Secondary Metabolites from Streptomyces araujoniae S-03 Show Biocontrol Potential against Rhododendron Root Rot Caused by Phytophthora cinnamomi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Bacteria Identification
2.3. Efficacy of Inhibiting Phytophthora cinnamomi Growth on Agar
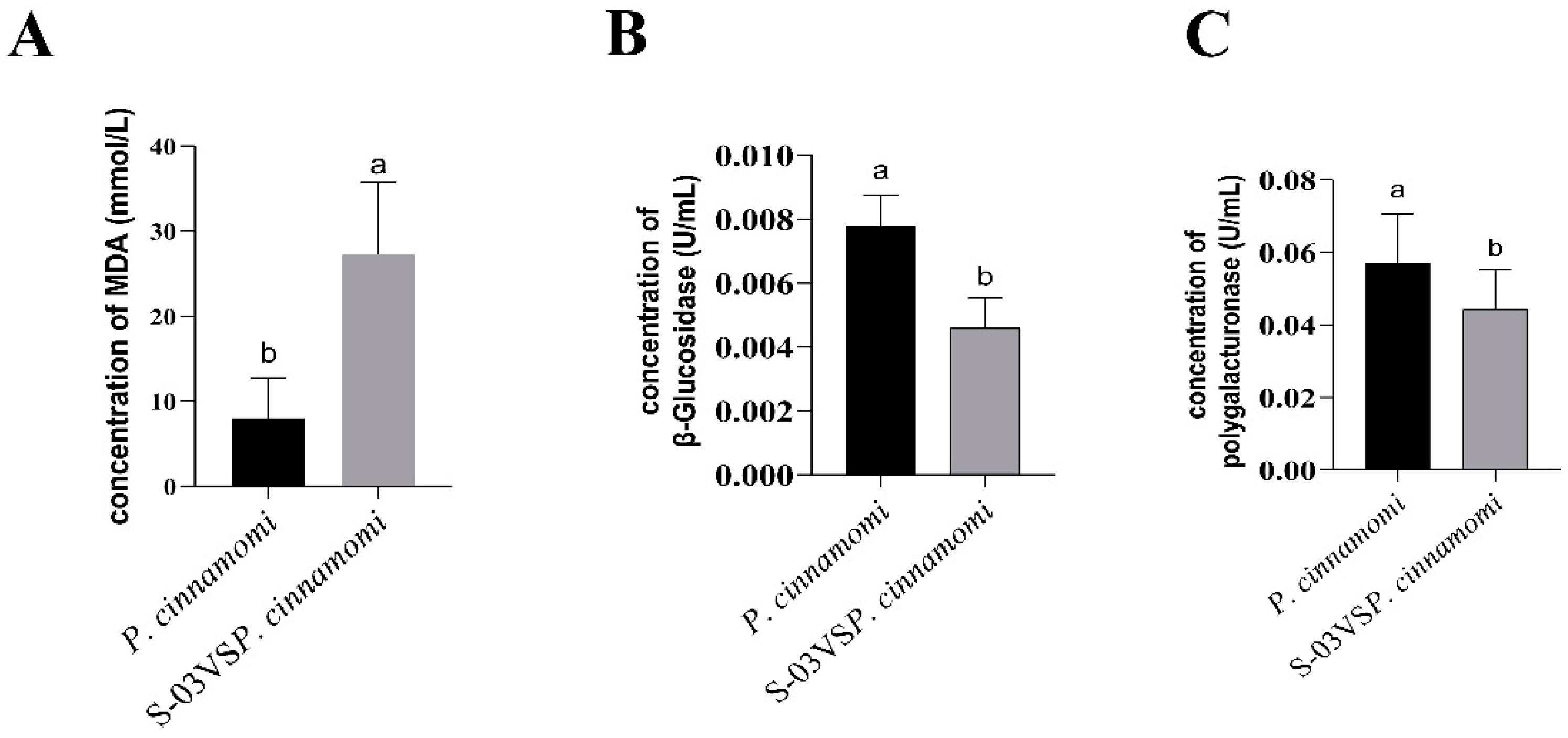
2.4. Effects of S-03 on Cell Membrane Permeability and Cell Wall-Degrading Enzyme Activities of Phytophthora cinnamomi
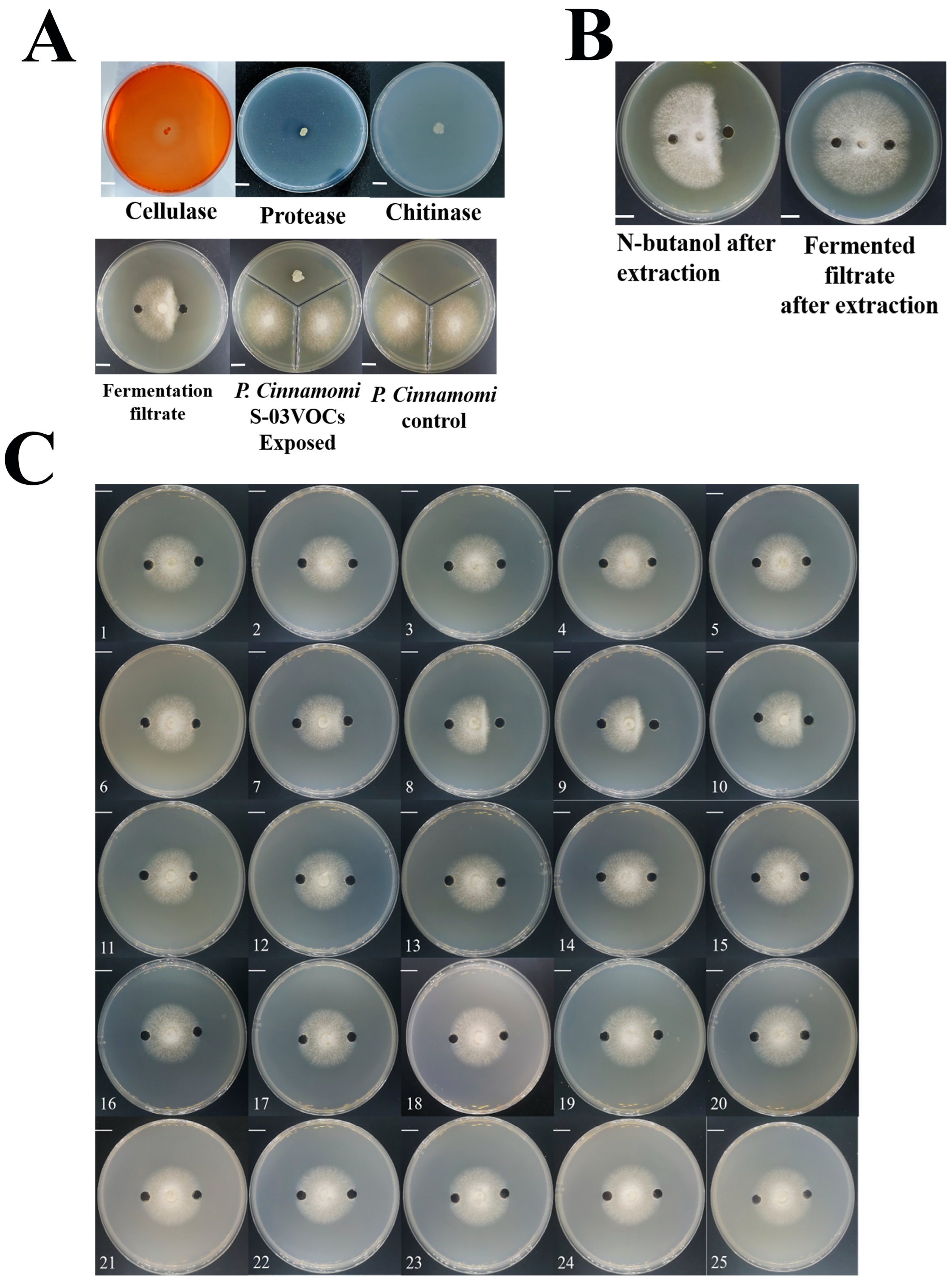
2.5. Detection of the Antagonistic Capacity of S-03
2.6. Extraction and Purification of Secondary Metabolites from S-03 Culture
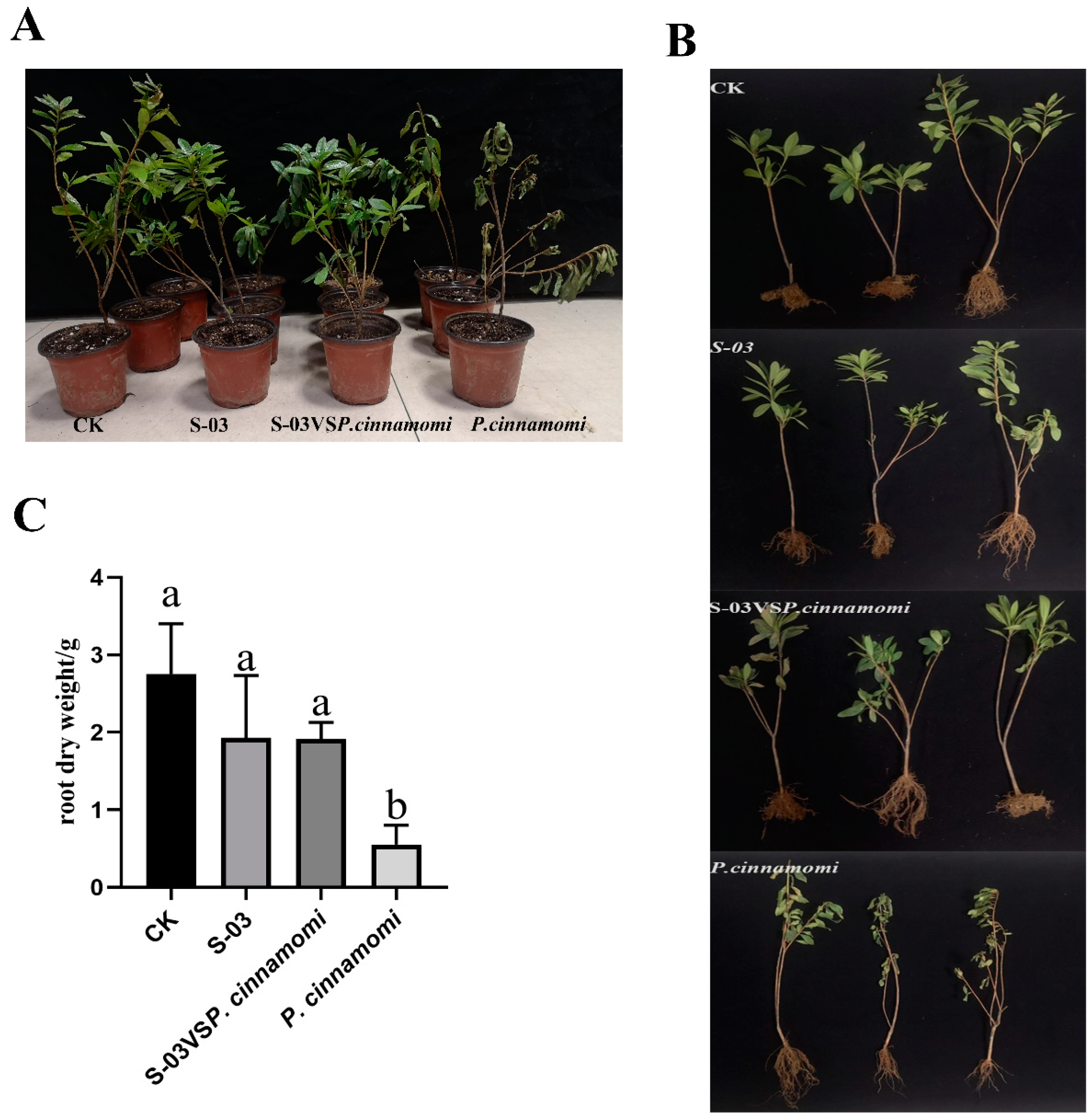
2.7. Biocontrol Effect of S-03 on Rhododendron Root Rot
3. Results
3.1. Molecular Biological Identification of Isolate S-03
3.2. Inhibition of Phytophthora cinnamomi by S-03
3.3. Effects of S-03 on Cell Membrane Permeability and Cell Wall-Degrading Enzyme Activities of Phytophthora cinnamomi
3.4. Detection of the Antifungal Capacity of S-03
3.5. Extraction and Purification of Secondary Metabolites from S-03 Culture
3.6. Biocontrol Effect of S-03 Fermentation Broth on Rhododendron Root Rot
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dai, T.; Wang, A.; Yang, X.; Yu, X.; Tian, W.; Xu, Y.; Hu, T. PHYCI_587572: An RxLR Effector Gene and New Biomarker in A Recombinase Polymerase Amplification Assay for Rapid Detection of Phytophthora cinnamomi. Forests 2020, 11, 306. [Google Scholar] [CrossRef]
- Andrade-Hoyos, P.; Victoria, H.; Romero-Arenas, O. Endophytic Trichoderma Species Isolated from Persea americana and Cinnamomum verum Roots Reduce Symptoms Caused by Phytophthora cinnamomi in Avocado. Plants 2020, 9, 1220. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Cantuarias-Avils, T.; Neto, H.B.; Filho, F.D.A.A.; Medina, R.B. Management of root rot in avocado trees. Rev. Bras. Frutic. 2016, 38, 175. [Google Scholar] [CrossRef]
- Hardham, A.R. Pathogen profile Phytophthora cinnamomi. Zhonghua Yi Xue Za Zhi 2005, 86, 3431–3434. [Google Scholar] [CrossRef]
- Ego, O.S.J.; Drenth, A.; Topp, B.; Henderson, J.; Akinsanmi, O.A. Prevalence of Phytophthora species in macadamia orchards in Australia and their ability to cause stem canker. Plant Pathol. 2020, 69, 1270–1280. [Google Scholar] [CrossRef]
- Reeksting, B.J.; Olivier, N.A.; Van Den Berg, N. Transcriptome responses of an ungrafted Phytophthora root rot tolerant avocado (Persea americana) rootstock to flooding and Phytophthora cinnamomi. BMC Plant Biol. 2016, 16, 205. [Google Scholar] [CrossRef] [PubMed]
- Loureno, D.; Branco, I.; Choupina, A. Phytopathogenic oomycetes: A review focusing on Phytophthora cinnamomi and biotechnological approaches. Mol. Biol. Rep. 2020, 47, 9179–9188. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Alguacil, M.; Pascual, J.A.; Wees, S. Phytohormone Profiles Induced by Trichoderma Isolates Correspond with Their Biocontrol and Plant Growth-Promoting Activity on Melon Plants. J. Chem. Ecol. 2014, 40, 804–815. [Google Scholar] [CrossRef]
- Aksoy, H.M.; Kaya, Y.; Ozturk, M.; Secgin, Z.; Onder, H.; Okumus, A. Pseudomonas putida—Induced Response in Phenolic Profile of Tomato Seedlings (Solanum lycopersicum L.) Infected by Clavibacter michiganensis subsp. michiganensis. Biol. Control 2016, 105, 6–12. [Google Scholar] [CrossRef]
- Srikhong, P.; Lertmongkonthum, K.; Sowanpreecha, R.; Rerngsamran, P. Bacillus sp. strain M10 as a potential biocontrol agent protecting chili pepper and tomato fruits from anthracnose disease caused by Colletotrichum capsici. Biocontrol 2018, 63, 833–842. [Google Scholar] [CrossRef]
- Brzezinska, M.S.; Jankiewicz, U.; Kalwasińska, A.; Witczak, J.; Ero, K. Characterization of chitinase from Streptomyces luridiscabiei U05 and its antagonist potential against fungal plant pathogens. J. Phytopathol. 2019, 167, 404–412. [Google Scholar] [CrossRef]
- Arfaoui, A.; Hadrami, A.E.; Adam, L.R.; Daayf, F. Combining Streptomyces hygroscopicus and phosphite boosts soybean’s defense responses to Phytophthora sojae. Biocontrol 2020, 65, 363–375. [Google Scholar] [CrossRef]
- Verma, V.C.; Singh, S.K.; Prakash, S. Bio-control and plant growth promotion potential of siderophore producing endophytic Streptomyces from Azadirachta indica A. Juss. J. Basic Microb. 2011, 51, 550–556. [Google Scholar] [CrossRef]
- Rubio, S.; Martínez-Cámara, S.; Fuente, J.; Rodríguez-Sáiz, M.; Barredo, J.L. Strain Improvement Program of Streptomyces roseosporus for Daptomycin Production; Humana: New York, NY, USA, 2021; Volume 2296, pp. 351–363. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, X.; Wu, C.; Chen, Z.; Dai, T. Whole-Genome Sequence Resource of Phytophthora pini, the Causal Pathogen of Foliage Blight and Shoot Dieback of Rhododendron pulchrum. Mol. Plant-Microbe Interact. 2022, 35, 944–948. [Google Scholar] [CrossRef]
- Xu, J.; Yang, X.; Wu, C.; Chen, Z.; Dai, T. Recombinase Polymerase Amplification–Lateral Flow Dipstick Assay for Rapid Detection of Fusarium circinatum Based on a Newly Identified Unique Target Gene. Plant Dis. 2023, 107, 1067–1074. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, W.-L.; Wu, X.-Q.; Li, P.-S. Inhibitory Effects of Phenazine Compounds and Volatile Organic Compounds Produced by Pseudomonas aurantiaca ST-TJ4 Against Phytophthora cinnamomi. Phytopathology 2022, 112, 1867–1876. [Google Scholar] [CrossRef]
- Boukaew, S.; Plubrukam, A.; Prasertsan, P. Effect of volatile substances from Streptomyces philanthi RM-1-138 on growth of Rhizoctonia solani on rice leaf. Biocontrol 2013, 58, 471–482. [Google Scholar] [CrossRef]
- Chen, J.; Xue, Q.H.; McErlean, C.S.P.; Zhi, J.H.; Ma, Y.Q.; Jia, X.T.; Zhang, M.; Ye, X.X. Biocontrol potential of the antagonistic microorganism Streptomyces enissocaesilis against Orobanche cumana. Biocontrol 2016, 61, 781–791. [Google Scholar] [CrossRef]
- Sena, K.; Crocker, E.; Vincelli, P.; Barton, C. Phytophthora cinnamomi as a driver of forest change: Implications for conservation and management. For. Ecol. Manag. 2018, 409, 799–807. [Google Scholar] [CrossRef]
- Dunstan, W.A.; Rudman, T.; Shearer, B.L.; Moore, N.A.; Paap, T.; Calver, M.C.; Dell, B.; Hardy, G. Containment and spot eradication of a highly destructive, invasive plant pathogen (Phytophthora cinnamomi) in natural ecosystems. Biol. Invasions 2010, 12, 913–925. [Google Scholar] [CrossRef]
- Romero, M.A.; González, M.; Serrano, M.S.; Sánchez, M.E. Trunk injection of fosetyl luminium controls the root disease caused by Phytophthora cinnamomi on Quercus ilex woodlands. Ann. Appl. Biol. 2019, 174, 313–318. [Google Scholar] [CrossRef]
- Barrett, S.R.; Rathbone, D. Long-term phosphite application maintains species assemblages, richness and structure of plant communities invaded by Phytophthora cinnamomi. Aust. Ecol. 2018, 43, 360–374. [Google Scholar] [CrossRef]
- González, M.; Caetano, P.; Sánchez, M.E. Testing systemic fungicides for control of Phytophthora oak root disease. For. Pathol. 2017, 47, e12343. [Google Scholar] [CrossRef]
- Keast, D.; Tonkin, C.; San Fe Lieu, L. Effects of Copper Salts on Growth and Survival of Phytophthora cinnamomi in vitro and on the Antifungal Activity of Actinomycete Populations from the Roots of Eucalyptus marginata and Banksia grandis. Aust. J. Bot. 1985, 33, 115–129. [Google Scholar] [CrossRef]
- Neves, D.; Caetano, P.; Oliveira, J.; Maia, C.; Horta, M.; Sousa, N.; Salgado, M.; Dionísio, L.; Magan, N.; Cravador, A. Anti-Phytophthora cinnamomi activity of Phlomis purpurea plant and root extracts. Eur. J. Plant Pathol. 2014, 138, 835–846. [Google Scholar] [CrossRef]
- Silva, L.J.; Taketani, R.G.A.; Melo, I.S.; Goodfellow, M.; Zucchi, T.D. Streptomyces araujoniae sp. nov.: An actinomycete isolated from a potato tubercle. Antonie Van Leeuwenhoek 2013, 103, 1235–1244. [Google Scholar] [CrossRef]
- Silva, L.J.; Crevelin, E.J.; Souza, W.R.; Moraes, L.A.; Zucchi, T.D. Streptomyces araujoniae Produces a Multiantibiotic Complex with Ionophoric Properties to Control Botrytis cinerea. Phytopathology 2014, 104, 1298–1305. [Google Scholar] [CrossRef]
- Appiah, A.A.; West, P.V.; Osborne, M.C.; Gow, N. Potassium homeostasis influences the locomotion and encystment of zoospores of plant pathogenic oomycetes. Fungal Genet. Biol. 2005, 42, 213–223. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Y.; Lu, J.; Liang, M.; Huang, S. Identification and Evaluation of UV-Tolerant Streptomyces araujoniae Strain Isolated from Tibet Plateau Soil as Biocontrol Agent Against Rice Blast. Rice Sci. 2020, 27, 359–362. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Wang, Q.; Li, Y.; Shen, Z.; Han, X.; Chen, P.; Shao, L.; Fan, B.; Zhao, Y. Secondary Metabolites from Streptomyces araujoniae S-03 Show Biocontrol Potential against Rhododendron Root Rot Caused by Phytophthora cinnamomi. Forests 2023, 14, 2054. https://doi.org/10.3390/f14102054
Sun Z, Wang Q, Li Y, Shen Z, Han X, Chen P, Shao L, Fan B, Zhao Y. Secondary Metabolites from Streptomyces araujoniae S-03 Show Biocontrol Potential against Rhododendron Root Rot Caused by Phytophthora cinnamomi. Forests. 2023; 14(10):2054. https://doi.org/10.3390/f14102054
Chicago/Turabian StyleSun, Zhimin, Qiuqin Wang, Yulong Li, Zizhu Shen, Xingshan Han, Peng Chen, Lin Shao, Ben Fan, and Yinjuan Zhao. 2023. "Secondary Metabolites from Streptomyces araujoniae S-03 Show Biocontrol Potential against Rhododendron Root Rot Caused by Phytophthora cinnamomi" Forests 14, no. 10: 2054. https://doi.org/10.3390/f14102054
APA StyleSun, Z., Wang, Q., Li, Y., Shen, Z., Han, X., Chen, P., Shao, L., Fan, B., & Zhao, Y. (2023). Secondary Metabolites from Streptomyces araujoniae S-03 Show Biocontrol Potential against Rhododendron Root Rot Caused by Phytophthora cinnamomi. Forests, 14(10), 2054. https://doi.org/10.3390/f14102054





