Abstract
The increasing demand for wood, fiber, and pulp, coupled with efforts to mitigate greenhouse gas emissions, has placed immense importance on the development of forest plantations. The rapidly growing human population faces shortages of food, particularly in the developing world where agricultural productivity is generally low. The taungya system, an age-old agroforestry practice involving the intercropping of crops with trees on the same unit of land, is opined as a win-win strategy to meet the need for wood products and food at the same time. In recent years, the taungya system has gained increasing attention from large forest companies as a tool and an opportunity to contribute to the social well-being of the local community. However, the effects of intercropping on the tree component are largely unexplored. Thus, this study was conducted to examine whether intercropping after 2 and 7 years has an effect on the root system of trees, thereby generating knowledge that supports evidence-based plantation management decisions involving the taungya system. To characterize the root system architecture, trenches were made on six young trees in both a pure Eucalyptus camaldulensis monoculture and intercropped stands (1111 trees/ha in both stands). To quantitatively estimate root biomass, a total of 324 soil cores (6 stands × 6 trees × 3 distances × 3 soil depths) were collected, and roots were sorted and dried to constant mass in an oven at 60 °C for 48 h. The root dry mass data were subjected to analysis of variance to examine the significant effects of intercropping, spacing, and stand age. The results show that the root system of E. camaldulensis was mainly confined to shallow depth but well elongated horizontally in both pure and intercropped stands with 4–6 thick lateral roots. The intercropping of rice/cassava with eucalypt had no effect on the total root dry mass of the tree component (p > 0.05) irrespective of the plantation spacing (5 m × 2 m or 9 m × 1 m); however, root biomass decreased with increasing horizontal distance from the tree base and in deeper soil layers, particularly for trees in young stands. The effects of spacing between trees, wide (5 m × 2 m) versus narrow (9 m × 1 m), on root dry mass were dependent on the horizontal and vertical distribution of the root system, and root biomass appeared to be higher at 40 cm soil depth for the stand with wide spacing between trees than for stands with narrow spacing. Root biomass was larger for older rather than younger trees in both monoculture and intercropped stands, suggesting the lack of a carry-over effect of intercropping on root biomass. In conclusion, this study provides evidence in support of intercropping as a win-win strategy to meet the short-term needs of food production while producing wood in the end. As root biomass varies with horizontal distribution, further research is recommended to test buffer zones between trees and crops other than 1m, which is currently used.
1. Introduction
The increasing demand for wood, fiber, and pulp, coupled with efforts to mitigate greenhouse gas emissions, has placed immense importance on the development of forest plantations. As a result, a considerable surge in areas of new forest plantations has been observed over the past decades [1]. In particular, planting short-rotation trees (e.g., Eucalyptus, Acacia), long recognized as sources of raw material for bioenergy [2], pulp and paper industries, as well as to potentially sequester carbon, is actively pursued in the tropical world. The global extent of forest plantations has been increasing by an average of 2% annually, and today there are about 140 million ha of forest plantations, of which nearly 110 million ha have been established primarily for wood production [3,4]. The proportion of the world’s industrial wood sourced from forest plantations is expected to continue to increase to nearly 50% by 2040 so as to meet the demand for wood products from the growing human population [5]. This demand can be met with the intensive management of planted forests, as intensively managed planted forests are highly productive, and hence the owner invests sustainably over the life of the forest to optimize wood production [5]. The scale of intensively managed planted forests can vary from tens to hundreds of thousands of hectares to smallholders as little as 0.1 ha [5]. The nature of ownership also varies from big forest corporations or governments to the estates of larger-scale private landowners. Nowadays, big forest companies, such as Stora Enso Lao Co., Ltd. (SEL) and Burapha Agroforestry Co., Ltd. (BAFCO), are moving to the south (the tropics) to establish commercial short-rotation forest plantations to supply wood and fiber industries with the desired quality and quantity of raw materials in the shortest possible time.
The rapidly growing human population faces not only a shortage of wood products but also a shortage of food. Food security is indeed a key issue today, particularly in developing countries, where there is scarcity of arable land, on the one hand, and high population pressure and low means of production, on the other. Therefore, an approach that balances wood and food demands must be sought. Over the past several decades, agroforestry systems have been proven to deliver a win-win solution for producing both wood and food at smallholder farm size [6]. Agroforestry is a land management technique that provides both food and timber as the agriculture and forest management is combined on the same unit of land [7]. Although the taungya system is an age-old practice, it has gained increasing attention in recent years as a tool and as an opportunity for external private forest owners to contribute towards the wellbeing of local communities; these owners have a moral obligation to fulfil corporate social responsibility [8]. Many scientists have stated that the use of the taungya system influences the afforestation cost in a positive way at the same time as fulfilling short-term goals such as food production [9]. Intercropping trees with crops secures the food and energy resources, reduces the reforestation cost, creates more job opportunities, and increases the participation of local people in the reforestation procedure [10,11]. Agroforestry can also play an important role in poverty alleviation and as a tool for third world countries to fulfil the Millennium Development Goals [9]. It can be seen as a means of financial security for local communities/families to have a wood resource in the case of failed agricultural results [12,13].
Stora Enso Lao Co., Ltd. (SEL) is one of the forest companies involved in the establishment of intensively managed short-rotation plantations in Asia, including China and Laos. SEL has worked toward a forestry practice that can play a greater role in the work of mitigating the conditions and the situation of poverty in Laos. Through good forest management and communication with the people in those areas, SEL sees good opportunities in having an efficient agricultural practice and being able to grow trees in the same area. SEL has therefore devised an agroforestry project in the remote parts of the Savannakhet and Saravanh provinces to enhance the agricultural productivity meanwhile maintaining short rotation forestry. The plantation model adopted by SEL is based on wide spacing between trees, 9 m × 1 m (1111 seedlings per hectare), and instead of undertaking the traditional shifting cultivation system the project provides the possibility of planting rice and cassava between the tree rows [14]. The intercropping model (modified taungya system) is improving the welfare of local communities by increasing both the yield of rice and cassava, but also allowing for commercial wood production. The planted areas consist of mainly Eucalyptus spp. (Eucalyptus camaldulensis hybrids) and in some cases Acacia spp. Between the tree rows, agriculture crops, such as rice and cassava, are planted and managed until the tree canopy closes, usually two to three years after planting. The trees must have a one-meter buffer zone free from crops on both sides of the trunk, meaning that the roots have two meters of undisturbed soil. The villagers can therefore plant in the seven meters between the tree rows [15].
However, it is not well known whether the current taungya system is beneficial for the trees, as the soils are disturbed a few times each year and the harvest residuals of the crops are ploughed down in the soil, or if the practice has no influence on the root development at all. Tree–crop interactions are not constant and vary depending on species combinations, planting density, climatic and edaphic conditions, and management regimes [16]. For instance, a positive effect on crop yield was observed when nitrogen fixing species, such as Gliricidia sepium (Jacq.) Steud., and Leucaena leucocephala (Lam.) de Wit were combined with crops [17,18]. Similarly, intercropping palisade grass (Urochloa brizantha (Hochst. ex A.Rich.) R.Webster) with Eucalyptus urograndis W.Hill showed belowground synergies with increasing tree root growth in intermediary inter-row positions and emerging new fungal symbiosis [19]. A complementary use of soil resources was observed in Eucalyptus deglupta Blume and coffee interplanting in Costa Rica, with most of the coffee roots close to the coffee rows and most of the tree roots in the inter-row spaces between E. deglupta and the coffee plants [20]. In this case, it was reported that the concentration of roots in the upper soil profile caused intense belowground competition for resources [21,22]. Spacing also influences root structural development [23], with wide spacing expected to favor better root growth than narrow spacing as it determines the extent of competition for belowground resources [24]. A study on the effects of spacing on biomass distribution of Eucalyptus species showed an increase in allocation to roots with increasing spacing [25]. Similarly, the roots of Eucalyptus globulus were more concentrated in soils with sufficient water than in dry soil [26], which is partly related to wider spacing that lowers the competition for water. Both vertical and horizontal anisotropy in the distribution of the fine roots of Eucalyptus species established with 4 × 4.7 m spacing was observed in the soil profiles, with root density decreasing sharply with depth and increasing with distance from the stump [27].
Although several studies on yield and yield components of the crops under crop–tree intercropping systems are available [28,29], there is no study on the root systems of the tree components and their development in this new plantation model. Thus, knowledge about the horizontal and vertical distribution of tree roots with taungya is highly needed to make evidence-based decisions regarding the future establishment of Eucalyptus plantations. This includes short- and long-term effects, negative and positive, from current establishment methods on the root systems of planted Eucalyptus trees. Thus, this study is aimed at examining how the integration of agriculture practices into short rotation tree plantations affects the development of tree roots, thereby generating knowledge for the improved implementation of the taungya system in commercial forest plantations. The fact that SEL is using wide spacing and have their main focus on rice and cassava as agricultural crops in the plantations with Eucalyptus means that tree spacing and crop type may influence the development of the root systems of trees. In this research, the age factor was also considered to investigate the carry-over effects of intercropping on root development after cropping had ceased. The key research questions addressed were: (1) does the intercropping of rice/cassava with Eucalyptus influence the horizontal and vertical distribution of the root biomass of trees compared with monoculture plantations of different spacing? (2) does spacing (5 m × 2 m versus 9 m × 1 m) influence the horizontal and vertical distribution of the root biomass of trees intercropped with rice? and (3) does root biomass differ between young (2 years old) and mature (7 years old) intercropped stands with 9 m × 1 m spacing compared with a monoculture plantation (1111 trees/ha in both monoculture and intercropped stands)? If so, does the effect vary in relation to distance from the tree and soil depth? The corresponding hypotheses of the study were that root biomass would be reduced in intercropped rather than pure eucalyptus stands, root biomass would be greater in the stand with wider rather than narrower spacing, and root biomass would be lower in young rather than mature intercropped stands as the root system is farther from the tree and at a greater soil depth.
2. Materials and Methods
2.1. Study Site
The study was carried out at four different sites/villages (Kean Luang, Ban Takor, Ban Sang and Sabong Kokhai) in Saravanh and Savannakhet provinces in southern Laos, close to the border of Vietnam (Figure 1) in September 2014 to coincide with the rainy period (June to September), because the soil was easier to work with at that time of the year (the end of wet season). The plantations were all established with 9 m × 1 m and 5 m × 2 m spacing, with or without intercropping with rice or cassava under similar silvicultural prescription and in agreement with the local people living in the areas. The tropical climate in Laos with its Southeast Asia monsoons produces distinct seasons. The rainy season generally starts in May and continues to the end of September, but the rain can continue well into October, especially in the mountains. December to January have the lowest temperatures with big diurnal variation; sometimes the air temperature can be as low as 5 °C and frost can occur in some areas. February to April is the hottest season with temperatures up to 40 °C (http://theredddesk.org/sites/default/files/fs_2020.pdf, accessed on 15 April, 2022). The mean annual precipitation is between 1800–2500 mm and approximately 90% falls during the wet season (May–September). The mean monthly temperature in the area can vary between 14–35 °C and the average temperature of the Savannakhet region is 26 °C [14].
Figure 1.
Map of Laos and location of the study area, Pakxe (red rectangle). Source: http://www.worldatlas.com/webimage/countrys/asia/laos/lalatlog.htm, accessed on 4 July 2022.
2.2. Design of the Study
For this study, three intercropped and three pure Eucalyptus monoculture stands were selected. The stands were established with 5 m × 2 m spacing in 2007 and 9 m × 1 m in 2007 and 2012. The 5 m × 2 m stand was intercropped with rice for the first two years; the 9 m × 1 m stand established in 2007 was intercropped with rice while the 9 × 1 m stand established in 2012 was intercropped with cassava. The stands established in 2007 were 7 years old and considered hereafter as mature stands as the rotation period is between 5–7 years while stands established in 2012 were 2 years old and considered hereafter as young stands. All trees were planted on ripped lines with homogenous soil properties in the plantation spots chosen. The soil preparation is conducted either with harrowing or ripping depending on soil depth and soil conditions. Before planting, approximately 330 kg of Dolomite fertilization per hectare was added, followed by 220 kg one year after establishment. During one rotation of the tree growth (about 7 years), a total of 550 kg/ha Dolomite fertilizer was added to all plantations. Weeding was conducted at least once a year, firstly by hand around the seedlings and then by machine as the trees grew. Mortality is negligibly small (about 1%).
The study was conducted by using two different methods; namely, trenching and core sampling. Trenching was conducted to gain insights into the root architecture and to guide subsequent soil coring (where and how to conduct it) for quantitatively determining root production. For trenching, six healthy and dominant trees in terms of height, determined visually and excluding edge trees, were subjectively selected since trenching is destructive and time-consuming. In addition, dominant trees normally have a more developed root system than suppressed trees and fully utilize their growth potential. Once the tree was selected, a rectangular area of 2.5 m × 1.0 m to a depth of 0.5 m was dug out (Figure 2A), the root systems were photographed, and the number of main and lateral roots counted both in situ and from the photos. Trenching was completed close to the tree, about 75 cm away from the stem base, and on two sides of the selected trees (Figure 2A).
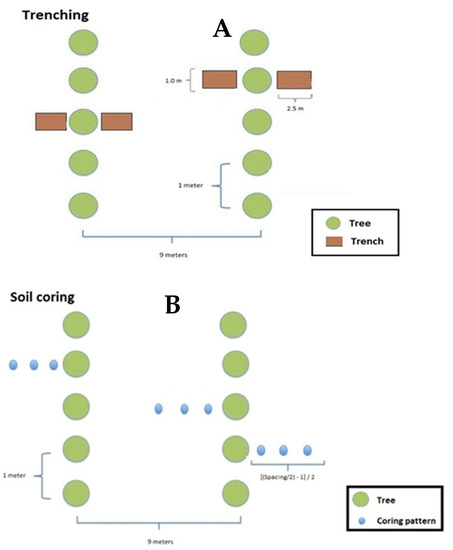
Figure 2.
Illustration of the trenching procedure in 9 m × 1 m plantations (A) and soil coring procedure (B).
For core sampling, 36 sample trees were subjectively selected from stands established in 2007 and 2012 with 9 m × 1 m and 5 m × 2 m that were intercropped with rice/cassava and monoculture. For each sample tree, nine core samples were taken at different soil depths (0–20 cm, 20–40 cm, and 40–60 cm) and at three distances from the sample trees (Figure 2B). The core samples were taken with a soil corer with a cylinder volume of 1570 cm3 (100mm internal tube diameter). As the volume of the cylinder is the same for all samples, the volume of the soil core device was not considered. The first distance was 100 cm from the tree base, and the subsequent distances were determined using the formula, [((Spacing/2) − 1)/2]. Thus, for a 5 m × 2 m stand, the two subsequent distances were set at 75 cm intervals (i.e., 175 cm and 250 cm from the tree base) while for the 9 m × 1 m stand the two subsequent distances were set at 175 cm intervals (i.e., 275 cm and 450 cm from the base of the sample tree).
2.3. Collection of Root Samples
For each individual sample tree, root samples were collected using a stainless steel soil corer (100 mm internal tube diameter). During the sampling, a single tree within the row was selected, excluding the edge trees (end of the row), as those trees will benefit from the edge effect on three sides instead of two sides as with most of the trees. The tree chosen was always one of the dominant trees in terms of height. Soil cores were sampled stepwise for each soil depth at 20 cm intervals down to 60 cm. A total of 324 soil cores (6 stands × 6 trees × 3 distances × 3 soil depths) were collected and packed separately in plastic bags. To be able to distinguish Eucalyptus roots in the field, visual root characteristics, morphology, color and the architecture were used as an identification kit. Before drying, all roots were carefully washed under a gentle flow of tap water to remove adhering soil. Thereafter, the roots were dried in an oven at 60 °C for 48 h until the root dry mass remained constant.
2.4. Statistical Analysis
For each sample tree, total root dry mass was computed by summing up root dry mass from different soil depths, and two-way ANOVA was computed to determine the effects of intercropping and distance from the tree base for each stand separately. To examine the variation in root dry mass across soil depth in relation to intercropping and distance from the tree base, three-way ANOVA was performed. To examine the effects of spacing (5 m × 2 m versus 9 m × 1 m), distance from the tree base, and soil depth on root dry mass, three-way ANOVA was performed for stands intercropped with rice and established in 2007. Three-way ANOVA was also performed to examine the effects of stand age (young versus mature stands with 9 m × 1 m), distance from the tree base, and soil depth on root dry mass. As the 9 m × 1 m stands were intercropped with rice (mature stands) and cassava (young stands), three-way ANOVA was performed for the intercropped and monoculture stands separately to disentangle the confounding effects of crop type. The results of the statistical analyses were considered significant if p < 0.05 and showed tendencies if 0.05 < p < 0.10. Means that exhibited significant differences were compared using Tukey’s test. All statistical analyses were performed using SPSS software (SPSS, version 20.0, Inc., and Chicago, IL, USA).
3. Results
3.1. Overview of Root System Architecture
The typical root system of a two-year-old E. camaldulensis tree in the 9 m × 1 m spacing plantations is shown in Figure 3. The root system was located in the upper parts of the soil (shallow depth) but well elongated horizontally in both pure and intercropped stands. The number of main lateral roots (>5 cm) ranged from four to five in intercropped stands and from five to six in pure Eucalyptus stands. The corresponding number of secondary lateral roots (<5 cm) was 12–22 in pure Eucalyptus stands (Figure 3A) and 12–13 in intercropped stands (Figure 3B).

Figure 3.
Typical root system of a two-year-old Eucalyptus camaldulensis tree in monoculture (A) and intercropping (B).
3.2. Effects of Intercropping and Distance from the Tree Base on Root Dry Mass
Two-way ANOVA revealed a lack of significant differences in the total root dry mass of seven-year-old E. camaldulensis trees planted in 2007 with 5 m × 2 m spacing between tree stands, intercropping with rice (p = 0.711), and the monoculture stands (Figure 4A). Total root dry mass tended (p = 0.097) to decrease with increasing distance from the tree base (Figure 4B). There was also no significant interaction effect (p = 0.155) of intercropping and distance from the tree base on total root dry mass. For mature stands established in 2007 with 9 m × 1 m spacing, root dry mass was unaffected by intercropping compared with monoculture (p = 0.502) as well as among distances from the tree base (p = 0.123; Figure 4); the interaction effect was also insignificant (p = 0.187). However, for the young stands established in 2012 with 9 m × 1 m spacing, total root dry mass significantly (p = 0.001) decreased with increasing distance from the tree base (Figure 4); although total root dry mass did not differ significantly between intercropped and pure stands (p = 0.956) and for the interaction between intercropping and distance from the tree base (p = 0.194).
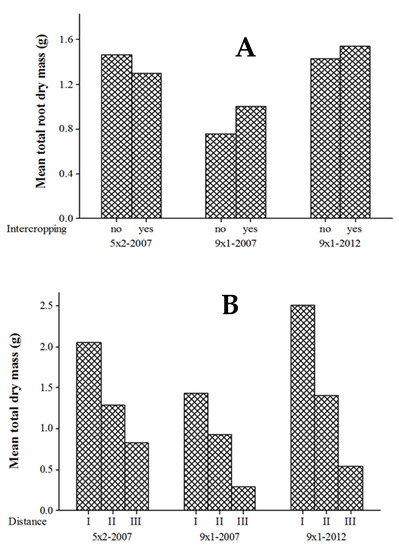
Figure 4.
Main effects of intercropping (A) and distance (B) from the tree on total root dry mass of E. camaldulensis trees in stands established with 5 m × 2 m spacing in 2007 and 9 m × 1 m spacing in 2007 and 2012.
Root dry mass at different soil depths in relation to intercropping and distance from the tree base showed significant variation in the stands with 5 m × 2 m spacing, showed tendency in young stands with 9 m × 1 m spacing, but remained unaffected in mature 9 m × 1 m stands (Table 1). For seven-year-old 5 m × 2 m stands, the mean root dry mass at 40 cm soil depth and at a distance of 175 cm away from the tree base was higher than the pure Eucalyptus stand. In contrast, the root dry mass at 20 cm soil depth was higher in the pure Eucalyptus stand than in the intercropped stand at the same 100 cm distance from the tree base (Figure 5). The mean root dry mass of a 7 years old 9 m × 1 m stand was higher at 20 cm and 60 cm soil depths for the intercropped stand rather than the pure Eucalyptus stand at a 275 cm distance from the tree base. The root dry mass at 60 cm tended to be higher for pure Eucalyptus stands than for intercropped at 275 cm distance from the tree base. For the young pure 9 m × 1 m stand, root dry mass at 20 cm soil depth was the highest at the second farthest distance from the tree base (275 cm) compared to the farthest distance (450 cm) across all soil depths and at 60 cm soil depth in the first 100 cm from the tree base. For the young intercropped stand, root dry mass at nearly all soil depths was higher in the first 100 cm distance from the tree base than the farthest distance from the tree base.

Table 1.
ANOVA results for variations in root dry mass of E. camaldulensis at different soil depths as affected by intercropping and distance from the tree for stands with 5 m × 2 m and 9 m × 1 m spacing.
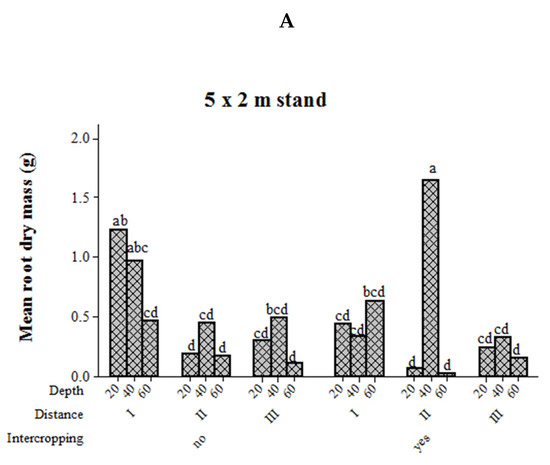
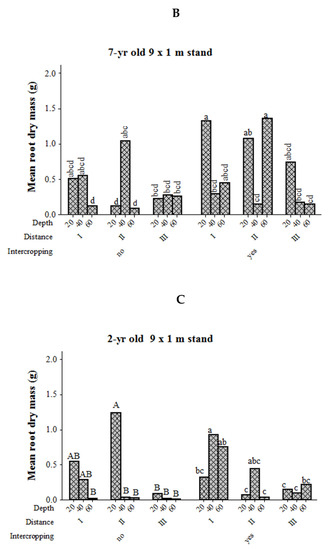
Figure 5.
Mean root dry mass (g) of E. camaldulensis trees at different soil depths in stands with or without intercropping, and at different distances from the tree base. Bars with the same lower case letter (s) are not significantly different among intercropped stands at different distance from the tree base and soil depth at 5% probability. Bars followed by different uppercase letter are significantly different among distances from the tree base and soil depth within monoculture stands. 5 m × 2 m stand (A), 7 years old 9 m × 1 m stand (B), 2 years old 9 m × 1 m stand (C).
3.3. Effects of Spacing and Distance from the Tree Base on Root Dry Mass
Root dry mass remained unaffected by tree spacing and soil depth, but it was significantly decreased with distance from the tree base for rice intercropped stands that were established in 2007. There were also significant interaction effects of spacing, depth, and distance (Table 2). The mean root dry mass at 40 cm soil depth was significantly higher for the 5 m × 2 m stand than for the 9 m × 1 m stand at the second farthest distance from the tree base, whereas root dry mass at a depth of 20 cm and 60 cm was higher for the 9 m × 1 m stand than for the other spacing at a distance of 275 cm away from the tree base (Figure 6). In addition, root dry mass at a soil depth of 20 cm was significantly higher at 100 cm distance away from the tree base than other soil depths.

Table 2.
ANOVA results for the effects of spacing, distance from the tree, and soil depth on root dry mass of E. camaldulensis intercropped with rice at the age of 7 years.
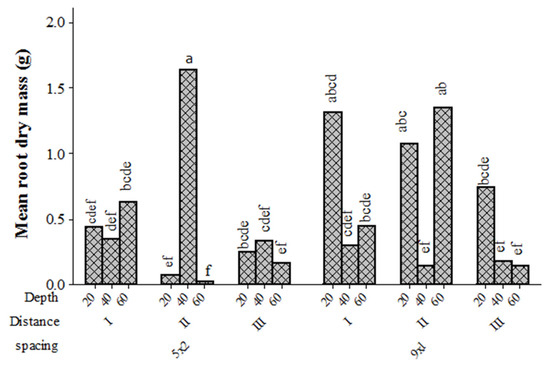
Figure 6.
Mean root dry mass (g) of E. camaldulensis in relation to spacing (5 m × 2 m versus 9 m × 1m), distance from the tree, and soil depth for stands established in 2007 with rice intercropping. Bars with the same letter (s) are not significantly different at 5% probability.
3.4. Effects of Stand Age and Distance from the Tree Base on Root Dry Mass
The effects of stand age and distance from the tree base on root dry mass at different soil depths were studied separately for pure and intercropped stands that were established using 9 m × 1 m spacing. For intercropped stands, stand age, distance to the tree base, and the interaction between stand age and depth had significant effects on root dry mass, while the interaction effect of stand age and distance from the tree base was marginally significant (Table 3). The overall mean root dry mass was lower in younger (0.33 ± 0.08 g) rather than mature stands (0.64 ± 0.15 g). Trees in mature stands had higher root dry mass at 60 cm soil depths and at 275 cm distance away from the tree base than the young stands, whereas the root dry mass in the young stands was high in the first 100 cm distance away the tree compared to other distances (Figure 7). Similar analysis on pure stands revealed significant differences in root dry mass between stand ages, soil depths, and their interaction, as well as three-way interaction between stand age, distance from the tree base, and soil depth (Table 3). For the young stands, root dry mass at 20 cm soil depth and at 275 cm distance away from the tree base was high compared to 60 cm depth. For mature stands, root dry mass at 40 cm depth and at 275 m away from the tree base was higher than at 20 cm and 60 cm depths across all distances from the tree base of young stands (Figure 7).

Table 3.
ANOVA results for the effects of stand age, distance from the tree, and soil depth on root dry mass of E. camdulensis in monoculture and intercropped stands.
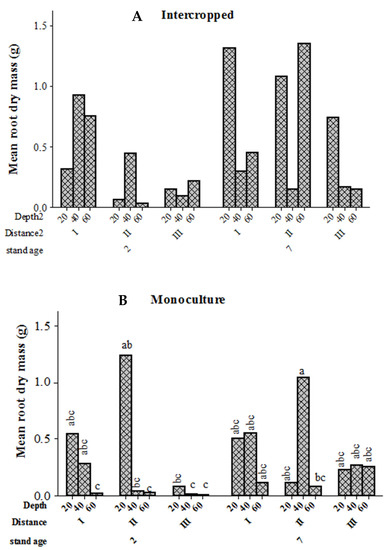
Figure 7.
Mean root dry mass (g) of E. camaldulensis in relation to stand age (2 years versus 7 years old), distance from the tree, and soil depth for stands established with 9 m × 1 m spacing, which were either intercropped or pure monoculture. Bars with the same letter (s) are not significantly different at 5% probability. Intercropping (A), Monoculture (B).
4. Discussion
Increased root production (root biomass) is the front line adaptation reaction to below-ground resource limitation to optimize resource acquisition [30]. In the present study, the number of main and lateral roots of eucalypt trees in monoculture and intercropped stands is nearly similar. This phenomenon could be related to tree size, as tree size has been shown to affect traits such as crown width and diameter [31], and root traits such as the number of roots per root system [32]. Taller neighbor trees apparently restricted focal tree root length and extent, suggesting that asymmetric competition for light between crowns had a negative effect on the root system. This might explain why trees in monoculture, having height diversity, have a higher number of secondary roots than trees in intercropped stands due to asymmetric competition [33]. Previous studies have shown that trees demonstrated reduced root systems but grew more roots when growing in monoculture or growing in stands of high soil nitrogen and in a greater depth to water table [34]. As the trees in the 2 years old stands are similar in height and the stands received similar amounts of fertilizer, the difference in root numbers between monoculture and intercropped stands appears to be similar in the present study.
Interspecific below-ground interactions can consist of competition or facilitation; the latter denotes that one crop positively modifies the environment of the other so as to benefit the growth of the other species [35]. In addition, Belter and Cahill [36] proposed two root “behavioral” strategies: size dependency and location dependency. Size dependency involves the horizontal reduction of root systems to avoid competition with neighbors while location dependency refers to spatial plasticity in root placement in response to neighbor presence. In the present study, intercropping had no significant effect on the root biomass of the tree component, but distance from the tree base and soil depths appeared to play a major role. The highest root dry mass was found at the depth of 40 cm in this study. There was a tendency of greater root biomass closer to the tree base and with the root mass decreasing with increasing distance from the tree base, particularly at a deeper soil layer. This suggests that there exists a plasticity of root traits and complementarity through niche differentiation [37]. In a coffee–Eucalyptus agroforestry system, root partitioning has been observed [20], which provides credence to the niche differentiation theory. It is generally hypothesized that a greater lateral deployment of roots results in asymmetric interspecific facilitation, while the compatibility of the spatial root distribution of intercropped species contributes to symmetric interspecific facilitation as observed in the fava bean/maize intercropping system [38]. Thus, the decrease in the horizontal distribution of eucalypt trees with distance from the base indicates that complementarity through niche differentiation could be a mechanism used by eucalypt trees to horizontally distribute their roots.
In addition, the root systems may sense competition at the top soil layer as they approach the crops, resulting in the investing of carbon in other parts of the root. It should be noted that the carbon cost for the increased production of both a large root biomass and longer roots simultaneously might be high for the plant. Consequently, the plant has to make a “decision” on resource allocation, either producing a larger root biomass or longer roots to optimize nutrient capture [39]. In Gliricidia and Peltophorum, maize hedgerow intercropping systems, the tree component is rooted more deeply than the crop component and hence intercepts leaching nitrogen and demonstrates improved nitrogen-use efficiency [40]. Another explanation is that the disk harrow that is usually used in the gaps between tree rows in pure stands creates ditches that can serve as water bodies, which might promote the growth of roots towards the water. It is worth noting that Eucalyptus trees need more water and exploit a greater amount of soil water [41]. The decrease in root biomass at a deeper soil layer might be due to compaction and poor site conditions, such as the availability of rocks and big stones at a deeper soil layer [42,43]. During sample collection, big stones or rocks were observed at lower depths, which might have restricted root growth. Soil compaction is one of the most limiting factors for good tree establishment by limiting root elongation rates [44]. In the plantations included in this study, the soil was relatively compact, especially in the pure monoculture compared to the intercropped sites. The crops may have loosened the soil slightly, and in so doing enhanced root elongation, as evidenced from the interaction effect of intercropping and soil depth. Furthermore, the nature of the root growth pattern of E. camaldulensis may account for the low root biomass production at a deeper soil surface. As evidenced from the analysis of the root system architecture, the species produces several thicker but relatively shorter lateral roots that are confined to shallow depths. Azam and others [45] also found that root elongation correlates negatively with soil penetration resistance, especially for E. camaldulensis. Their study further concluded that both primary and lateral roots respond to soil compaction and that their diameter increased significantly with compaction. As a whole, the root biomass appears to be confined to the upper soil depths, which in turn could be related to nutrient availability at this depth, as also observed in other studies [46,47]. The findings from this study are consistent with previous studies from other plantations and species [48,49,50,51,52,53,54].
When comparing root dry mass between the wide (5 m × 2 m) and narrow (9 m × 1 m) spacing of trees, the study revealed that higher root biomass was recorded in the 5 m × 2 m stand, particularly at 40 cm soil depth, rather than in the 9 m × 1 m stand. This could be attributed to competition between trees, which are expected to be lower as the spacing between trees is wider (2 m vis-à-vis 1 m). In plantations with wider spacing, the roots deeply penetrate into the soil because of greater growth space, while in narrow-spacing plantations they seem to penetrate into the shallow soil surface for the root-available resources [55]. Our results are consistent with a previous study that demonstrated better root growth in wider-spacing than in narrow-spacing plantations [56]. Stand age also has an influence on root biomass; i.e., the older the trees are, the larger the root biomass may be. This difference is more evident for intercropped than monoculture plantations, suggesting that there is no carry-over effect of intercropping on root biomass. It should be noted that the seven-year-old plantation in this study was intercropped during the first two years only, whereas the younger 2 years old plantation was being actively intercropped during the time that the study was carried out. Thus, the observed differences in root biomass could be attributed to ontogenic difference and the preferential deployment of roots towards the cropped spots due to the temporary boost in nutrient release from the decomposition of crop harvest residues incorporated after the cessation of cropping in older rather than younger stands. The incorporation of the crop harvest residue creates nutrient “hot-spots” in older stands more so than in the young stands, thereby facilitating root proliferation. This is evident in the older stands, wherein root biomass was high, even at 60 cm soil depths. Several studies have shown that plant roots respond to localized soil nutrient patches through root proliferation [57,58]. By doing so, roots acquire more soil nutrients in a heterogeneous rather than homogeneous environments by producing finer, more laterals roots, high root biomass, and specific root lengths in nutrient rich patches rather than elsewhere [59,60,61].
As a whole, the taungya models practiced today in commercial Eucalyptus plantations in Laos appear to have a minor influence on the total root biomass of the tree component. Thus, the new plantation model will play a significant role in effectively rehabilitating forests on degraded lands in order to meet the growing demands for wood and food, as well as in the creation of employment opportunity for landless people. From a methodological point of view, the coring method for estimating the root dry mass is a reasonable and good approach, though uprooting the whole tree and quantifying the root biomass would be more informative. However, the latter is destructive and time-consuming. Throughout the sample collection, we hit several objects (stones, large roots, etc.) that limited the coring procedure. Soil cores give an objective estimation of the root biomass at different depths and distances from the tree base. The influence is of minor importance due to the low amount of large objects being hit in comparison to the large sample collected.
5. Conclusions
This study was conducted to examine the effects of intercropping, spacing, and stand age on the horizontal and vertical distribution of root biomass in commercial eucalyptus plantations, integrating the taungya system in order to generate knowledge that supports evidence-based plantation management decisions. Based on the findings, the following conclusions can be drawn. The root system of E. camaldulensis is mainly confined to shallow depths but is well elongated horizontally in both pure and intercropped stands, with 4–6 thick lateral roots. The intercropping of rice/cassava with eucalypt has no effect on the total root dry mass of the tree component, irrespective of the plantation models (5 m × 2 m or 9 m × 1m). However, root biomass decreases with increasing horizontal distance from the tree base and in deeper soil layers, particularly for trees in young stands. This is partly because of the shallow root system of E. camaldulensis and the poor soil quality at deeper soil layers, and partly due to possible competition between tree and crop roots. The effects of spacing between tree components, wide (5 m × 2 m) versus narrow (9 m × 1 m), on root dry mass, are dependent on the horizontal and vertical distribution of the root system. Root biomass appears to be higher at 40 cm soil depth for the stands with wide spacing between trees as opposed to stands with narrow spacing, which might be attributed to between-tree competition in the narrow spacing. Root biomass differs based on stand age, with older trees having larger root biomass in both monoculture and intercropped stands, the difference being more evident in the latter than in the former. As a whole, the study provides evidence in support of incorporating the taungya system in commercial Eucalyptus plantations as a win-win strategy to meet the short-term needs for food production while also producing wood in the end. However, to acquire a complete picture of the pros and cons of the taungya system, the survival and growth of the trees should be studied. From the visual observation of the plantations, Eucalyptus with or without intercropping seems to grow equally well. Further research is recommended on spacing and tree–crop combinations in order to benefit both farmers and the forestry sector optimally. In particular, testing buffer zone between trees and crops other than 1 m (which is currently used) may be an important aspect to consider in future research. It is important to bear in mind that optimal spacing and different combinations of crops and trees are dependent on site conditions such as soil properties, and therefore site-specific tests need to be considered.
Author Contributions
Conceptualization, S.E. and M.T.; methodology, S.E, M.T., P.C.O.; software, S.E. and M.T.; validation, M.T.; formal analysis, S.E. and M.T.; investigation, S.E.; resources, S.E., M.T. and P.C.O.; data curation, S.E.; writing—original draft preparation, S.E.; writing—review and editing, M.T. and P.C.O.; visualization, S.E. and M.T.; supervision, M.T. and P.C.O.; project administration, P.C.O.; funding acquisition, S.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data used in this study can be made available by the authors upon reasonable request.
Acknowledgments
We thank Emma Sandell Festin, Former R&D assistant at Stora Enso Laos, for her support in providing background information and material about the on-going spacing projects in Savannakhet and Saravanh Provinces, Lao PDR and establishing contact with Stora Enso Laos. Our thanks go to Somphanith Soyochan (R&D specialist), Sinlakhone Siakkhachan (Pollination controller), Mid Saly (Field assistant) and Sayyanh Keophavong, (R&D officer) for their help in the field. We would like to give a special thanks to Juha Anttila (Regional Manager Stora Enso), Peter Fogde (CEO Stora Enso/Burapha), Andreas Magnusson (Head of Planning Burapha) for their support during the field work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Global Forest Resource Assessment 2015: How Are the World’s Forests Changing? Food and the Agricultural Organization: Rome, Italy, 2015; p. 47. [Google Scholar]
- Shepherd, M.; Bartle, J.; Lee, D.J.; Brawner, J.; Bush, D.; Turnbull, P.; Macdonel, P.; Brown, T.R.; Simmons, B.; Henry, R. Eucalypts as a biofuel feedstock: Review. Biofuels 2011, 2, 639–657. [Google Scholar] [CrossRef]
- Payn, T.; Carnus, J.-M.; Freer-Smith, P.; Kimberley, M.; Kollert, W.; Liu, S.; Orazio, C.; Rodriguez, L.; Silva, L.N.; Wingfield, M.J. Changes in planted forests and future global implications. For. Ecol. Manag. 2015, 352, 57–67. [Google Scholar] [CrossRef]
- Del Lungo, A.; Ball, J.; Carle, J. Global Planted Forests Thematic Study: Results and Analysis; Planted Forests and Trees Working Paper 38; Food and Agricultural organization: Rome, Italy, 2006; p. 174. [Google Scholar]
- Kanowski, P.; Murray, H. Intensively Managed Planted Forests: Toward Best Practice; A TFD Publication: New Haven, CT, USA, 2008; pp. 1–64. [Google Scholar]
- Kuyah, S.; Whitney, C.W.; Jonsson, M.; Sileshi, G.W.; Öborn, I.; Muthuri, C.W.; Luedeling, E. Agroforestry delivers a win-win solution for ecosystem services in sub-Saharan Africa. A meta-analysis. Agron. Sustain. Dev. 2019, 39, 47. [Google Scholar] [CrossRef]
- Alexander, T.G.; Sobhana, K.; Balagopalan, M.; Mary, M.V. Taungya in relation to soil properties, soil erosion and soil management. In KFRI Research Report; Kerala Forest Research Institute: Kerala, India, 1980; Volume 4, p. 24. [Google Scholar]
- Jordan, J.; Gajaseni, J.; Watanabe, H. Taungya: Forest Plantations with Agriculture in Southeast Asia; CAB International: Wallingford, UK, 1992. [Google Scholar]
- Victor, A.J.; Bakare, Y. Rural Livelihood Benefits from Participation in the Taungya Agroforestry System in Ondo State, Nigeria. Small-Scale For. Econ. Manag. Policy 2004, 3, 131–138. [Google Scholar]
- Schlonvoigt, A.; Beer, J. Initial growth of pioneer timber tree species in a Taungya system in the humid lowlands of Costa Rica. Agrofor. Syst. 2001, 51, 97–108. [Google Scholar] [CrossRef]
- Kalame, F.B.; Aidoo, R.; Nkem, J.; Ajayie, O.C.; Kanninen, M.; Luukkanen, O.; Idinoba, M. Modified taungya system in Ghana: A win-win practice for forestry and adaptation to climate change? Environ. Sci. Policy 2011, 14, 519–530. [Google Scholar] [CrossRef]
- Garrity, D.P. Agroforestry and the achievement of the Millennium Development Goals. Agrofor. Syst. 2004, 61, 5–17. [Google Scholar]
- Soto-Pinto, L.; Armijo-Florentino, C. Changes in Agroecosystem Structure and Function along a Chronosequence of Taungya System in Chiapas, Mexico. J. Agric. Sci. 2014, 6, 43–57. [Google Scholar] [CrossRef]
- Anonymous. Growing Food & Planting Trees; Annual Report 2007; Stora Enso Report: Stockholm, Finland, 2007. [Google Scholar]
- Anonymous. Environmental Impact Assessment of Stora Enso’s Commercial Plantations in Savannakhet and Saravanh Provinces, Lao PDR; Swedish University of Agricultural Sciences, Department of Soil and Environment: Uppsala, Sweden, 2008. [Google Scholar]
- Imo, M.; Timmer, V.R. Vector competition analysis of a Leucaena—maize alley cropping system in western Kenya. For. Ecol. Manag. 2000, 126, 255–268. [Google Scholar] [CrossRef]
- Young, A. Agroforestry for Soil Management; CABI: Wallingford, UK; ICRAF: Nairobi, Kenya, 1997; p. 320. [Google Scholar]
- Reyes, T.; Quiroz, R.; Luukkanen, O.; De Mendiburu, F. Spice crops agroforestry systems in the East Usambara Mountains, Tanzania: Growth analysis. Agrofor. Syst. 2009, 76, 513–523. [Google Scholar] [CrossRef]
- Bieluczyk, W.; de Cassia Piccolo, M.; Pereira, M.G.; Lambais, G.R.; de Moraes, M.T.; Soltangheisi, A.; de Campos Bernardi, A.C.; Pezzopane, J.R.M.; Bosi, C.; Cherubin, M.R. Eucalyptus tree influence on spatial and temporal dynamics of fine-root growth in an integrated crop-livestock-forestry system in southeastern Brazil. Rhizosphere 2021, 19, 100415. [Google Scholar] [CrossRef]
- Schaller, M.; Schroth, G.; Beer, J.; Jiménez, F. Species and site characteristics that permit the association of fast.growing trees with crops: The case of Eucalyptus deglupta as coffee shade in Costa Rica. For. Ecol. Manag. 2003, 175, 205–215. [Google Scholar] [CrossRef]
- Chamshama, S.A.O.; Mugasha, A.G.; Klovstad, A.; Haveraaen, O.; Maliondo, S.M.S. Growth and yield of maize alley cropped with Leucaena leucocephala and Faidherbia albida in Morogoro, Tanzan. Agrofor. Syst. 1998, 40, 215–225. [Google Scholar] [CrossRef]
- Rao, M.R.; Sharma, M.M.; Ong, C.K. A tree-crop interface design and its use for evaluating the potential of hedge row intercropping. Agrofor. Syst. 1991, 13, 143–158. [Google Scholar] [CrossRef]
- Atkinson, C.; Webster, A. The influence of the development of temperate fruit tree species on the potential for their uptake of radionuclides. J. Environ. Radioact. 2001, 52, 131–146. [Google Scholar] [CrossRef]
- Marziliano, P.A.; Coletta, V.; Menguzzato, G.; Nicolaci, A.; Pellicone, G.; Veltri, A. Effects of planting density on the distribution of biomass in a Douglas-fir plantation in southern Italy. Iforest 2015, 8, 368. [Google Scholar] [CrossRef]
- Bernardo, A.L.; Reis, M.G.F.; Reis, G.G.; Harrison, R.B.; Firme, D.J. Effect of spacing on growth and biomass distribution in Eucalyptus camaldulensis, E. pellita and E. urophylla plantations in southeastern Brazil. For. Ecol. Manag. 1998, 104, 1–13. [Google Scholar]
- Fabião, A.; Madeira, M.; Steen, E. Effect of Water and Nutrient Supply on Root Distribution in an Eucalyptus Globulus Plantation. In Management of Nutrition in Forests under Stress; Zöttl, H.W., Hüttl, R.F., Eds.; Springer: Dordrecht, The Netherland, 1991. [Google Scholar] [CrossRef]
- Laclau, J.-P.; Arnaud, M.; Bouillet, J.-P.; Ranger, J. Spatial distribution of Eucalyptus roots in a deep sandy soil in the Congo: Relationships with the ability of the stand to take up water and nutrients. Tree Physiol. 2001, 21, 129–136. [Google Scholar] [CrossRef]
- Nadir, S.W.; Ng’etich, W.K.; Kebeney, S.J. Performance of crops under Eucalyptus tree-crop mixtures and its potential for adoption in agroforestry systems. Aust. J. Crop Sci. 2018, 12, 1231–1240. [Google Scholar] [CrossRef]
- Ahlawat, K.S.; Daneva, V.; Sirohi, C.; Dalal, V. Production Potential of Agricultural Crops under Eucalyptus tereticornis Based Agrisilviculture System in Semi-Arid Region of Haryana. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2319–7706. [Google Scholar] [CrossRef]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 15–187. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, L.; Bongers, F. Architecture of 54 moist-forest tree species: Traits, trade-offs, and functional groups. Ecology 2006, 87, 1289–1301. [Google Scholar] [CrossRef]
- Zanetti, C.; Vennetier, M.; Mériaux, P.; Provansal, M. Plasticity of tree root system structure in contrasting soil materials and environmental conditions. Plant Soil 2015, 387, 21–35. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Syrjänen, K.; Kalliola, R. Structure of a pristine Picea abies forest in northeastern Europe. J. Veg. Sci. 1998, 9, 563–574. [Google Scholar] [CrossRef]
- Madsen, C.; Potvin, C.; Hall, J.; Katherine, S.; Turner, B.L.; Schnabel, F. Coarse root architecture: Neighbourhood and abiotic environmental effects on five tropical tree species growing in mixtures and monocultures. For. Ecol. Manag. 2020, 460, 117851. [Google Scholar] [CrossRef]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecology, Individuals, Populations and Communities, 3rd ed.; Blackwell Science: London, UK, 1996. [Google Scholar]
- Belter, P.R.; Cahill, J.F. Disentangling root system responses to neighbours: Identification of novel root behavioural strategies. AoB PLANTS 2015, 7, plv059. [Google Scholar] [CrossRef]
- Homulle, Z.; George, T.S.; Karley, A.J. Root traits with team benefits: Understanding belowground interactions in intercropping systems. Plant Soil 2022, 471, 1–26. [Google Scholar] [CrossRef]
- Li, L.; Sun, J.; Zhang, F.; Guo, T.; Bao, X.; Smith, F.A.; Smith, S.E. Root distribution and interactions between intercropped species. Oecologia 2006, 147, 280–290. [Google Scholar] [CrossRef]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Rowe, E.C.; van Noordwijk, M.; Suprayogo, D.; Hairiah, K.; Giller, K.E.; Cadisch, G. Root distributions partially explain 15N uptake patterns in Gliricidia and Peltophorum hedgerow intercropping systems. Plant Soil 2001, 235, 167–179. [Google Scholar] [CrossRef]
- Robinson, N.; Harper, R.J.; Smettem, K.R.J. Soil water depletion by Eucalyptus spp. Integrated into dryland agricultural systems. Plant Soil 2006, 286, 141–151. [Google Scholar]
- Goss, M.J. Effects of mechanical impedance on root growth in barley (Hordeum vulgare L.): I. Effects of the elongation and branching of seminal root axes. J. Exp. Bot. 1977, 28, 96–111. [Google Scholar] [CrossRef]
- Hebblethwaite, P.D.; McGowan, M. The effects of soil compaction on the emergence, growth and yield of sugar beet and peas. J. Sci. Food Agric. 1980, 31, 1131–1142. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Soil compaction and growth of woody plants. Scand. J. For. Res. 1999, 14, 596–619. [Google Scholar] [CrossRef]
- Azam, G.; Grant, D.C.; Murray, S.R.; Nuberg, K.I.; Misra, K.R. Comparison of the penetration of primary and lateral roots of pea and different tree seedlings growing in hard soils. Soil Res. 2014, 52, 87–96. [Google Scholar] [CrossRef]
- Montani, T.; Fernandez, O.A.; Busso, C.A.; Flemmer, A.C. Root growth, appearance and disappearance in perennial grasses: Effects of the timing of water stress with or without defoliation. Can. J. Plant Sci. 2002, 82, 539–547. [Google Scholar]
- Savadogo, P.; Santi, S.; Dayamba, S.D.; Nacro, H.B.; Sawadogo, L. Seasonal variation in fire temperature and influence on soil CO2 efflux, root biomass, and soil water properties in a Sudanian savanna–woodland, West Africa. Soil Res. 2012, 50, 195–206. [Google Scholar] [CrossRef]
- Carbon, B.A.; Bartle, G.A.; Murray, A.M.; Macpherson, D.K. The distribution of root length, and the limits to flow of soil water to roots in a dry sclerophyll forest. For. Sci. 1980, 26, 656–664. [Google Scholar]
- Nambiar, E.K.S. Root development and configuration in intensively managed radiate pine plantations. Plant Soil 1983, 71, 37–47. [Google Scholar] [CrossRef]
- Livesley, S.J.; Gregory, P.J.; Buresh, R.J. Competition in tree row agroforestry systems. 1. Distribution and dynamics of fine root length and biomass. Plant Soil 2000, 227, 149–161. [Google Scholar] [CrossRef]
- Bouillet, J.P.; Laclau, J.P.; Arnaud, M.; Thongo M’Bou, A.; Laurent, S.A.; Jourand, C. Changes with age in the spatial distribution of roots in Eucalyptus clone in Congo. Impact on water and nutrient uptake. For. Ecol. Manag. 2002, 171, 43–57. [Google Scholar] [CrossRef]
- Macinnis-Ng, C.M.O.; Fuentes, S.; O’Grady, A.P.; Palmer, A.R.; Taylor, D.; Whitley, R.J.; Yunusa, I.; Zeppel, M.J.B.; Hardie, M.; Eamus, D. Root biomass distribution and soil properties of an open woodland on a duplex soil. Plant Soil 2010, 327, 377–388. [Google Scholar] [CrossRef]
- Gwenzi, W.; Veneklaas, E.J.; Holmes, K.W.; Bleby, T.M.; Phillips, I.R.; Hinz, C. Spatial analysis of fine root distribution on a recently constructed ecosystem in a water-limited environment. Plant Soil 2011, 348, 471–489. [Google Scholar] [CrossRef]
- Levillain, J.; Thongo M’Bou, A.; Deleporte, P.; Saint-André, L.; Jourdan, C. Is the simple auger coring method reliable for below-ground standing biomass estimation in Eucalyptus forest plantations? Ann. Bot. 2011, 108, 221–230. [Google Scholar] [CrossRef]
- Van Noordwijk, M.; Lawson, G.; Hairiah, K.; Wilson, J. Root Distribution of Trees and Crops: Competition and/or Complementarity Tree–Crop Interactions: Agroforestry in a Changing Climate; CABI: Wallingford, UK, 2015; pp. 221–257. [Google Scholar]
- Farooq, T.H.; Wu, W.; Tigabu, M.; Ma, X.; He, Z.; Rashid, M.H.U.; Gilani, M.M.; Wu, P. Growth, Biomass Production and Root Development of Chinese fir in Relation to Initial Planting Density. Forests 2019, 10, 236. [Google Scholar] [CrossRef]
- Johnson, H.A.; Biondini, M.E. Root morphological plasticity and nitrogen uptake of 59 plant species from the Great Plains grasslands, U.S.A. Basic Appl. Ecol. 2001, 2, 127–143. [Google Scholar] [CrossRef][Green Version]
- Nie, Y.P.; Chen, H.S.; Wang, K.L.; Ding, Y.L. Rooting characteristics of two widely distributed woody plant species growing in different karst habitats of southwest China. Plant Ecology 2014, 215, 1099–1109. [Google Scholar] [CrossRef]
- Wijesing, D.K.; John, E.A.; Beurskens, S.; Hutchings, M.J. Root system size and precision in nutrient foraging: Responses to spatial pattern of nutrient supply in six herbaceous species. J. Ecol. 2001, 89, 972–983. [Google Scholar] [CrossRef]
- He, Y.; Liao, H.; Yan, X. Localized supply of phosphorus induces root morphological and architectural changes of rice in split and stratified soil cultures. Plant Soil 2003, 248, 247–256. [Google Scholar] [CrossRef]
- Mou, P.; Jones, R.H.; Tan, Z.; Bao, Z.; Chen, H. Morphological and physiological plasticity of plant roots when nutrients are both spatially and temporally heterogeneous. Plant Soil 2013, 364, 373–384. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).