A Comparison of Mangrove Forest Structure and Ecosystem Services in Maputo Bay (Eastern Africa) and Príncipe Island (Western Africa)
Abstract
:1. Introduction
2. Materials and Methods
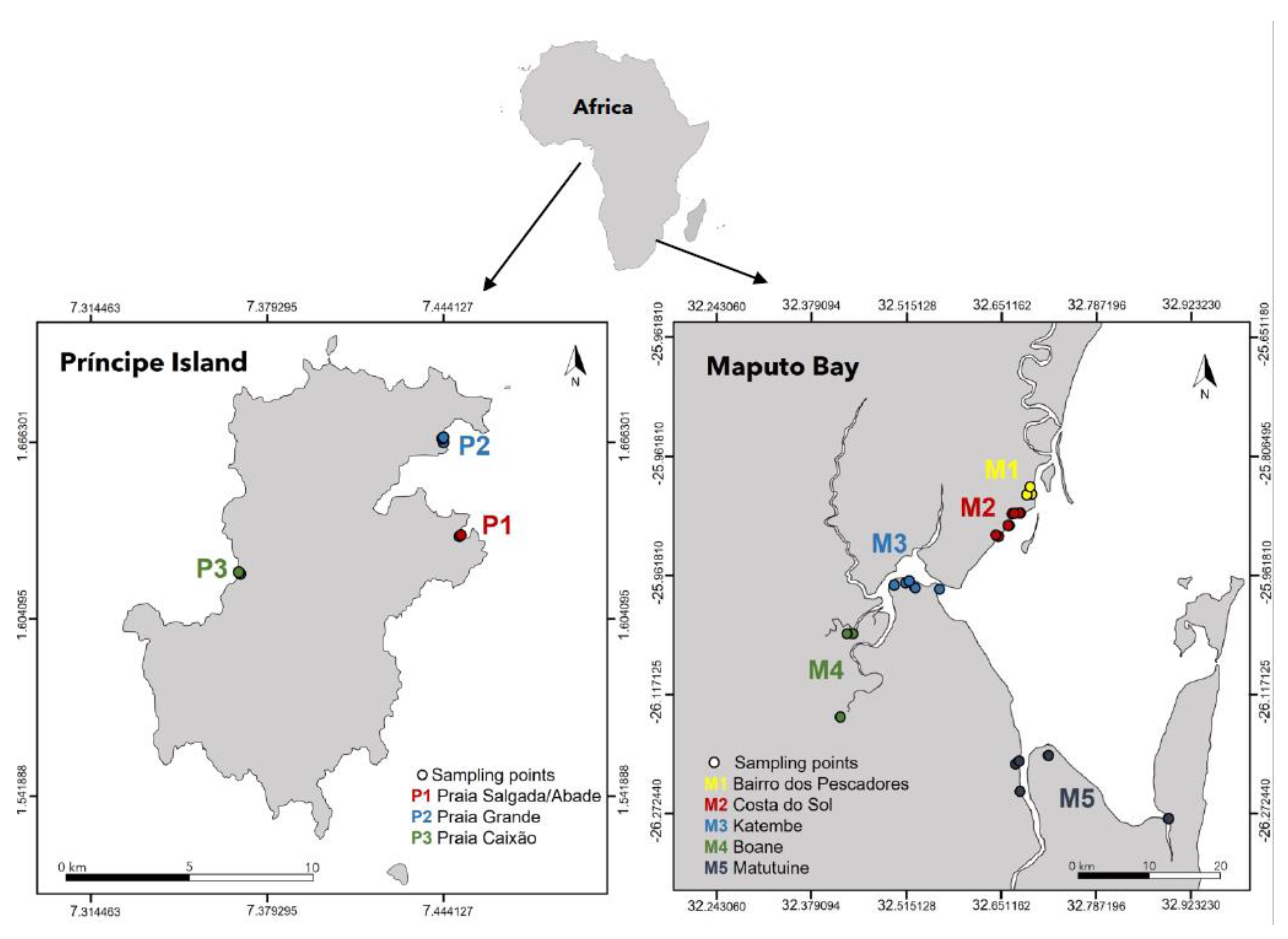
2.1. Study Area
2.1.1. Maputo Bay
2.1.2. Príncipe Island
2.2. Methods
2.2.1. Forest Structure Characterization
2.2.2. In-Depth Interviews with Community Members and Local Authorities
2.2.3. Data Treatment
3. Results
3.1. Study Area Characterization
3.2. Forest Structure
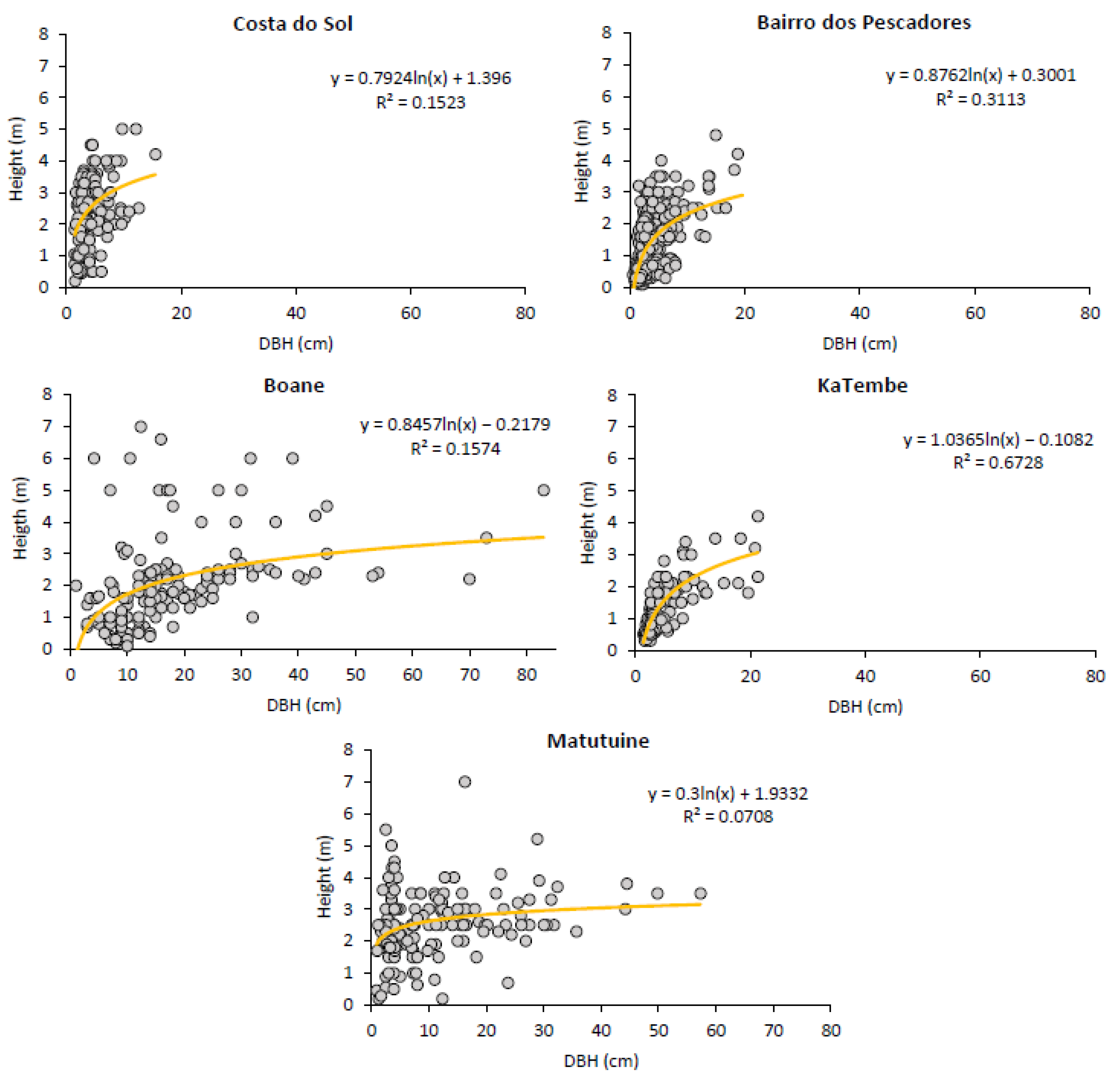
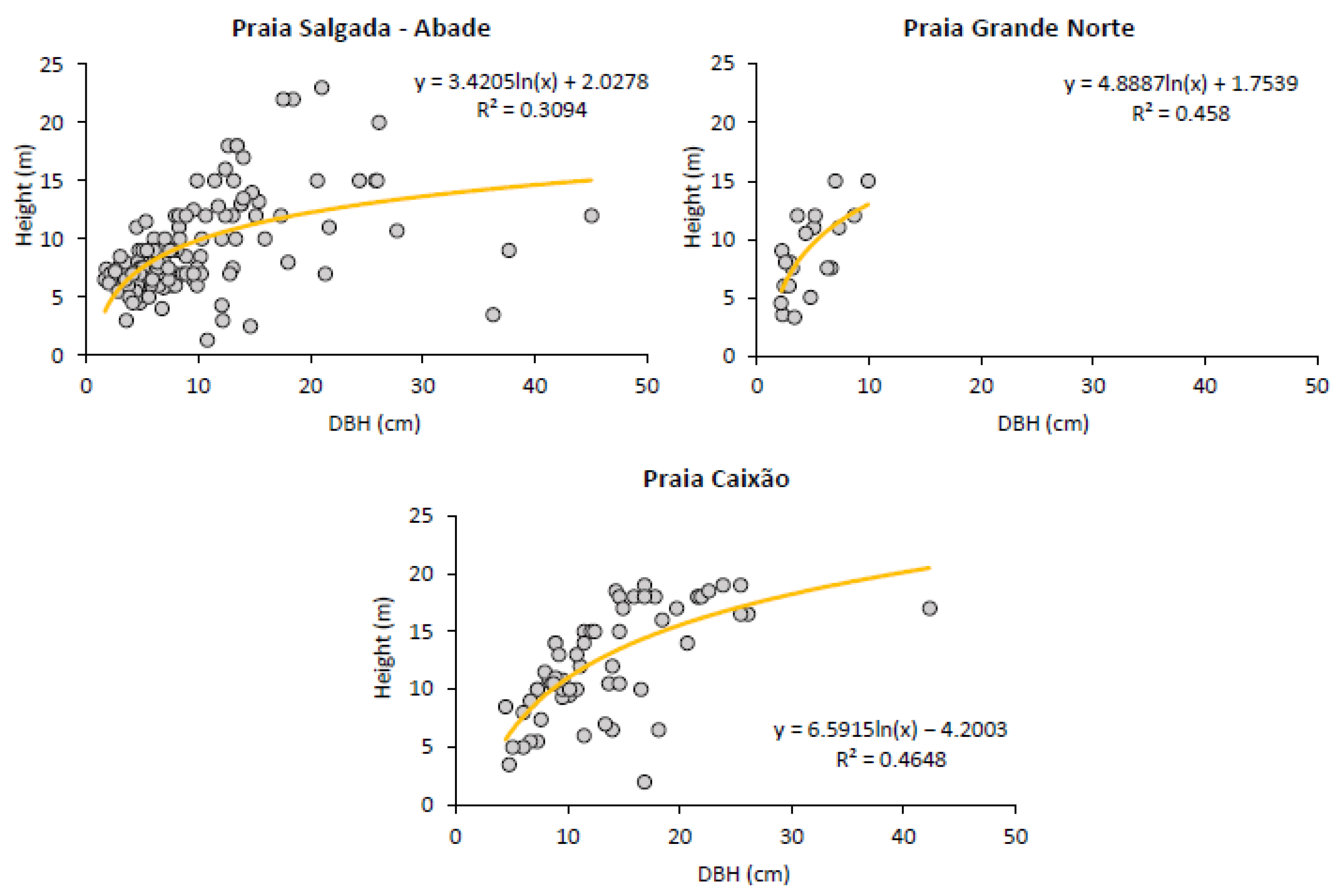
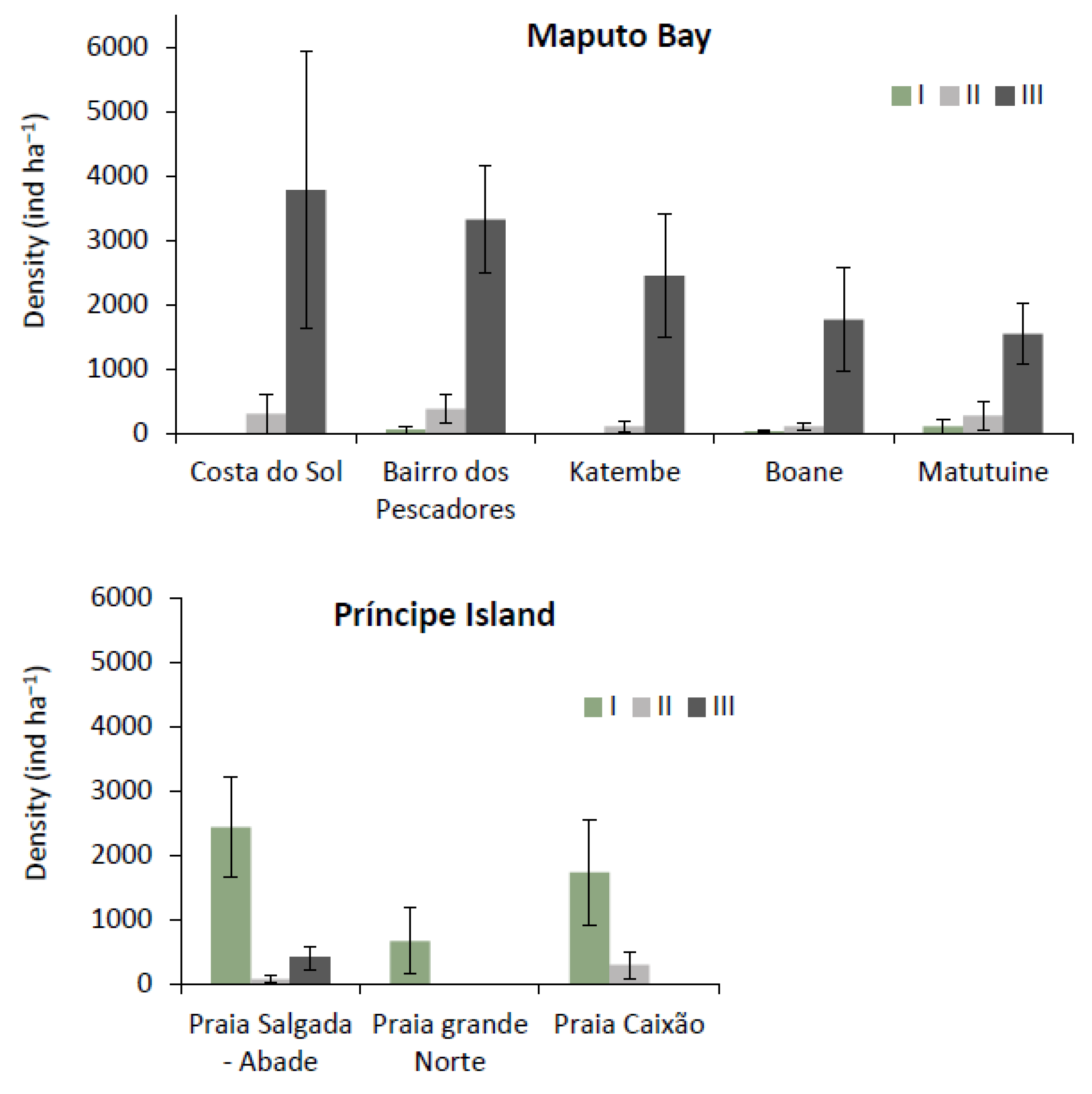
3.2.1. Trees’ DBH and Height Distribution
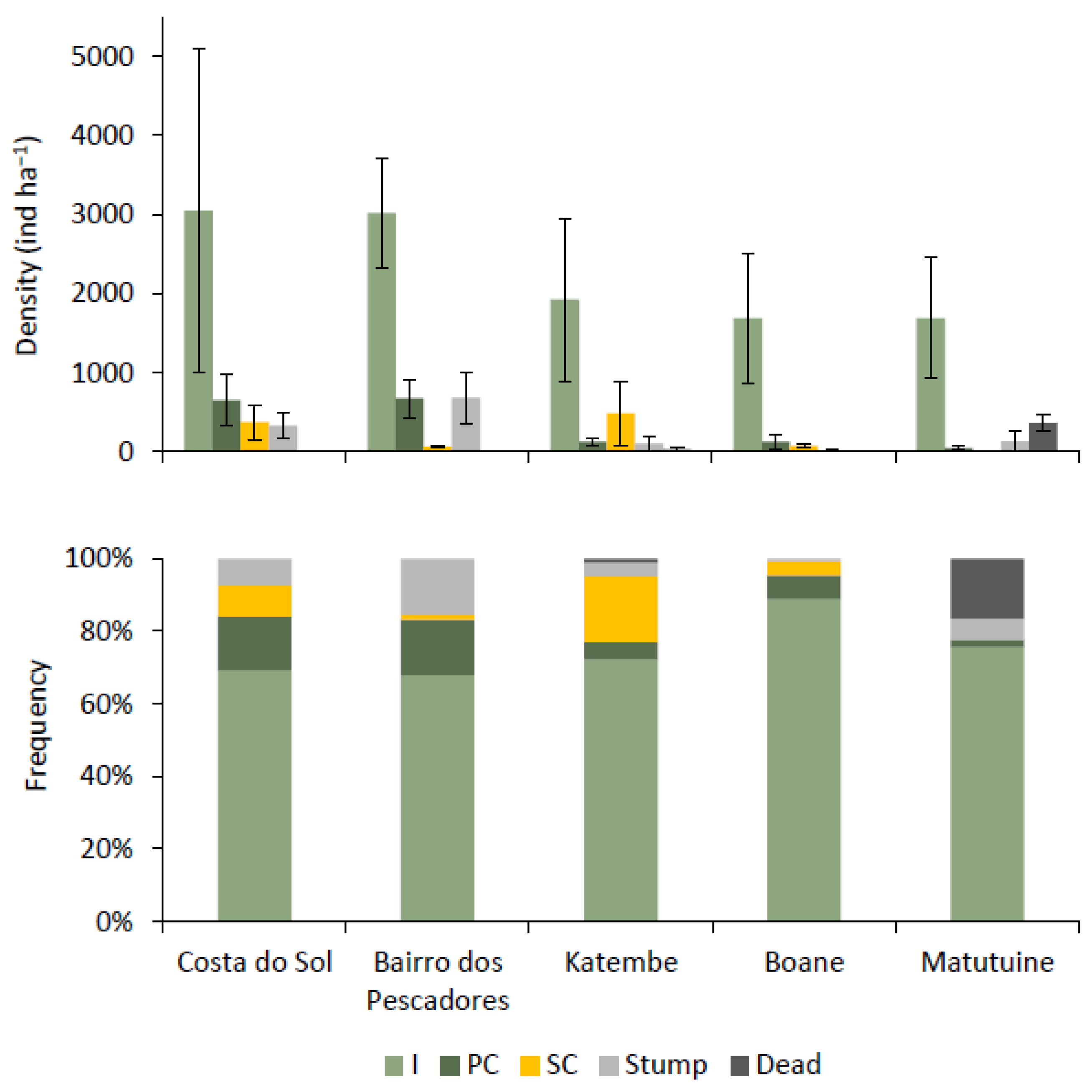
3.2.2. Tree Condition
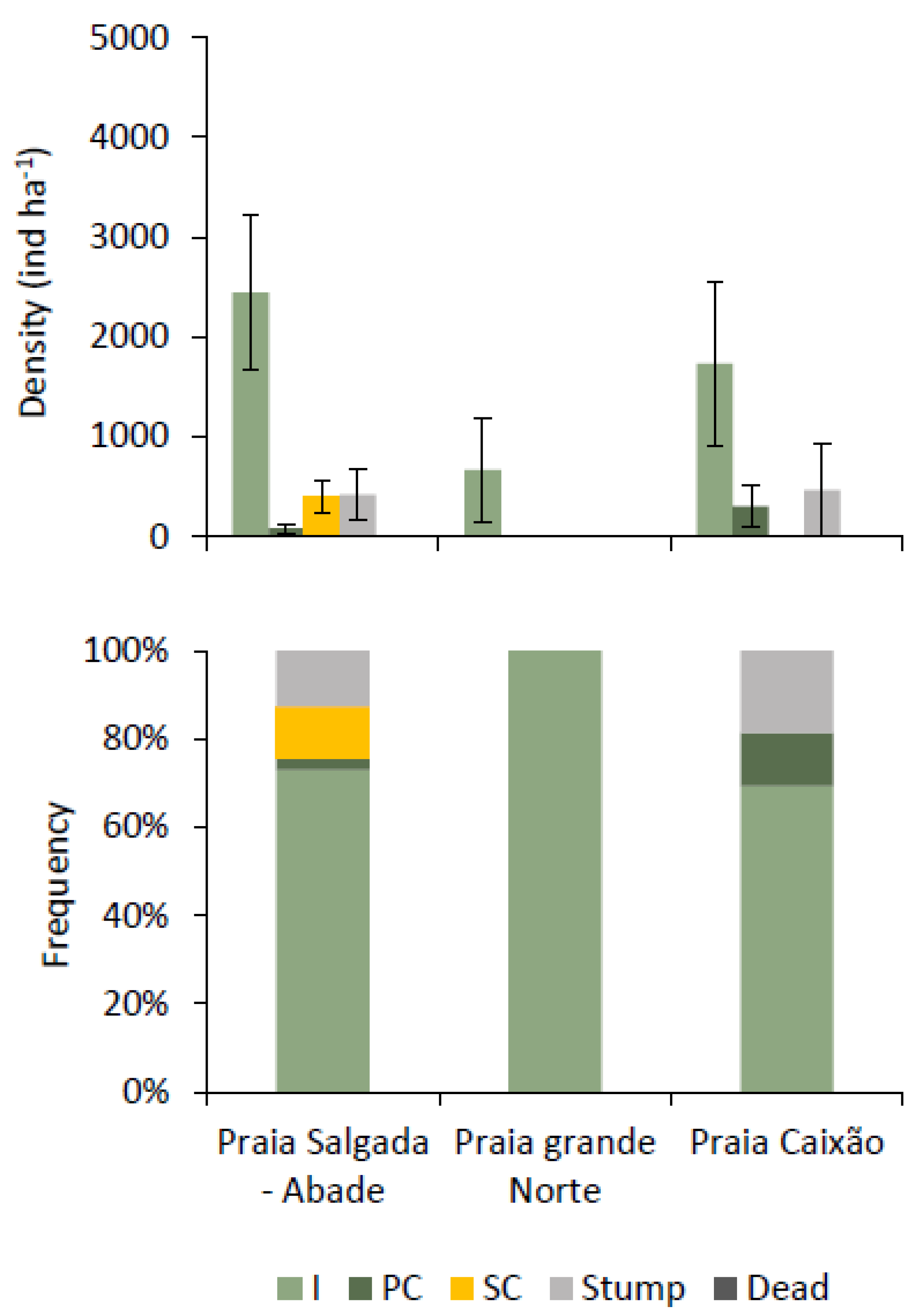
3.2.3. Stem Quality
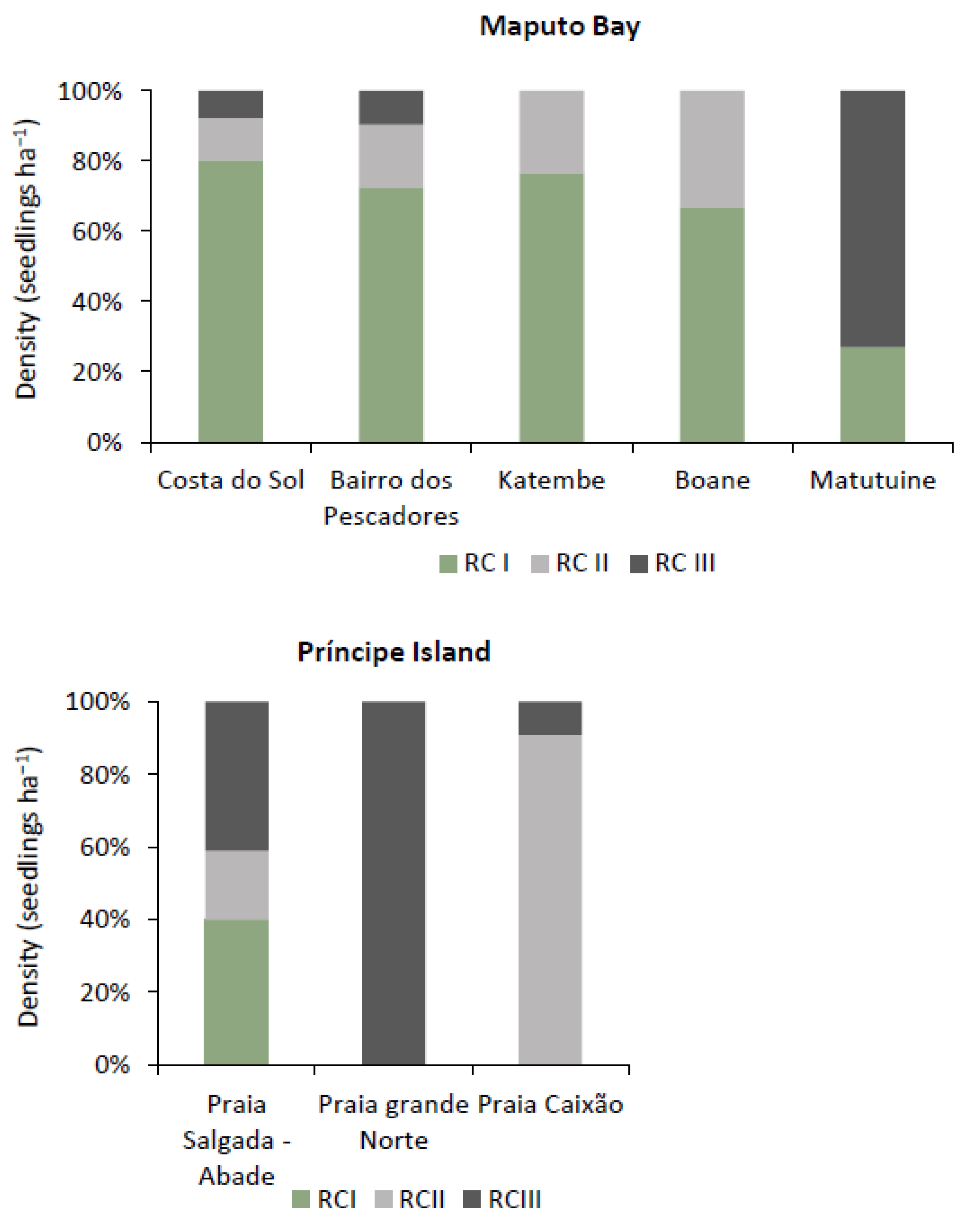
3.2.4. Regeneration Rate
3.3. Mangrove Ecosystem Services
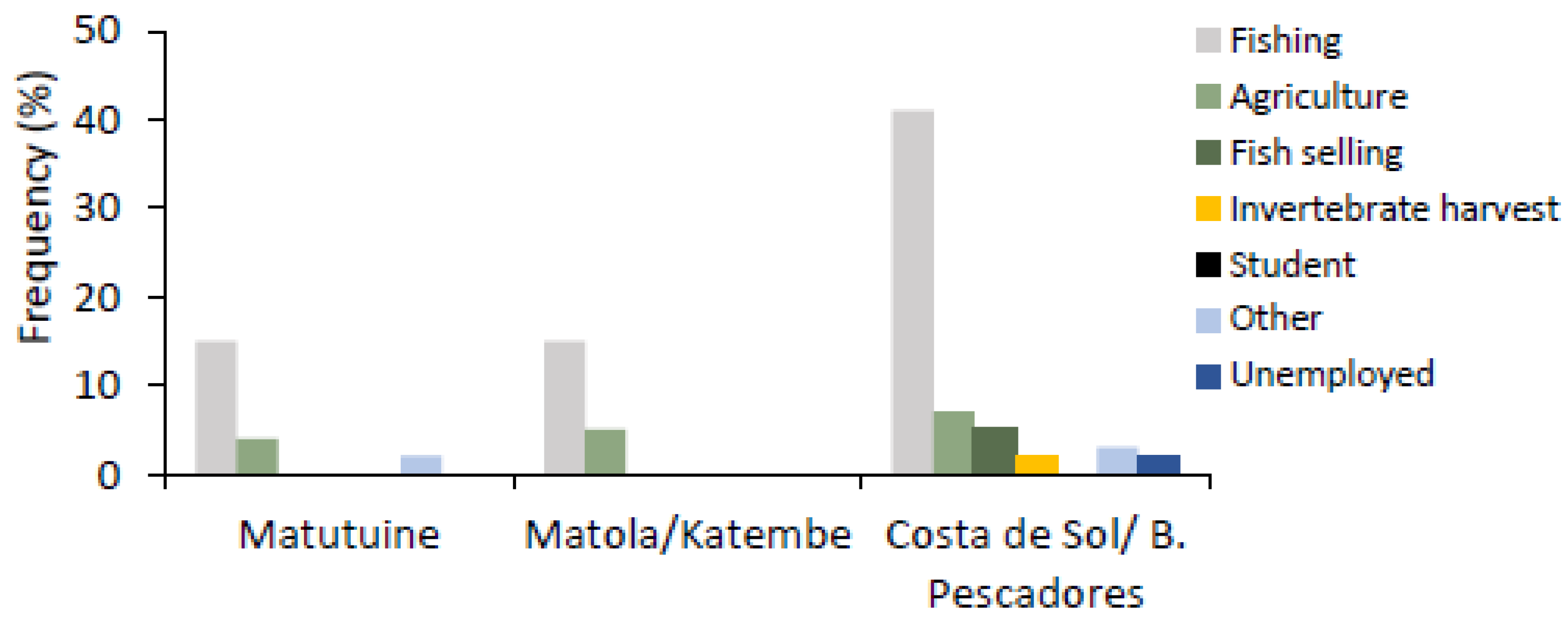
3.3.1. Mangrove Users
3.3.2. Ecosystem Services and Resource Uses
4. Discussion
4.1. Specific Composition and Abundance of Mangroves
4.2. Cut Level and Stem Quality
4.3. Mangrove Structure and Regeneration
4.4. Mangrove Conservation
4.5. Exploitation of Mangrove Ecosystem Resources
4.5.1. Resources Exploited
4.5.2. Income-Generating Activities in Maputo Bay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The World’s Mangroves 1980–2005. FAO Forestry Paper; FAO: Rome, Italy, 2007; Volume 153, p. 89. [Google Scholar]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Kathiresan, K.; Bingham, B.L. Biology of mangroves and mangrove ecosystems. Adv. Mar. Biol. 2001, 40, 81–251. [Google Scholar]
- Kauffman, J.B.; Donato, D.C. Protocols for the Measurement, Monitoring and Reporting of Structure, Biomass and Carbon Stocks in Mangrove Forests. Working Paper 86; CIFOR: Bogor, Indonesia, 2012. [Google Scholar]
- Taylor, M.; Ravilious, C.; Green, E.P. Mangroves of East Africa; UNEP, World Conservation Monitoring Centre: Cambridge, UK, 2003; p. 26. [Google Scholar]
- Walters, B.B.; Ronnback, P.; Kovacs, J.M.; Crona, B.; Hussain, S.A.; Badola, R.; Primavera, J.H.; Barbier, E.; Dahdouh-Guebas, F. Ethnobiology, socio-economics and management of mangrove forests: A review. Aquat. Bot. 2008, 89, 220–236. [Google Scholar]
- McLeod, E.; Salm, R.V. Managing Mangroves for Resilience to Climate Change; IUCN: Gland, Switzerland, 2006; p. 64. [Google Scholar]
- Spalding, M.D.; Blasco, F.; Field, C.D. World Mangrove Atlas; The International Ecosystems: Okinawa, Japan, 1997; p. 178. [Google Scholar]
- Fatoyinbo, T.E.; Simard, M.; Washington-Allen, R.A.; Shugart, H.H. Landscape-scale extent, height, biomass, and carbon estimation of Mozambique’s mangrove forests with Landsat ETM+ and Shuttle Radar Topography Mission elevation data. J. Geophys. Res. 2008, 113, 13. [Google Scholar] [CrossRef]
- Cohen, R.; Kaino, J.; Okello, J.A.; Bosire, J.O.; Kairo, J.G.; Huxham, M.; Mencuccini, M. Propagating uncertainty to estimates of above-ground biomass for Kenyan mangroves: A scaling procedure from tree to landscape level. For. Ecol. Manag. 2013, 310, 968–982. [Google Scholar] [CrossRef]
- UNEP. The Importance of Mangroves to People: A Call to Action; van Bochove, J., Sullivan, E., Nakamura, T., Eds.; United Nations Environment Programme World Conservation Monitoring Centre: Cambridge, UK, 2014; p. 128. [Google Scholar]
- Alongi, D.M. Present state and future of the world’s mangrove forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef]
- Bosire, J.O.; Mangora, M.M.; Bandeira, S.; Rajkaran, A.; Ratsimbazafy, R.; Appadoo, C.; Kairo, J.G. (Eds.) Mangroves of the Western Indian Ocean: Status and Management; WIOMSA: Zanzibar Town, Tanzania, 2016; p. 161. [Google Scholar]
- Page, S.E.; Rieley, J.O.; Banks, C.J. Global and regional importance of the tropical peatland carbon pool. Glob. Change Biol. 2011, 17, 798–818. [Google Scholar] [CrossRef]
- Spalding, M.; Kainuma, M.; Collins, L. World Atlas of Mangroves. A Collaborative Project of ITTO, ISME, FAO, UNEP-WCMC, UNESCOMAB, UNU-INWEH and TNC; Earthscan: London, UK, 2010; p. 319. [Google Scholar]
- Beentje, H.; Bandeira, S.O.; Williamson, J.; Moat, J.; Frith, R. Field guide to the mangrove trees of Africa and Madagascar. In Royal Botanic Gardens; Kew Publishing: Richmond, UK, 2007. [Google Scholar]
- Bandeira, S.; Paula, J. The Maputo Bay Ecosystem; WIOMSA: Zanzibar Town, Tanzania, 2014; p. 427. [Google Scholar]
- Fernando, S.; Bandeira, S. Litter fall and decomposition of mangrove species Avicennia marina and Rhizophora mucronata in Maputo Bay, Mozambique. West. Indian Ocean. 2009, 8, 172–182. [Google Scholar] [CrossRef]
- Langa, J.V. Problemas na Zona Costeira de Moçambique com Enfâse para a Costa de Maputo. Rev. De Restao Costeira Integr. 2017, 7, 33–44. [Google Scholar] [CrossRef]
- Barbosa, F.M.A.; Cuambe, C.C.; Bandeira, S.O. Status and Distribution of Mangroves in Mozambique. S. Afr. J. Bot. 2001, 67, 393–398. [Google Scholar] [CrossRef]
- Diniz, M.A.; Bandeira, S.; Martins, E.S. Flora e Vegetação da Província de Maputo: Sua Apropriação Pelas Populações; Instituto de Investigação Científica Tropical: Lisboa, Portugal, 2013; pp. 1–12. [Google Scholar]
- Macamo, C.; Bandeira, S.; Muando, S.; de Abreu, D.; Mabilana, H. Mangroves of Mozambique. In Mangroves of the Western Indian Ocean: Status and Management; Bosire, J.O., Mangora, M.M., Bandeira, S., Rajkaran, A., Ratsimbazafy, R., Appadoo, C., Kairo, J.G., Eds.; WIOMSA: Zanzibar Town, Tanzania, 2016; pp. 51–73. [Google Scholar]
- Afonso, F.; Félix, P.M.; Chainho, P.; Heumüller, J.A.; de Lima, R.F.; Ribeiro, F.; Brito, A.C. Assessing Ecosystem Services in Mangroves: Insights from São Tomé Island (Central Africa). Front. Environ. Sci. 2021, 9, 501673. [Google Scholar] [CrossRef]
- Herrero-Barrencua, A.; Haroun, R.; Abreu, A.D. Caracterización preliminar de los manglares de la Isla de Príncipe (São Tomé e Príncipe); IU-ECOAQUA, Univ. Las Palmas de Gran Canaria: Telde, Spain, 2017; p. 70. Available online: www.ecoaqua.eu (accessed on 5 July 2020).
- Vaz, H.; Oliveira, F. Relatório Nacional do estado geral da biodiversidade de S. Tomé e Príncipe; Ministério de Recursos Naturais e meio Ambiente, Direcção Geral de Ambiente, S. Tomé-RDSTP: São Tomé, República Democrática de S. Tomé e Príncipe, 2007. [Google Scholar]
- Macamo, C.; Balidy, H.; Bandeira, S.; Kairo, J.G. Mangrove transformation in the Incomati Estuary, Maputo Bay, Mozambique. WIO J. Mar. Sci. 2015, 14, 10–21. [Google Scholar]
- Macamo, C.; Balidy, H.; Bandeira, S. Incomati mangrove deforestation. In The Maputo Bay Ecosystem; Bandeira, S., Paula, J., Eds.; WIOMSA: Zanzibar Town, Tanzania, 2014; pp. 127–130. [Google Scholar]
- Afonso, F.; Félix, P.M.; Chainho, P.; Heumüller, J.A.; de Lima, R.F.; Ribeiro, F.; Brito, A.C. Community perceptions about mangrove ecosystem services and threats. Reg. Stud. Mar. Sci. 2021, 49, 102114. [Google Scholar] [CrossRef]
- Inácio, A.; Barros, P. Análise do Manancial e da Pescaria de Magumba, Hilsa kelee (Cuvier, 1829) na Baía de Maputo, Moçambique, no Período de 1992–2010. Rev. Moçambicana De Investig. Pesqueira (RIP) 2012, 31, 23–46. [Google Scholar]
- Ngale, A. Pesca Artesanal: A sua Contribuição no Rendimento dos Agregados Familiares da Cidade de Maputo–Estudo de caso das Comunidades de Pesca de Gwachene e de Marítimo. Dissertação Apresentada para a Obtenção do grau de Mestre em População e Desenvolvimento; Universidade Eduardo Mondlane: Maputo, Mozambique, 2012. [Google Scholar]
- Ferreira, M.A.; Bandeira, S. Maputo bay’s coastal habitats. In The Maputo Bay Ecosystem; Bandeira, S., Paula, J., Eds.; WIOMSA: Zanzibar Town, Tanzania, 2014; pp. 21–24. [Google Scholar]
- de Boer, W.F. The Rise and Fall of the Mangrove Forests in Maputo Bay, Mozambique. Wetl. Ecol. Manag. 2002, 10, 313–322. [Google Scholar] [CrossRef]
- Dias, J.M.; Canhanga, S. Tidal Characteristics of Maputo Bay, Mozambique. J. Mar. Syst. 2005, 58, 83–97. [Google Scholar]
- de Abreu, D.C.; Costa, A.; e Motta, H. (Eds.) . Levantamento Rápido no Arquipélago das Primeiras e Segundas, Contribuição para o Estabelecimento de um Programa de Monitoria; WWF: Maputo, Moçambique, 2008. [Google Scholar]
- Paula, J.; Macamo, C.; Bandeira, S. Mangroves of Maputo Bay. In The Maputo Bay Ecosystem; Bandeira, S., Paula, J., Eds.; WIOMSA: Zanzibar Town, Tanzania, 2014; pp. 109–146. [Google Scholar]
- Bonfim, F.; Carvalho, S. Fourth National Report on the Biodiversity (1st Draft); CBD Online Reporting Tool São Tomé, República Democrática de São Tomé e Príncipe, 2009. Available online: https://chm.cbd.int/database/record?documentID=201405 (accessed on 5 April 2022).
- Haroun, R.; Barrencua, A.H.; Abreu, A.D. Mangrove Habitats in São Tomé and Príncipe (Gulf of Guinea, Africa): Conservation and Management Status. In Threats to Mangrove Forests; Makowski, C., Finkl, C., Eds.; Coastal Research Library, Springer: Cham, Germany, 2018; Volume 25. [Google Scholar] [CrossRef]
- Chou, S.C.; de Arruda Lyra, A.; Gomes, J.L.; Rodriguez, D.A.; Alves Martins, M.; Costa Resende, N.; da Silva Tavares, P.; Pereira Dereczynski, C.; Lopes Pilotto, I.; Martins, A.M.; et al. Downscaling projections of climate change in Sao Tome and Principe Islands, Africa. Clim. Dyn. 2020, 54, 4021–4042. [Google Scholar] [CrossRef]
- Pisoni, T.; de Lima, R.F.; Brito, A.C.; Chainho, P.; Félix, P.M.; Caçador, I.; Carvalho, A. Planos de Gestão Participativa para dois Sítios de Mangal na Ilha de S. Tomé: Praia das Conchas e Malanza. Abordagem Ecossistémica Integrada para a Conservação e Gestão da Biodiversidade na zona Tados Parques Mpão Naturais obô de São Tomé e Príncipe; ALISEI: São Tomé, São Tomé e Príncipe, 2015; p. 79. [Google Scholar]
- NBSAP II. National Biodiversity Strategy and Action Plan 2015–2020, National Commission for Monitoring and Evaluation, Ministry of Infrastructure; Natural Resources and Environment: São Tomé, São Tomé and Príncipe, 2015; p. 120.
- Bandeira, S.O.; Macamo, C.C.F.; Kairo, J.G.; Amade, F.; Jiddawi, N.; Paula, J. Evaluation of mangrove structure and condition in two transboundary areas in the Western Indian Ocean. Aquat. Conserv. Mar. Freshw. Ecosyst. 2009, 19, 46–55. [Google Scholar] [CrossRef]
- Macamo, C.C.F.; Adams, J.B.; Bandeira, S.O.; Mabilana, H.A.; António, V.M. Spatial Dynamics and Structure of Human Disturbed Mangrove Forests in Contrasting Coastal Communities in Eastern Africa. Wetlands 2018, 38, 509–523. [Google Scholar] [CrossRef]
- Nicolau, D.K.; Macamo, C.C.; Bandeira, S.O.; Tajú, A.; Mabilana, H. Mangrove change detection, structure, and condition in a protected area of eastern Africa: The case of Quirimbas National Park, Mozambique. WIO J. Mar. Sci. 2017, 16, 47–60. [Google Scholar]
- Servino, R.N.; Gomes, L.E.D.O.; Bernardino, A.F. Bernardino. Extreme weather impacts on tropical mangrove forests in the Eastern Brazil Marine Ecoregion. Sci. Total Environ. 2018, 628–629, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Finch, H.; Lewis, J. Focus groups. In Qualitative Research Practice; Ritchie, J., Lewis, J., Eds.; Sage Publications: London, UK, 2003. [Google Scholar]
- Greenbaum, T.L. The Handbook of Focus Group Research, 2nd ed.; Sage Publications: London, UK, 1998. [Google Scholar]
- Rosales, R.M.; Pomeroyb, R.; Calabioc, I.J.; Batongd, M.; Cedoe, K.; Escaraf, N.; Facunlag, V.; Gulayanh, A.; Narvadezi, M.; Sarahadilj, M.; et al. Value chain analysis and small-scale fisheries management. Mar. Policy 2017, 83, 11–21. [Google Scholar] [CrossRef]
- Tuan, V.Q. Valuation of Mangrove Ecosystems along the Coast of the Mekong Delta in Vietnam: An Approach Combining Socio-Economic and Remote Sensing Methods. Doctoral Thesis, University of Kiel, Kiel, Germany, 2013. [Google Scholar]
- Brander, L.; Baggethun, E.G.; López, B.M.; Verma, M. The economics of valuing ecosystem services and biodiversity. In The Ecological and Economic Foundations; Kiel University: Kiel, Germany, 2010. [Google Scholar]
- Zafar, M.; Ahsan, N. Marketing and Value Chain Analysis of Mud Crab (Scylla sp.) in the Coastal Communities of Bangladesh; Institute of Marine Sciences, Chittagong University: Chittagong, Bangladesh, 2006. [Google Scholar]
- Fatoyinbo, T.E.; Simard, M. Height and biomass of mangroves in Africa from ICESat/GLAS and SRTM. Int. J. Remote Sens. 2013, 34, 668–681. [Google Scholar] [CrossRef]
- Cravo, M.; Almeida, A.J.; Lima, H.; e Silva, J.A.; Bandeira, S.; Machava-António, V.; Paula, J. Fish Assemblages in a Small Mangrove System on Príncipe Island, Gulf of Guinea. Front. Mar. Sci. 2021, 8, 721692. [Google Scholar] [CrossRef]
- Sitoe, C. Análise da Sustentabilidade do mangal no Âmbito da Construção de Infra-estruturas na Zona Costeira: Caso Costa do Sol. Master’s Thesis, University Eduardo Mondlane, Maputo, Mozambique, 2018; p. 35. [Google Scholar]
- Trettin, C.C.; Dai, Z.; Tang, W.; Lagomasino, D.; Thomas, N.; Lee, S.K.; Simard, M.; Ebanega, M.O.; Stoval, A.; Fatoyinbo, T.E. Mangrove carbon stocks in Pongara National Park, Gabon, Estuarine. Coast. Shelf Sci. 2021, 259, 107432, ISSN 0272-7714. [Google Scholar] [CrossRef]
- Shapiro, A.; Trettin, C.; Küchly, H.; Alavinapanah, S.; Bandeira, S. The Mangroves of the Zambezi Delta: Increase in Extent Observed via Satellite from 1994 to 2013. Remote Sens. 2015, 7, 16504–16518. [Google Scholar] [CrossRef]
- FAO. Mangrove Forest Management Guidelines. (FAO Forestry Paper 117); Food & Agriculture Org.: Rome, Italy, 1994. [Google Scholar]
- Aye, W.N.; Wen, Y.; Marin, K.; Thapa, S.; Tun, A.W. Contribution of Mangrove Forest to the Livelihood of Local Communities in Ayeyarwaddy Region, Myanmar. Forests 2019, 10, 414. [Google Scholar] [CrossRef]
- Kusmana, C.; Sukristijiono, S. Mangrove Resources Uses by Local Community in Indonesia. J. Pengelolaan Sumberd. Alam Dan Lingkung. 2016, 6, 217–224. [Google Scholar] [CrossRef]
- Bandeira, S.; Macamo, C.; Mabilana, H.; Muhanzule, R. Lesson learnet of mangrove restoration–implemented in several provinces by MICOA/MITADER (Danida). Intern. Report 2016. [Google Scholar]
- Olaleye, S.M.; Omokhua, G.E. Women involvement, empowerment and control of non-timber mangrove forest products in Rivers State. Niger. J. Agric. Food Environ. 2012, 8, 9–13. [Google Scholar]
- Acharya, G. Life at the Margins: The Social, Economic and Ecological Importance of Mangroves. Instituto de Ecologia, A.C. Xalapa, México. 2015. Available online: http://www.redalyc.org/articulo.oa?id=61709803 (accessed on 4 April 2022).
- Teka, O.; Houessou, G.; Lougbegnon, O.T.; Oumorou, M.; Sinsin, B. Mangrove Degradation and Endogenous Strategies for Participatory Restoration and Conservation in Benin. 2012. Rufford final report.
- Numbere, A.O. Perception of Mangrove Forest Protection and Utilization amongst Residents in Some Coastal Communities in the Niger Delta, Nigeria. Curr. Trends For. Res. 2019, 3, 1039. [Google Scholar]
- Mojiol, A.R.; Guntabid, J.; Lintangah, W.; Ismenyah, M.; Kodoh, J.; Chiang, L.K.; Sompud, S. Contribution of mangrove forest and socio-economic development of local communities in Kudat district, Sabah Malaysia. J. Trop. Resour. Sustain. Sci. 2016, 4, 38–41. [Google Scholar]
| Location | Soil Composition | Inundation Classes | Soil Type | Forest Type | |
|---|---|---|---|---|---|
| Maputo Bay | Costa do Sol | Clay | All spring tides High spring tide Extreme spring tide | Firm Intermediate | Basin Dwarf mangroves |
| Bairro dos Pescadores | Clay Sand/clay Peat/clay | All the neap tides All spring tides High spring tide | Firm Intermediate | Basin Fringe | |
| Katembe | Sand Clay Sand/clay Peat Peat/clay | All the neap tides All spring tides High spring tide Extreme spring tide | Firm Intermediate | Basin Dwarf mangroves Estuarine | |
| Boane | Sand/clay Peat/clay | All the neap tides All spring tides High spring tide | Firm Intermediate | Basin Riverine | |
| Matutuine | Sand/clay | All spring tides High spring tide | Firm Intermediate | Basin Dwarf mangroves | |
| Príncipe Island | Praia Salgada/Abade | Sand/clay | All the neap tides | Firm Intermediate | Basin Riverine |
| Praia Grande | Sand/clay Peat/clay | All the neap tides | Firm Intermediate | Basin Riverine | |
| Praia Caixão | Sand Clay Sand/clay | All the neap tides | Firm Intermediate | Basin Riverine | |
| Location | Species | Relative Values | ||||
|---|---|---|---|---|---|---|
| Density | Dominance | Frequency | IIV | |||
| Maputo Bay | Costa do Sol | A. marina C. tagal | 98.86 1.14 | 98.89 1.11 | 80.0 20.0 | 277.75 22.25 |
| Bairro dos Pescadores | A. marina B. gymnorhiza C. tagal R. mucronata | 94.17 0.38 2.81 2.63 | 96.00 0.33 2.70 0.97 | 63.16 5.26 21.05 10.53 | 253.33 5.96 26.58 14.13 | |
| Boane | A.marina R. mucronata X. granatum | 82.24 17.11 0.66 | 63.82 35.84 0.34 | 77.78 11.11 11.11 | 223.84 64.06 12.11 | |
| Katembe | A. marina B. gymnorhiza C. tagal R. mucronata | 86.17 0.53 9.63 3.74 | 80.24 5.78 9.13 4.85 | 0.86 7.69 30.77 15.38 | 204.17 14.00 49.53 23.98 | |
| Matutuine | A. marina B. gymnorhiza C. tagal R. mucronata | 47.09 12.87 32.75 7.02 | 73.31 4.84 16.57 5.27 | 53.85 15.38 15.38 15.38 | 174.25 33.02 65.10 27.63 | |
| Príncipe Island | Praia Salgada-Abade | R. harrisonii | 100 | 100 | 100 | 300 |
| Praia Grande Norte | R. harrisonii | 100 | 100 | 100 | 300 | |
| Praia Caixão | R. harrisonii | 100 | 100 | 100 | 300 | |
| Parameters | Maputo Bay | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Costa do Sol | Bairro dos Pescadores | Boane | Katembe | Matutuíne | ||||||
| Sampling | N = 176 | Q = 4 | N = 532 | Q = 12 | N = 152 | Q = 8 | N = 187 | Q = 7 | N = 171 | Q = 9 |
| Mean DBH (cm) ± SE | 4.37 ± 0.19 | 3.94 ± 0.13 | 18.09 ± 1.07 | 4.58 ± 0.28 | 10.44 ± 0.78 | |||||
| Mean Height (m) ± SE | 2.42 ± 0.08 | 1.31 ± 0.05 | 2.00 ± 0.12 | 1.24 ± 0.064 | 2.50 ± 0.09 | |||||
| N° of species | 2 | 4 | 3 | 4 | 4 | |||||
| Mean Density (ind·ha−1) ± SE | 4400.00 ± 2595.5 | 4433.33 ± 841.57 | 1900.00 ± 791.9 | 2671.43 ± 945.09 | 1911.11 ± 650.5 | |||||
| Basal area (m2·ha−1) | 0.34 | 1.09 | 5.98 | 0.62 | 3.00 | |||||
| Complexity index | 0.07 | 0.25 | 0.68 | 0.08 | 0.57 | |||||
| Parameters | Principe Island | |||||
|---|---|---|---|---|---|---|
| Praia Salgada-Abade | Praia Grande Norte | Praia Caixão | ||||
| Sampling | N = 168 | Q = 5 | N = 20 | Q = 3 | N = 75 | Q = 3 |
| Mean DBH (cm) ± SE | 9.5 ± 0.53 | 4.65 ± 0.52 | 13.31 ± 0.73 | |||
| Mean Height (m) ± SE | 8.93 ± 0.32 | 8.72 ± 0.78 | 12.24 ± 0.58 | |||
| N° of species | 1 | 1 | 1 | |||
| Mean Density (ind·ha−1) ± SE | 3340 ± 603.82 | 667 ± 517.47 | 2500 ± 1357.7 | |||
| Basal area (m2·ha−1) | 12.83 | 0.17 | 5.11 | |||
| Complexity index | 3.83 | 0.01 | 1.56 | |||
| Location | Occupation | |
|---|---|---|
| Men | Women | |
| Maputo Bay | Fishing | Invertebrate collection |
| Timber extraction | Fish selling | |
| Firewood extraction | ||
| Príncipe Island | Tannin production | - |
| Fishing | - | |
| Goods/Services | Uses | Location | |
|---|---|---|---|
| Maputo Bay | Príncipe Island | ||
| Non–extractable | Coastal protection | √ | |
| Tide flooding control | √ | ||
| Water purification | √ | √ | |
| Aesthetical beauty | |||
| Nursery ground | √ | √ | |
| Fishing ground | √ | √ | |
| Extractable | Firewood | √ | |
| Charcoal production | √ | ||
| Timber | √ | ||
| Shrimp fishing | √ | ||
| Invertebrates’ collection | √ | ||
| Tannin production | √ | √ | |
| Uses | Site | % | |
|---|---|---|---|
| Tannin extraction | Costa do Sol/B. Pescadores Katembe/Boane | 4.5% 7.7% | |
| Honey extraction | Matutuine | 4.8% | |
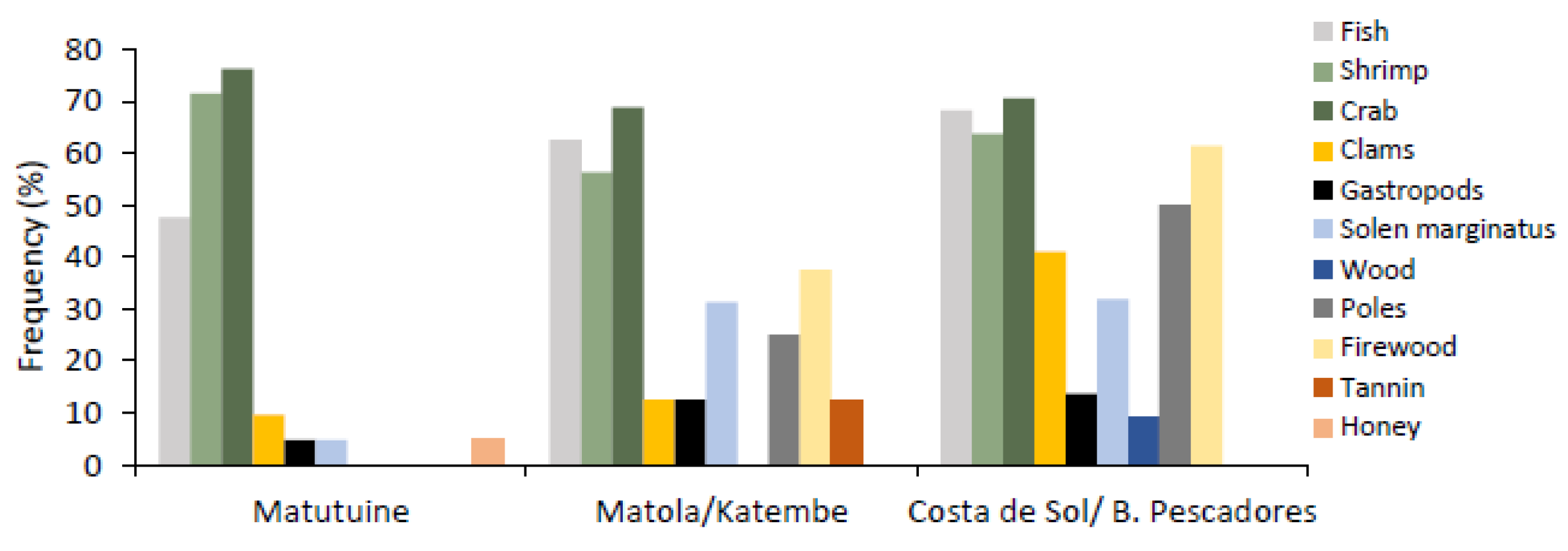
| Fishery resources | Crabs | Costa do Sol/B. Pescadores Katembe/Boane Matutuíne | 70.5% 42.3% 76.2% |
| Shrimp | Costa do Sol/B. Pescadores Katembe/Boane Matutuine | 64.6% 34.6% 71.4% | |
| Fish | Costa do Sol/B. Pescadores Katembe/Boane Matutuine | 68.2% 38.5% 47.6% |
| Scientific Name (Common Name) | Area | Main Uses |
| Avicennia marina (White mangrove) | Costa do sol, Katembe/Boane and Matutuine |
|
| Ceriops tagal (Indian mangrove) | Costa do sol, Katembe/Boane and Matutuine |
|
| Rhizophora mucronata (Red mangrove) | Katembe/Boane and Matutuine |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machava-António, V.; Fernando, A.; Cravo, M.; Massingue, M.; Lima, H.; Macamo, C.; Bandeira, S.; Paula, J. A Comparison of Mangrove Forest Structure and Ecosystem Services in Maputo Bay (Eastern Africa) and Príncipe Island (Western Africa). Forests 2022, 13, 1466. https://doi.org/10.3390/f13091466
Machava-António V, Fernando A, Cravo M, Massingue M, Lima H, Macamo C, Bandeira S, Paula J. A Comparison of Mangrove Forest Structure and Ecosystem Services in Maputo Bay (Eastern Africa) and Príncipe Island (Western Africa). Forests. 2022; 13(9):1466. https://doi.org/10.3390/f13091466
Chicago/Turabian StyleMachava-António, Vilma, Alberto Fernando, Mariana Cravo, Mágda Massingue, Hamilton Lima, Célia Macamo, Salomão Bandeira, and José Paula. 2022. "A Comparison of Mangrove Forest Structure and Ecosystem Services in Maputo Bay (Eastern Africa) and Príncipe Island (Western Africa)" Forests 13, no. 9: 1466. https://doi.org/10.3390/f13091466
APA StyleMachava-António, V., Fernando, A., Cravo, M., Massingue, M., Lima, H., Macamo, C., Bandeira, S., & Paula, J. (2022). A Comparison of Mangrove Forest Structure and Ecosystem Services in Maputo Bay (Eastern Africa) and Príncipe Island (Western Africa). Forests, 13(9), 1466. https://doi.org/10.3390/f13091466







