The Sink–Source Relationship Regulated Camellia oleifera Flower Bud Differentiation by Influencing Endogenous Hormones and Photosynthetic Characteristics
Abstract
1. Introduction
2. Materials and Methods
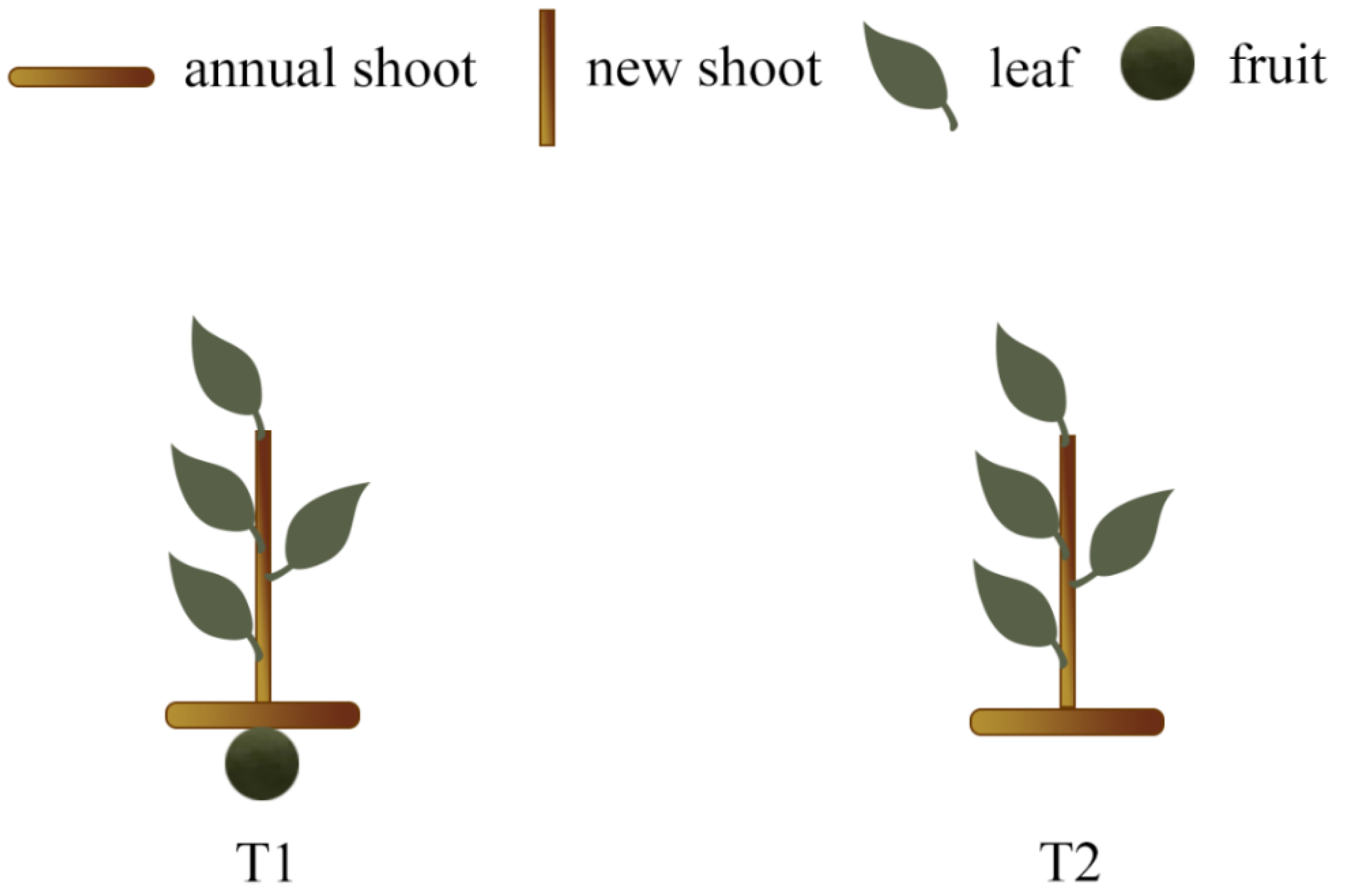
2.1. Experimental Site Description and Plant Materials
2.2. Investigation of Flower Bud Differentiation Rate
2.3. Determination of Endogenous Hormones
2.4. Determination of Carbohydrates
2.5. Determination of Photosynthetic Characteristics
2.6. 13C Labeling and Determination Analysis
2.7. Statistical Analysis
3. Results
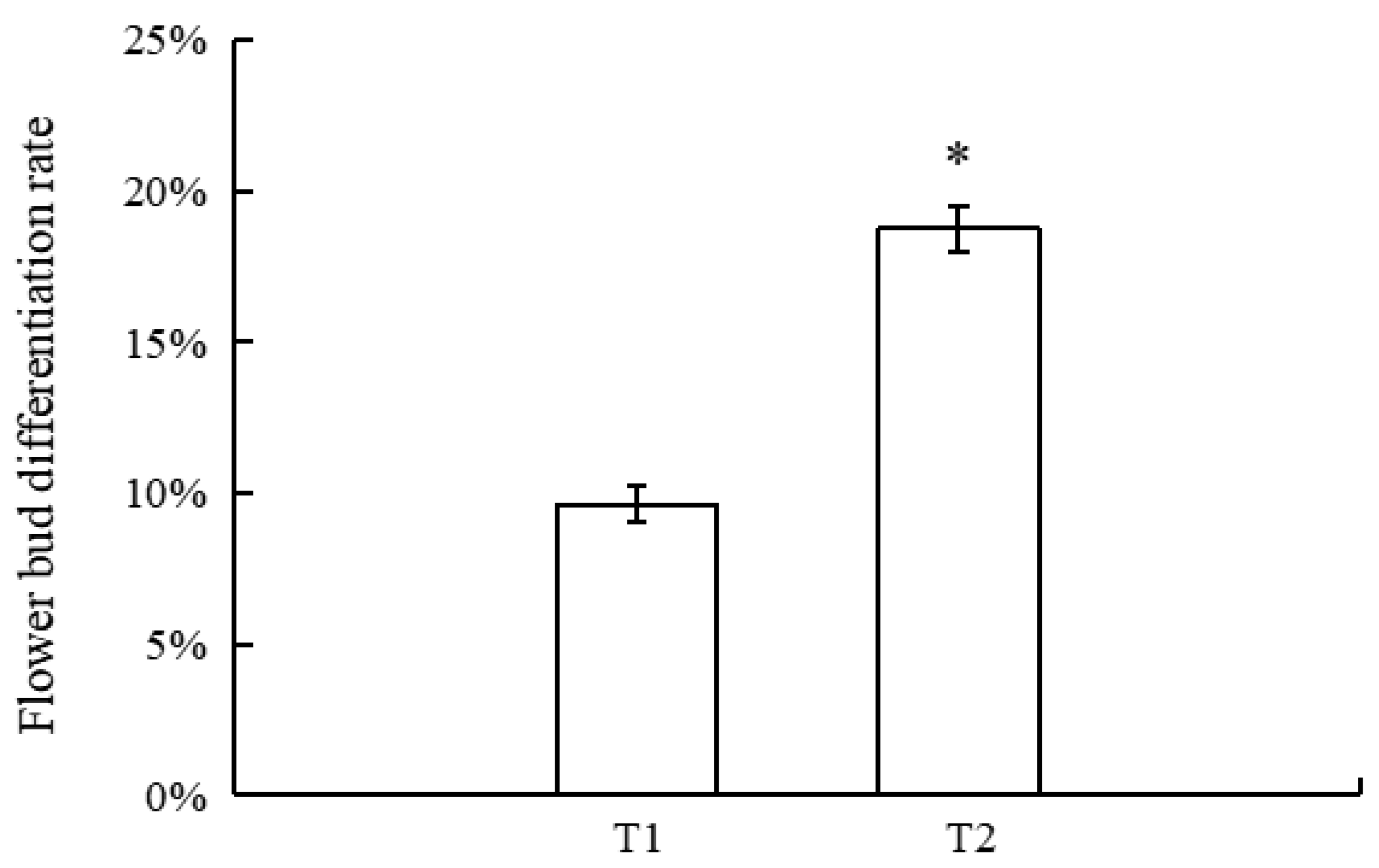
3.1. Flower Bud Differentiation Rate of New Shoots in Different Sink–Source Relationships at Late Flower Bud Differentiation
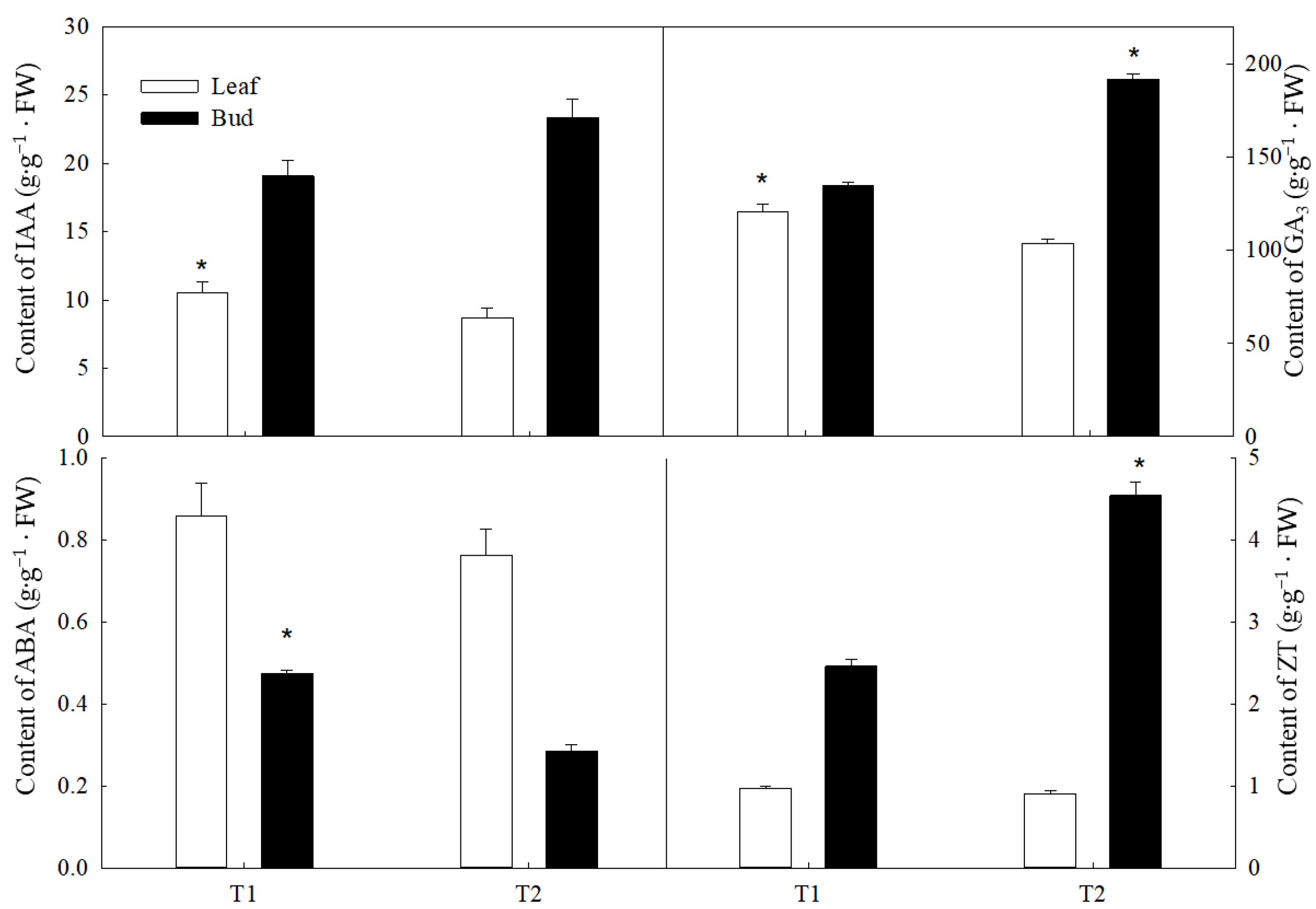
3.2. Endogenous Hormones of New Shoots in Different Sink–Source Relationships at the Preflower Bud Differentiation Stage
3.3. Soluble Sugar and Starch Content of New Shoots in Different Sink–Source Relationships at Different Stages
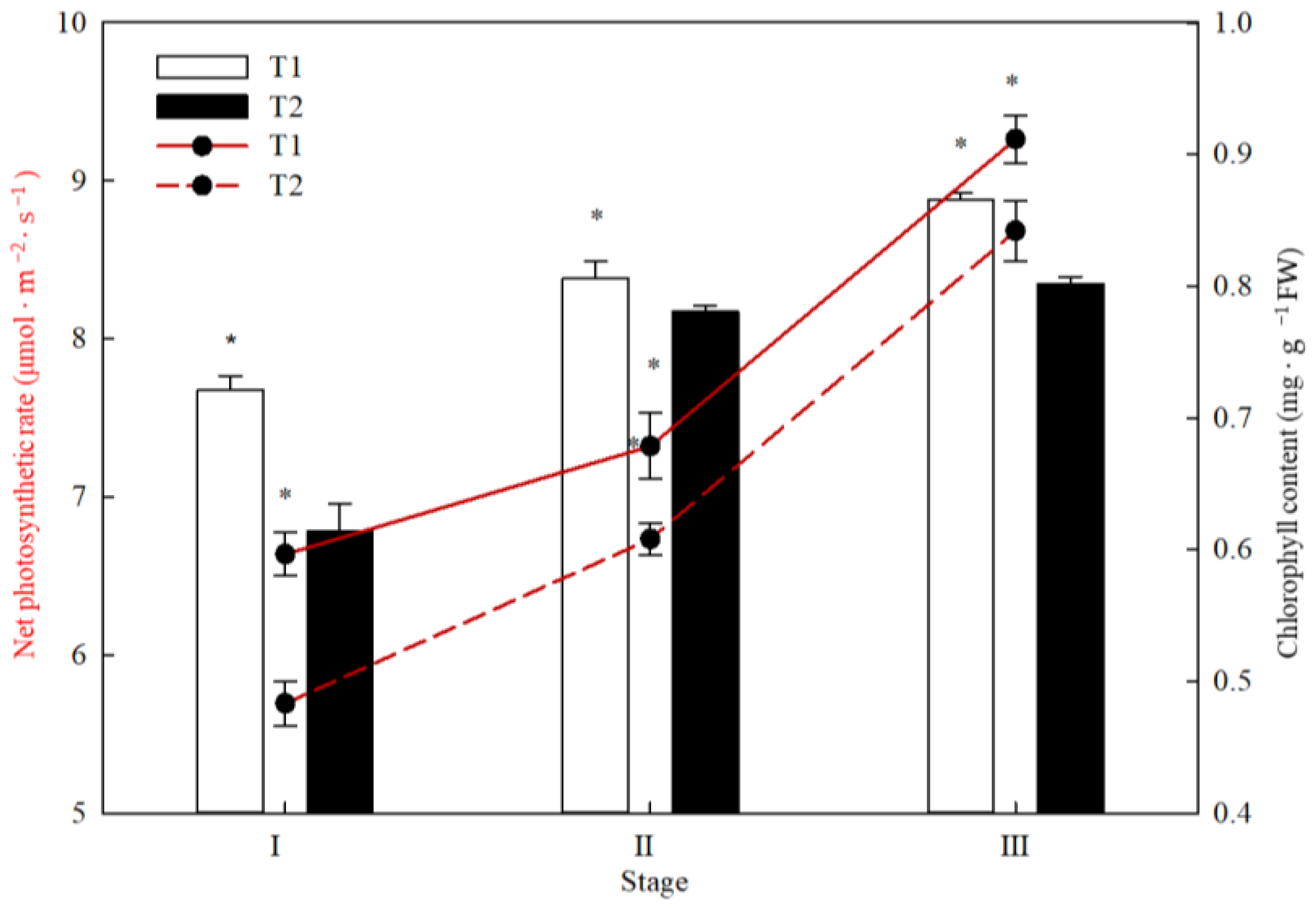
3.4. Chlorophyll Content and Net Photosynthetic Rate of New Shoots in Different Sink–Source Relationships at Different Stages
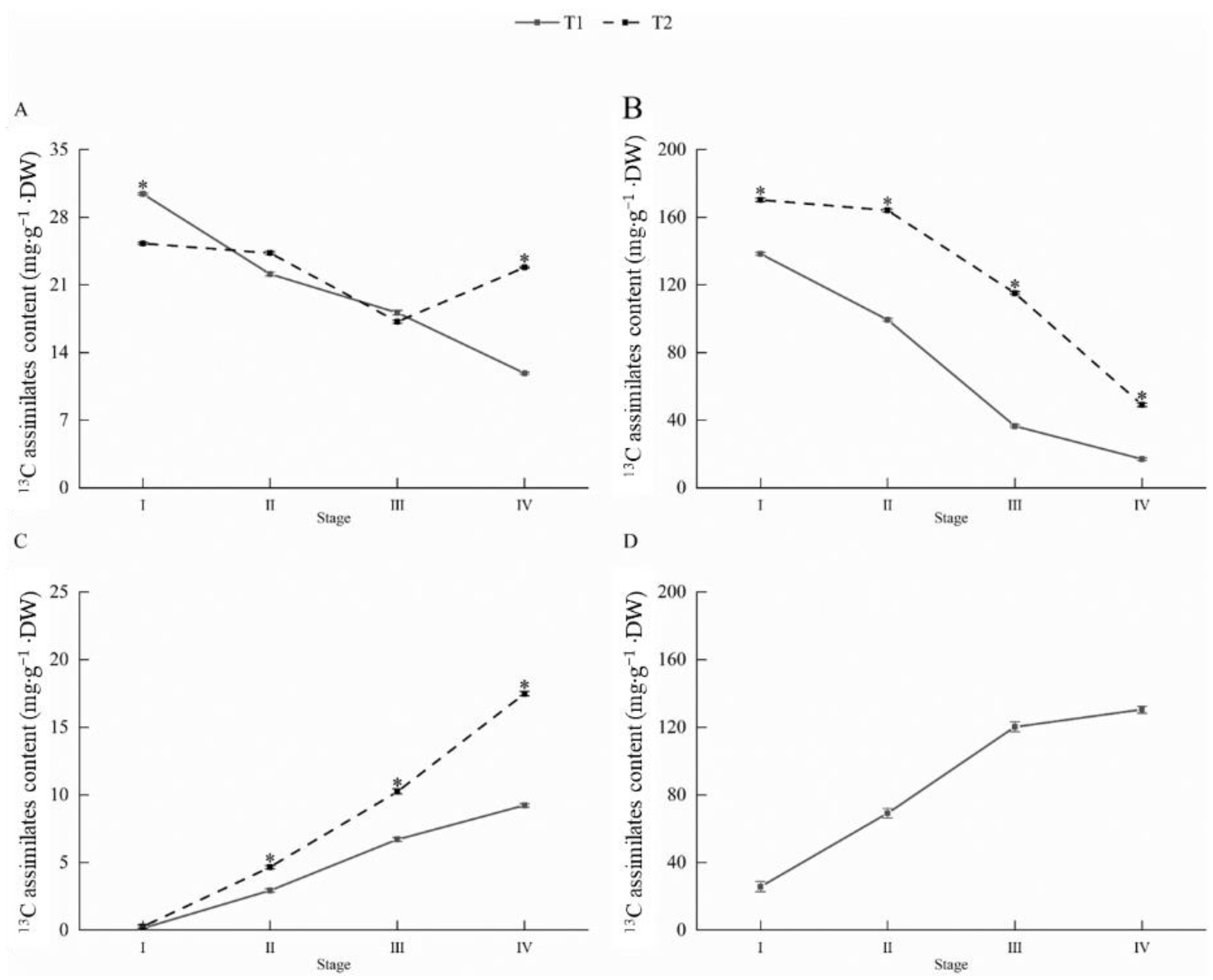
3.5. 13C Assimilate Accumulation and Allocation of New Shoots in Different Sink–Source Relationships at Different Stages
4. Discussion
4.1. Effect of Sink–Source Relationship on Endogenous Hormones and Its Relationship with Flower Bud Differentiation
4.2. Effect of the Sink–Source Relationship on Photosynthetic Characteristics and Its Relationship with Flower Bud Differentiation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heidari, H. Source-sink relationship in wheat as affected by defoliation and spikelet removal. Acta Fytotech. Zootech. 2022, 25, 40–45. [Google Scholar] [CrossRef]
- Moing, A.; Gutierrez, A.E.; Gaudillere, J.P. Modeling carbon export out of mature peach leaves. Plant Physiol. 1994, 106, 591–600. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Syvertsen, J.P.; Goñi, C.; Otero, A. Fruit load and canopy shading affect leaf characteristics and net gas exchange of ‘Spring’ navel orange trees. Tree Physiol. 2003, 23, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Lacointe, A.; Isebrands, J.G.; Host, G.E. A new way to account for the effect of source-sink spatial relationships in whole plant carbon allocation models. Can. J. For. Res. 2002, 32, 1838–1848. [Google Scholar] [CrossRef]
- Chang, T.G.; Zhu, X.G. Source–sink interaction: A century old concept under the light of modern molecular systems biology. J. Exp. Bot. 2017, 68, 4417–4431. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Bishnoi, C. Assimilate partitioning and distribution in fruit crops: A review. J. Pharmacogn. Phytochem. 2017, 6, 479–484. [Google Scholar]
- Pawar, R.; Rana, V.S. Manipulation of Source-Sink Relationship in Pertinence to Better Fruit Quality and Yield in Fruit Crops: A Review. Agric. Rev. 2019, 40, 1–8. [Google Scholar] [CrossRef]
- Nii, N. Changes of starch and sorbitol in leaves before and after removal of fruits from peach trees. Ann. Bot. 1997, 79, 139–144. [Google Scholar] [CrossRef]
- Domingo, J.I.; Ignacio, L.; Francisco, R.T.; Manuel, T. Regulation of photosynthesis through source: Sink imbalance in citrus is mediated by carbohydrate content in leaves. Physiol. Plant. 2002, 116, 563–572. [Google Scholar]
- Lv, X.; Zhang, Y.; Zhang, Y.; Fan, S.; Kong, L. Source-sink modifications affect leaf senescence and grain mass in wheat as revealed by proteomic analysis. BMC Plant Biol. 2020, 20, 1–17. [Google Scholar] [CrossRef]
- Kiseleva, I.S.; Kaminskaya, O.A. Hormonal regulation of assimilate utilization in barley leaves in relation to the development of their source function. Russ. J. Plant Physiol. 2002, 49, 534–540. [Google Scholar] [CrossRef]
- Guo, Y.F.; Ren, G.D.; Zhang, K.W.; Li, Z.H.; Miao, Y.; Guo, H.W. Leaf senescence: Progression regulation and application. Mol. Hortic. 2021, 1, 1–25. [Google Scholar] [CrossRef]
- Yang, S.Y. Study on Pruning Techniques of Camellia Oleifera Based on the Analysis of Canopy Microclimate. Dissertation’s Thesis, Beijing Forestry University, Beijing, China, 2015. [Google Scholar]
- Yuan, J.; Shi, B.; Wu, Z.L.; Tan, X.F. Response of fruit quality and leaf photosynthesis to different sink-source relationships in Camellia oleifera. Plant Physiol. J. 2015, 51, 1287–1292. [Google Scholar]
- Wen, Y.; Su, S.C.; Jia, T.T.; Wang, X.N. Allocation of photoassimilates in bud and fruit from different leaf nodes of Camellia oleifera. HortScience 2021, 56, 469–477. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, Y.; Xu, Y.; Wang, R.; Chen, L.; Ma, L.; Peng, S. Effects of sink-source manipulation on carbohydrate content and enzyme activity of Camellia oleifera leaves. J. Southwest For. Univ. 2018, 38, 41–45. [Google Scholar]
- Seung, D.; Risopatron, J.P.M.; Jones, B.J.; Marc, J. Circadian clock-dependent gating in ABA signalling networks. Protoplasma 2012, 249, 445–457. [Google Scholar] [CrossRef]
- Tsai, A.Y.L.; Gazzarrini, S. Trehalose-6-phosphate and SnRK1 kinases in plant development and signaling: The emerging picture. Front. Plant Sci. 2014, 5, 119. [Google Scholar] [CrossRef]
- Villar, L.; Lienqueo, I.; Llanes, A.; Rojas, P.; Perez, J.; Correa, F.; Almada, R. Comparative transcriptomic analysis reveals novel roles of transcription factors and hormones during the flowering induction and floral bud differentiation in sweet cherry trees (Prunus avium L. cv. Bing). PLoS ONE 2020, 15, e0230110. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Q.; Ni, J.; Gao, Y.; Tang, Y.; Bai, S.; Teng, Y. Early defoliation induces auxin redistribution, promoting paradormancy release in pear buds. Plant Physiol. 2022, 190, 2739–2756. [Google Scholar] [CrossRef]
- You, Y.G.; Li, Z.A.; Hong, Y.Y.; Miao, M.Y. Endogenous Hormones and Biochemical Changes during Flower Development and Florescence in the Buds and Leaves of Lycium ruthenicum Murr. Forests 2022, 13, 763. [Google Scholar]
- Lee, J.; Lee, I. Regulation and function of SOC1, a flowering pathway integrator. J. Exp. Bot. 2010, 61, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Adibi, M.; Yoshida, S.; Weijers, D.; Fleck, C. Correction: Centering the organizing center in the arabidopsis thaliana shoot apical meristem by a combination of cytokinin signaling and self-organization. PLoS ONE 2017, 12, e0174751. [Google Scholar] [CrossRef] [PubMed]
- Upreti, K.K.; Reddy, Y.T.N.; Prasad, S.S.; Bindu, G.V.; Jayaram, H.L.; Rajan, S. Hormonal changes in response to paclobutrazol induced early flowering in mango cv. Totapuri. Sci. Hortic. 2013, 150, 414–418. [Google Scholar] [CrossRef]
- Truernit, E. Plant physiology: The importance of sucrose transporters. Curr. Biol. 2001, 11, R169–R171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sawicki, M.; Jacquens, L.; Baillieul, F.; Clément, C.; Vaillant-Gaveau, N.; Jacquard, C. Distinct regulation in inflorescence carbohydrate metabolism according to grapevine cultivars during floral development. Physiol. Plant. 2015, 154, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Vaillant-Gaveau, N.; Maillard, P.; Wojnarowiez, G.; Gross, P.; Clément, C.; Fontaine, F. Inflorescence of grapevine (Vitis vinifera L.): A high ability to distribute its own assimilates. J. Exp. Bot. 2011, 62, 4183–4190. [Google Scholar] [CrossRef]
- Zhong, Y.; Gao, C.; Dong, Z.; Chen, N.; Wang, M. Determination of five endogenous hormones in wheat by high performance liquid chromatography. Chin. J. Chromatogr. 2013, 31, 800–803. [Google Scholar] [CrossRef]
- Li, H. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Eriksson, M.E.; Israelsson, M.; Olsson, O.; Moritz, T. Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat. Biotechnol. 2000, 18, 784–788. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Winter, C.M.; Wu, M.F.; Kanno, Y.; Yamaguchi, A.; Seo, M.; Wagner, D. Gibberellin acts positively then negatively to control onset of flower formation in Arabidopsis. Science 2014, 344, 638–641. [Google Scholar] [CrossRef]
- Chi, Z.H.; Wang, Y.Q.; Deng, Q.X.; Zhang, H.; Pan, C.P.; Yang, Z.W. Endogenous phytohormones and the expression of flowering genes synergistically induce flowering in loquat. J. Integr. Agric. 2020, 19, 2247–2256. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Xing, L.; Zhang, S.; Zhao, C.; Han, M. Effect of exogenous 6-benzylaminopurine (6-BA) on branch type, floral induction and initiation, and related gene expression in ‘Fuji’apple (Malus domestica Borkh). Plant Growth Regul. 2016, 79, 65–70. [Google Scholar] [CrossRef]
- Yan, B.; Hou, J.; Cui, J.; He, C.; Li, W.; Chen, X.; Li, M.; Wang, W. The effects of endogenous hormones on the flowering and fruiting of Glycyrrhiza uralensis. Plants 2019, 8, 519. [Google Scholar] [CrossRef]
- Baïram, E.; Lemorvan, C.; Delaire, M.; Buck-Sorlin, G. Fruit and leaf response to different source–sink ratios in apple, at the scale of the fruit-bearing branch. Front. Plant Sci. 2019, 10, 1039. [Google Scholar] [CrossRef]
- Yang, J.; Jeong, B.R. Side lighting enhances morphophysiology by inducing more branching and flowering in chrysanthemum grown in controlled environment. Int. J. Mol. Sci. 2021, 22, 12019. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Su, S.C.; Ma, L.Y.; Yang, S.Y.; Wang, Y.W.; Wang, X.N. Effects of gibberellic acid on photosynthesis and endogenous hormones of Camellia oleifera Abel. in 1st and 6th leaves. J. For. Res. 2018, 23, 309–317. [Google Scholar] [CrossRef]
- Rossetto, M.R.M.; Purgatto, E.; Nascimento, J.R.O.D.; Lajolo, F.M.; Cordenunsi, B.R. Effects of gibberellic acid on sucrose accumulation and sucrose biosynthesizing enzymes activity during banana ripening. Plant Growth Regul. 2003, 41, 207–214. [Google Scholar] [CrossRef]
- Kępczyńska, E.; Zielińska, S. Regulation of Medicago sativa L. somatic embryos regeneration by gibberellin A3 and abscisic acid in relation to starch content and α-amylase activity. Plant Growth Regul. 2006, 49, 209–217. [Google Scholar] [CrossRef]
- Liang, D.; Huang, X.; Shen, Y.; Shen, T.; Zhang, H.; Lin, L.; Xia, H. Hydrogen cyanamide induces grape bud endodormancy release through carbohydrate metabolism and plant hormone signaling. BMC Genom. 2019, 20, 1–14. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, X.; Yan, J.; Fan, L.; Rong, C.; Mo, C.; Zhang, M. Effect of exogenous spermidine on floral induction, endogenous polyamine and hormone production, and expression of related genes in ‘Fuji’ apple (Malus domestica Borkh.). Sci. Rep. 2019, 9, 12777. [Google Scholar] [CrossRef]
- Lijun, A.; Liang, J.; Chunqin, Y.; Tianhong, L. Effect and Functional Mechanism of Exogenous Gibberellin on Flowering of Peach. Sci. Agric. Sin. 2009, 42, 605–611. [Google Scholar]
- Shuliang, Z.; Yarui, W.; Hongguang, P.; Yinan, D.; Jianfeng, X.; Yingli, L.; Haixia, Z.; Jianguang, Z.; Yuxing, Z. Effect of exogenous hormone and leaves removing on pear flower bud differentiation. China Fruits 2020, 3, 61–64. [Google Scholar]
| Leaf | Bud | |||||||
|---|---|---|---|---|---|---|---|---|
| IAA | GA3 | ABA | ZT | IAA | GA3 | ABA | ZT | |
| Flower bud differentiation rate | 0.6 | 0.567 | 0.666 | 0.641 | 0.567 | 0.777 * | 0.566 | 0.817 * |
| Stage | Type of New Shoot | Starch Content (mg·g−1 DW) | Soluble Sugar Content (mg·g−1 DW) | ||||
|---|---|---|---|---|---|---|---|
| Leaf | Flower Bud | Fruit | Leaf | Flower Bud | Fruit | ||
| Preflower bud differentiation stage | T1 | 22.73 ± 0.13 * | 12.99 ± 0.04 | 17.12 ± 0.17 | 44.35 ± 0.26 * | 27.08 ± 0.63 | 14.51 ± 0.18 |
| T2 | 15.65 ± 0.09 | 15.92 ± 0.09 * | — | 41.64 ± 0.21 | 29.88 ± 0.27 * | — | |
| Flower bud differentiation period | T1 | 16.00 ± 0.19 * | 14.23 ± 0.16 | 29.61 ± 0.08 | 47.56 ± 0.20 * | 34.47 ± 0.13 | 25.64 ± 0.19 |
| T2 | 11.15 ± 0.07 | 19.10 ± 0.08 * | — | 44.11 ± 0.15 | 38.56 ± 0.28 * | — | |
| Late flowering bud differentiation | T1 | 10.19 ± 0.10 | 6.86 ± 0.13 | 34.95 ± 0.08 | 39.85 ± 0.28 | 22.05 ± 0.33 | 35.76 ± 0.25 |
| T2 | 10.23 ± 0.36 | 6.71 ± 0.14 | — | 39.58 ± 0.19 | 22.66 ± 0.14 | — | |
| Stage | Leaf | Flower Bud | ||
|---|---|---|---|---|
| Soluble Sugar | Starch | Soluble Sugar | Starch | |
| Preflower bud differentiation stage | 0.662 | 0.580 | 0.854 * | 0.837 * |
| Flower bud differentiation period | 0.667 | 0.569 | 0.691 | 0.588 |
| Late flowering bud differentiation | 0.628 | 0.608 | 0.640 | 0.629 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, Y.; Wen, Y.; Ye, H.; Jia, T.; Hao, Z.; Su, S.; Wang, X. The Sink–Source Relationship Regulated Camellia oleifera Flower Bud Differentiation by Influencing Endogenous Hormones and Photosynthetic Characteristics. Forests 2023, 14, 1965. https://doi.org/10.3390/f14101965
Si Y, Wen Y, Ye H, Jia T, Hao Z, Su S, Wang X. The Sink–Source Relationship Regulated Camellia oleifera Flower Bud Differentiation by Influencing Endogenous Hormones and Photosynthetic Characteristics. Forests. 2023; 14(10):1965. https://doi.org/10.3390/f14101965
Chicago/Turabian StyleSi, Yuanyuan, Yue Wen, Honglian Ye, Tingting Jia, Zhichao Hao, Shuchai Su, and Xiangnan Wang. 2023. "The Sink–Source Relationship Regulated Camellia oleifera Flower Bud Differentiation by Influencing Endogenous Hormones and Photosynthetic Characteristics" Forests 14, no. 10: 1965. https://doi.org/10.3390/f14101965
APA StyleSi, Y., Wen, Y., Ye, H., Jia, T., Hao, Z., Su, S., & Wang, X. (2023). The Sink–Source Relationship Regulated Camellia oleifera Flower Bud Differentiation by Influencing Endogenous Hormones and Photosynthetic Characteristics. Forests, 14(10), 1965. https://doi.org/10.3390/f14101965






