Water Vapor Sorption Kinetics of Beech Wood Modified with Phenol Formaldehyde Resin Oligomers
Abstract
1. Introduction
2. Materials and Methods
2.1. Wood Materials
2.2. Wood Modifier
2.3. Wood Treatment
2.4. Dynamic Vapor Sorption (DVS)
3. Results
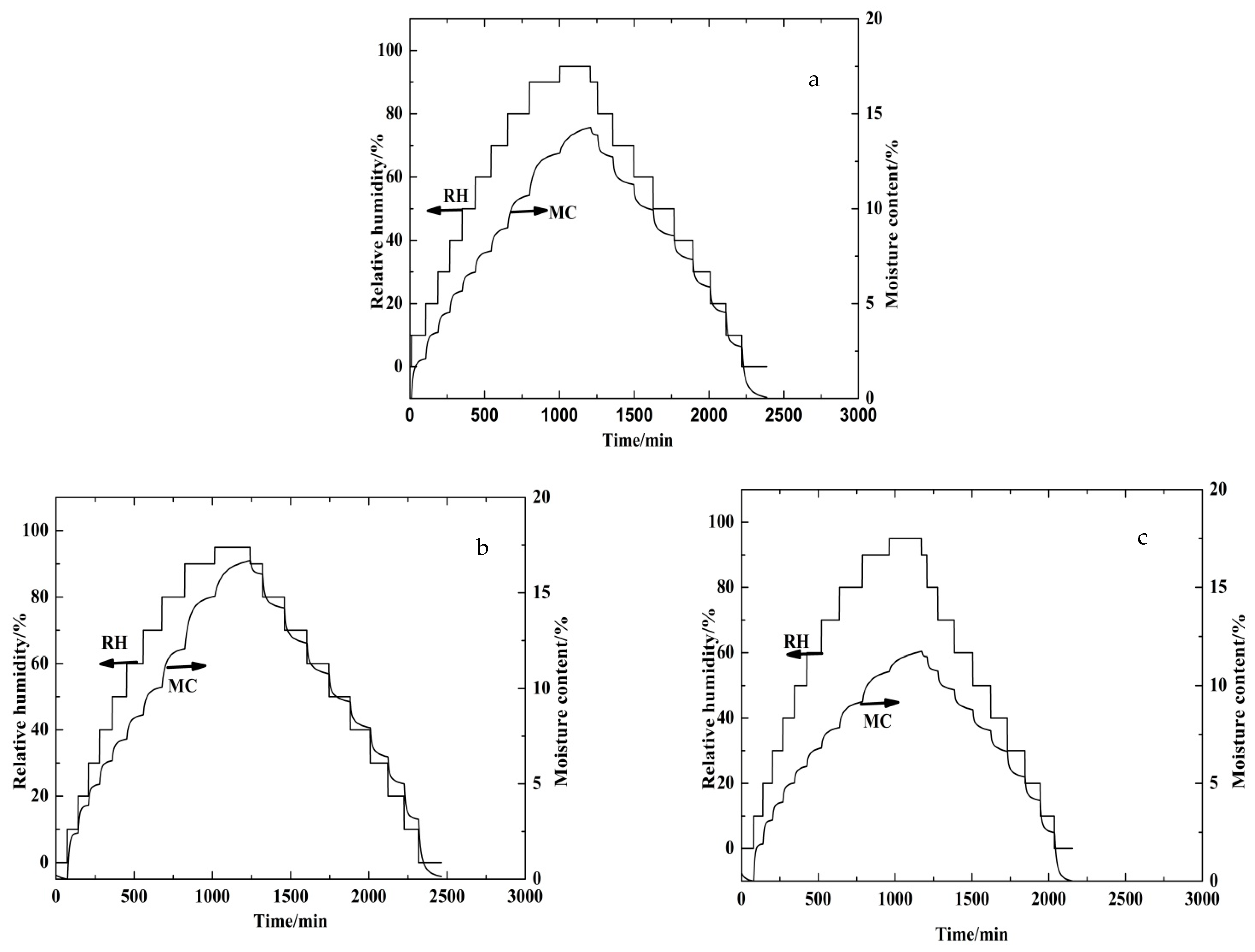
3.1. Dynamic Vapor Sorption (DVS) Behavior of Beech Wood
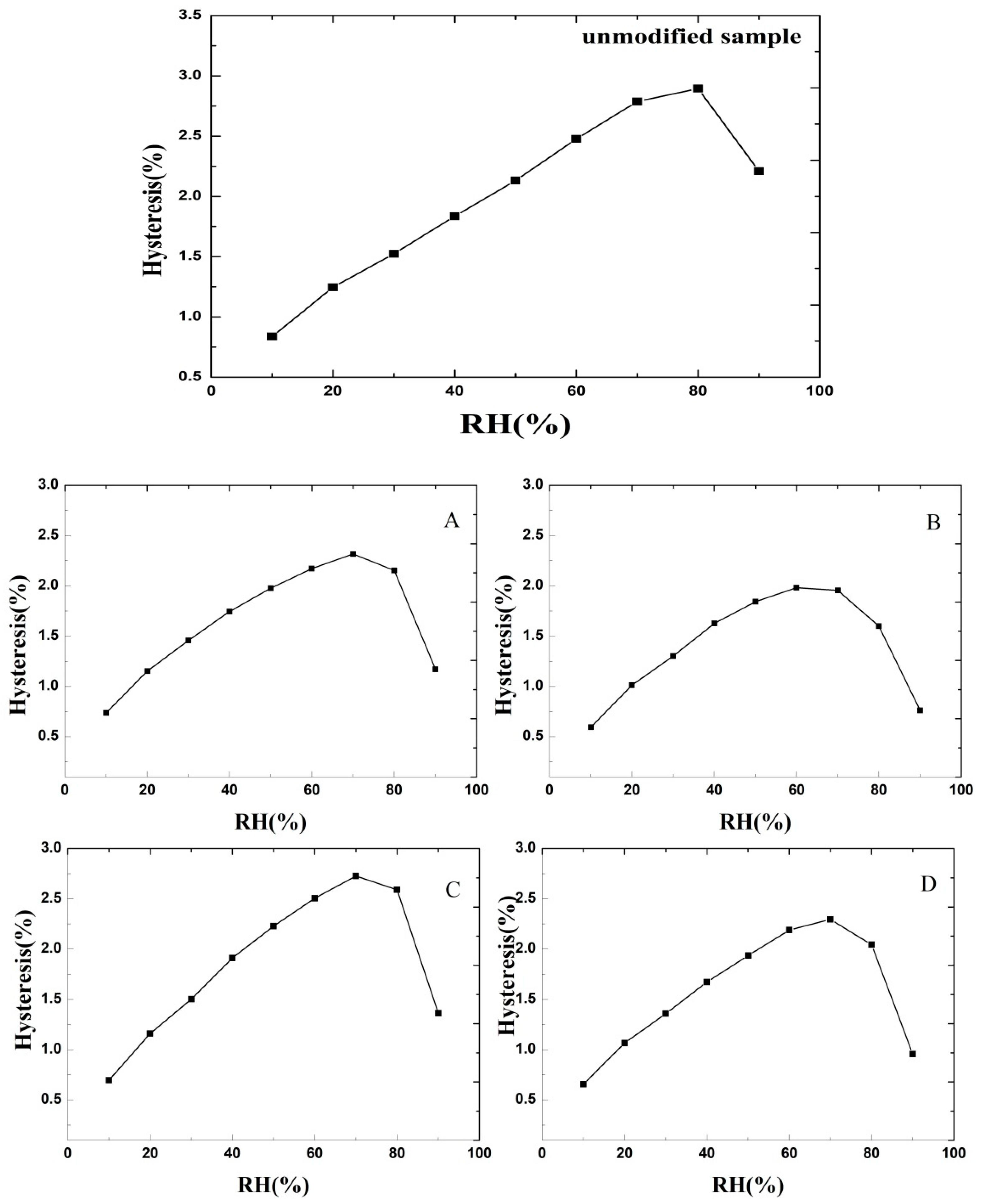
3.2. DVS Isotherm Hysteresis Plot
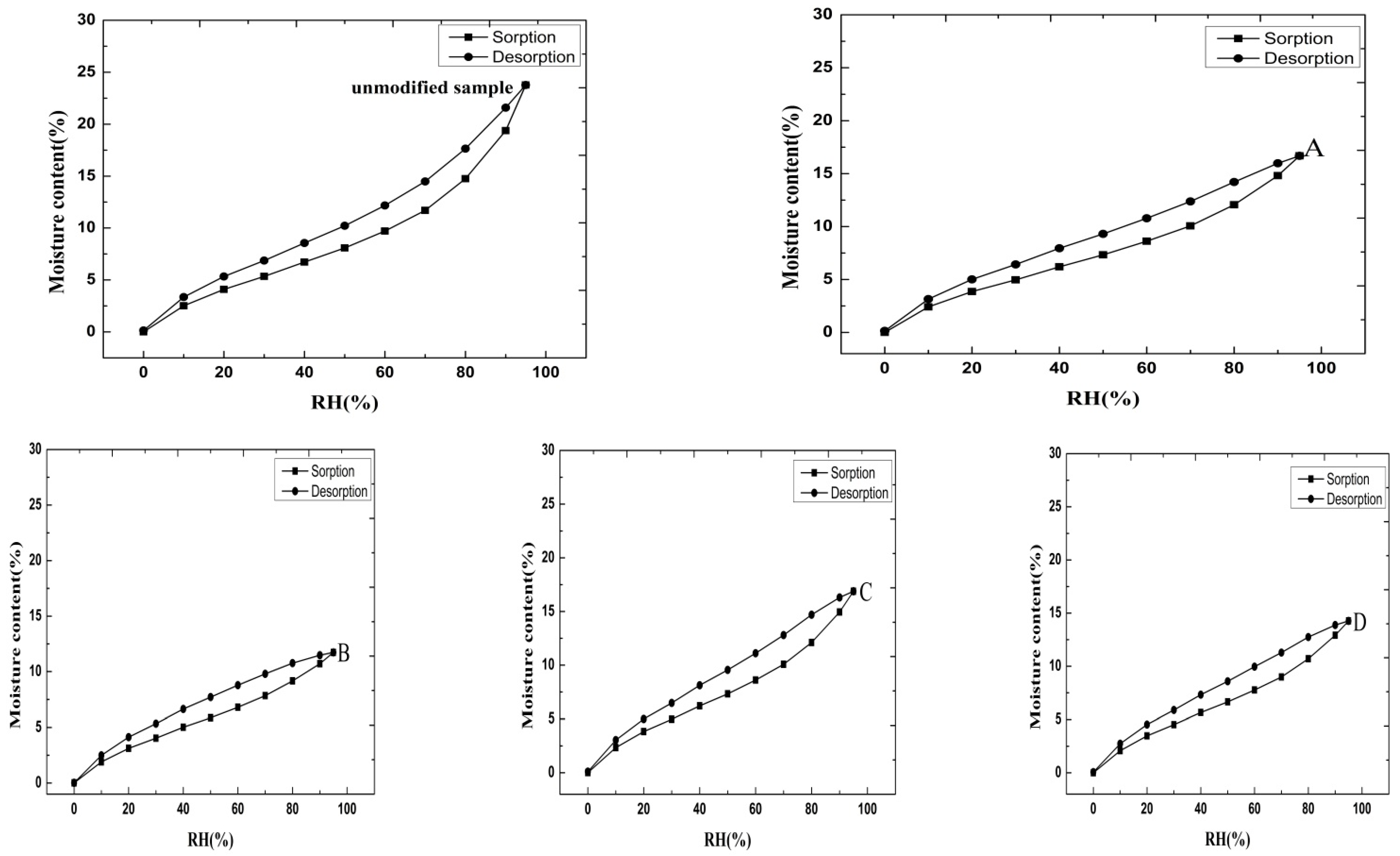
3.3. DVS Isotherm Adsorption and Desorption Plot
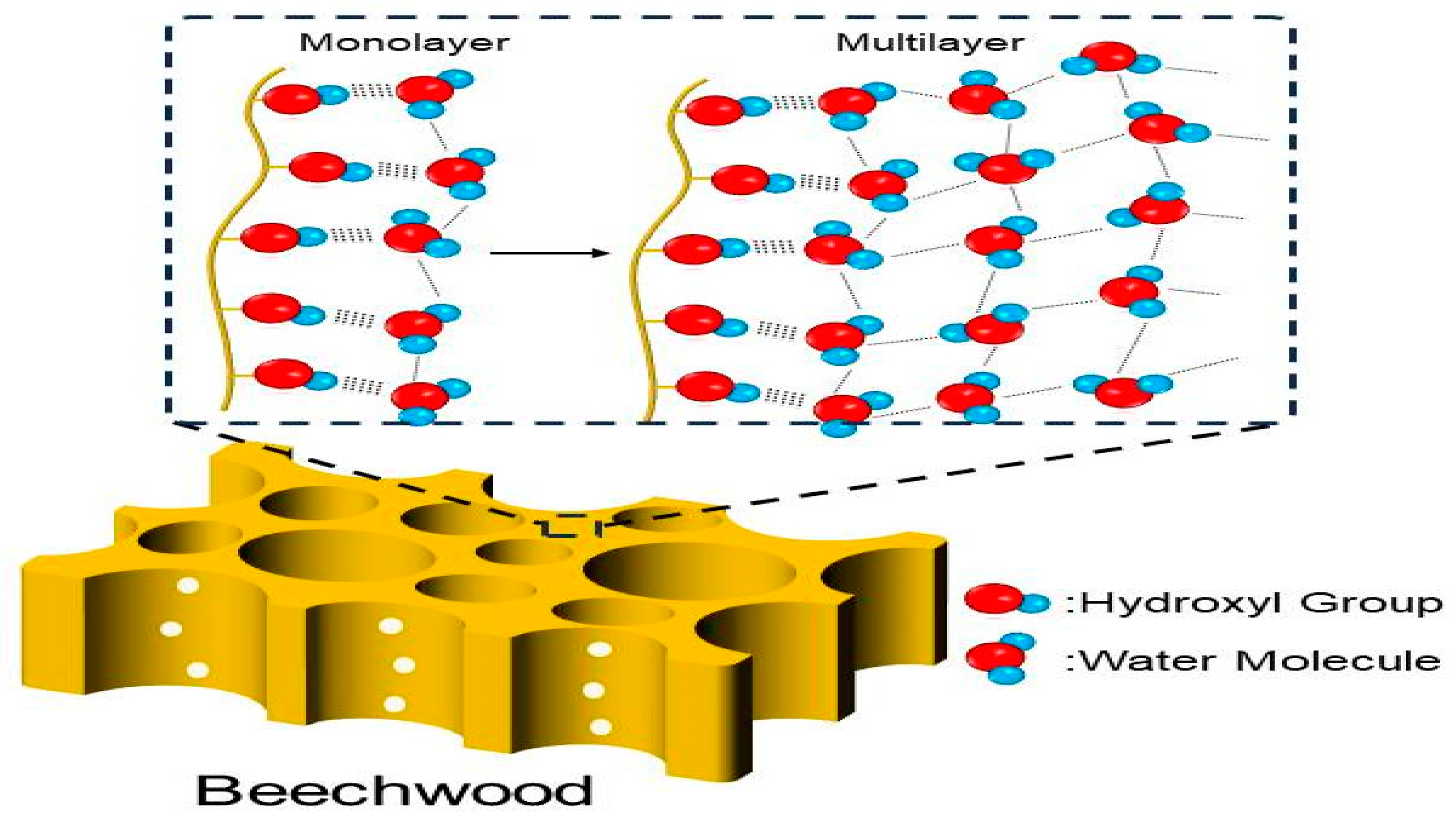
3.4. Mechanism of Adsorption and Desorption of Beech Wood Modified with PF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, Y.; Hill, C.A.; Xiao, Z.; Jalaludin, Z.; Militz, H.; Mai, C. Water vapor sorption kinetics of wood modified with glutaraldehyde. J. Appl. Polym. Sci. 2010, 117, 1674–1682. [Google Scholar] [CrossRef]
- Beck, G.; Strohbusch, S.; Larnøy, E.; Militz, H.; Hill, C. Accessibility of hydroxyl groups in anhydride modified wood as measured by deuterium exchange and saponification. Holzforschung 2018, 72, 17–23. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.; Xiao, Z.; Mai, C.; Militz, H. Dynamic water vapour sorption properties of wood treated with glutaraldehyde. Wood Sci. Technol. 2011, 45, 49–61. [Google Scholar] [CrossRef][Green Version]
- Gabrielli, C.P.; Kamke, F.A. Phenol–formaldehyde impregnation of densified wood for improved dimensional stability. Wood Sci. Technol. 2010, 44, 95–104. [Google Scholar] [CrossRef]
- Ghavidel, A.; Hosseinpourpia, R.; Militz, H.; Vasilache, V.; Sandu, I. Characterization of Archaeological European White Elm (Ulmus laevis P.) and Black Poplar (Populus nigra L.). Forests 2020, 11, 1329. [Google Scholar] [CrossRef]
- Aicher, S.; Zachary, C.; Maren, H. Rolling shear modulus and strength of beech wood laminations. Holzforschung 2018, 70, 773–781. [Google Scholar] [CrossRef]
- Gardiner, B.; Barnett, J.; Saranpää, P. The Biology of Reaction Wood; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Hill, C.; Moore, J.; Jalaludin, Z.; Leveneu, M.; Mahrdt, E. Influence of earlywood/latewood and ring position upon water vapour sorption properties of Sitka spruce. Int. Wood Prod. J. 2011, 2, 12–19. [Google Scholar] [CrossRef]
- Emmerich, L.; Altgen, M.; Rautkari, L.; Militz, H. Sorption behavior and hydroxyl accessibility of wood treated with different cyclic N-methylol compounds. J. Mater. Sci. 2020, 55, 16561–16575. [Google Scholar] [CrossRef]
- Kurkowiak, K.; Emmerich, L.; Militz, H. Sorption behavior and swelling of citric acid and sorbitol (SorCA) treated wood. Holzforschung 2021, 75, 1136–1149. [Google Scholar] [CrossRef]
- Hailwood, A.J.; Horrobin, S. Absorption of water by polymers: Analysis in terms of a simple model. Trans. Faraday Soc. 1946, 42, B084–B092. [Google Scholar] [CrossRef]
- Hill, C.A.; Norton, A.; Newman, G. The water vapor sorption behavior of flax fibers—Analysis using the parallel exponential kinetics model and determination of the activation energies of sorption. J. Appl. Polym. Sci. 2010, 116, 2166–2173. [Google Scholar] [CrossRef]
- Krabbenhoft, K.; Damkilde, L. A model for non-Fickian moisture transfer in wood. Mater. Struct. 2004, 37, 615–622. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.; Jalaludin, Z.; Sun, D. The water vapour sorption behaviour of three celluloses: Analysis using parallel exponential kinetics and interpretation using the Kelvin-Voigt viscoelastic model. Cellulose 2011, 18, 517–530. [Google Scholar] [CrossRef]
- Murakami, S.; Maeda, H.; Imamura, S. Fuzzy decision analysis on the development of centralized regional energy control system. IFAC Proc. Vol. 1983, 16, 363–368. [Google Scholar] [CrossRef]
- Rodwell, M.J.; Rowell, D.P.; Folland, C.K. Oceanic forcing of the wintertime North Atlantic Oscillation and European climate. Nature 1999, 398, 320–323. [Google Scholar] [CrossRef]
- Kajita, H.; Imamura, Y. Improvement of physical and biological properties of particleboards by impregnation with phenolic resin. Wood Sci. Technol. 1991, 26, 63–70. [Google Scholar] [CrossRef]
- Shams, M.I.; Yano, H.; Endou, K. Compressive deformation of wood impregnated with low molecular weight phenol formaldehyde (PF) resin I: Effects of pressing pressure and pressure holding. J. Wood Sci. 2004, 50, 337–342. [Google Scholar] [CrossRef]
- Xiao, Z.; Xie, Y.; Militz, H.; Mai, C. Modification of wood with glutaraldehyde. In Proceedings of the 4th European Conference on Wood Modification, Stockholm, Sweden, 13 May 2009. [Google Scholar]
- Hill, C.A.; Norton, A.; Newman, G. The water vapor sorption behavior of natural fibers. J. Appl. Polym. Sci. 2009, 112, 1524–1537. [Google Scholar] [CrossRef]
- Lu, Y.; Pignatello, J.J. Demonstration of the “conditioning effect” in soil organic matter in support of a pore deformation mechanism for sorption hysteresis. Environ. Sci. Technol. 2002, 36, 4553–4561. [Google Scholar] [CrossRef]
- Yasuda, R.; Minato, K. Chemical modification of wood by non-formaldehyde cross-linking reagents: Part 3. Mechanism of dimensional stabilization by glyoxal treatment and effect of the addition of glycol. Wood Sci. Technol. 1995, 29, 243–251. [Google Scholar] [CrossRef]
- Hosseinpourpia, R.; Adamopoulos, S.; Holstein, N.; Mai, C. Dynamic vapour sorption and water-related properties of thermally modified Scots pine (Pinus sylvestris L.) wood pre-treated with proton acid. Polym. Degrad. Stab. 2017, 138, 161–168. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Liu, M.; Guo, F.; Wang, H.; Ren, W.; Cao, M.; Yu, Y. Highly stable wood material with low resin consumption via vapor phase furfurylation in cell walls. ACS Sustain. Chem. Eng. 2020, 8, 13924–13933. [Google Scholar] [CrossRef]
- Shen, X.; Guo, D.; Jiang, P.; Yang, S.; Li, G.; Chu, F. Water vapor sorption mechanism of furfurylated wood. J. Mater. Sci. 2021, 56, 11324–11334. [Google Scholar] [CrossRef]
- Lang, Q.; Biziks, V.; Militz, H. Influence of Phenol–Formaldehyde Resin Oligomer Molecular Weight on the Strength Properties of Beech Wood. Forests 2022, 13, 1980. [Google Scholar] [CrossRef]

| Resin | Molecular Weight Mn [g/mol] | Solid Content [%] | Catalyst | Amount of Formaldehyde [%] | Free Phenol [%] |
|---|---|---|---|---|---|
| A | 266 | 49.5 | NaOH | <1 | <4 |
| B | 286 | 49.8 | NaOH | <1 | <4 |
| C | 387 | 49.0 | NaOH | <1 | <4 |
| D | 410 | 58.4 | NaOH | 1.88 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, Q.; Biziks, V.; Militz, H. Water Vapor Sorption Kinetics of Beech Wood Modified with Phenol Formaldehyde Resin Oligomers. Forests 2023, 14, 2015. https://doi.org/10.3390/f14102015
Lang Q, Biziks V, Militz H. Water Vapor Sorption Kinetics of Beech Wood Modified with Phenol Formaldehyde Resin Oligomers. Forests. 2023; 14(10):2015. https://doi.org/10.3390/f14102015
Chicago/Turabian StyleLang, Qian, Vladimirs Biziks, and Holger Militz. 2023. "Water Vapor Sorption Kinetics of Beech Wood Modified with Phenol Formaldehyde Resin Oligomers" Forests 14, no. 10: 2015. https://doi.org/10.3390/f14102015
APA StyleLang, Q., Biziks, V., & Militz, H. (2023). Water Vapor Sorption Kinetics of Beech Wood Modified with Phenol Formaldehyde Resin Oligomers. Forests, 14(10), 2015. https://doi.org/10.3390/f14102015





