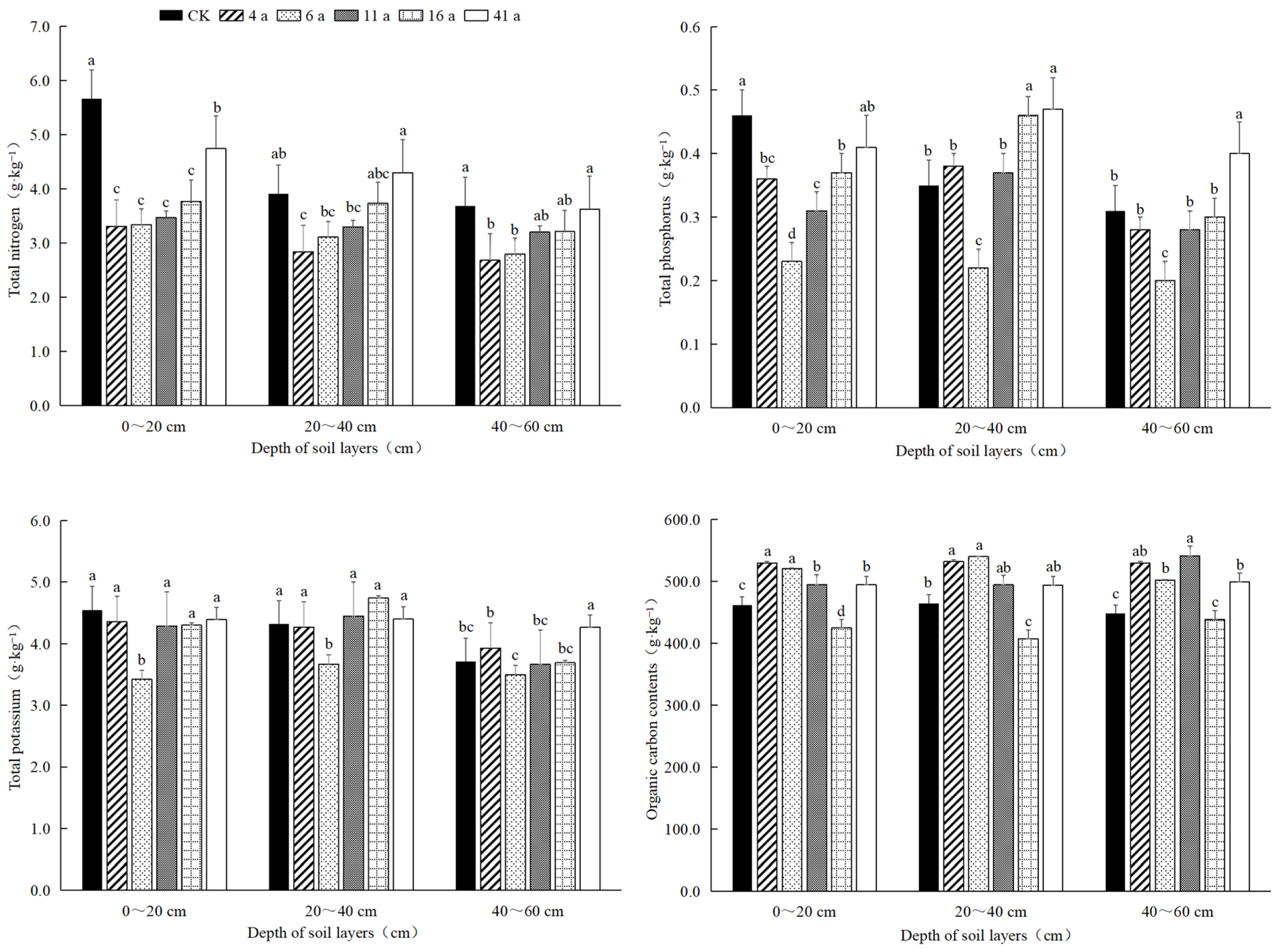
3.2.1. Total Nitrogen, Total Phosphorus, Total Potassium and Organic Carbon Content of Bamboo Whips
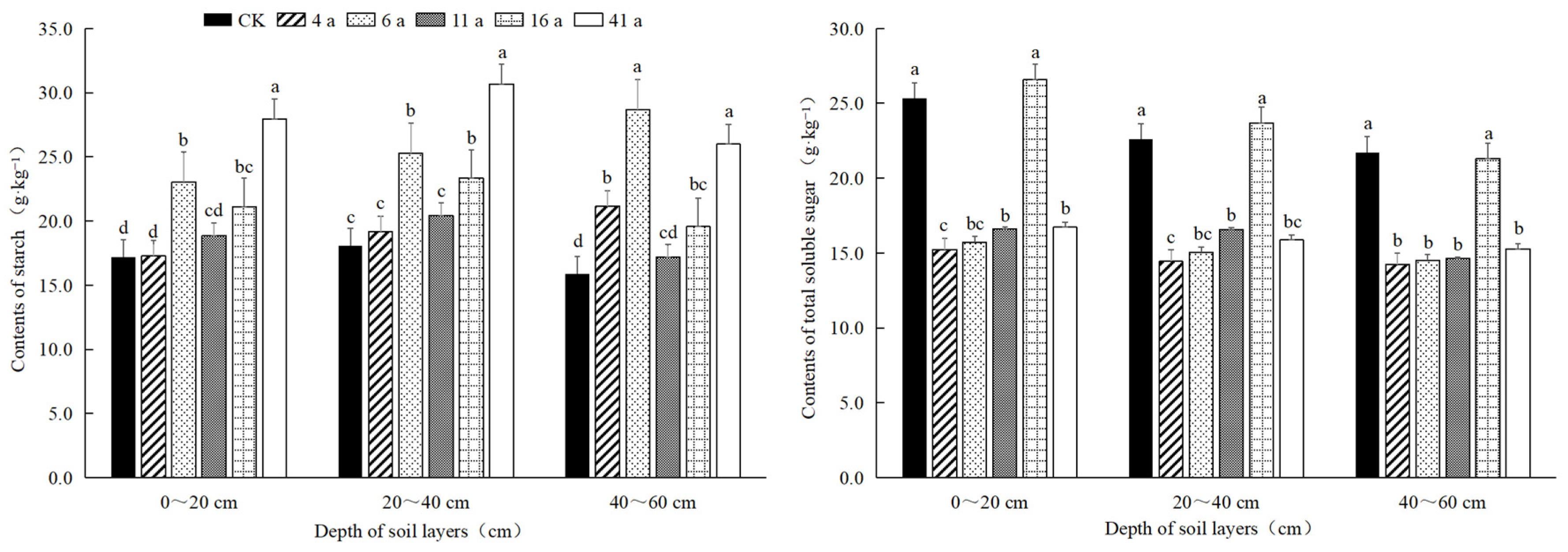
3.2.2. Starch and Soluble Sugar Content of Bamboo Whips
3.3. Correlation between Morphological Characteristics and Nutrient Content of Moso Bamboo Whips
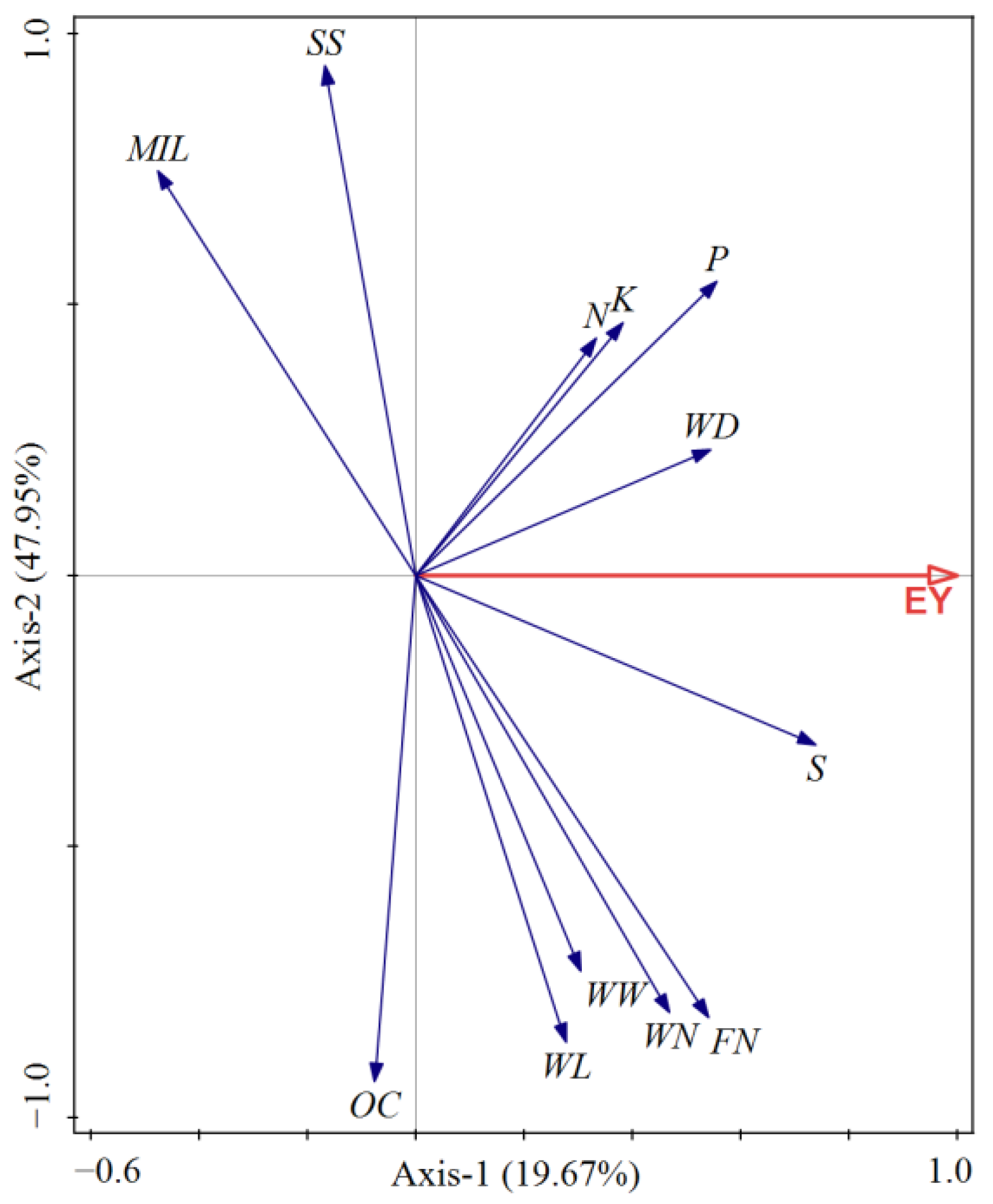
3.4. Redundancy Analysis between Quantitative Characteristics and Nutrient Content of Moso Bamboo Whips and Years of Closure
3.5. Principal Component Analysis and Evaluation of Quantitative Characteristics and Nutrient Content of Moso Bamboo Whips in Different Sequestration Years
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, Y.C.; Tang, H.P.; Xin, X.P.; Wang, X. Advances in research on the effects of exclosure on grasslands. Acta Ecol. Sin. 2009, 29, 5039–5046. [Google Scholar]
- Ping, L.; Xiong, G.M.; Xie, Z.Q. Characteristics and succession of Eucalyptus plantation after closing for afforestation in the three gorges reservoir area. J. Nat. Resour. 2009, 24, 1604–1615. [Google Scholar]
- Jing, Z.B.; Cheng, J.M.; Su, J.S.; Bai, Y.; Jin, J.W. Changes in plant community composition and soil properties under3-decade grazing exclusion in semiarid grassland. Ecol. Eng. 2014, 64, 171–178. [Google Scholar] [CrossRef]
- Qiu, L.P.; Wei, X.R.; Zhang, X.C.; Cheng, J.M. Ecosystem carbon and nitrogen accumulation after grazing exclusion in semiarid grassland. PLoS ONE 2013, 8, e55433. [Google Scholar] [CrossRef]
- Eddi, K.; Chaieb, M. Changes in soil properties and vegetation following livestock grazing exclusion in degraded arid environments of South Tunisia. Flora 2010, 205, 184–189. [Google Scholar]
- Cheng, J.M.; Jing, G.H.; Wei, L.; Jing, Z.B. Long-term grazing exclusion effects on vegetation characteristics, soil properties and bacterial communities in the semi-arid grasslands of China. Ecol. Eng. 2016, 97, 170–178. [Google Scholar] [CrossRef]
- Mofidi, M.; Rashtbari, M.; Abbaspour, H.; EBADI, A.; SHEIDAI, E.; MOTAMEDI, J. Impact of grazing on chemical, physical and biological properties of soils in the mountain rangelands of Sahand, Iran. Rangel. J. 2012, 34, 297–303. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Fan, W.Y.; Ge, L.L.; Cui, Y.Y.; Wu, T.; Ren, J.M. Investigation on effect of hillside closing and facilitate afforestation in fragile ecological zone in Daqing Mountain in Inner Mongolia. J. Arid Land Resour. Environ. 2008, 3, 178–182. [Google Scholar]
- Su, J.S.; Zhao, J.; Ji, G.H.; Wei, L.; Liu, J.; Cheng, J.M.; Zhang, J.E. Root pattern of Stipa plants in semiarid grassland after long-term grazing exclusion. Acta Ecol. Sin. 2017, 37, 6571–6580. [Google Scholar]
- Huang, Q.Q.; Zhang, S.F.; Xv, G.Q.; Li, X.; Xu, Z.Q.; Jia, Y.L. Effects of nitrogen addition and enclosure on growth of Larix principis-rupprechtii plantation and herb diversity. For. Res. 2023, 36, 149–156. [Google Scholar]
- Lv, P.; Zuo, X.A.; Yue, X.Y.; Zhang, J.; Zhao, S.L.; Cheng, Q.P. Temporal changes of vegetation characteristics during the long-term grazing exclusion in Horqin Sandy Land. Chin. J. Ecol. 2018, 37, 2880–2888. [Google Scholar]
- Fan, Y.M.; Wu, H.Q.; Jin, G.L.; Xie, Y. Effects of enclosure on stoichiometric characteristics of C, N, P in desert grassland ecosystem. Chin. J. Grassl. 2018, 40, 76–81. [Google Scholar]
- Zhang, J.; Zuo, X.; Zhou, X.; Liu, P.; Lian, J.; Yue, X. Long-term grazing effects on vegetation characteristics and soil properties in a semiarid grassland, northern China. Environ. Monit. Assess. 2017, 189, 216. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Su, J.S.; Cheng, J.M. Root biomass distribution of natural grasslands with different enclosing years in the Loess Plateau. Pratacultural Sci. 2013, 30, 1824–1830. [Google Scholar]
- Mommer, L.; Van, R.J.; De, C.H.; Smit-Tiekstra, A.E.; Wagemaker, C.A.M.; Ouborg, N.J.; Bögemann, G.M.; Van Der Weerden, G.M.; Berendse, F.; De Kroon, H. Unveiling below-ground species abundance in a biodiversity experiment:a test of vertical niche differentiation among grassland species. J. Ecol. 2010, 98, 1117–1127. [Google Scholar] [CrossRef]
- Genney, D.R.; Alexander, I.J.; Hartley, S.E. Soil organic matter distribution and below-ground competition between Calluna vulgaris and Nardus stricta. Funct. Ecol. 2002, 16, 664–670. [Google Scholar] [CrossRef]
- Shen, R.; Bai, S.B.; Zhou, G.M.; Wang, Y.X.; Wang, N.; Wen, G.S.; Chen, J. The response of root morphological plasticity to the expansion of a population of Phyllostachys edulis into a mixed needle-and broad-leaved forest. Acta Ecol. Sin. 2016, 36, 326–334. [Google Scholar]
- Xiong, Y.L.; Zhou, Y.F.; Li, B.; Tong, L.; Zhou, G.M.; Shi, Y.J.; Du, H.Q. Non-destructive detection by ground penetrating radar of growth characteristics and spatial structure of rhizomes in moso bamboo forest. Sci. Silvae Sin. 2020, 56, 19–27. [Google Scholar]
- Tong, R.; Chen, Q.B.; Zhou, B.Z.; Tang, Y.Q.; An, Y.F.; Ge, X.G.; Cao, Y.H.; Yang, Z.Y. Structure and biomechanical properties of underground system of moso bamboo and lei bamboo. Acta Ecol. Sin. 2020, 40, 2242–2251. [Google Scholar]
- LY/T 1270-1999; Determination of Total Nitrogen, Phosphorus, Potassium, Sodium, Calcium and Magnesium in Forest Plant and Forest Floor. National Forestry and Grassland Administration: Beijing, China, 1999.
- LY/T 1271-1999; Determination of Total Silicon, Iron, Aluminum, Calcium, Magnesium, Potassium, Sodium, Phosphorus, Sulfur, Manganese, Copper and Zinc in Forest Plant and Forest Floor. National Forestry and Grassland Administration: Beijing, China, 1999.
- LY/T 1237-1999; Determination of Forest Soil Organic Matter and Calculation of Carbon-Nitrogen Ratio. National Forestry and Grassland Administration: Beijing, China, 1999.
- Li, H.S.; Sun, Q.; Zhao, S.J.; Zhang, W.H. Principle and Technology of Plant Physiological and Biochemical Experiment; Higher Education Press: Beijing, China, 2003; pp. 195–197. [Google Scholar]
- Lin, Y.; Karim, A. Effects of salt tolerance on the content of soluble sugar, starch, proline of Pistachio. J. Xinjiang Agric. Univ. 2004, 27, 19–23. [Google Scholar]
- Dong, Y.W.; Chen, S.L.; Wang, S.P.; Guo, Z.W.; He, Y.Y.; Zhang, W. Morphology and biomass distribution of underground rhizome of Phyllostachys edulis during the succession of understory vegetation. For. Res. 2023, 36, 158–167. [Google Scholar]
- Ji, L.K.; Xie, J.Z.; Zhang, W.; Lu, P.; Zhang, L. Organic carbon allocation pattern and changes regulation in various organs of Phyllostachys violascens clone system in shooting period. Acta Ecol. Sin. 2016, 36, 7624–7634. [Google Scholar]
- Ying, Y.S.; Yang, L.T.; Cheng, J.X.; Lan, C.B.; Chen, S.L.; Guo, Z.W. Effect of habitats on the morphological and structural characteristic of rhizome roots of Pleioblastus amarus and its allometric growth. Acta Bot. Boreali-Occident. Sin. 2022, 42, 1583–1590. [Google Scholar]
- Zhao, X.D.; Zeng, Q.C.; An, S.S.; Fang, Y.; Ma, R.T. Ecological stoichiometric characteristics of grassland soils and plant roots relative to enclosure history on the Loess Plateau. Acta Pedol. Sin. 2016, 53, 1541–1551. [Google Scholar]
- Ren, S.L.; Wang, Y.J.; Jin, A.W.; Zhu, Q.G.; Ji, X.L.; Ma, Y.; Fang, W.L. Rhizome characteristics, stand structure and soil properties under Phyllostachys edulis expansion into coniferous and broadleaf forest. J. Northeast For. Univ. 2023, 51, 21–27. [Google Scholar]
- Wu, F.; Yang, W.; Lu, Y. Effects of dwarf bamboo (Fargesia denudata) density on biomass, carbon and nutrient distribution pattern. Acta Ecol. Sin. 2009, 29, 192–198. [Google Scholar] [CrossRef]
- Cai, X.; Wu, J.; Li, B.J.; Li, S.K.; Rong, J.D.; Chen, L.G.; He, T.Y.; Zheng, Y.S. Effect of moso bamboo forest density on rhizome characteristics and root activity of moso bamboo under long-period mother bamboo conservation mode. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2023, 52, 500–504. [Google Scholar]
- Zheng, Y.S.; Wang, S.F. Study on bamboo underground structure of mixed forest of chinese fir and bamboo. Sci. Silvae Sin. 2000, 36, 69–72. [Google Scholar]
- Li, T.C.; Chen, G.S.; Cao, G.M.; Zhang, D.G. Characteristics of mineral elements K, Ca, Mg in degraded grassland and enclosure grassland on the north bank of Qinghai Lake. Acta Agrestia Sin. 2011, 19, 752–759. [Google Scholar]
- Xu, M.P.; Jian, J.N.; Wang, J.Y.; Zhang, Z.J.; Yang, G.H.; Han, X.H.; Ren, C.J. Response of root nutrient resorption strategies to rhizosphere soil microbial nutrient utilization along Robinia pseudoacacia plantation chronosequence. For. Ecol. Manag. 2021, 489, 119053. [Google Scholar] [CrossRef]
- Nie, T.T.; Dong, B.Q.; Yang, H.; AstaiKen, H.; Zhou, S.J.; An, S.Z. Effects of enclosure on plant and soil stoichiometric characteristics in an Artemisia desert. J. Agric. Sci. Technol. 2023, 25, 178–187. [Google Scholar]
- Takahashi, T.; Seino, T.; Kohyama, T. Plastic changes of leaf mass per area and leaf nitrogen content in response to canopy opening in saplings of eight deciduous broad-leaved tree species. Ecol. Res. 2005, 20, 17–23. [Google Scholar] [CrossRef]
- Liu, X.M.; Rao, H.L.; Ding, X.X.; Guo, R.F.; Wu, C.Z.; Lin, Y.M.; Li, J. Effects of different mixed forest types on soil organic carbon and soil respiration in Phyllostachys edulis J. Houz forest. Chin. J. Appl. Environ. Biol. 2021, 27, 71–80. [Google Scholar]
- Li, W.; Huang, G.; Zhang, H. Enclosure increases nutrient resorption from senescing leaves in a subalpine pasture. Plant Soil 2020, 457, 269–278. [Google Scholar] [CrossRef]
- Hong, J.T.; Wu, J.B.; Wang, X.D. Root C: N: P stoichiometry of Stipa purpurea in apine steppe on the Northern Tibet. Mt. Res. 2014, 32, 467–474. [Google Scholar]
- Sun, H.; Cao, Z.H.; Wu, Z.N.; Fang, M.G.; Zhang, R.F.; Liu, J.; Miao, T.; Yan, C. Effects of mulching on soil nutrients, enzyme activities and microbial community in Phyllostachys edulis forest. Non-Wood For. Res. 2023, 41, 223–233. [Google Scholar]
- Hu, X.B.; Jiang, C.Q.; Wang, H.; Jiang, C.W.; Liu, J.Z.; Zang, Y.M.; Li, S.G.; Wang, Y.X.; Bai, Y.F. A Comparison of soil C, N, and P stoichiometry characteristics under different thinning intensities in a subtropical moso bamboo (Phyllostachys edulis) forest of China. Forests 2022, 13, 1770. [Google Scholar] [CrossRef]
- Zhou, X.; Guan, F.Y.; Zhang, X.; Li, C.J.; Zhou, Y. Response of moso bamboo growth and soil nutrient content to strip cutting. Forests 2022, 13, 1293. [Google Scholar] [CrossRef]
- Guo, C.R.; Yang, J.P.; Li, S.W.; Niu, B.; Ma, G.L.; Wu, J.S. Effects of grazing exclusion by fencing on soil mineral elements and plant community in alpine steppes of the northern Tibetan Plateau. Pratacultural Sci. 2022, 39, 645–659. [Google Scholar]
- Liu, Y.; Li, B.L.; Yuan, Y.C.; Qi, J.L.; Li, Y.; Li, R. Assessment of grazing exclusion on grassland restoration through the changes of plant community structure of alpine meadow in the Three River Headwater Region. Acta Ecol. Sin. 2021, 41, 7125–7137. [Google Scholar]
- Zhang, Y.J.; Zhao, J.; An, S.Z.; Sun, Z.J.; Hou, Y.R. Effects of fenced enclosure on nonstructural carbohydrate of Seriphidium transiliense. Xinjiang Agric. Sci. 2010, 47, 1182–1188. [Google Scholar]
- Wu, Y.P.; Wang, Y.R.; Hu, X.W.; Zhang, B.L. Effects of enclosing on non-structure carbohydrate of Cleistogenes songorica. Acta Bot. Boreali-Occident. Sin. 2007, 27, 2298–2305. [Google Scholar]
- Su, W.H.; Zeng, X.L.; Fan, S.H.; Ni, H.J. Effects of strip clear-cutting on the allocation of non-structural carbohydrates and aboveground biomass of Phyllostachys edulis. Chin. J. Ecol. 2019, 38, 2934–2940. [Google Scholar]
- Cai, Z.M.; Deng, Z.W.; Li, D.B.; Li, S.K.; Chen, L.G.; Wen, W.Q.; Zheng, Y.S.; Rong, J.D. Effects of strip logging on the biomass and root non-structural carbohydrates of Phyllostachys edulis. J. Cent. South Univ. For. Technol. 2023, 43, 33–42. [Google Scholar]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. United States Am. 2004, 101, 11001–11006. [Google Scholar] [CrossRef]
- Zhu, W.D.; Wang, Y.L.; Yang, C.; Zhao, N.; Zhao, X.Q.; Xu, S.X.; Sun, P. Effects of different grazing pattern on leaf characteristics of Festuca ovinain alpine meadow of Guinan county. Chin. J. Grassl. 2021, 43, 69–75. [Google Scholar]
- Chen, S.L.; Wu, B.L.; Wu, M.; Zhang, D.M.; Cao, Y.H.; Yang, Q.P. A study of the interannual succession rule and influential factors of young stands structures of Phyllostachys pubescens. J. Zhejiang A F Univ. 2004, 21, 393–397. [Google Scholar]

| Plots | Soil Moisture Content /% | Organic Matter /(g·kg−1) | Total Nitrogen /(g·kg−1) | Total Phosphorus /(g·kg−1) | Total Potassium /(g·kg−1) | Available Phosphorus /(mg·kg−1) | Available Potassium /(mg·kg−1) |
|---|---|---|---|---|---|---|---|
| CK | 20.7 | 32.5 | 1.0 | 0.2 | 21.7 | 1.0 | 31.3 |
| 4 | 35.7 | 35.5 | 1.3 | 0.2 | 15.4 | 1.2 | 41.9 |
| 6 | 39.5 | 43.4 | 1.6 | 0.2 | 19.1 | 1.3 | 42.2 |
| 11 | 42.3 | 47.2 | 1.7 | 0.2 | 17.9 | 1.5 | 45.6 |
| 16 | 45.7 | 52.3 | 2.4 | 0.2 | 21.0 | 1.5 | 46.3 |
| 41 | 60.5 | 61.9 | 3.3 | 0.2 | 19.7 | 2.5 | 47.6 |
| Plots | Plant Height/m | Diameter/cm | Density/(Plant·ha−2) | |||
|---|---|---|---|---|---|---|
| Moso Bamboo | Forest Tree | Moso Bamboo | Forest Tree | Moso Bamboo | Forest Tree | |
| CK | 15.4 | 11.8 | 2764 | |||
| 4 | 17.8 | 20.5 | 12.3 | 40.3 | 2123 | 45 |
| 6 | 14.9 | 6.5 | 11.9 | 8.7 | 4086 | 45 |
| 11 | 19.36 | 13.3 | 12.4 | 22.8 | 3365 | 95 |
| 16 | 14.28 | 11.8 | 12.2 | 15.5 | 5909 | 1172 |
| 41 | 20.48 | 13.0 | 12.3 | 20.0 | 2965 | 756 |
| Index | WN | WD | MIL | FN | WL | WW | N | P | K | OC | SS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WD | 0.196 | ||||||||||
| MIL | −0.877 ** | −0.128 | |||||||||
| FN | 0.908 ** | 0.021 | −0.918 ** | ||||||||
| WL | 0.786 ** | −0.221 | −0.696 ** | 0.883 ** | |||||||
| WW | 0.678 ** | −0.188 | −0.718 ** | 0.840 ** | 0.908 ** | ||||||
| N | −0.078 | 0.219 | −0.079 | −0.058 | −0.214 | 0.039 | |||||
| P | −0.210 | 0.384 | 0.155 | −0.103 | −0.130 | 0.104 | 0.596 ** | ||||
| K | −0.216 | 0.141 | 0.242 | −0.122 | −0.061 | 0.126 | 0.453 | 0.820 ** | |||
| OC | 0.701 ** | −0.164 | −0.592 ** | 0.693 ** | 0.822 ** | 0.691 ** | −0.464 | −0.479 * | −0.345 | ||
| SS | −0.813 ** | 0.050 | 0.680 ** | −0.827 ** | −0.870 ** | −0.730 ** | 0.467 | 0.410 | 0.320 | −0.906 ** | |
| S | 0.701 ** | 0.637 ** | −0.672 ** | 0.601 ** | 0.247 | 0.154 | −0.005 | −0.013 | −0.20 | 0.187 | 0.385 |
| Index | Principal Component | Eigenvector | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| Whip number | 0.948 | 0.243 | 0.041 | 0.380 | 0.151 | 0.031 |
| Whip diameter | −0.939 | 0.172 | −0.040 | −0.378 | 0.107 | −0.030 |
| Mean internode length | 0.924 | 0.209 | −0.185 | 0.372 | 0.130 | −0.140 |
| Flagella number | 0.907 | 0.044 | 0.365 | 0.365 | 0.027 | 0.277 |
| Whip length | −0.870 | −0.273 | 0.153 | −0.350 | −0.170 | 0.116 |
| Whip weight | 0.864 | −0.297 | 0.147 | 0.348 | −0.185 | 0.111 |
| Total N content | 0.805 | 0.257 | 0.497 | 0.324 | 0.160 | 0.377 |
| Total P content | −0.327 | 0.848 | 0.267 | −0.132 | 0.528 | 0.202 |
| Total K content | −0.271 | 0.731 | 0.122 | −0.109 | 0.456 | 0.092 |
| Organic carbon content | −0.306 | 0.683 | 0.492 | −0.123 | 0.426 | 0.373 |
| Soluble sugar content | 0.528 | 0.396 | −0.709 | 0.213 | 0.247 | −0.537 |
| Starch content | −0.023 | 0.581 | −0.677 | −0.009 | 0.362 | −0.513 |
| Eigenvalue | 6.164 | 2.575 | 1.751 | 6.164 | 2.575 | 1.751 |
| Contribution % | 51.36 | 21.46 | 14.59 | 51.36 | 21.46 | 14.59 |
| Cumulative contribution % | 51.36 | 72.82 | 87.41 | 51.36 | 72.82 | 87.41 |
| Treatment/(a) | Principal Component Score | Comprehensive Score | Sort | ||
|---|---|---|---|---|---|
| F1 | F2 | F3 | |||
| CK | 0.520 | 0.210 | −0.260 | 0.313 | 2 |
| 4 | −0.613 | −0.160 | 0.783 | −0.270 | 4 |
| 6 | −0.093 | −1.350 | −0.397 | −0.450 | 6 |
| 11 | 0.553 | −0.923 | 1.120 | 0.283 | 3 |
| 16 | −0.353 | 0.710 | −2.267 | −0.410 | 5 |
| 41 | −0.013 | 1.517 | 1.020 | 0.533 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, X.; Gao, T.; Zheng, S.; Jiang, R.; Zhang, Y.; Rong, J.; He, T.; Chen, L.; Zheng, Y. Effects of Enclosure Succession on the Morphological Characteristics and Nutrient Content of a Bamboo Whip System in a Moso Bamboo (Phyllostachys edulis) Forest on Wuyi Mountain, China. Forests 2023, 14, 2193. https://doi.org/10.3390/f14112193
Cai X, Gao T, Zheng S, Jiang R, Zhang Y, Rong J, He T, Chen L, Zheng Y. Effects of Enclosure Succession on the Morphological Characteristics and Nutrient Content of a Bamboo Whip System in a Moso Bamboo (Phyllostachys edulis) Forest on Wuyi Mountain, China. Forests. 2023; 14(11):2193. https://doi.org/10.3390/f14112193
Chicago/Turabian StyleCai, Xing, Tianyu Gao, Suyun Zheng, Ruiyi Jiang, Yirong Zhang, Jundong Rong, Tianyou He, Liguang Chen, and Yushan Zheng. 2023. "Effects of Enclosure Succession on the Morphological Characteristics and Nutrient Content of a Bamboo Whip System in a Moso Bamboo (Phyllostachys edulis) Forest on Wuyi Mountain, China" Forests 14, no. 11: 2193. https://doi.org/10.3390/f14112193
APA StyleCai, X., Gao, T., Zheng, S., Jiang, R., Zhang, Y., Rong, J., He, T., Chen, L., & Zheng, Y. (2023). Effects of Enclosure Succession on the Morphological Characteristics and Nutrient Content of a Bamboo Whip System in a Moso Bamboo (Phyllostachys edulis) Forest on Wuyi Mountain, China. Forests, 14(11), 2193. https://doi.org/10.3390/f14112193






