Chlorophyll Pigment and Leaf Macronutrient Trait Variation of Four Salix Species in Elevated CO2, under Soil Moisture Stress and Fertilization Treatments
Abstract
1. Introduction
2. Materials and Methods
3. Plant Material, Growing Conditions and Treatment Delivery
4. Chlorophyll Determinations—2017 and 2018
5. Leaf Nutrient Analyses—2017 and 2018
6. Statistical Analysis
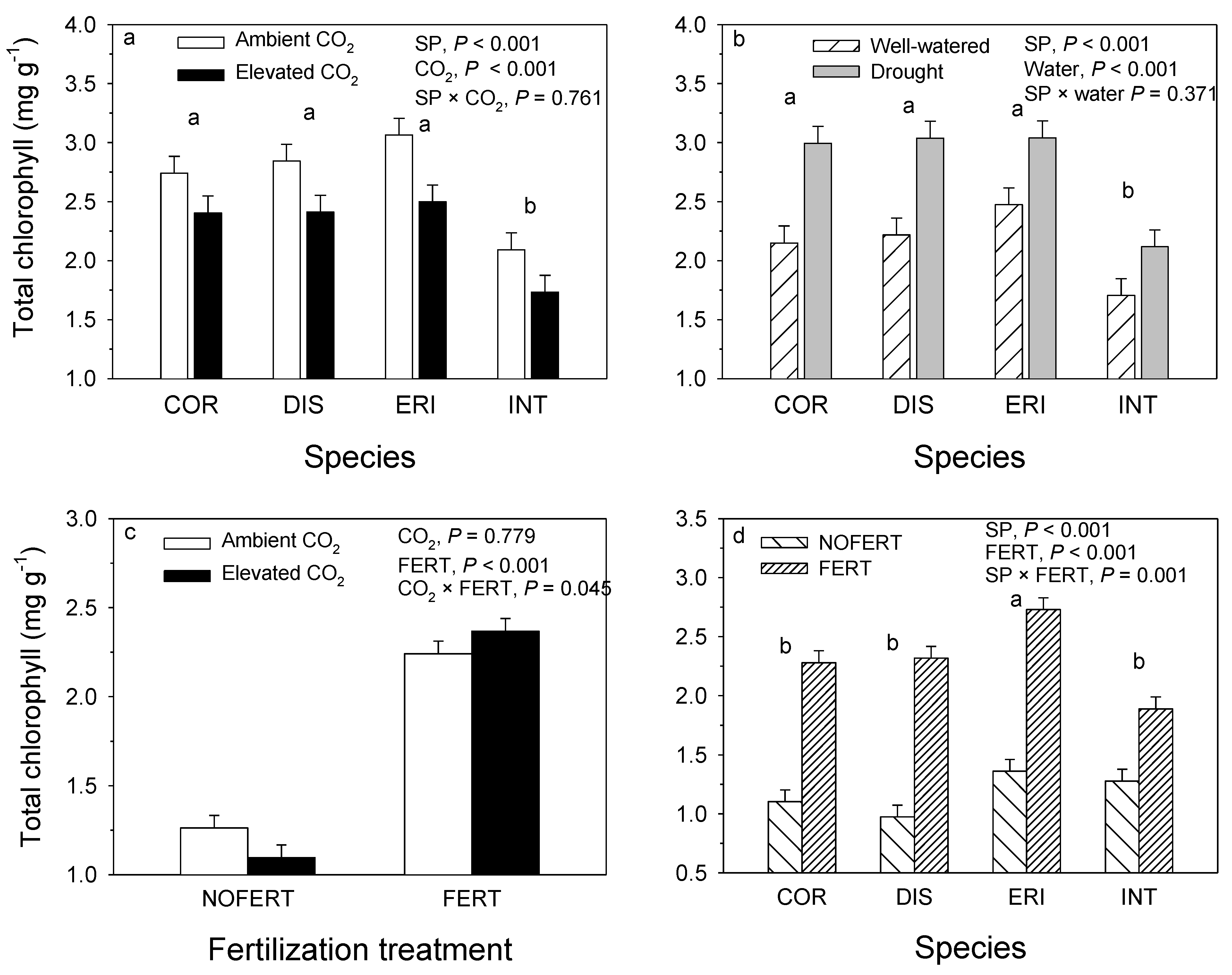
7. Results
8. Chlorophyll and Carotenoid Content
9. Carotenoid Content
10. Chlorophyll a:b and Chlorophyll to Carotenoid Ratio
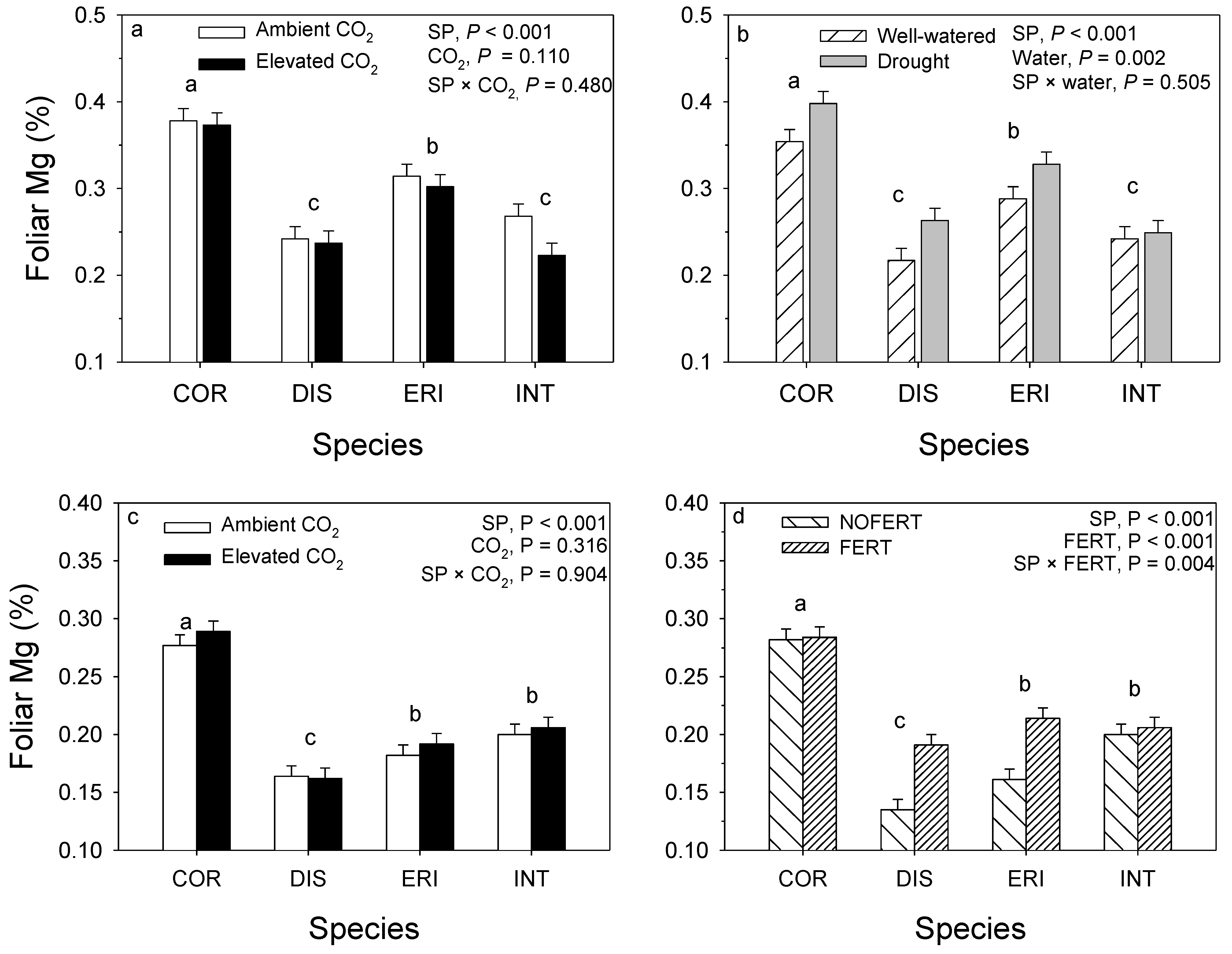
11. Clonal Variation
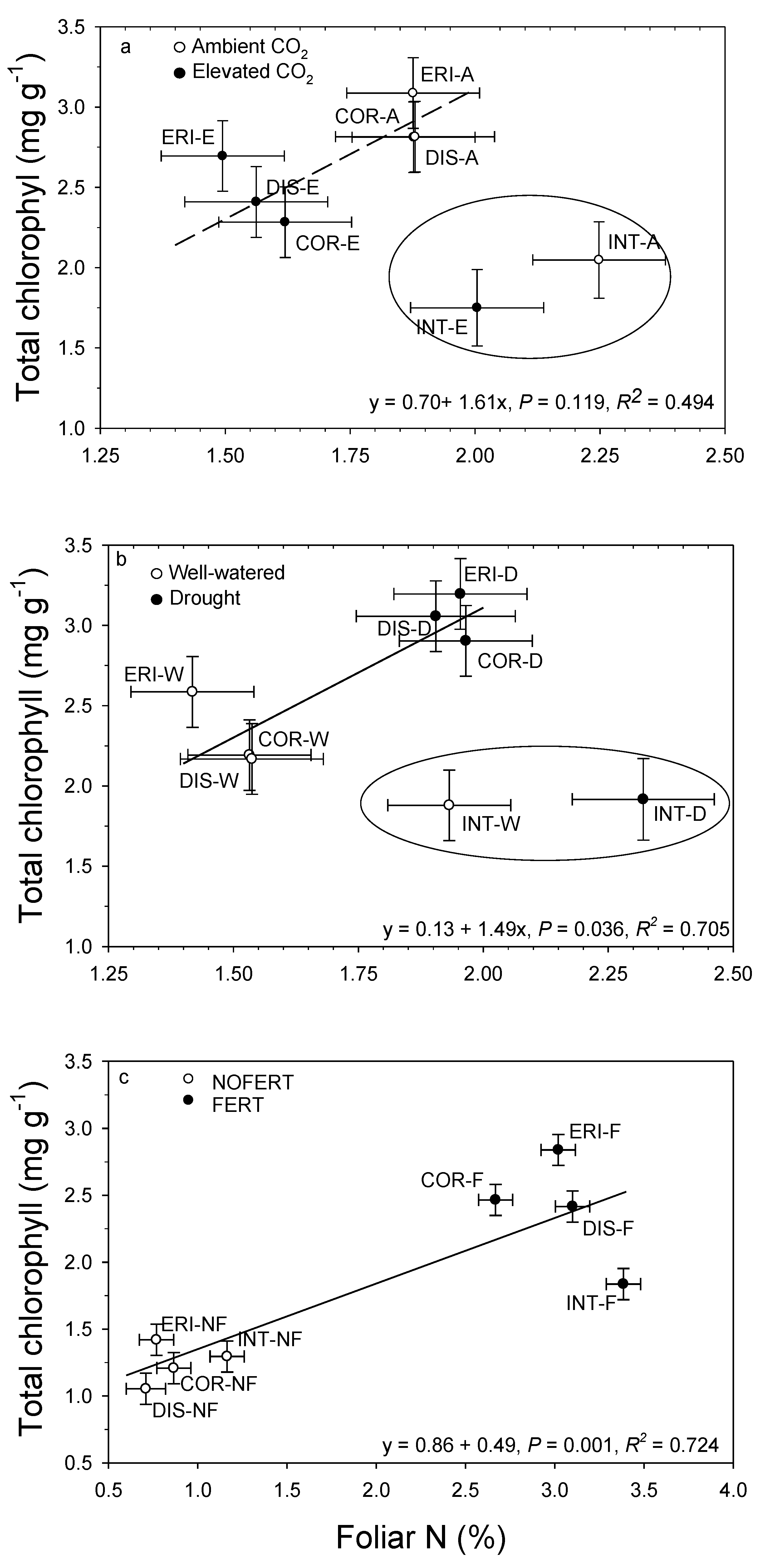
12. Leaf Macronutrients
13. Interrelationships
14. Discussion
15. Application
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jach, M.E.; Ceulemans, R. Effects of season, needle age and elevated atmospheric CO2 on photosynthesis in Scots pine (Pinus sylvestris). Tree Physiol. 2000, 20, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Barsi, D.C.; Major, J.E.; Mosseler, A.; Campbell, M. Genetic variation and control of chloroplast pigment concentrations and related needle-level traits in Picea rubens, Picea mariana, and their hybrids: Moisture and light environmental effects. Trees 2009, 23, 555–571. [Google Scholar] [CrossRef]
- Minocha, R.; Martinez, G.; Lyons, B.; Long, S. Development of a standardized methodology for quantifying total chlorophyll and carotenoids from foliage of hardwoods and conifer tree species. Can. J. For. Res. 2009, 39, 849–861. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Malavasi, U.C.; Malavasi, M.M. Leaf characteristics and chlorophyll concentration of Schyzolobium parahybum and Hymenaea stilbocarpa seedlings grown in different light regimes. Tree Physiol. 2001, 21, 701–703. [Google Scholar] [CrossRef]
- Behera, R.; Mishra, P.C.; Choudhury, N.K. High irradiance and water stress induce alterations in pigment composition and chloroplast activities of primary wheat leaves. J. Plant Physiol. 2002, 159, 967–973. [Google Scholar] [CrossRef]
- Wertin, T.M.; McGuire, M.A.; Teskey, R.O. The influence of elevated temperature, elevated atmospheric CO2 concentration and water stress on net photosynthesis of loblolly pine (Pinus taeda L.) at northern, central and southern sites in its native range. Glob. Chang. Biol. 2010, 16, 2089–2103. [Google Scholar] [CrossRef]
- Ellsworth, D.S.; Thomas, R.; Crous, K.Y.; Palroth, S.; Ward, E.; Maier, C.; Delucia, E.; Oren, R. Elevated CO2 affects photosynthetic responses in canopy pine and subcanopy deciduous trees over 10 years: A synthesis from Duke FACE. Glob. Chang. Biol. 2012, 18, 223–242. [Google Scholar] [CrossRef]
- Ellsworth, D.S.; Reich, P.B.; Nauburg, E.S.; Koch, G.W.; Kubiske, M.E.; Smith, S.D. Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated pCO2 across four free-air CO2 enrichment experiments in forest, grassland and desert. Glob. Chang. Biol. 2004, 10, 2121–2138. [Google Scholar] [CrossRef]
- Crous, K.Y.; Walters, M.B.; Ellsworth, D.S. Elevated CO2 concentration affects leaf photosynthesis—Nitrogen relationships in Pinus teada over nine years in FACE. Tree Physiol. 2008, 28, 607–614. [Google Scholar] [CrossRef]
- Major, J.E.; Mosseler, A.; Malcolm, J.W. Genetic variation among pines and spruces in assimilation efficiencies and photosynthetic regulation under elevated CO2. Trees Struct. Funct. 2018, 32, 215–229. [Google Scholar] [CrossRef]
- Wang, X.; Wei, X.; Wu, G.; Chen, S. High Nitrate or Ammonium Applications Alleviated Photosynthetic Decline of Phoebe bournei Seedlings under Elevated Carbon Dioxide. Forests 2020, 11, 293. [Google Scholar] [CrossRef]
- Luomala, E.M.; Laitinen, K.; Kellomaki, S.; Vapavuori, E. Variable photosynthetic acclimation in consecutive cohorts of Scots pine needles during 3 years of growth at elevated CO2 and elevated temperature. Plant Cell Environ. 2003, 26, 645–660. [Google Scholar] [CrossRef]
- Zhou, Y.-M.; Wang, C.-G.; Han, S.-J.; Cheng, X.-B.; Li, M.-H.; Fan, A.-N.; Wang, X.-X. Species-specific and needle age-related responses of photosynthesis in two Pinus species to long-term exposure to elevated CO2 concentration. Trees 2011, 25, 163–173. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.; van der Schreir, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Attia, Z.; Domec, J.-C.; Oren, R.; Way, D.A.; Moshelion, M. Growth and physiological responses of isohydric and anisohydric poplars to drought. J. Expt. Bot. 2015, 66, 4373–4381. [Google Scholar] [CrossRef]
- Teskey, R.O.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef]
- Duan, H.; O’Grady, A.P.; Duursma, R.A.; Choat, B. Drought responses of two gymnosperm with contrasting stomatal regulation under elevated CO2 and temperature. Tree Physiol. 2015, 35, 756–770. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Major, J.E.; Barsi, D.C.; Mosseler, A.; Campbell, M. Genetic variation and control of chloroplast pigment content in Picea rubens, Picea mariana, and their hybrids. I. Under ambient and elevated CO2 environments. Tree Physiol. 2007, 27, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Finzi, A.C.; Borby, R.J.; Calfapietra, C. Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proc. Natl. Acad. Sci. USA 2007, 104, 14014–14019. [Google Scholar] [CrossRef] [PubMed]
- Major, J.E.; Mosseler, A.; Barsi, D.C.; Campbell, M. Comparative nutrient economy, stable isotopes, and related adaptive traits in Picea rubens, Picea mariana, and their hybrids. Trees 2007, 21, 677–692. [Google Scholar] [CrossRef]
- Mellert, K.H.; Gottlein, A. Comparison of new foliar nutrient thresholds derived from van den Burg’s literature compilation with established central European references. Eur. J. For. Res. 2012, 131, 1461–1472. [Google Scholar] [CrossRef]
- Lukac, M.; Calfapietra, C.; Lagomarsino, A.; Loreto, F. Global climate change and tree nutrition: Effects of elevated CO2 and temperature. Tree Physiol. 2010, 30, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Mosseler, A.; Zsuffa, L.; Stoehr, M.U.; Kenney, W.A. Variation in biomass production, moisture content, and specific gravity in some North American willows (Salix L.). Can. J. For. Res. 1988, 18, 1535–1540. [Google Scholar] [CrossRef]
- Mosseler, A.; Major, J.E. Clonal variation in coppiced and uncoppiced growth, root sprout stem formation, and biomass partitioning in Salix interior on two highly disturbed site types. Can. J. For. Res. 2022, 52, 148–157. [Google Scholar] [CrossRef]
- Major, J.E.; Mosseler, A.; Malcolm, J.W. Salix species variation in gas exchange, leaf sodium and nutrient parameters at three levels of salinity. Can. J. For. Res. 2017, 47, 1045–1055. [Google Scholar] [CrossRef]
- Major, J.E.; Mosseler, A.; Malcolm, J.W.; Heartz, S. Salinity tolerance of three Salix species: Survival, biomass yield and allocation, and biochemical efficiencies. Biomass Bioenergy 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Silvola, J.; Ahlholm, U. Photosynthesis in willows (Salix x dasyclados) grown at different CO2 concentrations and fertilization levels. Oecologia 1992, 91, 208–213. [Google Scholar] [CrossRef]
- Major, J.E.; Mosseler, A.; Malcolm, J.W. Assimilation efficiencies and gas exchange responses of four Salix species in elevated CO2, under soil moisture stress and fertilization treatments. Forests 2022, 13, 776. [Google Scholar] [CrossRef]
- Johnson, J.D.; Tognetti, R.; Paris, P. Water relations and gas exchange in poplar and willow under water stress and elevated atmospheric CO2. Physiol. Plant 2002, 115, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Dai, S.; Wang, R.; Guo, J.; Ding, Y.; Xu, Y. Combined effects of elevated CO2 and Cd-contaminated soil on the growth, gas exchange, antioxidant defense and Cd accumulation of poplars and willows. Environ. Expt. Bot. 2015, 115, 1–10. [Google Scholar] [CrossRef]
- Mosseler, A.; Major, J.E. Phytoremediation efficacy of Salix discolor and S. eriocephella on adjacent acidic clay and shale overburden on a former mine site: Growth, soil and foliage traits. Forests 2017, 8, 475. [Google Scholar] [CrossRef]
- Moran, R.; Porath, D. Chlorophyll determination in intact tissues using N,N-Dimethylformamide. Plant Physiol. 1980, 65, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- McKeague, J.A. (Ed.) Manual on Soil Sampling and Methods of Analysis, 2nd ed.; Canadian Society of Soil Science: Ottawa, ON, Canada, 1978; 212p. [Google Scholar]
- Major, J.E.; Mosseler, A. Chlorophyll pigment and needle macronutrient responses and interactions to soil moisture and atmospheric CO2 treatments of eight pine and spruce species. Trees Struct. Funct. 2021, 35, 2069–2085. [Google Scholar] [CrossRef]
- Hicks, C.R. Fundamental Concepts in Design of Experiments, 3rd ed.; Hold, Reinhart and Winston: New York, NY, USA, 1982. [Google Scholar]
- Teskey, R.O. Combined effects of elevated CO2 and air temperature on carbon assimilation of Pinus taeda trees. Plant Cell Environ. 1997, 20, 373–380. [Google Scholar] [CrossRef]
- Mandre, M.; Lukjanova, A.; Parn, H.; Koresaar, K. State of Scots pine (Pinus sylvestris L.) under nutrient and water deficit on coastal dunes of the Baltic Sea. Trees 2010, 24, 1073–1085. [Google Scholar] [CrossRef]
- Dorn, R.D. A synopsis of American Salix. Can. J. Bot. 1976, 54, 2769–2789. [Google Scholar] [CrossRef]
- Brunsfeld, S.J.; Soltis, D.E.; Soltis, P.S. Patterns of genetic variation in Salix section Longifoliae (Salicaceae). Am. J. Bot. 1991, 78, 855–869. [Google Scholar] [CrossRef]
- Brunsfeld, S.J.; Soltis, D.E.; Soltis, P.S. Evolutionary patterns and processes in Salix sect. Longifoliae: Evidence from chloroplast DNA. Syst. Bot. 1992, 17, 239–256. [Google Scholar] [CrossRef]
- Briggs, M. Riparian Ecosystem Recovery in Arid Lands: Strategies and References; University of Arizona Press: Tucson, AZ, USA, 1996. [Google Scholar]
- Bhusal, N.; Han, S.-G.; Yoon, T.-M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus x domestica Borkh.). Sci. Hort. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Major, J.E.; Johnsen, K.H. Shoot water relations of mature black spruce families displaying a genotype x environment interaction in growth rate. II. Temporal trends and response to varying soil water conditions. Tree Physiol. 1999, 19, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Limousin, J.M.; Roussel, A.; Rodríguez-Calcerrada, J.; Torres-Ruiz, J.M.; Moreno, M.; de Jalon, L.G.; Ourcival, J.-M.; Simioni, G.; Cochard, H.H.; Martin-St Paul, N. Drought acclimation of Quercus ilex leaves improves tolerance to moderate drought but not resistance to severe water stress. Plant Cell Environ. 2022, 45, 1967–1984. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A.; Nelson, R.; Long, S.P. Testing the “source-sink” hypothesis of down-regulation of photosynthesis in elevated CO2 in the field with single gene substitutions in Glycinie max. Agric. For. Meteorol. 2004, 122, 85–94. [Google Scholar] [CrossRef]
- Oren, R.; Ellsworth, D.S.; Johnsen, K.H.; Phillips, N.; Ewers, B.E.; Maier, C.; Schäfer, K.V.; McCarthy, H.; Hendrey, G.; Katul, G.G.; et al. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 2001, 411, 469–472. [Google Scholar] [CrossRef]
- Fabio, E.S.; Leary, C.J.; Smart, L.b. Tolerance of novel inter-specific shrub willow hybrids to water stress. Trees 2019, 33, 1015–1026. [Google Scholar] [CrossRef]
- Weih, M.; Bonosi, L.; Ghelardini, L.; Ronnberg-Wastljung, A.C. Optimizing nitrogen economy under drought: Increased leaf nitrogen is an acclimation to water stress in willow (Salix spp.). Ann. Bot. 2011, 108, 1347–1353. [Google Scholar] [CrossRef]
- Hirose, T. Nitrogen use efficiency in growth of Polygonum cuspidatum Sieb. et Zucc. Ann. Bot. 1984, 54, 695–704. [Google Scholar] [CrossRef]
- Schieving, F. Plato’s Plant: On the Mathematical Structure of Simple Plants and Canopies; Backhuys Publishers: Leiden, The Netherlands, 1998. [Google Scholar]
- Farquhar, G.D.; Buckley, T.N.; Miller, J.M. Optimal stomatal control in relation to leaf area and nitrogen content. Silva Fenn. 2002, 36, 625–637. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M. Least-cost input mixtures of water and nitrogen for photosynthesis. Am. Nat. 2003, 161, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pezeshki, S.R.; Goodwin, S.; Shields, F.D., Jr. Physiological responses of black willow (Salix nigra) cuttings to a range of soil moisture regimes. Photosynthetica 2004, 42, 585–590. [Google Scholar] [CrossRef]
- Ditmarova, L.; Kurjak, D.; Palmroth, S.; Kmet, J.; Strelcova, K. Physiological responses of Norway spruce (Picea abies) seedlings to drought stress. Tree Physiol. 2009, 30, 205–213. [Google Scholar] [CrossRef]
- Suvorova, G.G.; Oskorbina, M.V.; Kopytova, L.D.; Yan’kova, L.S.; Popova, E.V. Seasonal changes in photosynthetic activity and chlorophylls in Scots pine and Siberian spruce with optimal or insufficient moistening. Contemp. Probl. Ecol. 2011, 4, 626–633. [Google Scholar] [CrossRef]
- Mosseler, A.; Major, J.E.; Ostaff, D. Distribution of genetic variation in five coppice growth traits among natural populations of seven North American willow (Salix) species. Can. J. For. Res. 2017, 47, 36–46. [Google Scholar] [CrossRef]
- Bigras, F.J.; Bertrand, A. Responses of Picea mariana to elevated CO2 concentration during growth, cold hardening: Phenology, cold tolerance, photosynthesis and growth. Tree Physiol. 2006, 26, 875–888. [Google Scholar] [CrossRef]
- Luomala, E.M.; Laitinen, K.; Sutinen, S.; Kellomaki, S.; Vapavuori, E. Stomatal density, anatomy and nutrient concentrations of Scots pine needles are affected by elevated CO2 and temperature. Plant Cell Environ. 2005, 28, 733–749. [Google Scholar] [CrossRef]
- Salazar-Tortosa, D.; Castro, J.; Villar-Salvador, P.; Binegla, B.; Matias, L.; Michelsen, A.; Rubio de Casas, R.; Querejeta, J. The “isohydric trap”: A proposed feedback between water shortage, stomatal regulation, and nutrient acquisition drives differential growth and survival of European pines under climatic dryness. Glob. Change Biol. 2018, 24, 4069–4083. [Google Scholar] [CrossRef]
- Jose, S.; Merritt, S.; Ramsey, C.L. Growth nutrition, photosynthesis and transpiration responses of longleaf pine seedlings to light, water and nitrogen. For. Ecol. Manag. 2003, 180, 335–344. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Mosseler, A.; Major, J.E.; Labrecque, M. Genetic by environment interactions of two North American Salix species assessed for coppice yield and components of growth on three sites of varying quality. Trees Struct. Funct. 2014, 28, 1401–1411. [Google Scholar] [CrossRef]
- Doty, S.L.; Oakley, B.; Xin, G.; Kang, J.W.; Singleton, G.; Khan, Z.; Vajzovic, A.; Staley, J.T. Diazotrophic endophytes of native black cottonwood and willow. Symbiosis 2009, 47, 23–33. [Google Scholar] [CrossRef]
- Kandel, S.L.; Firrincieli, A.; Joubert, P.M.; Okubara, P.A.; Leston, N.D.; McGeorge, K.M.; Mugnozza, G.S.; Harfouche, A.; Kim, S.-H.; Doty, S.L. An In vitro Study of Bio-Control and Plant Growth Promotion Potential of Salicaceae Endophytes. Front. Microbiol. 2017, 8, 386. [Google Scholar] [CrossRef]
- Mosseler, A.; Major, J.E. Biomass and root stem production of a colony-forming willow (Salix interior) on highly disturbed, low fertility sites. Biomass Bioenergy 2015, 74, 202–212. [Google Scholar] [CrossRef]





| 2017 | 2018 | ||||||
|---|---|---|---|---|---|---|---|
| Species | Clone ID | Provenance | Lat-Long | Chlorophyll | Nutrients | Chlorophyll | Nutrients |
| S. cordata | BIG-C4m | Big Sandy Bay, ON | 44°06′ N–77°43′ W | X | X | X | |
| BIG-C5f | Big Sandy Bay, ON | 44°06′ N–77°43′ W | X | X | |||
| NOR-C4f | North Beach Park, ON | 43°56′ N–77°31′ W | X | X | X | X | |
| OUT-C1m | Outlet Beach, ON | 43°53′ N–77°13′ W | X | X | X | ||
| S. discolor | HAW-D4m | Hawkesbury, ON | 45°36′ N–74°36′ W | X | X | X | |
| HAW-D5f | Hawkesbury, ON | 45°36′ N–74°36′ W | X | X | X | ||
| MON-D1f | Montmagny, QC | 46o58′ N–70o33′ W | X | X | X | X | |
| RIC-D2m | Richmond Fen, ON | 45°07′ N–75°42′ W | X | X | |||
| S. eriocephala | ALL-E2m | Allumette Island, QC | 45°54′ N–77°06′ W | X | X | X | |
| FRE-E1f | Fredericton, NB | 45°57′ N–66°38′ W | X | X | X | ||
| GRE-E1f | Green River, NB | 47°34′ N–68°19′ W | X | X | X | X | |
| WAI-E1m | Wainfleet, ON | 42°55′ N–79°20′ W | X | X | |||
| S. interior | LAF-I2f | Ottawa, ON | 45°25′ N–75°41′ W | X | X | X | X |
| LAF-I12m | Ottawa, ON | 45°25′ N–75°41′ W | X | X | |||
| WAI-I1m | Wainfleet, ON | 42°55′ N–79°20′ W | X | X | X | ||
| WAI-I2f | Wainfleet, ON | 42°55′ N–79°20′ W | X | X | X | ||
| Treatment | Organic Matter (%) | Carbon (%) | Nitrogen (%) | Potassium (meq/100 g) | Calcium (meq/100 g) | Magnesium (meq/100 g) | Phosphorus (ppm) |
|---|---|---|---|---|---|---|---|
| NOFERT | 0.67 ± 0.40 a | 0.39± 0.02 a | 0.085 ± 0.009 a | 0.04 ± 0.01 b | 1.21 ± 0.08 a | 0.050 ± 0.004 b | 6.50 ± 0.50 b |
| FERT | 0.63 ± 0.40 a | 0.37 ± 0.02 a | 0.090 ± 0.009 a | 0.16 ± 0.01 a | 1.08 ± 0.08 a | 0.068 ± 0.004 a | 9.50 ± 0.50 a |
| Treatment | Sodium (%) | Sulfur (%) | pH | C:N ratio | |||
| NOFERT | 0.11 ± 0.01 a | 0.020 ± 0.007 a | 6.8 ± 0.1 a | 4.7 ± 0.2 a | |||
| FERT | 0.11 ± 0.01 a | 0.013 ± 0.007 a | 6.2 ± 0.1 b | 3.9 ± 0.2 a | |||
| Source of Variation | df | Chlorophyll a (mg·g−1) | Chlorophyll b (mg·g−1) | Carotenoids (mg·g−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MS | VC (%) | p-Value | MS | VC (%) | p-Value | MS | VC (%) | p-Value | ||
| Block | 1 | 0.15 | 0.4 | 0.343 | 0.023 | 0.4 | 0.365 | 0.008 | 0.7 | 0.165 |
| SP 1 | 3 | 2.165 | 17.0 | <0.001 | 0.449 | 20.1 | <0.001 | 0.076 | 21.2 | <0.001 |
| CO2 | 1 | 3.049 | 8.0 | <0.001 | 0.516 | 7.7 | <0.001 | 0.089 | 8.2 | <0.001 |
| Water | 1 | 7.149 | 18.7 | <0.001 | 1.135 | 17.0 | <0.001 | 0.227 | 21.0 | <0.001 |
| SP × CO2 | 3 | 0.067 | 0.5 | 0.750 | 0.010 | 0.5 | 0.777 | 0.001 | 0.2 | 0.927 |
| SP × Water | 3 | 0.169 | 1.3 | 0.387 | 0.032 | 1.4 | 0.334 | 0.005 | 1.3 | 0.323 |
| Water × CO2 | 1 | 1.786 | 4.7 | 0.002 | 0.331 | 4.9 | 0.001 | 0.034 | 3.2 | 0.005 |
| SP × Water × CO2 | 3 | 0.210 | 1.6 | 0.290 | 0.037 | 1.6 | 0.277 | 0.006 | 1.7 | 0.215 |
| Clone(SP) | 12 | 0.279 | 8.7 | 0.089 | 0.047 | 8.4 | 0.090 | 0.008 | 8.4 | 0.047 |
| Clone(SP) × CO2 | 12 | 0.145 | 4.5 | 0.571 | 0.025 | 4.4 | 0.564 | 0.004 | 4.4 | 0.444 |
| Clone(SP) × water | 12 | 0.122 | 3.8 | 0.707 | 0.021 | 3.7 | 0.698 | 0.003 | 3.2 | 0.708 |
| Clone(SP) × water * CO2 | 12 | 0.120 | 3.8 | 0.717 | 0.021 | 3.7 | 0.707 | 0.003 | 3.6 | 0.621 |
| Error | 63 | 0.164 | 27.0 | 0.028 | 26.1 | 0.004 | 22.9 | |||
| R2 | 0.731 | 0.740 | 0.772 | |||||||
| Source of variation | df | Total chlorophyll (mg·g−1) | Chlorophyll a:b ratio | Chlorophyll: carotenoids ratio | ||||||
| MS | VC (%) | p-value | MS | VC (%) | p-value | MS | VC (%) | p-value | ||
| Block | 1 | 0.291 | 0.4 | 0.348 | 0.000 | <0.1 | 0.905 | 0.034 | 0.1 | 0.769 |
| SP | 3 | 4.581 | 17.9 | <0.001 | 0.156 | 36.3 | <0.001 | 3.887 | 20.5 | <0.001 |
| CO2 | 1 | 6.075 | 7.9 | <0.001 | 0.002 | 0.2 | 0.500 | 0.091 | 0.2 | 0.630 |
| Water | 1 | 13.982 | 18.2 | <0.001 | 0.002 | 0.2 | 0.479 | 0.030 | 0.1 | 0.782 |
| SP × CO2 | 3 | 0.127 | 0.5 | 0.761 | 0.006 | 1.3 | 0.288 | 0.556 | 2.9 | 0.242 |
| SP × Water | 3 | 0.347 | 1.4 | 0.371 | 0.008 | 1.9 | 0.152 | 0.188 | 1.0 | 0.695 |
| Water × CO2 | 1 | 3.654 | 4.8 | 0.001 | 0.012 | 0.9 | 0.101 | 1.946 | 3.4 | 0.029 |
| SP × Water × CO2 | 3 | 0.420 | 1.6 | 0.287 | 0.005 | 1.2 | 0.347 | 0.235 | 1.24 | 0.614 |
| Clone(SP) | 12 | 0.548 | 8.6 | 0.093 | 0.026 | 24.5 | <0.001 | 0.611 | 12.9 | 0.123 |
| Clone(SP) × CO2 | 12 | 0.288 | 4.5 | 0.568 | 0.004 | 3.8 | 0.541 | 0.244 | 5.2 | 0.810 |
| Clone(SP) × water | 12 | 0.242 | 3.8 | 0.705 | 0.006 | 5.8 | 0.194 | 0.405 | 8.5 | 0.422 |
| Clone(SP) × water × CO2 | 12 | 0.239 | 3.7 | 0.714 | 0.002 | 2.0 | 0.921 | 0.055 | 1.2 | 1.000 |
| Error | 63 | 0.326 | 26.8 | 0.005 | 22.0 | 0.388 | 43.0 | |||
| R2 | 0.733 | 0.781 | 0.571 | |||||||
| Source of Variation | df | Chlorophyll a (mg·g−1) | Chlorophyll b (mg·g−1) | Carotenoids (mg·g−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MS | VC (%) | p-Value | MS | VC (%) | p-Value | MS | VC (%) | p-Value | ||
| Block | 1 | 0.430 | 2.8 | 0.002 | 0.089 | 3.6 | 0.001 | 0.007 | 2.9 | 0.008 |
| SP 1 | 3 | 0.326 | 6.4 | <0.001 | 0.068 | 8.2 | <0.001 | 0.008 | 10.3 | <0.001 |
| CO2 | 1 | 0.002 | 0.0 | 0.842 | 0.002 | 0.1 | 0.639 | 0.001 | 0.4 | 0.247 |
| FERT2 | 1 | 10.418 | 68.0 | <0.001 | 1.613 | 64.7 | <0.001 | 0.134 | 55.1 | <0.001 |
| SP × CO2 | 3 | 0.039 | 0.8 | 0.412 | 0.007 | 0.8 | 0.397 | 0.001 | 1.2 | 0.328 |
| SP × FERT | 3 | 0.252 | 4.9 | 0.001 | 0.042 | 5.1 | 0.002 | 0.005 | 6.6 | 0.001 |
| CO2 × FERT | 1 | 0.187 | 1.2 | 0.037 | 0.023 | 0.9 | 0.076 | 0.005 | 2.1 | 0.024 |
| SP × CO2 × FERT | 3 | 0.179 | 3.5 | 0.008 | 0.028 | 3.4 | 0.014 | 0.004 | 4.9 | 0.005 |
| Error | 47 | 0.040 | 12.4 | 0.007 | 13.3 | 0.001 | 16.5 | |||
| R2 | 0.876 | 0.867 | 0.835 | |||||||
| Source of variation | df | Total chlorophyll (mg·g−1) | Chlorophyll a:b ratio | Chlorophyll: carotenoids ratio | ||||||
| MS | VC (%) | p-value | MS | VC (%) | p-value | MS | VC (%) | p-value | ||
| Block | 1 | 0.912 | 3.0 | 0.002 | 0.014 | 1.4 | 0.283 | 3.254 | 3.9 | 0.002 |
| SP | 3 | 0.687 | 6.8 | <0.001 | 0.070 | 20.5 | 0.002 | 0.46 | 1.7 | 0.205 |
| CO2 | 1 | 0.006 | 0.0 | 0.779 | 0.002 | 0.2 | 0.669 | 5.13 | 6.2 | <0.001 |
| FERT | 1 | 20.228 | 67.2 | <0.001 | 0.127 | 12.4 | 0.002 | 56.886 | 68.7 | <0.001 |
| SP × CO2 | 3 | 0.080 | 0.8 | 0.405 | 0.008 | 2.4 | 0.554 | 0.09 | 0.3 | 0.817 |
| SP × FERT | 3 | 0.498 | 5.0 | 0.001 | 0.013 | 3.9 | 0.360 | 0.27 | 1.0 | 0.433 |
| CO2 × FERT | 1 | 0.343 | 1.1 | 0.045 | 0.023 | 2.2 | 0.174 | 0.357 | 0.4 | 0.272 |
| SP × CO2 × FERT | 3 | 0.348 | 3.5 | 0.009 | 0.006 | 1.8 | 0.687 | 0.355 | 1.3 | 0.310 |
| Error | 47 | 0.081 | 12.6 | 0.012 | 55.2 | 0.289 | 16.4 | |||
| R2 | 0.874 | 0.449 | 0.836 | |||||||
| Source of Variation | df | Leaf Nitrogen (%) | Leaf Phosphorus (%) | Leaf Potassium (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MS | VC (%) | p-Value | MS | VC (%) | p-Value | MS | VC (%) | p-Value | ||
| Block | 1 | 0.807 | 6.9 | 0.008 | 0.016 | 5.8 | 0.045 | 0.040 | 0.2 | 0.602 |
| SP 1 | 3 | 0.571 | 14.7 | 0.003 | 0.002 | 2.2 | 0.686 | 1.194 | 19.2 | <0.001 |
| CO2 | 1 | 1.526 | 13.1 | <0.001 | 0.003 | 1.1 | 0.352 | 0.019 | 0.1 | 0.718 |
| Water | 1 | 2.394 | 20.6 | <0.001 | 0.037 | 13.3 | 0.003 | 6.788 | 36.5 | <0.001 |
| CO2 × SP | 3 | 0.013 | 0.3 | 0.942 | 0.004 | 4.3 | 0.364 | 0.044 | 0.7 | 0.824 |
| Water × SP | 3 | 0.041 | 1.1 | 0.756 | 0.004 | 4.3 | 0.392 | 0.303 | 4.9 | 0.116 |
| Water × CO2 | 1 | 0.003 | 0.0 | 0.872 | 0.004 | 1.4 | 0.293 | 0.216 | 1.2 | 0.231 |
| Water × CO2 × SP | 3 | 0.051 | 1.3 | 0.691 | 0.003 | 2.9 | 0.544 | 0.022 | 0.4 | 0.928 |
| Error | 47 | 0.104 | 41.9 | 0.004 | 64.8 | 0.146 | 36.9 | |||
| R2 | 0.596 | 0.349 | 0.632 | |||||||
| Source of variation | df | Leaf calcium (%) | Leaf magnesium (%) | Leaf sodium (%) | ||||||
| MS | VC (%) | p-value | MS | VC (%) | p-value | MS | VC (%) | p-value | ||
| Block | 1 | 0.111 | 1.4 | 0.303 | 0.018 | 5.5 | 0.002 | 0.00009 | 1.4 | 0.338 |
| SP | 3 | 0.260 | 10.2 | 0.067 | 0.065 | 59.5 | <0.001 | 0.00046 | 21.0 | 0.006 |
| CO2 | 1 | 0.166 | 2.2 | 0.209 | 0.004 | 1.2 | 0.110 | 0.00002 | 0.3 | 0.653 |
| Water | 1 | 0.588 | 7.7 | 0.020 | 0.018 | 5.5 | 0.002 | 0.00001 | 0.2 | 0.701 |
| CO2 × SP | 3 | 0.103 | 4.0 | 0.398 | 0.001 | 1.2 | 0.480 | 0.00001 | 0.5 | 0.963 |
| Water × SP | 3 | 0.109 | 4.3 | 0.374 | 0.001 | 1.2 | 0.505 | 0.00008 | 3.8 | 0.471 |
| Water × CO2 | 1 | 0.517 | 6.8 | 0.029 | 0.002 | 0.6 | 0.285 | 0.00007 | 1.1 | 0.419 |
| Water × CO2 × SP | 3 | 0.016 | 0.6 | 0.922 | 0.001 | 1.2 | 0.522 | 0.00001 | 0.6 | 0.935 |
| Error | 47 | 0.102 | 62.8 | 0.002 | 23.9 | 0.00010 | 71.2 | |||
| R2 | 0.373 | 0.763 | 0.293 | |||||||
| Source of Variation | df | Leaf Nitrogen % | Leaf Phosphorus % | Leaf Potassium % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MS | VC (%) | p-Value | MS | VC (%) | p-Value | MS | VC (%) | p-Value | ||
| Block | 1 | 0.353 | 0.4 | 0.059 | 0.0008 | 0.3 | 0.617 | 0.522 | 4.0 | 0.020 |
| SP 1 | 3 | 0.678 | 2.6 | <0.001 | 0.0018 | 2.2 | 0.620 | 1.913 | 43.9 | <0.001 |
| CO2 | 1 | 0.481 | 0.6 | 0.028 | 0.0002 | 0.1 | 0.820 | 0.107 | 0.8 | 0.280 |
| FERT 2 | 1 | 71.339 | 89.6 | <0.001 | 0.0716 | 29.4 | <0.001 | 0.956 | 7.3 | 0.002 |
| CO2 × SP | 3 | 0.019 | 0.1 | 0.892 | 0.0020 | 2.4 | 0.580 | 0.009 | 0.2 | 0.960 |
| FERT × SP | 3 | 0.244 | 0.9 | 0.063 | 0.0045 | 5.6 | 0.222 | 0.291 | 6.7 | 0.030 |
| CO2 × FERT | 1 | 0.066 | 0.1 | 0.408 | 0.0004 | 0.2 | 0.716 | 0.480 | 3.7 | 0.025 |
| CO2 × FERT × SP | 3 | 0.054 | 0.2 | 0.636 | 0.0018 | 2.2 | 0.622 | 0.049 | 1.1 | 0.651 |
| Error | 47 | 0.094 | 5.6 | 0.003 | 57.6 | 0.090 | 32.3 | |||
| R2 | 0.945 | 0.424 | 0.677 | |||||||
| Source of variation | df | Leaf calcium (%) | Leaf Magnesium (%) | Leaf sodium (%) | ||||||
| MS | VC (%) | p-value | MS | VC (%) | p-value | MS | VC (%) | p-value | ||
| Block | 1 | 0.075 | 0.5 | 0.412 | 0.00019 | 0.1 | 0.598 | 0.000564 | 17.0 | <0.001 |
| SP | 3 | 1.045 | 22.8 | <0.001 | 0.04318 | 68.3 | <0.001 | 0.000281 | 25.3 | <0.001 |
| CO2 | 1 | 0.037 | 0.3 | 0.566 | 0.00069 | 0.4 | 0.316 | 0.000002 | <0.1 | 0.824 |
| FERT | 1 | 3.866 | 28.1 | <0.001 | 0.01351 | 7.1 | <0.001 | 0.000039 | 1.2 | 0.268 |
| CO2 × SP | 3 | 0.248 | 5.4 | 0.093 | 0.00013 | 0.2 | 0.904 | 0.000014 | 1.3 | 0.717 |
| FERT × SP | 3 | 0.216 | 4.7 | 0.131 | 0.00344 | 5.4 | 0.004 | 0.000002 | 0.1 | 0.985 |
| CO2 × FERT | 1 | 0.066 | 0.5 | 0.443 | 0.00150 | 0.8 | 0.141 | 0.000127 | 3.8 | 0.049 |
| CO2 × FERT × SP | 3 | 0.010 | 0.2 | 0.966 | 0.00066 | 1.1 | 0.406 | 0.000081 | 7.3 | 0.063 |
| Error | 47 | 0.110 | 37.4 | 0.00067 | 16.6 | 0.000031 | 44.0 | |||
| R2 | 0.626 | 0.834 | 0.560 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Major, J.E.; Mosseler, A.; Malcolm, J.W. Chlorophyll Pigment and Leaf Macronutrient Trait Variation of Four Salix Species in Elevated CO2, under Soil Moisture Stress and Fertilization Treatments. Forests 2023, 14, 42. https://doi.org/10.3390/f14010042
Major JE, Mosseler A, Malcolm JW. Chlorophyll Pigment and Leaf Macronutrient Trait Variation of Four Salix Species in Elevated CO2, under Soil Moisture Stress and Fertilization Treatments. Forests. 2023; 14(1):42. https://doi.org/10.3390/f14010042
Chicago/Turabian StyleMajor, John E., Alex Mosseler, and John W. Malcolm. 2023. "Chlorophyll Pigment and Leaf Macronutrient Trait Variation of Four Salix Species in Elevated CO2, under Soil Moisture Stress and Fertilization Treatments" Forests 14, no. 1: 42. https://doi.org/10.3390/f14010042
APA StyleMajor, J. E., Mosseler, A., & Malcolm, J. W. (2023). Chlorophyll Pigment and Leaf Macronutrient Trait Variation of Four Salix Species in Elevated CO2, under Soil Moisture Stress and Fertilization Treatments. Forests, 14(1), 42. https://doi.org/10.3390/f14010042




