Abstract
The short- to long-distance dispersal (SDD and LDD) of propagules is critical for the regeneration of mangrove forests. Mark–recapture experiments are considered to be a good tool for assessing such dispersal patterns. However, dense mangrove roots, exposed mudflats, shallow water, etc. often limit the number of recaptured propagules and their implication studies. Therefore, a combination of hydrodynamic and particle tracking models, together with a mark–recapture experiment, were applied to identify the dispersal behavior of Rhizophora mucronata Lamk. propagules in a coastal lagoon of Setiu Wetlands, Malaysia. The transport trajectories revealed that the dispersal of propagules is leptokurtic, with majority of them confined to very-short-distance dispersal (VSDD; 3–5 m) to SDD (1400 m). While higher obstacle density (e.g., mangrove roots) constrained the propagule dispersal, weaker tidal currents that coupled with less upstream discharge increased their retention time and settlement closer to the point of release. Under this scenario, the chances for propagules to exit from the lagoon mouth and their entry into the open waters for LDD are very limited. These results can explain the abundance of Rhizophora spp. in the northern sector of the Setiu Wetlands and be useful for species-level conservation/management.
1. Introduction
Mangroves in the tropical, subtropical and warm temperate latitudes are well-known for their regeneration through viviparous hydrochorous propagules [1,2,3,4,5]. Thriving in the tide dominated areas, mangrove propagules experience short- to long-distance dispersal (hereafter referred to as SDD and LDD, respectively), before their establishment and growth under suitable physico-chemical conditions elsewhere [6,7,8]. Meanwhile, some propagules also get stranded among roots and shrubs close to where they originated [8,9,10,11]. While propagule movement in the water is governed by tides, current, winds and waves [12], their subsequent survival as juveniles depends on the other factors such as herbivorous crabs and rodents, insects, barnacle infestation, etc. [13,14,15,16,17].
As mangroves have a patchy distribution [18,19], the LDD is considered to be an advantageous mechanism for their colonization of distant areas [2]. However, as mentioned before, the propagule dispersal and establishment dynamics are influenced by a combination of biological and environmental settings. For instance, high water levels and proper tidal inundations can release some stranded propagules and subject them to SDD or LDD [13,20]. A study on the buoyancy of Rhizophoraceae species (Bruguiera gymnorrhiza (L.) Sav., Ceriops tagal (Perr.) C.B. Robinson and Rhizophora mucronata Lamk.) found that the fate of each propagule and its capability to escape the local forest and reach open water is determined by the interaction between propagule traits and surface water conditions as well as its landscape matrix and forest type [21]. The dispersal pattern of the mangrove propagules also varies in relation to their size, shape, weight, buoyancy, longevity and speed of root-growth characteristics between the species [3,4,22,23,24]. Through a field-based tracking experiment, De Ryck et al. [23] found that smaller C. tagal propagules with low density and high agility disperse much more rapidly than the larger R. mucronata propagules with more starch reserves to benefit their LDD. Further, Robert et al. [25] documented that larger R. mucronata propagules were less vulnerable to dehydration than those of C. tagal, which confirms their best fit for the LDD. Apart from mangrove propagule traits, its initial distribution is also susceptible to predation and retention. While predation results in a loss of propagules in several locations (e.g., coastal Louisiana, USA; [12]), its retention by roots/vegetation as trapping agents limit their dispersal (e.g., Pambala–Chilaw Lagoon Complex, Sri Lanka) [10].
Setiu Wetlands in Peninsular Malaysia (Figure 1) is a unique place where several coastal features such as mudflats, islands, lagoon, estuary, rivers, beach, sea, seagrass meadows and the mangroves are interconnected [26,27,28]. These wetlands are receiving a good deal of scientific attention in recent years, and several researchers have focused on hydrodynamics [29,30], distributions of mangrove flora and fauna [27,31,32], vegetation mapping based on remote sensing [28], sea-level rise [33], food web, microplastics and heavy metal pollution [34,35,36]. However, to date, there were no scientific investigations of mangrove propagule dispersal, which therefore makes this study highly relevant.
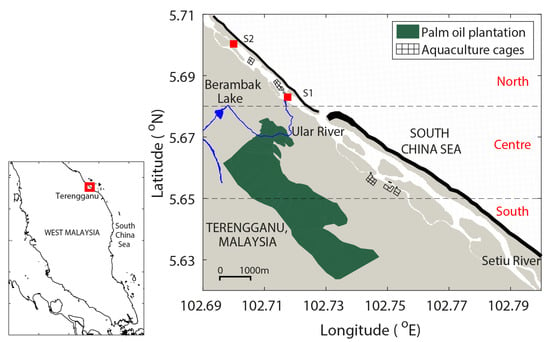
Figure 1.
The map of Setiu Wetlands with a narrow lagoon, in Terengganu, Malaysia. The Setiu River and Berambak Lake supply the freshwater into the lagoon. The water exchange between the Setiu Lagoon and the South China Sea occurs through a small inlet. The gray patches indicate the mangrove-dominated areas, S1 marks the location of the current meter deployment, and S2 marks the release point of the mangrove propagules. Black band in between the lagoon and the sea represents the beach. The study area was divided into three different sectors, namely north, centre and south, for the interpretation of the results.
At Setiu Wetlands, the mangrove propagules are likely to show three dispersal patterns: very-short-distance dispersal (VSDD) to SDD by the ones inside the lagoon and LDD by those that exit from the lagoon mouth and enter into the open waters (South China Sea). We hypothesise that (1) the shallow and narrow landscape of the lagoon limits the propagules to VSDD and SDD and (2) the majority of the propagules are entrapped by the vegetation due to subdued water circulation. The specific objectives of this study were to identify maximum dispersal distances of R. mucronata propagules through a combination of hydrodynamic and particle tracking models and to validate the results via mark–recapture experiment. The findings revealed coherent transport trajectories for R. mucronata propagules in the Setiu Lagoon.
2. Materials and Methods
2.1. Study Area
The Setiu Wetlands, located in Terengganu State on the east coast of Peninsular Malaysia (Figure 1), are dominated by mangroves and have supported the local communities through various goods and services for ages [34]. This is one of the most diverse ecosystems in Malaysia with >30 exclusive mangrove species including hybrids such as Bruguiera × hainesii C.G. Rogers, Bruguiera × rhynchopetala (W.C. Ko) X.-J. Ge & N.C. Duke, Rhizophora × annamalayana Kathir. and Sonneratia × hainanensis W.C. Ko, E.Y. Chen & W.Y. Chen [37,38,39]. The 14-km long Setiu Lagoon has a total water surface area of 880 ha [34] and depth between 0.3 and 3.2 m [29,40]. The freshwater input in the lagoon comes from Setiu River on the south and Berambak Lake, via the Ular River, on the north (Figure 1). In between, the seawater intrusion brings mixed tidal conditions in the lagoon [41]. Mangroves with different species associations are found along the lagoon [27,42], like interior basin mangroves in the northern sector, tide-dominant fringing mangroves in the central sector and riverine mangroves in the southern sector (Figure 1). Small islets scattered in the lagoon are colonised by Rhizophora, Avicennia, Sonneratia and Nypa spp. [34,40]. The direct and indirect use-value of the Setiu Wetlands was estimated to be around MYR 1452—10,700 per hectare (MYR: Malaysian ringgits [43]).
Setiu Lagoon is influenced by the northeast monsoon (November to March), which is characterised by heavy rainfall, and a dry period of the southwest monsoon (May to September) [44,45,46]. The two inter-monsoon periods in April and October are often linked with convective rains [47].
2.2. In Situ Data Collection
A total of 90 matured R. mucronata propagules were collected for the mark–recapture experiment. The criterion used for collecting these propagules was their easy detachment from the parent trees when their branches were shaken or hand-plucked with a gentle pull. Propagules already available on the ground were ignored due to the chances of their exposure to the osmotic effect with the stranded conditions [22]. All propagules were first weighed (using a portable balance) and then marked using an aerosol spray paint (as 3” band around the hypocotyl) before releasing them into the water (17 October 2015). The point of release chosen was ca. 5 m away from Rhizophora stands in the core mangrove area (S2 location in northern sector, Figure 1) to avoid root entrapment and have less impact of human activities and boat passages (Figure 1). Further, the release location was chosen based on the distribution of Rhizophora mucronata in Setiu Wetlands, which can only be found in the vicinity of S2 [48]. Meanwhile, a Valeport® current meter (Model 308, Totnes, UK) with 10-min data recording intervals (e.g., tidal height, current speed, direction, etc.) was moored in a suitable depth (min. 1.5 m) in the lagoon for 1 week (21–28 October 2015; S1 location in northern sector; Figure 1). Rhizophora mucronata was chosen for this study because of its abundance as well as long floating and viable propagules [25], which can easily be identified in the field compared with other species’ propagules. The released propagules were tracked for a period of 1 month to record their dispersal locations (using a handheld Garmin® 45 GPS (Taipei, Taiwan), accuracy 5–10 m). These results were later used to validate the findings of the particle tracking simulation, whereas the current meter data were used to validate the hydrodynamic simulation. Bathymetric data of the entire lagoon were collected in June 2014, November 2015 and August 2017 using a Garmin® GPSmap echosounder (Model 298, Southampton, UK) and SeaSTAR 3200 LR12 DGPS Receiver (Amsterdam, The Netherlands).
2.3. Numerical Modelling
A two-dimensional surface hydrodynamic model of the Setiu Wetlands was generated through Delft3D FLOW module (using Delft3D v. 4.0.3 software developed by Deltares and Delft University of Technology, Delft, The Netherlands) in WGS84/UTM 48 N projection coordinate system. The bathymetry of the coastal waters was interpolated through a combination of the extracted General Bathymetric Chart of the Oceans (GEBCO) data and the bathymetric survey results obtained from the lagoon. The spatial resolution of ~100 m applied to the open waters (= South China Sea) was increased to ~36 m for the coast and lagoon. A time step of 15 sec was applied to the model due to the complex nature of the Setiu Lagoon. In the present study, 13 tidal constituents for the water level or current were imposed to the coastal boundary (sea). Meanwhile, daily wind velocity and direction obtained from the European Center for Medium-Range Weather Forecast (ECMWF) were used to force the hydrodynamic model. After the first 3 days of model warm-up time, several numerical experiments were carried out to test the model sensitivity, for which a Manning coefficient of 0.03 and the horizontal eddy viscosity of 1.0 m2/s were found to be most effective values for the Setiu Lagoon hydrodynamic modelling.
For simulating the transport of R. mucronata propagules, the particle tracking module (D-WAQ PART) was chosen since it has the capacity to follow particles as individuals while assigning them specific properties [10]. The initial weight of each propagule was specified as 50 g referring to their average weight measured in the field. A decay constant of 0.007 was imposed to the particles as recommended by Kamal et al. [49]. Through the calibration, a settling velocity of 5 m/d (equivalent to 0.0058 cm/s) was assigned to the propagules that ultimately produced the most reliable output. The dispersal trajectories of R. mucronata propagules in Setiu Lagoon were projected for 43 days and produced graphical representations by using the MATLAB software (Version 2015 by MathWorks, Torrance, CA, USA).
2.4. Validation of the Models
A set of statistical parameters (i.e., correlation coefficient, root mean square and index of the agreement) was applied to the in situ data to test the capability of the hydrodynamic model. A perfect agreement between the simulation-based results and the field-based observations produced an index of 1, whereas complete disagreement produced an index of 0. In the case of particle tracking, the model results were validated by comparing them with the mark–recapture experiment data.
3. Results
3.1. Hydrodynamic Characteristics in Setiu Lagoon
A total of 44 high tide events, which is also referred as flood time, were recorded for the period of hydrodynamic simulation (17 October–30 November 2015; Figure 2a). The amplitude of the water current in the lagoon followed a general pattern of the tidal flow, implying the domination of mixed tides in this area (Figure 2b). The high tide events were found to be associated with strong current speeds ranging between 0.1 to 0.3 m/s (Figure 2b). The magnitude of the water current speed was higher during spring tides than during the neap tides (Figure 2b and Figure 3). There were two main water current directions observed; one moved from the inlet towards the northern and southern sectors during the flooding (Figure 3a,c), while the other moved from the northern and southern areas towards the inlet during the ebbing (Figure 3b,d), which reflected a bidirectional flow. Additionally, the general circulation of the coastal water current followed the same trend for spring and neap tides (Figure 3).
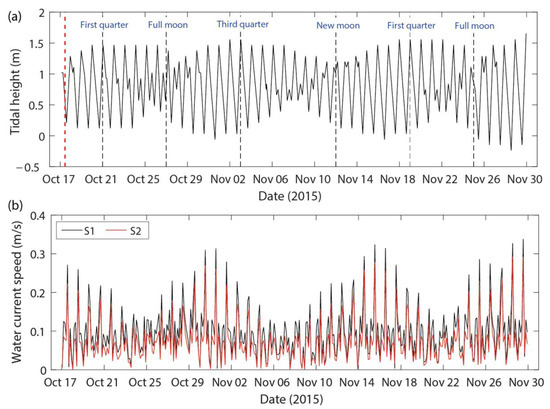
Figure 2.
Hydrodynamic simulations made for Setiu Lagoon: (a) tidal height superimposed by different lunar phases; (b) water current speed at the places of current meter deployment (S1; black line) and mangrove propagules released point (S2; red line). Figure 1 shows S1 and S2 locations.

Figure 3.
Water current circulation during the (a) neap high tide, (b) neap low tide, (c) spring high tide and (d) spring low tide, in Setiu Lagoon and the adjacent coastal waters.
3.2. Simulation-Based Rhizophora Propagules Dispersal in Setiu Lagoon
The particle tracking simulation shows that the dispersal of >90% propagules (87 out of 90) was confined to the northern sector, whereas 2 propagules had a chance to exit from the lagoon mouth on Days 6 and 7 (Figure 4 and Figure A1). Another propagule that managed to enter the central sector on Day 8 reached the southern sector only on Day 30 (Figure 4 and Figure A1). In terms of the dispersal distances, R. mucronata propagules travelled in both northward and southward directions from the point of release, corresponding to the bidirectional tidal current in the lagoon (Figure 3). The two propagules that managed to go out of the lagoon, however, remained closer to the coastline throughout the period of simulation (Figure 5 and Figure A1). Overall, the dispersal behaviour of R. mucronata propagules in Setiu Lagoon was leptokurtic (VSDD to SDD), limited by a few meters to less than 2.5 km (Figure 6). The average dispersal distance was 1.7 km, though a few propagules travelled over 5 km after receiving 20 times flooded conditions (Figure 6). Propagules in the northern sector have settled down at distances of 26–957 m from the point of release (Figure 6).

Figure 4.
Simulation-based observations of the numbers of propagules dispersed within north, central and southern sectors of Setiu Lagoon (please follow Figure 1 for sectors) and exited from the lagoon mouth.
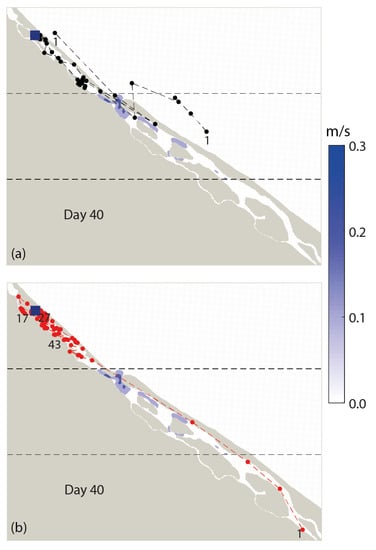
Figure 5.
Spatial distribution of water current speeds (m/s) and dispersal trajectories of Rhizophora mucronata propagules in the Setiu Lagoon based on particle tracking simulation for 40 days. The blue square indicates the point of propagules released. The black and red points represent all recorded particle positions. (a) Propagules exiting the Setiu Lagoon and (b) propagules remaining in the lagoon.
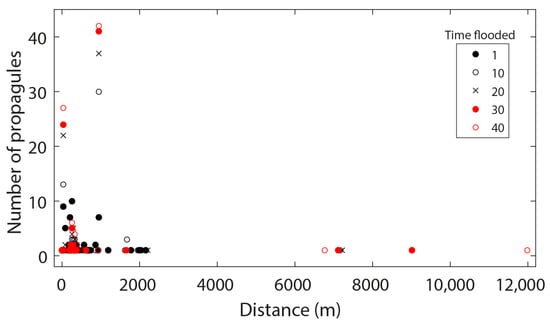
Figure 6.
Dispersal distances of Rhizophora mucronata propagules based on the particle tracking simulation in the Setiu Lagoon.
3.3. Mark–Recapture Experiment in Setiu Lagoon
Despite the long-term tracking of the marked R. mucronata propagules in the lagoon, only 42% (38 propagules) of their total count were found. The majority of those recaptured propagules landed in sites that consist of dense Rhizophora prop roots and/or Avicennia pneumatophores adjacent to the waterways. A few propagules that went southwards in groups also got distanced and stranded among the roots. While some propagules remained within 3–5 m radius from the point of release (S2; VSDD), the others travelled up to 300 m northwards and 1400 m southwards as their maximum SDD within the northern sector of the lagoon.
3.4. Accuracy of Hydrodynamic and Particle Tracking Models
The higher index of agreement for the simulation-based results confirms that both water level and current component variations in the hydrodynamic model were accurately reproduced (Table 1). Furthermore, the pattern of R. mucronata propagule’s VSDD and SDD between simulation-based and field-based observations within the northern sector confirms that the particle tracking model chosen for Setiu Lagoon produced the closest results to the natural conditions (Figure 7).

Table 1.
Statistical assessment of the hydrodynamic model used for Setiu Lagoon.

Figure 7.
Comparison between field-based and simulation-based dispersals of Rhizophora mucronata propagules in Setiu Lagoon.
4. Discussion
The results of this study indicate that the majority of R. mucronata propagules in Setiu Lagoon show only VSDD to SDD and settle down within the mangrove stands nearby. In this context, the higher density of Rhizophora prop roots along the waterways and Avicennia/Sonneratia pneumatophores on the exposed mudflats were found to restrict their movement. Earlier, Di Nitto et al. [10] also noticed these inhibiting factors for R. mucronata propagules, causing their movement to be limited and concentrated near the parental trees, at Pambala–Chilaw Lagoon in Sri Lanka. In fact, the root structural complexity of mangroves remains as one of the potential barriers to the propagules’ dispersal [12,23]. The dispersal characteristics of different mangrove propagules (R. mucronata, C. tagal and Heritiera littoralis Dryand) and a mangrove seed (Xylocarpus granatum König) in relation to different barrier densities were unveiled by Van der Stocken et al. [20].
Riverine and tidal processes are very important for any mangrove ecosystem, and undoubtedly, tidal circulation is the cause of water movement inside the mangrove habitats. In addition to the root persuaded retention of mangrove propagules, the hydrodynamics also play a crucial role in their SDD and LDD. Consistent with the previous (e.g., Zainol et al. [29,30]) as well as present findings on the hydrodynamic characteristics of the Setiu Lagoon, the bidirectional movement of R. mucronata propagules with the incoming flood and the outgoing ebb conditions was evident. This clearly shows the influence of daily tides on the dispersal and establishment of mangrove propagules in the vicinity. However, the weaker water current speed in the northern sector during low tides (~0.15 m/s; with a reduced flow velocity by 5–28 times than high tides) was found to be insufficient for pushing the propagules towards the lagoon mouth, especially before the intrusion of the next high tide waters. Although water current changes seasonally, the previous studies also revealed similar conditions (less water current magnitude, higher residence time) for the northern portion of the Setiu Lagoon [29,30,39]. Thus, propagules are largely distributed within the same area closer to the point of release. According to Van der Stocken et al. [20], higher water current speed will intensify the kinetic energy and reduce the friction that could help the propagules to move freely. Under these circumstances, an increased freshwater discharge (e.g., from the Ular River) would be able to facilitate wider propagules dispersion in the Setiu Lagoon [10,50,51]. Although there were no salinity data available in this research, Zainol et al. [29] managed to document the presence of slightly more saline water in the northern sector than in the central and southern sectors, which evidenced the low riverine input in this area. Reduced upstream discharge into the lagoon was found to be associated with increased oil palm plantations and other agriculture/aquaculture activities, including settlement areas, in the vicinity [40,52,53]. A combination of low freshwater input and shifted (Setiu) river mouth (ca. 5 km southwards in 2013–2014) are causing poor water circulation/exchange mechanisms [39]. As a consequence, much of the sand brought by the tidal current is accumulating inside the lagoon as well as mangrove areas (personal observation). While local authorities are regularly carrying out sand dredging operations, such activities are known to cause several negative impacts on the bottom sediment, benthic flora/fauna, etc. [54,55].
Meanwhile, the landscape features of the Setiu Wetlands were also found influencing the mangrove propagules’ dispersal. Due to inadequate freshwater discharge in the northern sector, some shallow and narrow channels are completely exposed during the ebb tides. The basin mangrove in the northern sector with denser root system increased the retention of the released propagules, limiting their dispersal pattern considerably [56]. Although a few propagules were able to settle down as juvenile vegetation at some corners of the mangrove islands (where sediment accretion is still active), the majority of them remain unproductive due to long-term stranded conditions in the lagoon, entrapment inside the roots and unfavourable (sandy) substratum. In addition, the presence of obstacles such as fish cages (in about 5000 sq m area with a production over 1460 metric tons per year) are further hampering the mangrove propagules’ dispersal in the lagoon. In fact, Setiu Lagoon was recognised as an ‘aquaculture industry area’ by the State Government of Terengganu, and the demand for cage aquaculture is persistently increasing year by year [57].
In addition to the general agreement between particle tracking simulation and mark–recapture experiment, the differences in relation to exit of two propagules from the Setiu Lagoon mouth and entry of a propagule into the southern sector are understandable from the point of difficulties to trace them in the field. On the other hand, this could be a limitation of the present simulation due to no cage culture barriers. Given the fact that the voluminous shape and smooth surface of R. mucronata propagules assist in preventing their dehydration and buoyancy loss for longer periods [23,25], their exit from the lagoon mouth or travel up to southern sector cannot be ruled out. According to Tonné et al. [21], the propagules released directly into the tidal creeks have good opportunities for LDD, but not in the case of Setiu under the present hydrodynamic conditions. Propagules that went out of the lagoon mouth, however, remained afloat in the coastal waters due to small-scale (local) eddies that are known to occur with the onset of the northeast monsoon. The structure of mangroves can reflect the responses to geomorphology and habitat changes [58,59], where the confinement of the released propagules to the northern sector of the lagoon reveals the abundance of Rhizophora stands in this core mangrove area of the Setiu Wetlands.
Overall, the studies on mangrove propagule dispersal are not new but are limited. More than four decades ago, Rabinowitz [60] identified the propagule dispersal properties of six mangrove species (Laguncularia, Avicennia, Rhizophora and Pelliciera spp.) in relation to their land or seaward zones of occurrence. In recent times, the field-based experiments conducted by Di Nitto et al. [10], Van der Stocken et al. [20], De Ryck et al. [23], Robert et al. [25], etc., were constructive. As Wang et al. [61] suggest that mangrove propagule dispersal is not a passively buoyant process controlled by water current alone, the physical barriers remain important. In the lagoon systems like Pambala–Chilaw (Sri Lanka) and Setiu (Malaysia), extensive mangrove roots itself found to act as natural barriers (cf. [10], present study). The dispersal distance of R. mucronata propagules largely within 500 m to 1 km radius in the present study also coincides with the observations of Clarke [62]. In addition to the local environmental conditions, LDD of the mangrove propagules tends to change with climate change and seawater density [63], which require further investigations at both local and regional scales.
5. Conclusions
The findings of this study clearly indicate that R. mucronata propagules in the Setiu Wetlands are subjected to VSDD and SDD and are concentrating over the northern sector. Along with the shallow and narrow features of the lagoon, the basin mangrove with dense root system in this sector was responsible for the leptokurtic dispersal of the propagules. The subdued water circulation due to weaker tidal current and low freshwater discharge is further enhancing the retention period of propagules entrapped by Rhizophora, Avicennia and Sonneratia root systems. To overcome these restricted hydrodynamic conditions, measures to improve freshwater discharge (with the help of the Dept. of Irrigation and Drainage authorities) are necessary. Such key initiatives should not only restore water circulation in the Setiu Lagoon but also help mangroves to be healthy and continue benefiting the local communities with various goods and services. Otherwise, incidents such as higher salinity and sand accumulation will continue to bring tremendous changes to the local topography as well as species composition, especially in the case of the low- to moderate-salinity-preferring Sonneratia, Aegiceras, Nypa and Lumnitzera spp. Improved hydrodynamics can also put a halt to the sand dredging activity. The present study recommends more propagules and longer periods of tracking for both simulation and mark–recapture experiments to avoid uncertainties pertaining to the LDD. The future particle tracking simulations in Setiu Lagoon should also focus on the cage culture barriers at the (sub)surface of the water to draw more precise conclusions on the propagules’ dispersal. The present findings are extremely useful to the local authorities for possible conservation/management measures for the mangrove species at Setiu Wetlands.
Author Contributions
Conceptualization, Z.Z., A.D.P., M.F.A., B.S. and F.D.-G.; methodology, M.F.A., B.S. and F.D.-G.; software, Z.Z. and M.F.A.; validation, Z.Z., M.F.A., B.S. and F.D.-G.; formal analysis, Z.Z. and A.D.P.; investigation, Z.Z. and A.D.P.; data curation, Z.Z. and A.D.P.; writing—original draft, Z.Z. and B.S.; writing—review and editing, M.F.A., N.H.A.R., B.S. and F.D.-G.; visualization, Z.Z., M.F.A. and B.S.; supervision, M.F.A., B.S. and F.D.-G.; funding acquisition, M.F.A., B.S. and F.D.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Systems Ecology and Resource Management Research Unit (ULB, Belgium) as part of the “mangrove propagules buoyancy and viability” experimentation, at the Institute of Oceanography and Environment (UMT, Malaysia). Further, this research was also supported by the Malaysia Higher Institution Centre of Excellence (HICoE) in Marine Science Fund with grant number 66928.
Data Availability Statement
Not applicable.
Acknowledgments
Special thanks are due to every colleague who helped us in carrying out both field and lab-based observations successfully.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A


Figure A1.
Spatial distributions of the water current speed (m/s), direction and trajectories for propagules on (a) Day 5, (b) Day 10, (c) Day 15, (d) Day 20, (e) Day 25, (f) Day 30, (g) Day 35 and (h) Day 40.
References
- Tomlinson, P.B. The Botany of Mangroves, 2nd ed.; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Van der Stocken, T.; Menemenlis, D. Modelling mangrove propagule dispersal trajectories using high-resolution estimates of ocean surface winds and currents. Biotropica 2017, 49, 472–481. [Google Scholar] [CrossRef]
- Van der Stocken, T.; Carroll, D.; Menemenlis, D.; Simard, M.; Koedam, N. Global-scale dispersal and connectivity in mangroves. Proc. Natl. Acad. Sci. USA 2019, 116, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Van der Stocken, T.; Wee, A.K.S.; De Ryck, D.J.R.; Vanschoenwinkel, B.; Friess, D.A.; Dahdouh-Guebas, F.; Simard, M.; Koedam, N.; Webb, E.L. A general framework for propagule dispersal in mangroves. Biol. Rev. 2019, 94, 1547–1575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xin, K.; Sheng, N.; Xie, Z.; Liao, B. The regenerative capacity of eight mangrove species based on propagule traits in Dongzhai Harbor, Hainan Province, China. Glob. Ecol. Conserv. 2021, 30, e01788. [Google Scholar] [CrossRef]
- Di Nitto, D.; Dahdouh-Guebas, F.; Kairo, J.G.; Decleir, H.; Koedam, N. Digital terrain modelling to investigate the effects of sea level rise on mangrove propagule establishment. Mar. Ecol. Prog. Ser. 2008, 356, 175–188. [Google Scholar] [CrossRef][Green Version]
- Krauss, K.W.; Lovelock, C.E.; McKee, K.L.; López-Hoffman, L.; Ewe, S.M.L.; Sousa, W.P. Environmental drivers in mangrove establishment and early development: A review. Aquat. Bot. 2008, 89, 105–127. [Google Scholar] [CrossRef]
- Peterson, J.M.; Bell, S.S. Saltmarsh boundary modulates dispersal of mangrove propagules: Implications for mangrove migration with sea-level rise. PLoS ONE 2015, 10, e0119128. [Google Scholar] [CrossRef]
- Alleman, L.K.; Hester, M.W. Reproductive ecology of Black Mangrove (Avicennia germinans) along the Louisiana Coast: Propagule production cycles, dispersal limitations, and establishment elevations. Estuaries Coasts 2011, 34, 1068–1077. [Google Scholar] [CrossRef]
- Di Nitto, D.; Erftemeijer, P.L.A.; Van Beek, J.K.L.; Dahdouh-Guebas, F.; Higazi, L.; Quisthoudt, K.; Jayatissa, L.P.; Koedam, N. Modelling drivers of mangrove propagule dispersal and restoration of abandoned shrimp farms. Biogeosciences 2013, 10, 5095–5113. [Google Scholar] [CrossRef]
- Simpson, L.T.; Osborne, T.Z.; Feller, I.C. Establishment and biomass allocation of black and red mangroves: Response to propagule flotation duration and seedling light availability. J. Coast. Res. 2017, 33, 1126–1134. [Google Scholar] [CrossRef]
- Yando, E.S.; Jones, S.F.; Hester, M.W. Limited mangrove propagule retention at a latitudinal range limit: Spatiotemporal patterns at the patch scale. Estuaries Coasts 2020, 44, 834–845. [Google Scholar] [CrossRef]
- Cannicci, S.; Burrows, D.; Fratini, S.; Smith, T.J.; Offenberg, J.; Dahdouh-Guebas, F. Faunal impact on vegetation structure and ecosystem function in mangrove forests: A review. Aquat. Bot. 2008, 89, 186–200. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Koedam, N.; Satyanarayana, B.; Cannicci, S. Human hydrographical changes interact with propagule predation behaviour in Sri Lankan mangrove forests. J. Exp. Mar. Biol. Ecol. 2011, 399, 188–200. [Google Scholar] [CrossRef]
- Satyanarayana, B.; Koedam, N.; De Smet, K.; Di Nitto, D.; Bauwens, M.; Jayatissa, L.P.; Cannicci, S.; Dahdouh-Guebas, F. Long-term mangrove forest development in Sri Lanka: Early predictions evaluated against outcomes using VHR remote sensing and VHR ground-truth data. Mar. Ecol. Prog. Ser. 2011, 443, 51–63. [Google Scholar] [CrossRef]
- Jenoh, E.M.; Robert, E.M.R.; Lehmann, I.; Kioko, E.; Bosire, J.O.; Ngisiange, N.; Dahdouh-Guebas, F.; Koedam, N. 2016. Wide ranging insect infestation of the pioneer mangrove Sonneratia alba by two insect species along the Kenyan coast. PLoS ONE 2016, 11, e0154849. [Google Scholar] [CrossRef] [PubMed]
- Langston, A.K.; Kaplan, D.A. Modelling the effects of climate, predation, and dispersal on the poleward range expansion of black mangrove (Avicennia germinans). Ecol. Model. 2020, 434, 109245. [Google Scholar] [CrossRef]
- Duke, N.C.; Maynecke, J.O.; Dittmann, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; et al. A world without mangroves? Science 2007, 317, 41–43. [Google Scholar] [CrossRef]
- Curnick, D.J.; Pettorelli, N.; Amir, A.A.; Balke, T.; Barbier, E.B.; Crooks, S.; Dahdouh-Guebas, F.; Duncan, C.; Endsor, C.; Friess, D.A.; et al. The value of small mangrove patches. Science 2019, 363, 239. [Google Scholar] [CrossRef]
- Van der Stocken, T.; De Ryck, D.J.R.; Vanschoenwinkel, B.; Deboelpaep, E.; Bouma, T.J.; Dahdouh-Guebas, F.; Koedam, N. Impact of landscape structure on propagule dispersal in mangrove forests. Mar. Ecol. Prog. Ser. 2015, 524, 95–106. [Google Scholar] [CrossRef]
- Tonné, N.; Beeckman, H.; Robert, E.M.R.; Koedam, N. Towards an unknown fate: The floating behaviour of recently abscised propagules from wide ranging Rhizophoraceae mangrove species. Aquat. Bot. 2017, 140, 23–33. [Google Scholar] [CrossRef]
- Clarke, P.J.; Kerrigan, R.A.; Westphal, C.J. Dispersal potential and early growth in 14 tropical mangroves: Do early life history traits correlate with patterns of adult distribution? J. Ecol. 2001, 89, 648–659. [Google Scholar] [CrossRef]
- De Ryck, D.J.R.; Robert, E.M.R.; Schmitz, N.; Van der Stocken, T.; Di Nitto, D.; Dahdouh-Guebas, F.; Koedam, N. Size does matter, but not only size: Two alternative dispersal strategies for viviparous mangrove propagules. Aquat. Bot. 2012, 103, 66–73. [Google Scholar] [CrossRef]
- Van der Stocken, T.; López-Portillo, J.; Koedam, N. Seasonal release of propagules in mangroves—Assessment of current data. Aquat. Bot. 2017, 138, 92–99. [Google Scholar] [CrossRef]
- Robert, E.M.R.; Oste, J.; Van der Stocken, T.; De Ryck, D.J.R.; Quisthoudt, K.; Kairo, J.G.; Dahdouh-guebas, F.; Koedam, N.; Schmitz, N. Viviparous mangrove propagules of Ceriops tagal and Rhizophora mucronata, where both Rhizophoraceae show different dispersal and establishment strategies. J. Exp. Mar. Biol. Ecol. 2015, 468, 45–54. [Google Scholar] [CrossRef]
- Suratman, S.; Hussein, A.N.A.R.; Latif, M.T.; Weston, K. Reassessment of physico-chemical water quality in Setiu Wetland, Malaysia. Sains Malays. 2014, 43, 1127–1131. [Google Scholar]
- Jamilah, M.S.; Faridah, M.; Rohani, S. Setiu: More than a wetland. In Setiu Wetlands: Species, Ecosystem and Livelihoods; Penerbit Universiti Malaysia Terengganu (UMT): Terengganu, Malaysia, 2015. [Google Scholar]
- Ruwaimana, M.; Satyanarayana, B.; Otero, V.; Muslim, A.M.; Syafiq, A.M.; Ibrahim, S.; Raymaekers, D.; Koedam, N.; Dahdouh-Guehbas, F. The advantages of using drones over space-borne imagery in the mapping of mangrove forests. PLoS ONE 2018, 13, e0200288. [Google Scholar] [CrossRef] [PubMed]
- Zainol, Z.; Akhir, M.F.; Abdullah, S. Hydrodynamics, nutrient concentrations, and phytoplankton biomass in a shallow and restricted coastal lagoon under different tidal and monsoonal environmental drivers. Reg. Stud. Mar. Sci. 2020, 38, 101376. [Google Scholar] [CrossRef]
- Zainol, Z.; Akhir, M.F.; Zainol, Z. Pollutant transport and residence time of a shallow and narrow coastal lagoon estimated using a numerical model. Mar. Pollut. Bull. 2021, 164, 112011. [Google Scholar] [CrossRef]
- Mutalib, A.H.A.; Fadzly, N.; Foo, R. Striking a balance between tradition and conservation: General perceptions and awareness level of local citizens regarding turtle conservation efforts based on age factors and gender. Ocean. Coast. Manag. 2013, 78, 56–63. [Google Scholar] [CrossRef]
- Hidir, A.; Aaqillah-Amr, M.A.; Noordiyana, M.N.; Ikhwanuddin, M. Diet and internal physiological changes of female orange mud crabs, Scylla olivacea (Herbst, 1796) in different ovarian maturation stages. Anim. Reprod. Sci. 2018, 195, 216–229. [Google Scholar] [CrossRef]
- Culver, S.J.; Leorri, E.; Mallinson, D.J.; Corbett, D.R.; Shazili, N.A.M. Recent coastal evolution and sea-level rise, Setiu Wetland, Peninsular Malaysia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 417, 406–421. [Google Scholar] [CrossRef]
- Le, Q.D.; Haron, N.A.; Tanaka, K.; Ishida, A.; Sano, Y.; Dung, L.V.; Shirai, K. Quantitative contribution of primary food sources for a mangrove food web in Setiu lagoon from East coast of Peninsular Malaysia, stable isotopic (δ13C and δ15N) approach. Reg. Stud. Mar. Sci. 2017, 9, 174–179. [Google Scholar] [CrossRef]
- Siau, Y.F.; Le, D.Q.; Suratman, S.; Jaaman, S.A.; Tanaka, K.; Kotaro, S. Seasonal variation of total mercury transfer through a tropical mangrove food web, Setiu Wetlands. Mar. Pollut. Bull. 2021, 162, 111878. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, S.R.; Altrawneh, R.S.; Anuar, S.T.; Khalik, W.M.A.W.M.; Kolandhasamy, P.; Ibrahim, Y.S. Ingestion of microplastics by the estuarine polychaete, Namalycastis sp. in the Setiu Wetlands, Malaysia. Mar. Pollut. Bull. 2021, 170, 112617. [Google Scholar] [CrossRef] [PubMed]
- Razali, M.S.; Zulkifli, M.K.F.; Wan Ahmad, W.J. Distribution and rarity of mangrove and coastal plants in developing indicators of hotspots in Setiu Wetlands. In Proceedings of the Setiu Wasteland Scientific Expedition Seminar “Connecting Science to Society”, Universiti Malaysia Terengganu, Terengganu, Malaysia, 13 October 2016. [Google Scholar]
- Bakar, N.H.A.; Jusoh, A.; Ahmad, M.F.; Noor, M.J.M.M.; Norzilah, A. A spatial nutrient distribution due to seabass aquaculture activities at Setiu, Terengganu, Malaysia. J. Fish. Aquat. Sci. 2016, 11, 332–348. [Google Scholar] [CrossRef][Green Version]
- Zainol, Z.; Akhir, M.F. Temporal variability of phytoplankton biomass in relation to salinity and nutrients in a shallow coastal lagoon. Malays. J. Anal. Sci. 2019, 23, 1090–1106. [Google Scholar]
- Poh, S.C.; Ng, N.C.W.; Suratman, S.; Mathew, D.; Mohd Tahir, N. Nutrient availability in the Setiu Wetland Lagoon, Malaysia: Trends, possible causes and environmental impacts. Environ. Monit. Assess. 2019, 191, 3. [Google Scholar] [CrossRef]
- Zainol, Z.; Akhir, M.F.; Joseph, J. Tidal circulation in Bay of Chagar Hutang, Malaysia. Indian J. Geo-Mar. Sci. 2015, 44, 1–9. [Google Scholar]
- Mohd Azmi, M.I. Valuing the potential economic value of mangroves resources in Setiu Wetlands, Terengganu, Malaysia: A preliminary findings. Int. J. Educ. Res. 2014, 2, 487–504. [Google Scholar]
- Jamilah, M.S.; Nik Kamil, N.F.; Komoo, I. Managing Setiu Wetlands for ecosystem services. In Setiu Wetlands: Species, Ecosystem and Livelihoods; Penerbit Universiti Malaysia Terengganu (UMT): Terengganu, Malaysia, 2015. [Google Scholar]
- Zainol, Z.; Akhir, M.F. Coastal upwelling at Terengganu and Pahang coastal waters: Interaction of hydrography, current circulation and phytoplankton biomass. J. Teknol. 2016, 78, 11–27. [Google Scholar] [CrossRef][Green Version]
- Johari, A.; Akhir, M.F. Exploring thermocline and water masses variability in southern South China Sea from the World Ocean Database (WOD). Acta Oceanol. Sin. 2019, 38, 38–47. [Google Scholar] [CrossRef]
- Johari, A.; Akhir, M.F.; Satar, M.N.; Zainol, Z.; Guo, J. Inter-annual changes of water temperature in the Southern South China Sea’s continental shelf: The influence of ENSO on Malaysian waters. J. Mar. Sci. Technol. 2021, 29, 569–581. [Google Scholar] [CrossRef]
- Suhaila, J.; Deni, S.M.; Zawiah Zin, W.A.N.; Jemain, A.A. Trends in Peninsular Malaysia rainfall data during the southwest monsoon and northeast monsoon seasons: 1975–2004. Sains Malays. 2010, 39, 533–542. [Google Scholar]
- Nor, S.M.M.; Sahari, M.S.I.; Razali, N.A.M.; Salam, M.R.; Wan Mustaffa, W.F.; Stephen, E.R.; Yusof, R.M.; Jamaludin, P.M.; Jamludin, P.N.; Mokhetar, N. Mangrove floristic composition dataset of the Setiu Lagoon, Terengganu Malaysia. Data Brief 2022, 42, 108020. [Google Scholar]
- Kamal, A.H.M.; Hoque, M.M.; Idris, M.H.; Ahmed, O.H.; Bhuiyan, M.K.A.; Billah, M.M.; Hoque, M.N.; Rosli, Z. Decay of Rhizophora apiculata (Blume) and Xylocarpus granatum (Koenig) detrital sources in the Sarawak Mangrove, Malaysia. J. For. Res. 2020, 31, 613–623. [Google Scholar] [CrossRef]
- Mahanty, M.M.; Mohanty, P.K.; Pattnaik, A.K.; Panda, U.S.; Pradhan, S.; Samal, R.N. Hydrodynamics, temperature/salinity variability and residence time in the Chilika lagoon during dry and wet period: Measurement and modeling. Cont. Shelf Res. 2016, 125, 28–43. [Google Scholar] [CrossRef]
- Otero, V.; Lucas, R.; Van De Kerchove, R.; Satyanarayana, B.; Mohd-Lokman, H.; Dahdouh-Guebas, F. Spatial analysis of early mangrove regeneration in the Matang Mangrove Forest Reserve, Peninsular Malaysia, using geomatics. For. Ecol. Manag. 2020, 472, 118213. [Google Scholar] [CrossRef]
- Suratman, S.; Latif, M.T. Reassessment of nutrient status in Setiu Wetland, Terengganu, Malaysia. Asian J. Chem. 2015, 27, 239–242. [Google Scholar] [CrossRef]
- Lola, M.S.; Ramlee, M.N.A.; Isa, H.; Abdullah, M.I.; Hussin, M.F.; Zainuddin, N.H.; Rahman, M.N. Forecasting towards planning and sustainable development based on a system dynamic approach: A case study of the Setiu district, state of Terengganu, Malaysia. Open J. Stat. 2016, 6, 931–950. [Google Scholar] [CrossRef]
- Buraschi, G.V.; Gallo, M.N. Hydro-morphodynamics of a dredged tidal canal, Fundão Canal-RJ, Brazil. J. S. Am. Earth Sci. 2021, 112, 103603. [Google Scholar] [CrossRef]
- Liu, H.; Xu, K.; Wilson, C.; Bentley, S.J.; Xue, Z.; Zhang, Z. Geomorphologic change and patchy mud infilling in a sandy dredge pit in eastern Ship Shoal, Louisiana shelf, USA. Geomorphology 2022, 396, 107983. [Google Scholar] [CrossRef]
- Kathiresan, K. Mangroves: Types and Importance. In Mangroves: Ecology, Biodiversity and Management; Springer: Singapore, 2021; pp. 1–3. [Google Scholar]
- Alipiah, R.M.; Raffaelli, D.; Smart, J.; Mohd Kamil, N.F.N. Modelling the impact of aquaculture on Setiu wetland ecosystems using bayesian belief network approach. J. Sustain. Sci. Manag. 2017, 12, 183–193. [Google Scholar]
- Cunha-Lignon, M.; Coelho, C., Jr.; Almeida, R.; Menghini, R.; Correa, F.; Schaeffer-Novelli, Y.; Cintrón-Molero, G.; Dahdouh-Guebas, F. Mangrove Forests and Sedimentary Processes on the South of Coast of São Paulo State (Brazil). J. Coast. Res. 2009, 56, 405–409. [Google Scholar]
- Cunha-Lignon, M.; Coelho, C., Jr.; Almeida, R.; Menghini, R.P.; Schaeffer-Novelli, Y.; Cintrón, G.; Dahdouh-Guebas, F. Characterisation of mangrove forest types in view of conservation and management: A review of mangals at the Cananéia region, São Paulo State, Brazil. J. Coast. Res. 2011, 64, 349–353. [Google Scholar]
- Rabinowitz, D. Dispersal properties of mangrove propagules. Biotropica 1978, 10, 47–57. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Wang, M. Propagule dispersal determines mangrove zonation at intertidal and estuarine scales. Forests 2019, 10, 245. [Google Scholar] [CrossRef]
- Clarke, P.J. Dispersal of grey mangrove (Avicennia marina) propagules in southeastern Australia. Aquat. Bot. 1993, 45, 195–204. [Google Scholar] [CrossRef]
- Van der Stocken, T.; Vanschoenwinkel, B.; Carroll, D.; Cavanaugh, K.C.; Koedam, N. Mangrove dispersal disrupted by projected changes in global seawater density. Nat. Clim. Chang. 2022, 12, 685–691. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).