Abstract
Aims: Microbial residue deposition is considered an important part of soil carbon sequestration. However, there is still a lack of understanding of the link between tree species composition and diversity and microbial carbon deposition, which hampers the rational selection and allocation of tree species for artificial carbon sequestration afforestation in northern China. Methods: In this study, plots from temperate planting forests (>60 years) were examined for the importance values of tree species, mycorrhizal types, tree diversity, and soil properties. Soil amino sugar was used as the biomarker to indicate the accumulation of fungi- and bacteria-derived carbon. Results: We found that tree species diversity and the importance values of tree species and mycorrhizal types were significantly positively correlated with soil microbial residual carbon. Hierarchical partitioning modeling showed that three groups of variables significantly affected soil microbial residual carbon, accounting for a total of 26.75% of the variation. Among them, tree species diversity accounted for the largest proportion (11.5%), and tree species diversity and importance values had a high joint impact (9.74%). The importance values of all AM-associated species constituted one of the most significant individual factors and could independently account for 10.9% of the variation in microbial residues. The findings of piecewise structural equation modeling showed that the importance of tree species had a large direct impact on GluN, GalN, and the GluN/MurN ratio. By influencing soil properties, the importance values of tree species also had indirect effects on soil microbial residual carbon. Conclusions: We suggest that an increase in the importance values of AM-associated tree species, such as Acer negundo L., will be accompanied by an increase in the total importance value of AM-associated tree species, which can significantly increase soil microbial residual carbon.
1. Introduction
Forest carbon sinks are widely recognized as an important way to address climate change and reduce carbon dioxide concentrations in the atmosphere, and they can promote indirect emission reduction [1,2]. Forest carbon sequestration refers to how forests absorb carbon dioxide, convert carbon dioxide into carbohydrates, and store them in the form of biomass through photosynthesis. In the terrestrial carbon cycle, forest ecosystems are a large carbon pool; if forests continue to be damaged and degraded, it will lead to an intensification of climate change and the loss of forest resources, so curbing forests destruction and degradation is a pressing need. One of the most important strategies for halting the destruction and decline of forests is to plant new trees. In addition to producing logs Tian, 2009 [3], protecting and restoring plant diversity Gogoi et al., 2021 [4], and increasing forest carbon sinks [5,6], plantations also improve soil carbon sequestration capacity. According to some studies, increasing the number of plantations on the planet is an efficient way to lower atmospheric carbon dioxide [7,8]. It is a low-cost and efficient approach to carbon sequestration and emission reduction. In recent years, China has begun to pay greater attention to planted forests, and many effective measures have been taken. The area of artificial forests in China is 69 million hectares, with a storage capacity of 2.483 billion cubic meters, according to the findings of the 8th National Forest Resources Inventory (State Forestry Administration of China, 2014 [9]), placing it first in the world in terms of artificial forest area. Meanwhile, China is the first country in the world to establish forestry carbon sinks and promote their development in private areas. It is predicted that by 2050, China’s plantations will reach 1.58 million square kilometers Qian et al., 2018 [10].
The soil carbon storage varies with the different tree species planted in the plantations, which may be related to the characteristics of tree species and their associated microbial communities. In recent years, the issue of microbial control of SOC retention has been a prevalent topic, especially in the field of ectomycorrhizal fungi and arbuscular mycorrhizal fungi. The mycorrhizal types have been highlighted as significant predictive factors for underground nutrient cycling and carbon storage in numerous studies [11,12,13] and have a significant impact on the capacity of forest soils to store carbon. Clemmensen et al. [14,15] suggested that most of the carbon stored in the SOM of the boreal forest system comes from roots and fungi, and that the input of mycorrhizal fungal necrotic substances was of great significance for the global carbon cycle model. Also taken into consideration were ectomycorrhizal fungi, which significantly influence the distribution and release of carbon to soil as well as the microbial biomass and respiration of soil [16]. Arbuscular mycorrhizal (AM) fungi were thought to, in some cases, directly encourage the decomposition of organic matter through the “priming effect,” promoting the activity of free-living saprophytes and thereby increasing soil organic matter [17,18]. Similarly, the plant diversity in plantations may lead to different soil carbon sequestration capabilities. Some studies have highlighted that the management of plantations needs to promote species richness to sustain forest carbon storage gains Li et al., 2022 [19], while other studies have suggested that there is no clear relationship between plant diversity and carbon storage Kirby et al., 2007 [20], and that a greater carbon stock may not always be associated with higher diversity Banik et al., 2018 [21]. In summary, there is an ongoing debate over whether the diversity of tree species has a sizable effect on carbon sequestration in planted forests.
Forest soils are regarded as essential carbon storage sites, and long-lasting and stable forest carbon sinks are vital to the terrestrial carbon cycle and the mitigation of climate change. Numerous studies have demonstrated that microbial residues are an important component of long-lasting organic carbon in forest soil. According to Simpson et al., 2007 [22], microorganisms can contribute up to over 50% of the total extractable SOM, 45% of the humin fraction, and over 80% of the soil’s nitrogen. SOC sequestration largely depends on microbial community dynamics, through their control of the balance between the formation and degradation of microbial residues Huang et al., 2019 [23]. In an incubation experiment, Kallenbach et al., 2016 [24] noted the massive formation of microbially derived soil organic matter. Other researchers have also hypothesized that, with forest succession, amino sugar accumulation is faster than SOC accumulation, possibly indicating that the generation and retention of microbial residues are necessary prerequisites for SOC retention Shao et al., 2017 [25]. In addition, microbial residues in the mineral soil layer were particularly vulnerable to the protection combined with silt and clay minerals Craig et al., 2018 [26]. Numerous studies on the effects of microorganisms and their derivatives on SOC have been published recently. Shao et al., 2017 [25], for instance, measured the variation in amino sugar content (biomarker of microbial residues) with successional gradients to assess the contribution of microbial residues to SOC accumulations over time. According to some studies [27,28], soil microbial residues may change due to the application of external nitrogen sources and ongoing drought. This may change how much microbial residues contribute to soil organic carbon (SOC). However, little consideration has been given to the biological elements that influence microbial residual carbon. Therefore, we used amino sugars as biomarkers to link plant diversity, the importance value of trees’ mycorrhizal types, and microbial residues, and we compared soil amino sugar contents in 30 plots with different plant diversity gradients and dominant tree species, aiming to reveal (1) whether diverse afforestation by selecting trees’ mycorrhizal type can affect microbial-derived carbon sequestration in plantation forests in Northeast China, and (2) how the importance value of mycorrhizal type and species diversity influence microbial residual carbon accumulation.
2. Methods
2.1. Study Site
Our research was carried out at the Northeast Forestry University’s experimental forest farm in Harbin City (126.62° E, 45.76° N) (Figure 1). On average, this area is 142 m above sea level. A private Belarussian botanical garden was constructed in 1930, and the experimental forest farm was divided into various sections for experimental afforestation in the 1950s and 1960s. It has natural soil, or zonal chernozem, with flat topography and ideal moisture levels. There were no changes in land use after afforestation. It was a good location for this experiment due to the topography, which is a flat plain, and because detailed historical information about this area exists. In-depth descriptions of tree planting and species composition have been provided in earlier studies [29,30]. With high summer temperatures and significant summertime precipitation, but a chilly, dry winter, Harbin has a mid-temperate continental monsoon climate. The annual cumulative averages of both temperature and precipitation are 3.5 °C and 534 mm, respectively.
Figure 1.
Study location (a), the green block indicates the experimental sample area. Forest site surroundings (b).
2.2. Experimental Design and Field Survey
Using the guidelines for large field plot settings Shi et al., 2021 [31], we divided the 7.2 ha (300 m × 240 m) large field into 720 10 m × 10 m plots. In each plot, all trees with a diameter greater than 1.0 cm at breast height were examined, and the coordinates, taxonomic names, and abundance were recorded. We measured 18,054 trees in the field from 28 different tree species and determined the relative importance of each species. Field survey data were clustered using functions of hierarchical clustering in R 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria) (Appendix A, Figure A1). Based on the results of cluster analysis, we again selected 30 plots belonging to 9 different main cluster regions to create a gradient of tree species diversity. Using a 200 cm3 cutting ring, soil samples were taken at the surface of the 0–20 cm layer. In each plot, three soil samples were combined to form an average soil sample.
2.3. Soil Properties and Amino Sugar Determination
The heated dichromate titration method was used to determine the SOC content. The semi-micro Kjeldahl method was used to calculate the total nitrogen in the soil. Using an acidity meter (PHSJ-3F; Shanghai, China) and an EC meter (DDS-307; Shanghai, China), the pH and electrical conductivity (EC) of the soil were measured in a solution of 1 g soil: 5 mL deionized water Bao, 2000 [32].
The soil amino sugars were measured according to the method of Zhang and Amelung, 1996 [33]. Specifically, 0.4 g of soil was hydrolyzed with 10 mL of HCl (6 mol·L−1) at 105 °C, filtered, and then 100 μL of inositol (internal standard) was added. After drying, the pH was adjusted to 6.6–6.8, the supernatant was centrifuged, and the sample was freeze-dried; the residue was dissolved with anhydrous methanol, centrifuged, transferred to a derivatization bottle, and blown dry with N2 at 45 °C; then, 1 mL of ultrapure water and 100 μL of internal standard 2 (N-methylglucosamine, MGlcN) were added, and the sample was freeze-dried, derivatized, and determined by gas chromatography Jia et al., 2021 [34].
Amino sugars include glucosamine, galactosamine, mannosamine, and muramic acid. Muramic acid is thought to be unique to bacteria, while glucosamine is more prevalent in the cell walls of fungal organisms than in bacteria Guggenberger et al., 1999 [35]. Mannosamine and galactosamine are widely present. According to Liang et al., 2016 [36], the ratio of glucosamine to muramic acid (GlcN/MurA) represents the proportion of residues derived from fungi as opposed to bacteria. In Appendix A, Table A1, a list of the analyzed biomarkers, their ratios, and their interpretations is provided.
2.4. Calculation of Tree Diversity and Species Importance Value
According to the method of Ma, 1995 [37], the tree species diversity indexes of each sample plot were calculated as follows:
Pi refers to the proportion of the number of individuals of one woody plant in the sample plot to the number of individuals of all species, and S refers to the species richness of woody plants.
According to Chen, 2022 [29], the following formulas were used to determine the significance of the dominant tree species:
In this study, the importance value of the AM mycorrhizal type was determined by adding the importance values of all the tree species that are associated with AM fungi in each sample plot. Similarly, we can determine the importance value of the ECM mycorrhizal type by adding the importance values of the tree species that are associated with the ECM fungi in each sample plot.
Tree species richness was ≤3 species in the lowest 25% of the data, 4–7 species in the middle 25%–75%, and ≥8 species in the highest 25% of the data, which were categorized as low, medium, and high, respectively.
2.5. Data Statistics and Analysis
R 4.1.3 (R Development Core Team 2022) and the packages “ggplot2,” “corrplot,” “ggpubr,” “nlme,” “vegan,” and “ggrepel” were used for all statistical analyses. Using the ‘ks.test’ function in the R library, the Kolmogorov-Smirnov test was performed on all raw data to determine whether the residue distribution was normal and whether the variance was homogeneous Jia et al., 2021 [34]. By using a two-tailed correlation test, Spearman correlations were used to examine relationships between amino sugar contents, ratios, and environmental factors Zhang et al., 2020 [38]. The significance level for correlations was set at p < 0.05. To compare the differences in microbial biomarker contents under low, medium, and high tree species richness, a one-way analysis of variance (ANOVA) with a t-test was used. A p value of less than 0.05 was also used to define the threshold for statistical significance.
To test the relationship between variations in fungi- and bacteria-derived residues and various environmental factors, the environmental factors were divided into three groups: (1) soil properties (electrical conductivity, pH, total organic carbon, and total nitrogen content), (2) tree species diversity indexes (Patrick, Shannon-Wiener, Simpson, and Pielou indexes), and (3) importance value of tree species (Pinus sylvestris, Fraxinus mandshurica, Phellodendron amurense, Quercus mongolica, Juglans mandshurica, Ulmus pumila, Lonicera maackii, Pinus tabuliformis var. mukdensis, Acer negundo). To eliminate arbitrary measurement units, the raw data were normalized into the range [0, 1].
Using the ‘rda’ function in the “vegan” package, redundancy analysis (RDA) was carried out to separate the relative contribution of environmental factors to variations in fungi- and bacteria-derived residues. Prior to RDA, the original data were normalized into the [0, 1] range. Two collinearity environment variables (Pielou and Simpson) were deleted through the ‘vif.cca’ function in the vegan package of R 4.1.3. Redundancy analysis used the ‘envfit’ function of the vegan package to perform 999 randomizations based on the significant variables. Conditional term effects were tested using the ‘anova’ function based on 999 permutations Kong et al., 2022 [39].
We used hierarchical partitioning modeling to quantify the relative importance of individual groups and individual environmental factors for variations in fungi- and bacteria-derived residues by using the ‘rdacaa.hp’ function in the rdacca.hp package Lai et al., 2022 [40]. These frameworks were made more inclusive by the development of hierarchical partitioning, which calculates the variable importance from all subset models and produces an unordered assessment of importance Pecuchet et al., 2022 [41]. The nine most important environmental factors were used to test the contribution of individual environmental factors with the ‘envfit’ function of RDA. The “UpSetVP” package in R was used to visualize the results of the hierarchical partitioning analysis.
We used “piecewise SEM” to further evaluate the direct and indirect effects of soil properties, tree species diversity, and the importance value of tree species on variations in GluN (a), GlaN (b), MurN (c), and GluN/MurN (d) [42,43] All environmental variables included in these models were also first divided into three groups, as in the upper Mantel test. In the linear mixed effect model of environmental group effects, we assumed that the importance values of tree species affect tree species diversity and then soil properties. We placed soil properties, tree species diversity, and the importance values of tree species in sequence. The models were carried out using the “piecewise SEM” package for linear mixed effect models based on the “nlme” package. We modified our models with the significance (P0.05) and the model goodness using Fisher’s C-test (when 0.05P1.00), which we used to confirm the accuracy of the modeling results Chen, 2017 [44].
3. Results
3.1. Relationships of Fungi- and Bacteria-Derived Residues and Environmental Factors
Glucosamine, galactosamine, and muramic acid content were positively correlated with TN, SOC, EC, Shannon-Wiener, Patrick-R, Simpson, and the importance values of AM and L. maa. Meanwhile, glucosamine, galactosamine, and muramic acid content also were negatively correlated with the importance values of EcM and P. tab (p < 0.05; Table 1). In addition, glucosamine content was positively correlated with the importance values of J. man and P. aum, galactosamine content was positively correlated with We added genes & Latin binomial name & statistical variable through all the paper, please confirm. of J. man and A. neg, and muramic acid content was positively correlated with the importance values of A. neg (p < 0.05; Table 1). The ratio of glucosamine and muramic acid content was positively correlated with the importance values of J. man and AM and was negatively correlated with the importance values of Q. mon.

Table 1.
Spearman correlation coefficients (r) of fungi- and bacteria-derived residues contents with environmental variables in the northeastern forests of China (n = 30; *, **, and *** indicate p < 0.05, 0.01, and 0.001, respectively).
For each independent variable, we divided the number of tree species into low, medium, and high groups. To compare the differences in microbial biomarker contents under low, medium, and high tree species richness groups, a one-way analysis of variance (ANOVA) with a t-test was used. From the low to the high tree species richness gradients, the contents of glucosamine, galactosamine, and muramic acid showed an increasing trend (p < 0.05; Figure 2), but the ratio of glucosamine to muramic acid did not change significantly.
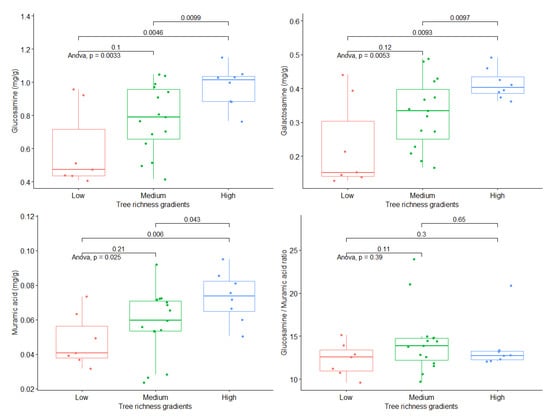
Figure 2.
Significance of the effects of tree species richness on fungi- and bacteria-derived residues by one-way analysis of variance with a t-test. The p values of the t-test were marked in the graph; p value < 0.05 was defined as statistical significance.
3.2. Separate Effects of Environmental Factors on Fungi- and Bacteria-Derived Residues
Based on the results of the correlation analysis, we discovered a strong relationship between the residues produced by fungi and bacteria and soil characteristics, the diversity of tree species, and the importance values of tree species. To reveal the variations in the relationships of fungi- and bacteria-derived residues with environmental factors, RDA models were also carried out. The first axis explained 82.77% of the variation in the variances of fungi- and bacteria-derived residues, and the second axis explained 14.06% (Figure 3). For the conditional effect, TN, AM, and SOC were the main drivers of the variations in fungi- and bacteria-derived residues according to ANOVA based on 999 permutations (p < 0.05) (Appendix A, Table A3). The explanatory amount for TN, AM, and SOC was 22.30% (Appendix A, Table A2).
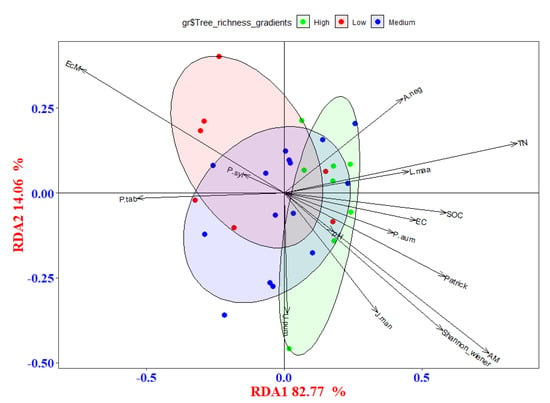
Figure 3.
The redundancy analysis (RDA) for fungi- and bacteria-derived residues and the environment factors. The effect variables are represented by grey arrows. Green dots and circle coverage, blue dots and circle coverage, and red dots and circle coverage show amino sugar contents at high-, medium-, and low- diversity gradients, respectively. EC: soil conductivity; pH: soil pH value; SOC: soil organic carbon content; TN: total soil nitrogen content; Patrick, Shannon-Wiener, Simpson, and Pielou represent the different diversity indexes in different plots. P. syl, F. man, P. amu, Q. mon, J. man, U. pum, L. maa, P. tab, and A. neg representhe importance values of different tree species in different plots.
To determine the contribution of the individual groups or individual environmental factors to the variations in the fungi- and bacteria-derived residues, we conducted hierarchical partitioning analysis (Figure 4). The results showed that species diversity, tree species’ importance values and soil properties significantly affected fungi- and bacteria-derived residues (p < 0.05), and three group variables accounted for a total of 26.75% of the variation. Tree species diversity and the importance values of tree species had a high proportion of common effects (9.47%).

Figure 4.
Hierarchical partitioning of the nine most significant environmental factors and three groups of environmental variables. Each row in the dot-matrix plot to the right represents a different environmental factor. The top column diagram shows the percentage of variation explained by each component (from variation partitioning), with the isolated black dot for each column representing the marginal effect of each environmental factor and the lines connecting multiple dots representing the common effect among these corresponding environmental factors. The hierarchical partitioning column diagram on the left displays the individual effects of each environmental factor; each effect’s value is equal to the sum of its marginal effect and its average combined effect with other environmental factors. p < 0.05.
TN was the most important individual factor and could independently explain 23.07% of the variation in fungi- and bacteria-derived residues, followed by AM, which explained 10.9% of the variation (p < 0.05). The ten most important environmental factors (Appendix A, Table A3) accounted for 67.03% of the variation in the fungi- and bacteria-derived residues (Figure 4).
We verified the contribution of single environmental factors to the variations in three different components of the bacteria-derived residues by hierarchical partitioning. TN, SOC, AM, EcM, Shanno-Wiener, Patrick, and P. tab were the environmental factors driving variations in GluN, GalN, and MurN. TN and AM had significant contributions, driving 23.65% and 15.52% of the variation in GluN, respectively (p < 0.05). TN, AM, and ECM were important environmental factors driving 19.52%, 13.4%, and 11.52% of the variations in GalN, respectively (p < 0.05). TN and SOC were important environmental factors driving 37.58% and 11.77% of the variation in MurN, respectively (p < 0.05); AM and EcM had only weak effects on the variations in MurN (p > 0.05) (Figure 5).

Figure 5.
Effects of individual environmental factors on the variations in GluN, GlaN, and MurN by hierarchical partitioning. *, p < 0.05. EC: soil conductivity; pH: soil pH value; SOC: soil organic carbon content; TN: total soil nitrogen content; Patrick, Shannon-Wiener, Simpson, and Pielou represent different diversity indexes in different plots. P. syl, F. man, P. amu, Q. mon, J. man, U. pum, L. maa, P. tab, and A. neg represent the importance values of different tree species in different plots.
We performed an SEM analysis using the tree species diversity indexes, theimportance value of tree species, and the soil properties to reveal their direct and indirect impacts on the soil amino sugars (Figure 6). The results showed that the importance values of tree species had a significant direct effect on GluN (0.33, p < 0.05), GalN (0.464, p < 0.01), and the GluN/MurN ratio (0.458, p < 0.05). Additionally, tree species diversity and soil properties were directly affected by the importance value of tree species (Figure 6a–c). Soil properties could directly affect GluN (0.562, p < 0.001), GalN (0.68, p < 0.001), and MurN (0.562, p < 0.001). Overall, importance values of tree species and the soil properties had a significant direct impact on soil amino sugar accumulation, but tree species diversity ony had a weak effect on soil amino sugars. We speculate that, in addition to the direct effect of tree species on amino sugar content, the importance value of tree species can promote increases in GluN, GalN, and MurN content by directly affecting soil physicochemical properties.
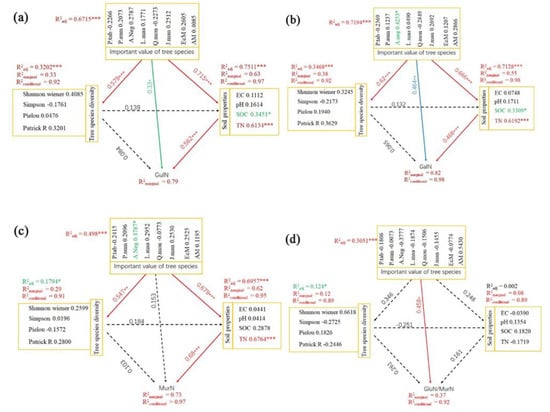
Figure 6.
Structure equation modeling of the driving paths of environmental factors’ effects on fungi- and bacteria-derived residues. Piecewise SEM accounted for the direct and indirect effects of soil properties, tree species diversity, and the importance values of tree species on variations in GluN (a), GlaN (b), MurN (c), and GluN/MurN (d). Numbers adjacent to arrows are path coefficients (partial regression) which represent the directly standardized effect size of the relationship. The marginal and conditional R2 values represent the proportion of variance explained by all predictors either without or with accounting for random effects. Significance levels for each predictor were * p < 0.05, ** p < 0.01, *** p < 0.001.
4. Discussion
The importance values of tree species, the importance values of the mycorrhizal types, and plant diversity all have varying degrees of influence on the soil microbial residual carbon. According to the global forests and tree species richness gradient experiment, species richness was positively correlated with the amount of carbon stored by forest ecosystems [45,46]. Microbial residue-derived carbon, as an important fraction of soil organic carbon, was also closely related to species richness. In the present study, we used soil amino sugars as biomarkers of microbial residual carbon and divided tree species richness into three gradients (low, medium, and high). We found that the contents of glucosamine, galactosamine, and muramic acid increased with the increasing richness gradient. It has been repeatedly reported that higher tree species richness leads to stronger plant productivity, higher microbial secretion diversity, and more microbial residues [34,47], which is consistent with our findings. The reason could be that, as tree species diversity rises, dead branches and leaves are added. This may also improve the availability of soluble organic matter and nitrogen, which in turn encourages microbial pathways for SOC accumulation and, ultimately, raises the concentration of amino sugars present. Our findings indicated that the ratio of glucosamine to muramic acid did not show a significant change with variation in the tree species richness gradients. Thus, we speculate that the increase in tree species richness will not have a significant impact on the ratio of soil fungal and bacterial residues.
Similarly, the soil amino sugar contents and microbial residual carbon vary depending on the tree species in the plantation. Many studies have observed the widespread expansion of N-fixing tree species (such as alder) in northern China and the subarctic region, which not only improves plant productivity, litter chemical properties [48,49], and soil nitrogen and phosphorus status, but may also alter the composition of microbial communities and have a significant impact on the quantity and composition of microbial residues [50,51]. Our data showed that P. tab was significantly negatively correlated with glucosamine, galactosamine, and muramic acid contents, though not the glucosamine/muramic acid ratio, but A. neg, L. maa and J. man were positively correlated with glucosamine, galactosamine, and muramic acid contents. Additionally, the results of SEM analysis showed that tree species could not only directly affect soil microbial residual carbon, but also directly cause variations in soil properties, thereby promoting soil microbial residual accumulation. Based on these results, it is not difficult to conclude that tree species play an essential role in soil microbial residual carbon accumulation, and that coniferous tree species (such as P. tab) are not beneficial for soil amino sugar accumulation, while broad-leaved tree species (such as the J. man. and A. neg.), with highlight-absorption efficiency and less intense intraspecific competition, are more conducive to soil amino sugar accumulation, which is consistent with previous conclusions.
Nearly all tree species in the forests of Northeast China are associated with ectomycorrhizal and arbuscular mycorrhizal fungi. The types of mycorrhizal tree species and the accumulation of microbial residual carbon in the soil may be connected. Different types of mycorrhizal tree species and their associated soil microorganisms can also cause different impacts on the formation and decomposition of SOM Craig et al., 2018 [26]. Increasing evidence has indicated that the oldest SOMs are mainly composed of unstable microbial residues, which are protected by binding with active sludge and clay in soil mineral layers. Consequently, Cotrufo, et al. 2013 [52] proposed the “MEMS” hypothesis, which suggests that mineral-stable SOMs can be formed under rapid decay conditions, and can also improve the production speed and efficiency of microbial biomass. In comparison to forests dominated by ECM-associated tree species, forests dominated by AM-associated tree species typically have higher nutrient availability and higher quality leaf litter [53,54]. AM-related roots can also explain most of the deep SOM and they decay faster as well [55,56]. In the present study, P. syl, Q. mon, and P. tab were the ECM-associated tree species, and F. man, P. amu, J. man, U. pum, L. maa, and A. neg were the AM-associated tree species. Through further research on the data, we found that AM-associated tree species have a significantly positive correlation with amino sugar contents, which is consistent with the observation of Craig et al., 2018 [26]. In contrast, we also observed that ECM-associated plants were significantly negative for soil amino sugar contents. In this regard, our research findings are consistent with the hypothesis that rapidly decomposing systems, such as the ecosystem of AM-dominated forests, promote stable SOM formation by enhancing microbial production Cotrufo et al., 2013 [52]. Our results also indicated that the high species diversity and high importance values of AM-associated tree species (such as L. maa, J. man, and A. neg) can lead to high amino sugar contents. Both have a common promoting effect on the accumulation of amino sugars. We speculate that this might be because an increased number of tree species in the high species diversity group were AM-associated tree species. Therefore, we highlighted that selecting tree species with certain mycorrhizal types to maintain higher plant diversity could better increase the accumulation of microbial residual carbon.
Forest carbon sinks are vital for atmospheric carbon reduction and addressing climate change Bäckstrand et al., 2006 [1]. Microbial residual carbon is one of the important sources of the forest soil carbon pool, and we can start from it and take measures to increase the soil carbon sink. The present research showed that tree species richness, the dominant tree species, and the types of plant mycorrhizae all have varying degrees of impact on soil microbial residual carbon, with AM-associated tree species having the greatest impact on microbial residual carbon. Selecting tree species with specific functions or mycorrhizal types to maintain a high level of diversity, rather than maximizing overall tree species richness, may be an effective measure to increase soil microbial-derived- carbon components and soil carbon sinks. In the future construction and transformation of planted forests, we note that tree species with special functions (such as N-fixing tree species), broad-leaved tree species with high absorption efficiency and low intraspecies competition, and AM-associated tree species with high nutrient utilization efficiency and high leaf quality should account for a large proportion of the tree species composition, in order to reasonably allocate the composition of forest tree species, improve the diversity of forest tree species, and promote an increase in soil amino sugars and microbial residual carbon contents, achieving the goal of increasing forest soil carbon sinks.
5. Conclusions
In this study, we used soil amino sugars as biomarkers for microbial residual carbon and synthesized the measurement results of soil amino sugar contents in 30 sampling plots with different tree species diversity gradients, dominant tree species, and soil properties. Our results indicated that tree species diversity in the sample plots accounted for the greatest contribution to the changes in soil amino sugar content, followed by the importance values of the dominant tree species, with both contributing more to amino sugar accumulation than soil properties (Figure 4). Tree species diversity and importance values had a higher common effect on microbial residual carbon. Although the soil amino sugars increased with the tree species richness gradients, simply maximizing tree species richness without considering the dominant tree species’ mycorrhizal types and the tree species composition of a sample plot would have little effect on the soil amino sugar content. Additionally, through RDA and hierarchical partitioning analysis, we found that TN, the importance values of AM-associated species, and SOC were the main drivers of microbial residual carbon accumulation. L. maa, J. man, and A. neg, which are the AM-associated species, had a significant positive effect on soil amino sugar contents, while P. tab, an ECM-associated species, had a significant negative effect on soil amino sugars contents. The soil amino sugars accumulated more in the AM-dominated forests than the ECM-dominated forests, which showed that AM-associated species were more beneficial for microbial residual carbon accumulation. The SEM analysis showed that the importance values of tree species have two impacts on microbial residual carbon, one directly affecting the accumulation of fungi- and bacterial-derived carbon, and the other causing differences in soil physicochemical properties and thereby promoting the accumulation of microbial residual carbon. All of these findings contribute to a better understanding of the connection between the abundance of tree species, the dominant tree species, and the accumulation of microbial residual carbon, and they offer a theoretical foundation for future tree species selection in diverse afforestation
Author Contributions
H.H. (Haiyan Huang): conceptualization, supervision, writing—original draft preparation; X.S.: validation, writing—review and editing; L.J. and Y.S.: investigation, validation, resources, software; Z.Z.: methodology, data curation, writing-original draft preparation; Z.T., W.W. and H.H. (Haisheng He): project administration, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Heilongjiang Province of China (grant number LH2021C003), the National Natural Science Foundation of China (grant number 41730641), and the Basic Research Fund for the Central University (grant number 2572021DT03).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
For their support in the determination of soil amino sugars, we are thankful to He Hongbo and Zhang Jianan of the Shenyang Institute of Applied Ecology, Chinese Academy of Sciences.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A
Figure A1.
Cluster analysis results of the plant data in sample plot.

Table A1.
Abbreviations.
Table A1.
Abbreviations.
| Tree Species | Abbreviations | Mycorrhizal Type |
|---|---|---|
| Pinus sylvestris var. mongolica Litv. | P. syl | Ectomycorrhiza |
| Fraxinus mandshurica Rupr. | F. man | Arbuscular mycorrhiza |
| Phellodendron amurense Rupr. | P. amu | Arbuscular mycorrhiza |
| Quercus mongolica Fisch. ex Ledeb. | Q. mon | Ectomycorrhiza |
| Juglans mandshurica Maxim. | J. man | Arbuscular mycorrhiza |
| Ulmus pumila L. | U. pum | Arbuscular mycorrhiza |
| Lonicera maackii (Rupr.) Maxim. | L. maa | Arbuscular mycorrhiza |
| Pinus tabuliformis var. mukdensis (Uyeki ex Nakai) Uyeki | P. tab | Ectomycorrhiza |
| Acer negundo L. | A. neg | Arbuscular mycorrhiza |
| Analyzed amino sugars | ||
| Glucosamine | GluN | |
| Galactosamine | GalN | |
| Muramic acid | MurN | |
| Glucosamine:Muramic acid | GluN:MurN | |
| Soil properties | ||
| Soil organic carbon | SOC | |
| Total nitrogen | TN | |
| Electrical conductivity | EC |

Table A2.
Conditional term effects in the redundancy analysis (RDA). Model: rda (formula = fungi-and bacteria-derived residues ~ TN + AM + SOC + L. maa + pH + Shannon_wiener + Patrick + EC + J. man + U. pum + P. syl + EcM + P. tab + P. aum + A. neg + F. man + Q. mon, data = environmental factors, scale = FALSE).
Table A2.
Conditional term effects in the redundancy analysis (RDA). Model: rda (formula = fungi-and bacteria-derived residues ~ TN + AM + SOC + L. maa + pH + Shannon_wiener + Patrick + EC + J. man + U. pum + P. syl + EcM + P. tab + P. aum + A. neg + F. man + Q. mon, data = environmental factors, scale = FALSE).
| Influence Factors | Explains (%) | F | p | Adj.r.Squared |
|---|---|---|---|---|
| TN | 16.6259 | 51.5933 | 0.001 *** | 0.4995107 |
| AM | 4.1134 | 12.7648 | 0.002 ** | 0.6183001 |
| SOC | 1.3267 | 4.1169 | 0.034 * | 0.6575433 |
| Shannon_wiener | 1.0081 | 3.1284 | 0.082 | |
| pH | 0.7106 | 2.205 | 0.134 | |
| F. man | 0.5762 | 1.7881 | 0.204 | |
| Patrick | 0.5676 | 1.7613 | 0.181 | |
| L. maa | 0.5103 | 1.5836 | 0.191 | |
| EC | 0.4501 | 1.3969 | 0.252 | |
| J. man | 0.4428 | 1.3741 | 0.269 | |
| P. aum | 0.36 | 1.1171 | 0.347 | |
| P. tab | 0.2378 | 0.738 | 0.46 | |
| EcM | 0.1656 | 0.5138 | 0.559 | |
| P. syl | 0.1437 | 0.446 | 0.594 | |
| Residual | 0.045115 |
Signif. codes: ‘***’ 0.001, ‘**’ 0.01, ‘*’ 0.05.

Table A3.
Importance of explanatory variables in RDA through envfit (permu = 999) function.
Table A3.
Importance of explanatory variables in RDA through envfit (permu = 999) function.
| RDA1 | RDA2 | r2 | p | |
|---|---|---|---|---|
| TN | 0.99822 | 0.05958 | 0.6579 | 0.001 *** |
| AM | 0.90208 | −0.43156 | 0.6903 | 0.001 *** |
| Patrick | 0.94653 | −0.32263 | 0.3688 | 0.001 *** |
| SOC | 0.99199 | −0.12633 | 0.3261 | 0.006 ** |
| EC | 0.98522 | −0.17132 | 0.2199 | 0.034 * |
| EcM | −0.9321 | 0.3622 | 0.6202 | 0.001 *** |
| Shannon_wiener | 0.88652 | −0.4627 | 0.4353 | 0.001 *** |
| J. man | 0.80795 | −0.58925 | 0.1974 | 0.065 |
| L. maa | 0.99929 | 0.03775 | 0.1886 | 0.062 |
| P. tab | −0.99915 | 0.04113 | 0.2628 | 0.027 * |
| P. aum | 0.96793 | −0.25121 | 0.1601 | 0.088 |
| A. neg | 0.92844 | 0.37148 | 0.2069 | 0.041 * |
| pH | 0.90331 | −0.42899 | 0.0407 | 0.55 |
| P. syl | −0.9557 | 0.29436 | 0.0227 | 0.75 |
| Q. mon | −0.788 | 0.61567 | 0.3287 | 0.01 ** |
| F. man | 0.66875 | −0.74349 | 0.0878 | 0.288 |
| U. pum | 0.13074 | −0.99142 | 0.0805 | 0.32 |
Signif. codes: ‘***’ 0.001, ‘**’ 0.01, ‘*’ 0.05.
References
- Bäckstrand, K.; Lövbrand, E. Planting Trees to Mitigate Climate Change: Contested Discourses of Ecological Modernization, Green Governmentality and Civic Environmentalism. Glob. Environ. Politic 2006, 6, 50–75. [Google Scholar] [CrossRef]
- Zhu, H.; Cai, Y.; Lin, H.; Tian, Y. Impacts of Cross-Sectoral Climate Policy on Forest Carbon Sinks and Their Spatial Spillover: Evidence from Chinese Provincial Panel Data. Int. J. Environ. Res. Public Health 2022, 9, 14334. [Google Scholar] [CrossRef]
- Tian, Y. Conservation of forest resources and the role of forest tree breeding. Breed Res. 2009, 11, 112–115. [Google Scholar]
- Gogoi, A.; Ahirwal, J.; Sahoo, U.K. Plant biodiversity and carbon sequestration potential of the planted forest in Brahmaputra flood plains. J. Environ. Manag. 2021, 280, 111671. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.D.; Zhang, G.Q. Impact Factors of Carbon Sequestration in Artificial Forest Carbon Stock and Its Impact Factors. World For. Res. 2009, 22, 34–38. [Google Scholar] [CrossRef]
- Shi, X.; Wang, T.; Lu, S.; Chen, K.; He, D.; Xu, Z. Evaluation of China’s forest carbon sink service value. Environ. Sci. Pollut. Res. 2022, 29, 44668–44677. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M.A. Above-and belowground ecosystem biomass and carbon pools in an age-sequence of temperate pine plantation forests. Agric. For. Meteorol. 2006, 140, 51–63. [Google Scholar] [CrossRef]
- Taylor, A.R.; Wang, J.R.; Chen, H.Y. Carbon storage in a chronosequence of red spruce (Picea rubens) forests in central Nova Scotia, Canada. Can. J. Forest Res. 2007, 37, 2260–2269. [Google Scholar] [CrossRef]
- State Forestry Administration of China. Results of the Eighth Forest Resource Inventory. For. Resour. Manag. 2014, 1–2. [Google Scholar]
- Qian, Y.; Sun, H.; Dong, R.; Jiang, J. Research Progress of Carbohydrates Allocation in Conifers. Sci. Silvae Sin. 2018, 54, 141–153. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Durling, M.B.; Michelsen, A.; Hallin, S.; Finlay, R.D.; Lindahl, B.D. A tipping point in carbon storage when forest expands into tundra is related to mycorrhizal recycling of nitrogen. Ecol. Lett. 2021, 24, 1193–1204. [Google Scholar] [CrossRef]
- Phillips, R.P.; Brzostek, E.; Midgley, M.G. The mycorrhizal-associated nutrient economy: A new framework for predicting carbon-nutrient couplings in temperate forests. New Phytol. 2013, 199, 41–51. [Google Scholar] [CrossRef]
- Steidinger, B.S.; Crowther, T.W.; Liang, J.; Van Nuland, M.E.; Werner, G.D.; Reich, P.B.; Nabuurs, G.J.; de-Miguel, S.; Zhou, M.; Zhou, N.; et al. Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature 2019, 569, 404–408. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and associated fungidrive long-term carbon sequestration in boreal forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Finlay, R.D.; Dahlberg, A.; Stenlid, J.; Wardle, D.A.; Lindahl, B.D. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol. 2015, 205, 1525–1536. [Google Scholar] [CrossRef]
- Fransson, P. Elevated CO2 impacts ectomycorrhiza-mediated forest soil carbon flow: Fungal biomass production, respiration and exudation. Fungal Ecol. 2012, 5, 85–98. [Google Scholar] [CrossRef]
- Frey, S.D. Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 237–259. [Google Scholar] [CrossRef]
- Hodge, A. Chapter 8—Accessibility of Inorganic and Organic Nutrients for Mycorrhizas. In Mycorrhizal Mediation of Soil; Elsevier: Amsterdam, The Netherlands, 2017; pp. 129–148. [Google Scholar] [CrossRef]
- Li, T.; Zou, Y.; Liu, Y.; Luo, P.; Xiong, Q.; Lu, H.; Lai, C.; Axmacher, J.C. Mountain forest biomass dynamics and its drivers in southwestern China between 1979 and 2017. Ecol. Indic. 2022, 142, 109289. [Google Scholar] [CrossRef]
- Kirby, K.R.; Potvin, C. Variation in carbon storage among tree species: Implications for the management of a small-scale carbon sink project. For. Ecol. Manag. 2007, 246, 208–221. [Google Scholar] [CrossRef]
- Banik, B.; Deb, D.; Deb, S.; Datta, B.K. Assessment of Biomass and Carbon Stock in Sal (Shorea robusta Gaertn.) Forests under Two Management Regimes in Tripura, Northeast India. J. For. Environ. Sci. 2018, 34, 209–223. [Google Scholar] [CrossRef]
- Simpson, A.J.; Simpson, M.J.; Smith, E.; Kelleher, B.P. Microbially derived inputs to soil organic matter: Are current estimates too low? Environ. Sci. Technol. 2007, 41, 8070–8076. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, C.; Duan, X.; Chen, H.; Li, D. Variation of microbial residue contribution to soil organic carbon sequestration following land use change in a subtropical karst region. Geoderma 2019, 353, 340–346. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Zhao, Y.; Zhang, W.; Hu, G.; Xie, H.; Yan, J.; Han, S.; He, H.; Zhang, X. Linkage of microbial residue dynamics with soil organic carbon accumulation during subtropical forest succession. Soil Biol. Biochem. 2017, 114, 114–120. [Google Scholar] [CrossRef]
- Craig, M.E.; Turner, B.L.; Liang, C.; Clay, K.; Johnson, D.J.; Phillips, R.P. Tree mycorrhizal type predicts within-site variability in the storage and distribution of soil organic matter. Glob. Change Biol. 2018, 4, 3317–3330. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, X.; Zhang, B. The Effects of N Addition on Soil Microbial Residues in Croplands and Forests: A Meta-analysis. J. Soil Sci. Plant Nutr. 2023, 23, 1449–1458. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L.; Zhou, G.; Zhou, H.; Lu, C.; Gu, Z.; Liu, R.; He, Y.; Du, Z.; Liang, X.; et al. Tradeoffs of fungal and bacterial residues mediate soil carbon dynamics under persistent drought in subtropical evergreen forests. Appl. Soil Ecol. 2022, 178, 104588. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; She, D.; Zhang, Z.; Zhou, Z.; Wang, H.; Wang, W. Effects of plant species diversity, dominant species importance, and soil properties on glomalin-related soil protein. Biodivers. Sci. 2022, 30, 21115. [Google Scholar] [CrossRef]
- Wang, W.; Lu, J.; Du, H.; Wei, C.; Wang, H.; Fu, Y.; He, X. Ranking thirteen tree species based on their impact on soil physiochemical properties, soil fertility, and carbon sequestration in Northeastern China. For. Ecol. Manag. 2017, 404, 214–229. [Google Scholar] [CrossRef]
- Shi, G.; Liu, F.; Chen, D.; Deng, Y.; Lin, L. Species composition and community classification of the 20-ha tropical seasonal rainforest dynamics monitoring plot in the Naban River, Yunnan. Biodivers. Sci. 2021, 29, 10–20. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Zhang, X.D.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Jia, Y.; Zhai, G.; Zhu, S.; Liu, X.; Schmid, B.; Wang, Z.; Ma, K.; Feng, X. Plant and microbial pathways driving plant diversity effects on soil carbon accumulation in subtropical forest. Soil Biol. Biochem. 2021, 161, 108375. [Google Scholar] [CrossRef]
- Guggenberger, G.; Frey, S.D.; Six, J.; Paustian, K.; Elliott, E.T. Bacterial and Fungal Cell-Wall Residues in Conventional and No-Tillage Agroecosystems. Soil Sci. Soc. Am. J. 1999, 63, 1188–1198. [Google Scholar] [CrossRef]
- Liang, C.; Kao-Kniffin, J.; Sanford, G.R.; Wickings, K.; Balser, T.C.; Jackson, R.D. Microorganisms and their residues under restored perennial grassland communities of varying diversity. Soil Biol. Biochem. 2016, 103, 192–200. [Google Scholar] [CrossRef]
- Ma, K.P.; Huang, J.H.; Yu, S.L.; Chen, L. Plant community diversity in Dongling Mountain, Beijing, China. II. Species richness, evenness and species diversities. Acta Ecol. Sin. 1995, 15, 268–277. [Google Scholar]
- Zhang, X.; Dai, G.; Ma, T.; Liu, N.; Hu, H.; Ma, W.; Zhang, J.-B.; Wang, Z.; Peterse, F.; Feng, X. Links between microbial biomass and necromass components in the top- and subsoils of temperate grasslands along an aridity gradient. Geoderma 2020, 379, 114623. [Google Scholar] [CrossRef]
- Kong, W.; Yao, Y.; Hou, L.; Bao, K.; Zhang, L.; Wei, X. Effects of vegetation presence on soil net N mineralization are independent of landscape position and vegetation type in an eroding watershed. Agric. Ecosyst. Environ. 2022, 325, 107743. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P.R. Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca.hp R package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Pecuchet, L.; Jørgensen, L.L.; Dolgov, A.V.; Eriksen, E.; Husson, B.; Skern-Mauritzen, M.; Primicerio, R. Spatio-temporal turnover and drivers of bentho-demersal community and food web structure in a high-latitude marine ecosystem. Divers. Distrib. 2022, 28, 2503–2520. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S. lme4: Linear mixed-efects models using Eigen and S4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2015, 7, 573–579. [Google Scholar] [CrossRef]
- Chen, W.; Xie, X.; Wang, J.; Pradhan, B.; Hong, H.; Bui, D.T.; Duan, Z.; Ma, J. A comparative study of logistic model tree, random forest, and classification and regression tree models for spatial prediction of landslide susceptibility. Catena 2017, 151, 147–160. [Google Scholar] [CrossRef]
- Augusto, L.; Boča, A. Tree functional traits, forest biomass, and tree species diversity interact with site properties to drive forest soil carbon. Nat. Commun. 2022, 13, 1097. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Y.; Zhang, Y.; Wang, J.; Freedman, Z.B.; Liu, P.; Cong, W.; Wang, J.; Zang, R.; Liu, S. Evenness of soil organic carbon chemical components changes with tree species richness, composition and functional diversity across forests in China. Glob. Change Biol. 2023, 29, 2852–2864. [Google Scholar] [CrossRef] [PubMed]
- Steinauer, K.; Chatzinotas, A.; Eisenhauer, N. Root exudate cocktails: The link between plant diversity and soil microorganisms? Ecol. Evol. 2016, 6, 7387–7396. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Zhang, X.; Song, C.; Wang, X.; Finnegan, P.M. Plant functional group controls litter decomposition rate and its temperature sensitivity: An incubation experiment on litters from a boreal peatland in northeast China. Sci. Total Environ. 2018, 626, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Lin, G.; Liu, B.; Ding, Y.; Li, S.; Mao, R. Contrasting responses of soil phosphorus pool and bioavailability to alder expansion in a boreal peatland, Northeast China. Catena 2022, 212, 106128. [Google Scholar] [CrossRef]
- Chen, G.J.; Shi, F.X.; Ying, Q.; Mao, R. Alder expansion increases soil microbial necromass carbon in a permafrost peatland of Northeast China. Ecol. Indic. 2022, 144, 109488. [Google Scholar] [CrossRef]
- Ramm, E.; Liu, C.; Mueller, C.W.; Gschwendtner, S.; Yue, H.; Wang, X.; Bachmann, J.; Bohnhoff, J.A.; Ostler, U.; Schloter, M.; et al. Alder-induced stimulation of soil gross nitrogen turnover in a permafrost-affected peatland of Northeast China. Soil Biol. Biochem. 2022, 172, 108757. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Lin, G.; Mccormack, M.L.; Ma, C.; Guo, D. Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytol. 2016, 213, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Midgley, M.G.; Brzostek, E.; Phillips, R.P. Decay rates of leaf litters from arbuscular mycorrhizal trees are more sensitive to soil effects than litters from ectomycorrhizal trees. J. Ecol. 2015, 103, 1454–1463. [Google Scholar] [CrossRef]
- Jacobs, L.M.; Sulman, B.N.; Brzostek, E.R.; Feighery, J.J.; Phillips, R.P. Interactions among decaying leaf litter, root litter and soil organic matter vary with mycorrhizal type. J. Ecol. 2018, 106, 502–513. [Google Scholar] [CrossRef]
- Rasse, D.P.; Rumpel, C.; Dignac, M. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).