Maximum Rooting Depth of Pinus thunbergii Parl. Estimated with Depth at the Center Point of Rotation in a Tree-Pulling Experiment in a Coastal Forest in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Tree-Pulling Experiments and Root System Excavation of P. thunbergii
2.3. Calculation of Dcp and Cp Displacement
2.4. Data Sets and Statistical Analysis
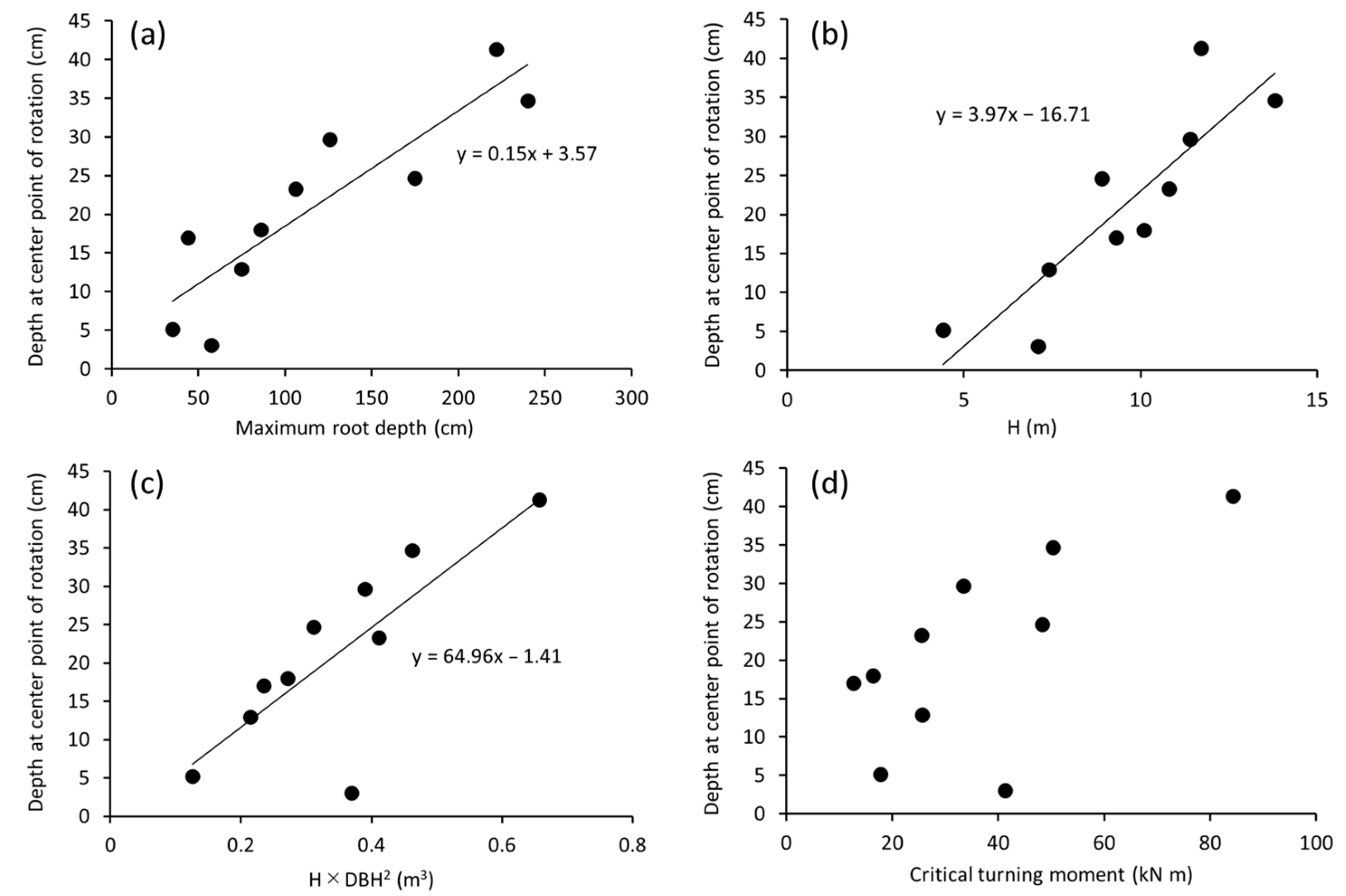
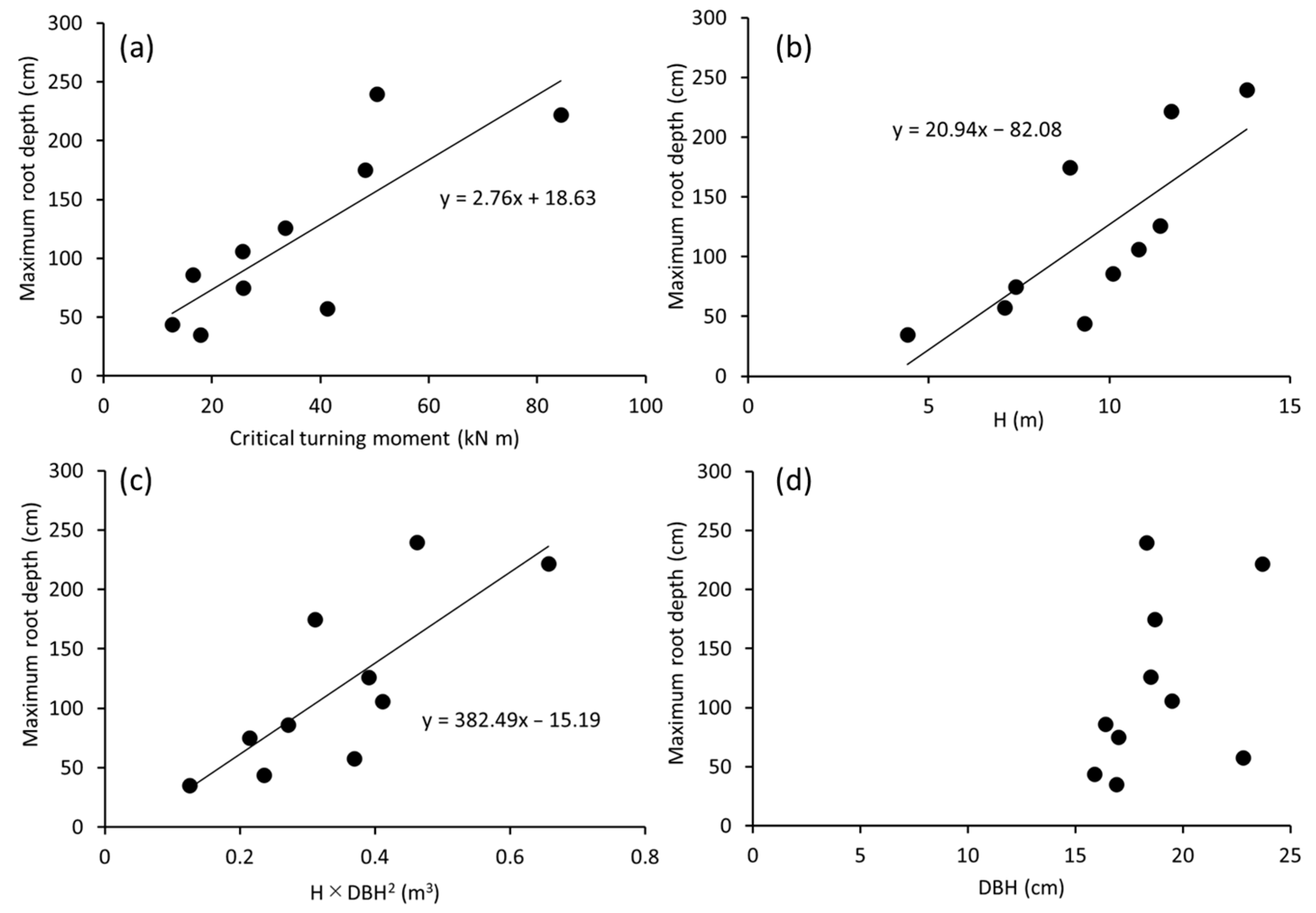
3. Results
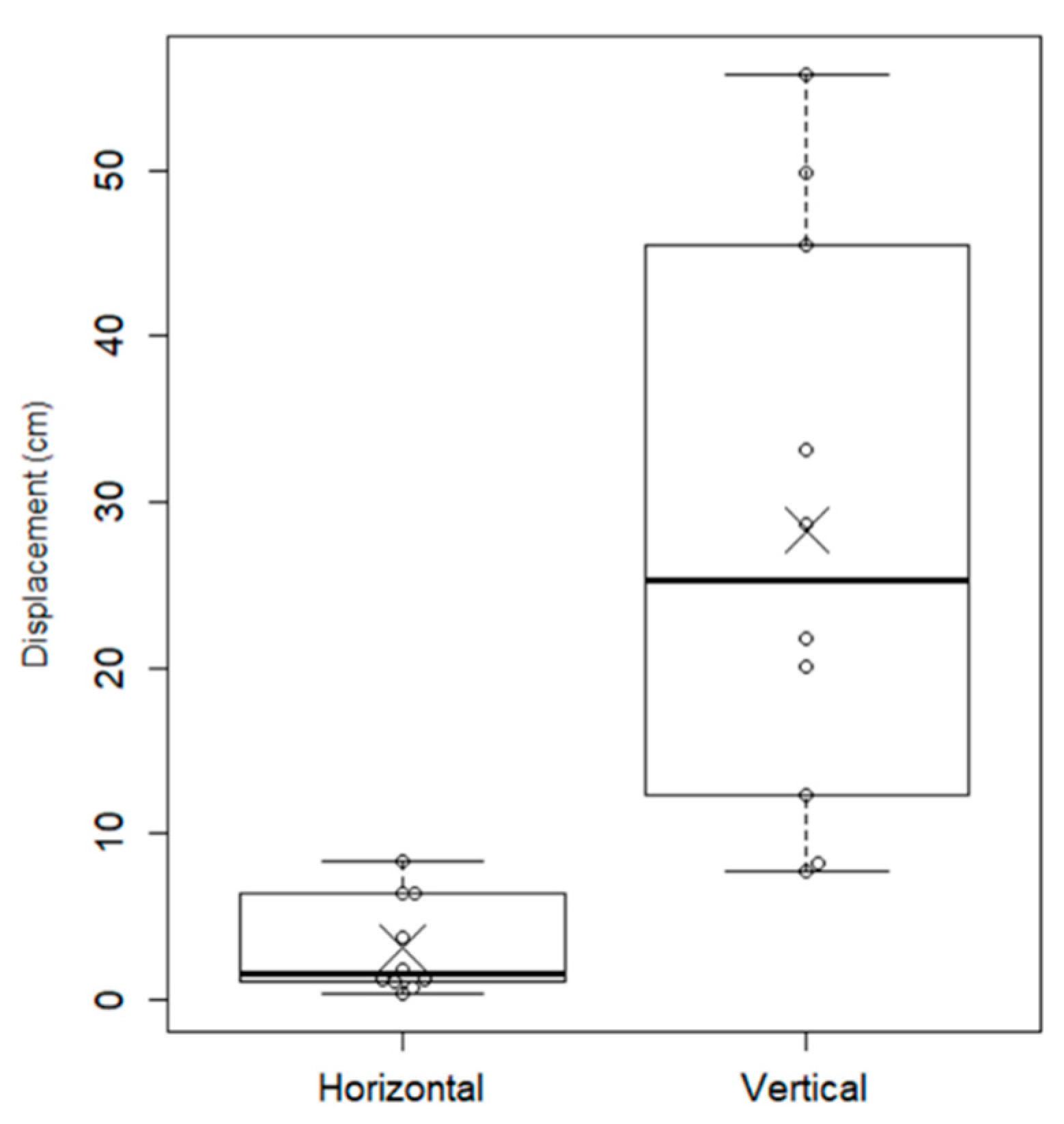
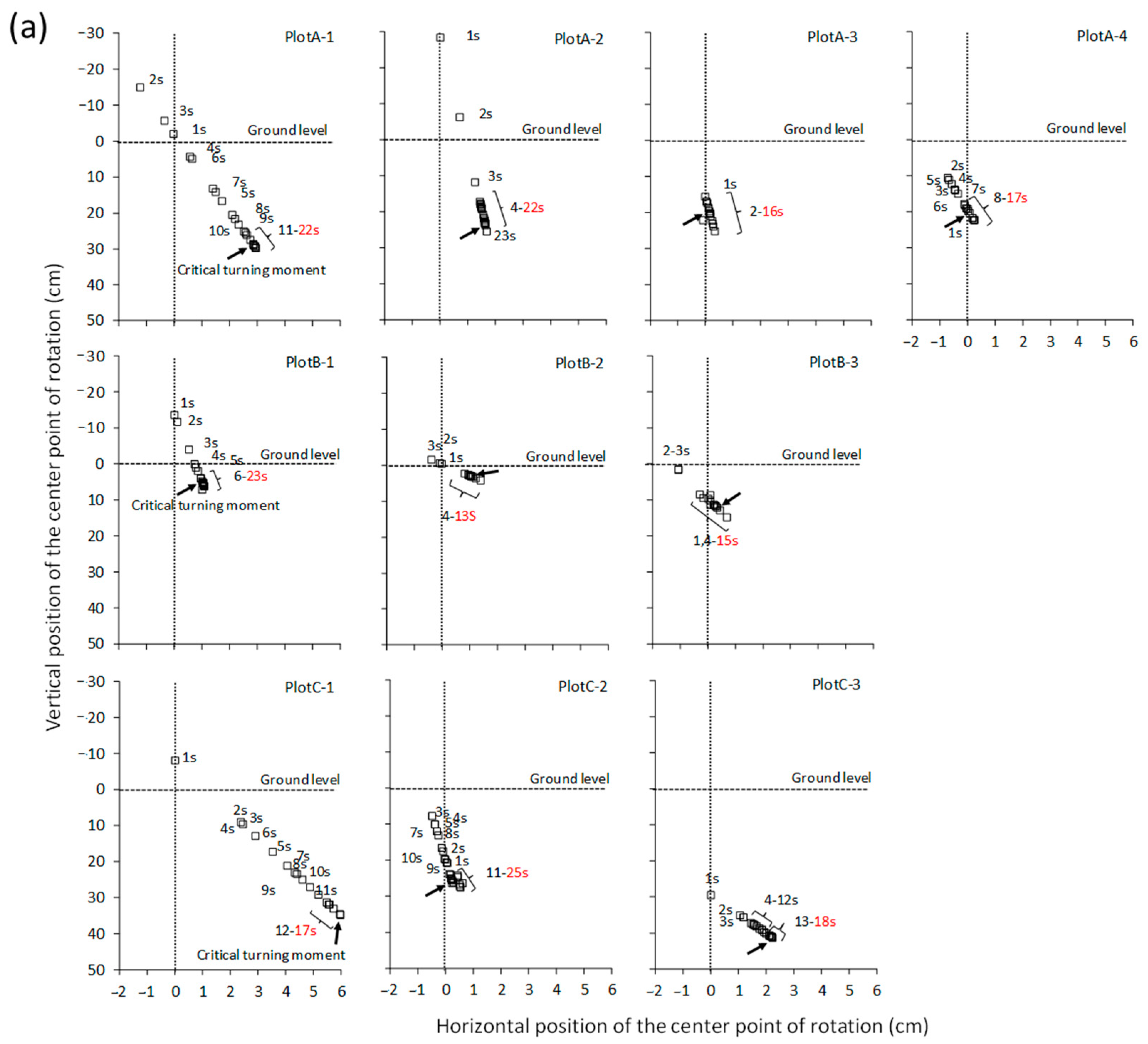
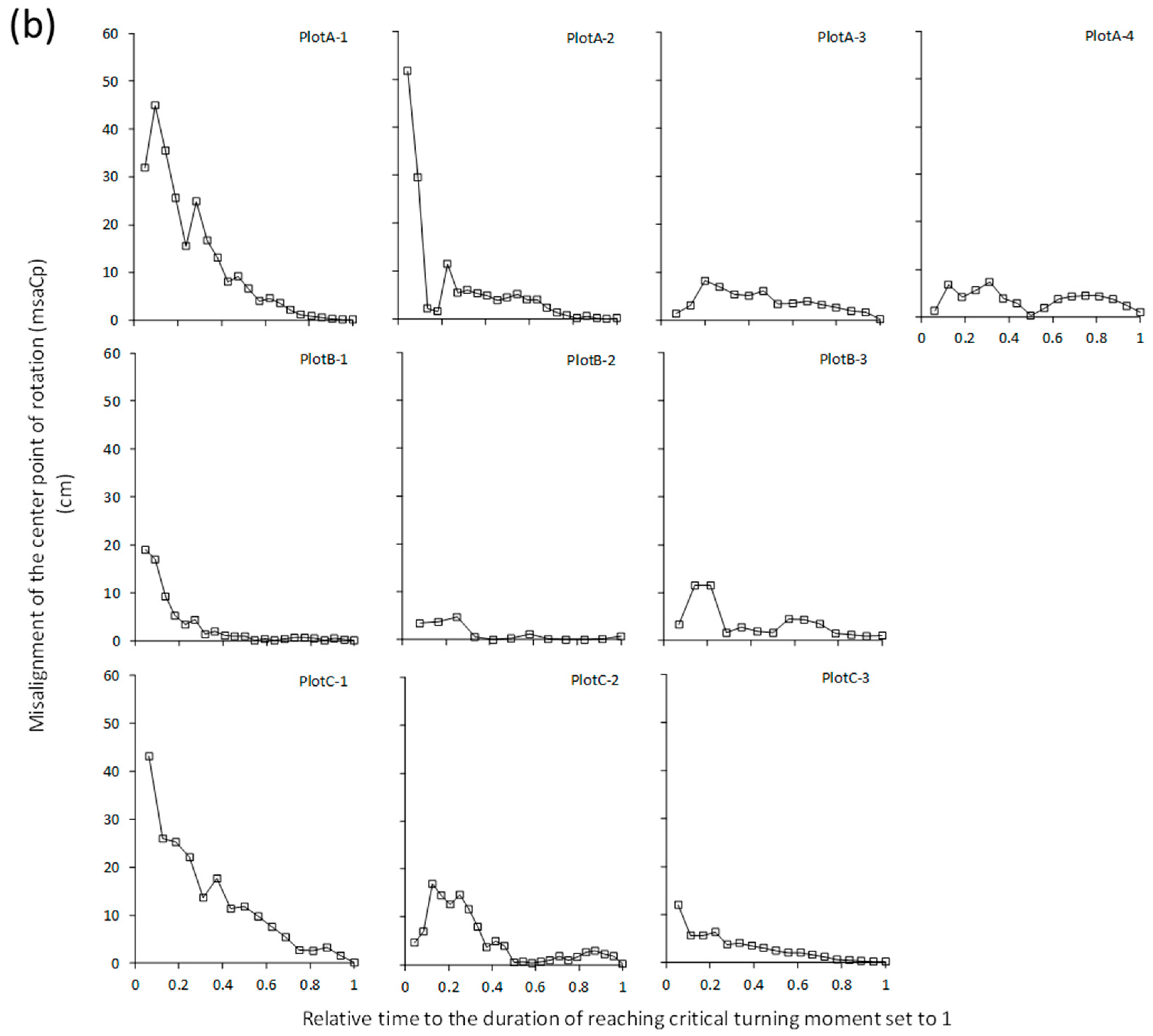
4. Discussion
4.1. Dcp as an Indicator of Maximum Root Depth
4.2. Relationship between Cp Displacement and Critical Turning Moment
4.3. Relationship between Cp Position and RSP
4.4. Relationship between Soil Type and Cp Displacement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murai, H.; Ishikawa, M.; Endo, J.; Tadaki, Y. The Coastal Forest in Japan; Soft Science Inc.: Tokyo, Japan, 1992; p. 513. (In Japanese) [Google Scholar]
- Zhu, J.; Matsuzaki, T.; Sakioka, K. Windspeeds within a single crown of Japanese black pine (Pinus thunbergii Parl.). For. Ecol. Manag. 2000, 135, 19–31. [Google Scholar] [CrossRef]
- Konta, F. The present conditions and functions of the coastal forests in Japan. J. Jpn. Soc. Coast. For. 2001, 1, 1–4, (In Japanese with English summary). [Google Scholar]
- Ogawa, M. Microbial flora in Pinus thunbergii forest of coastal sand dune. Bull. For. For. Prod. Res. Inst. 1979, 305, 107–124, (In Japanese with English summary). [Google Scholar]
- Forestry and Forest Products Research Institute. Regeneration of Coastal Forests Affected by Tsunami; Forestry and Forest Products Research Institute: Tsukuba, Japan, 2012; pp. 1–24. [Google Scholar]
- Karizumi, N. The Illustrations of Tree Roots; Seibundoshinko-sya: Tokyo, Japan, 1979; p. 1121. (In Japanese) [Google Scholar]
- Todo, C.; Tokoro, C.; Yamase, K.; Tanikawa, T.; Ohashi, M.; Ikeno, H.; Dannoura, M.; Miyatani, K.; Doi, R.; Hirano, Y. Stability of Pinus thunbergii between Two Contrasting Stands at Differing Distances from the Coastline. For. Ecol. Manag. 2019, 431, 44–53. [Google Scholar] [CrossRef]
- Hirano, Y.; Todo, C.; Yamase, K.; Tanikawa, T.; Dannoura, M.; Ohashi, M.; Doi, R.; Wada, R.; Ikeno, H. Quantification of the Contrasting Root Systems of Pinus thunbergii in Soils with Different Groundwater Levels in a Coastal Forest in Japan. Plant Soil. 2018, 426, 327–337. [Google Scholar] [CrossRef]
- Coutts, M.P. Components of Tree Stability in Sitka Spruce on Peaty Gley Soil. Forestry 1986, 59, 173–197. [Google Scholar] [CrossRef]
- Ray, D.; Nicoll, B.C. The Effect of Soil Water-Table Depth on Root-Plate Development and Stability of Sitka Spruce. Forestry 1998, 71, 169–182. [Google Scholar] [CrossRef]
- Moore, J.R. Differences in Maximum Resistive Bending Moments of Pinus radiata Trees Grown on a Range of Soil Types. For. Ecol. Manag. 2000, 135, 63–71. [Google Scholar] [CrossRef]
- Peltola, H.; Kellomäki, S.; Hassinen, A.; Granander, M. Mechanical Stability of Scots Pine, Norway Spruce and Birch: An Analysis of Tree-Pulling Experiments in Finland. For. Ecol. Manag. 2000, 135, 143–153. [Google Scholar] [CrossRef]
- Cucchi, V.; Meredieu, C.; Stokes, A.; Berthier, S.; Bert, D.; Najar, M.; Denis, A.; Lastennet, R. Root Anchorage of Inner and Edge Trees in Stands of Maritime Pine (Pinus pinaster Ait.) Growing in Different Podzolic Soil Conditions. Trees 2004, 18, 460–466. [Google Scholar] [CrossRef]
- Nicoll, B.C.; Achim, A.; Mochan, S.; Gardiner, B.A. Does Steep Terrain Influence Tree Stability? A Field Investigation. Can. J. For. Res. 2005, 35, 2360–2367. [Google Scholar] [CrossRef]
- Tanaka, N. Effectiveness and Limitations of Coastal Forest in Large Tsunami: Conditions of Japanese Pine Trees on Coastal Sand Dunes in Tsunami Caused by Great East Japan Earthquake. J. Jpn. Soc. Civ. Eng. Ser. B1 2012, 68, II_7–II_15. [Google Scholar] [CrossRef]
- Nicoll, B.C.; Gardiner, B.A.; Rayner, B.; Peace, A.J. Anchorage of Coniferous Trees in Relation to Species, Soil Type, and Rooting Depth. Can. J. For. Res. 2006, 36, 1871–1883. [Google Scholar] [CrossRef]
- Todo, C.; Yamase, K.; Tanikawa, T.; Ohashi, M.; Ikeno, H.; Dannoura, M.; Hirano, Y. Effect of Thinning on the Critical Turning Moment of Sugi (Cryptomeria japonica (L. f.) D. Don). J. Jpn. Soc. Reveget Tec. 2015, 41, 308–314, (In Japanese with English summary). [Google Scholar] [CrossRef]
- Nonoda, T.; Hayashi, S.; Kawabe, H.; Honda, K.; Koyabu, K. The Mechanism of the Tree-Uprooting Occurred by Pulling Down a Tree. J. Jpn. For. Soc. 1996, 78, 390–397, (In Japanese with English summary). [Google Scholar] [CrossRef]
- Okada, Y. Measuring the Critical Turning Moment of the Japanese Cedar (Cryptomeria japonica) in situ. J. For. Res. 2019, 24, 168–177. [Google Scholar] [CrossRef]
- Lundström, T.; Jonas, T.; Stöckli, V.; Ammann, W. Anchorage of Mature Conifers: Resistive Turning Moment, Root–Soil Plate Geometry and Root Growth Orientation. Tree Physiol. 2007, 27, 1217–1227. [Google Scholar] [CrossRef]
- Hale, S.E.; Gardiner, B.A.; Wellpott, A.; Nicoll, B.C.; Achim, A. Wind Loading of Trees: Influence of Tree Size and Competition. Eur. J. For. Res. 2012, 131, 203–217. [Google Scholar] [CrossRef]
- Tanikawa, T.; Ikeno, H.; Todo, C.; Yamase, K.; Ohashi, M.; Okamoto, T.; Mizoguchi, T.; Nakao, K.; Kaneko, S.; Torii, A.; et al. A Quantitative Evaluation of Soil Mass Held by Tree Roots. Trees 2021, 35, 527–541. [Google Scholar] [CrossRef]
- Bischetti, G.B.; Chiaradia, E.A.; Simonato, T.; Speziali, B.; Vitali, B.; Vullo, P.; Zocco, A. Root Strength and Root Area Ratio of Forest Species in Lombardy (Northern Italy). Plant Soil 2005, 278, 11–22. [Google Scholar] [CrossRef]
- Giambastiani, Y.; Preti, F.; Errico, A.; Sani, L. On the Tree Stability: Pulling Tests and Modelling to Assess the Root Anchorage. Procedia Environ. Sci. Eng. Manag. 2017, 4, 207–218. [Google Scholar]
- Giambastiani, Y.; Guastini, E.; Preti, F.; Censini, G. Laboratory Tests About Resistivity Variation in Soil, in Connection with Root Presence. Geophys. Res. 2021, 22, 46–61. [Google Scholar] [CrossRef]
- Giambastiani, Y.; Errico, A.; Preti, F.; Guastini, E.; Censini, G. Indirect Root Distribution Characterization Using Electrical Resistivity Tomography in Different Soil Conditions. Urban For. Urban Green. 2022, 67, 127442. [Google Scholar] [CrossRef]
- Nicoll, B.C.; Gardiner, B.A.; Peace, A.J. Improvements in Anchorage Provided by the Acclimation of Forest Trees to Wind Stress. Forestry 2008, 81, 389–398. [Google Scholar] [CrossRef]
- Yang, M.; Défossez, P.; Danjon, F.; Dupont, S.; Fourcaud, T. Which Root Architectural Elements Contribute the Best to Anchorage of Pinus species? Insights From In Silico Experiments. Plant Soil 2017, 411, 275–291. [Google Scholar] [CrossRef]
- Preti, F. Forest Protection and Protection Forest: Tree Root Degradation Over Hydrological Shallow Landslides Triggering. Ecol. Eng. 2013, 61, 633–645. [Google Scholar] [CrossRef]
- Giadrossich, F.; Schwarz, M.; Marden, M.; Marrosu, R.; Phillips, C. Minimum Representative Root Distribution Sampling for Calculating Slope Stability in Pinus radiata D.Don Plantations in New Zealand. N. Z. J. For. Sci. 2020, 50, 5. [Google Scholar] [CrossRef]
- Yamase, K.; Tanikawa, T.; Dannoura, M.; Ohashi, M.; Todo, C.; Ikeno, H.; Aono, K.; Hirano, Y. Ground-penetrating radar estimates of tree root diameter and distribution under field conditions. Trees 2018, 32, 1657–1668. [Google Scholar] [CrossRef]
- Danjon, F.; Stokes, A.; Bakker, M.R. Root Systems of Woody Plants. In Plant Roots; The Hidden Half; CRC Press: Boca Raton, FL, USA, 2013; p. 848. [Google Scholar]
- Kokutse, N.K.; Temgoua, A.G.T.; Kavazović, Z. Slope Stability and Vegetation: Conceptual and Numerical Investigation of Mechanical Effects. Ecol. Eng. 2016, 86, 146–153. [Google Scholar] [CrossRef]
- Yang, M.; Défossez, P.; Danjon, F.; Fourcaud, T. Analyzing Key Factors of Roots and Soil Contributing to Tree Anchorage of Pinus species. Trees 2018, 32, 703–712. [Google Scholar] [CrossRef]
- Dupuy, L.; Fourcaud, T.; Stokes, A. A Numerical Investigation into Factors Affecting the Anchorage of Roots in Tension. Eur. J. Soil Sci. 2005, 56, 319–327. [Google Scholar] [CrossRef]
- Morioka, N. Strength of Standing Trees for Logging Cable Support (II) Method to Find the Center of Moment of Force That Makes Tree Incline. J. Jpn. For. Soc. 1983, 65, 342–346. (In Japanese) [Google Scholar] [CrossRef]
- Morioka, N. Strength of Standing Trees for Logging Cable Support (III) on the Relation Between Moment of a Lateral Force and Stem Inclination. J. Jpn. For. Soc. 1984, 66, 160–163. (In Japanese) [Google Scholar] [CrossRef]
- Shimada, H.; Nonoda, T. Critical Turning Moment of sugi (Cryptomeria japonica (L.f.) D. Don) and Hinoki (Chamaecyparis obtusa (Siebold et Zucc.) Endl). In central Mie Prefecture. J. Jpn. Soc. Reveget. Technol. 2017, 43, 138–143, (In Japanese with English summary). [Google Scholar] [CrossRef][Green Version]
- Kamimura, K.; Kitagawa, K.; Saito, S.; Mizunaga, H. Root Anchorage of Hinoki (Chamaecyparis obtuse (Sieb. et Zucc.) Endl.) Under the Combined Loading of Wind and Rapidly Supplied Water on Soil: Analyses Based on Tree-Pulling Experiments. Eur. J. For. Res. 2012, 131, 219–227. [Google Scholar] [CrossRef]
- Fourcaud, T.; Ji, J.N.; Zhang, Z.Q.; Stokes, A. Understanding the Impact of Root Morphology on Overturning Mechanisms: A Modelling Approach. Ann. Bot. 2008, 101, 1267–1280. [Google Scholar] [CrossRef]
- Tanaka, J.; Nakazawa, H.; Satou, T. Case Study of Root Systems Growth of Pinus thunbergii Parlatore and Groundwater Level in the Nishinohama Coastal Forest, Tahara City, Aichi Prefecture. J. Jpn. Soc. Reveget Tec. 2017, 43, 298–301. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Todo, C.; Ikeno, H.; Yamase, K.; Tanikawa, T.; Ohashi, M.; Dannoura, M.; Kimura, T.; Hirano, Y. Reconstruction of Conifer Root Systems Mapped with Point Cloud Data Obtained by 3D Laser Scanning Compared with Manual Measurement. Forests 2021, 12, 1117. [Google Scholar] [CrossRef]
- Tahara City. Damage Investigation for Nankai Megathrust Earthquake in Tahara City. 2015, p. 26. Available online: http://www.city.tahara.aichi.jp/_res/projects/default_project/_page_/001/001/437/mayor/newsconference/pdf/1506/1506_1-1nankaitorafu-higaisoutei.pdf (accessed on 30 August 2022).
- Sakamoto, T.; Noguchi, H.; Gotoh, Y.; Suzuki, S.; Shimada, K. Management of Japanese Black Pine (Pinus thunbergii) Seedlings Caused by Natural Regeneration. J. Jpn. Soc. Coast. For. 2013, 12, 29–34, (In Japanese with English summary). [Google Scholar]
- Suzuki, Y.; Yoshizaki, S. On the natural generation process of Black pine forests at Nishinohama coastal forest of Tahara City, Aichi Pref., Japan. J. Jpn. Soc. Reveget Tech. 2016, 42, 256–359. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- FAO-UNESCO. Soil Map of the World. Revised Legend. Reprinted with Corrections. In World Soil Resources Report 60; Food and Agriculture Organization: Rome, Italy, 1990; p. 119. [Google Scholar]
- Japan Meteorological Agency. 2019. Available online: http://www.data.jma.go.jp/obd/stats/etrn/ (accessed on 12 August 2019).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 12 July 2020).
- Danjon, F.; Barker, D.H.; Drexhage, M.; Stokes, A. Using Three-Dimensional Plant Root Architecture in Models of Shallow-Slope Stability. Ann. Bot. 2008, 101, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Danjon, F.; Fourcaud, T.; Bert, D. Root Architecture and Wind-Firmness of Mature Pinus pinaster. New Phytol. 2005, 168, 387–400. [Google Scholar] [CrossRef]
- Ennos, A.R. The Mechanics of Root Anchorage. Adv. Bot. Res. 2000, 33, 133–157. [Google Scholar] [CrossRef]
- Crook, M.J.; Ennos, A.R.; Banks, J.R. The Function of Buttress Roots: A Comparative Study of the Anchorage Systems of Buttressed (Aglaia and Nephelium ramboutan species) and Non-buttressed (Mallotus wrayi) Tropical Trees. J. Exp. Bot. 1997, 48, 1703–1716. [Google Scholar] [CrossRef]
- Fraser, A.I. The soil and roots as factors in tree stability. Forestry 1962, 34, 117–127. [Google Scholar] [CrossRef]
- Hirano, Y.; Yamamoto, R.; Dannoura, M.; Aono, K.; Igarashi, T.; Ishii, M.; Yamase, K.; Makita, N.; Kanazawa, Y. Detection Frequency of Pinus thunbergii Roots by Ground-Penetrating Radar Is Related to Root Biomass. Plant Soil 2012, 360, 363–373. [Google Scholar] [CrossRef]
- Ohashi, M.; Ikeno, H.; Sekihara, K.; Tanikawa, T.; Dannoura, M.; Yamase, K.; Todo, C.; Tomita, T.; Hirano, Y. Reconstruction of Root Systems in Cryptomeria japonica Using Root Point Coordinates and Diameters. Planta 2019, 249, 445–455. [Google Scholar] [CrossRef]
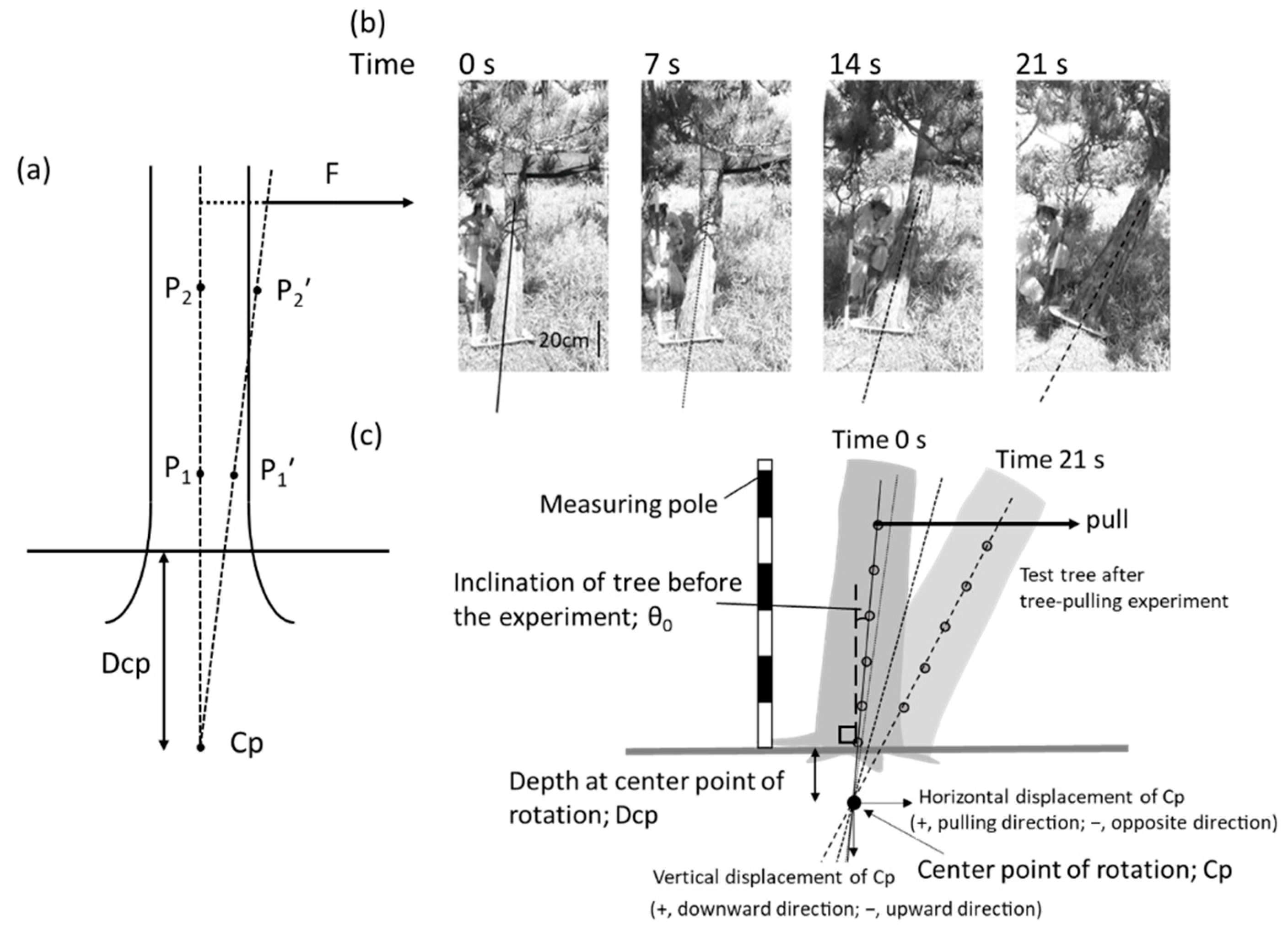
| Plot | Tree No. | Age (Years) | θ0 (Degrees) | H (m) | DBH (cm) | H × DBH2 (m3) | Critical Turning Moment (kN m) | Maximum Root Depth (cm) | Dcp (cm) | RSPRadius (cm) | RSP Radius/ Dcp |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plot A | 1 | 50 a | –5.1 | 11.4 a | 18.5 a | 0.39 | 33.4 | 126.0 a | 29.7 | 90.7 | 3.1 |
| 2 | 45 a | –0.09 | 10.8 b | 19.5 b | 0.41 | 25.5 a | 106.0 a | 23.3 | 61.9 | 2.7 | |
| 3 | 40 | –1.4 | 9.3 b | 15.9 b | 0.23 | 12.7 | 44.0 | 17.0 | 67.7 | 4.0 | |
| 4 | 51 | –3.1 | 10.1 b | 16.4 b | 0.27 | 16.4 | 86.0 | 18.0 | 58.8 | 3.3 | |
| Mean | 46.5 | –2.4 | 10.4 | 17.6 | 0.33 | 22.1 | 90.5 | 22.0 | 69.8 | 3.2 | |
| Plot B | 1 | 36 c | 7.5 | 7.4 c | 17.0 c | 0.21 c | 25.7 c | 75.0 c | 12.9 c | 73.4 c | 5.7 |
| 2 | 39 c | 16.2 | 7.1 c | 22.8 c | 0.37 c | 41.2 c | 57.5 c | 3.1 c | 104.4 c | 34.0 | |
| 3 | 44 c | 9.5 | 4.4 c | 16.9 c | 0.13 c | 17.8 c | 35.0 c | 5.2 c | 50.8 c | 9.8 | |
| Mean | 39.7 | 11.1 | 6.3 | 18.9 | 0.24 | 28.2 | 55.8 | 7.1 | 76.2 | 16.5 | |
| Plot C | 1 | 32 c | –11.2 | 8.9 c | 18.7 c | 0.31 c | 48.3 c | 175.0 c | 24.7 c | 58.4 c | 2.4 |
| 2 | 51 c | 3.7 | 13.8 c | 18.3 c | 0.46 c | 50.4 c | 240.0 c | 34.7 c | 43.3 c | 1.2 | |
| 3 | 58 c | –10.8 | 11.7 c | 23.7 c | 0.66 c | 84.3 c | 222.0 c | 41.3 c | 99.9 c | 2.4 | |
| Mean | 47.0 | –6.1 | 11.5 | 20.2 | 0.48 | 61.0 | 212.0 | 33.6 | 67.2 | 2.0 |
| Parameter | Maximum Root Depth (cm) | H (m) | H × DBH2 (m3) | Critical Turning Moment (kN m) |
|---|---|---|---|---|
| Allten P. thunbergii Trees | ||||
| Dcp (cm) | 0.92 *** | 0.90 *** | 0.77 * | 0.59 |
| Maximum root depth (cm) | − | 0.82 ** | 0.81 ** | 0.77 * |
| Seven P. thunbergii treeswith tap roots excluding three trees with plate root system | ||||
| Dcp (cm) | 0.93 ** | 0.75 | 0.89 * | 0.96 ** |
| Maximum root depth (cm) | − | 0.64 | 0.82 * | 0.96 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todo, C.; Yamase, K.; Ikeno, H.; Tanikawa, T.; Ohashi, M.; Hirano, Y. Maximum Rooting Depth of Pinus thunbergii Parl. Estimated with Depth at the Center Point of Rotation in a Tree-Pulling Experiment in a Coastal Forest in Japan. Forests 2022, 13, 1506. https://doi.org/10.3390/f13091506
Todo C, Yamase K, Ikeno H, Tanikawa T, Ohashi M, Hirano Y. Maximum Rooting Depth of Pinus thunbergii Parl. Estimated with Depth at the Center Point of Rotation in a Tree-Pulling Experiment in a Coastal Forest in Japan. Forests. 2022; 13(9):1506. https://doi.org/10.3390/f13091506
Chicago/Turabian StyleTodo, Chikage, Keitaro Yamase, Hidetoshi Ikeno, Toko Tanikawa, Mizue Ohashi, and Yasuhiro Hirano. 2022. "Maximum Rooting Depth of Pinus thunbergii Parl. Estimated with Depth at the Center Point of Rotation in a Tree-Pulling Experiment in a Coastal Forest in Japan" Forests 13, no. 9: 1506. https://doi.org/10.3390/f13091506
APA StyleTodo, C., Yamase, K., Ikeno, H., Tanikawa, T., Ohashi, M., & Hirano, Y. (2022). Maximum Rooting Depth of Pinus thunbergii Parl. Estimated with Depth at the Center Point of Rotation in a Tree-Pulling Experiment in a Coastal Forest in Japan. Forests, 13(9), 1506. https://doi.org/10.3390/f13091506





