Gap-Scale Disturbance Patterns and Processes in a Montane Pinus palustris Woodland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Methods
2.3. Analytical Methods
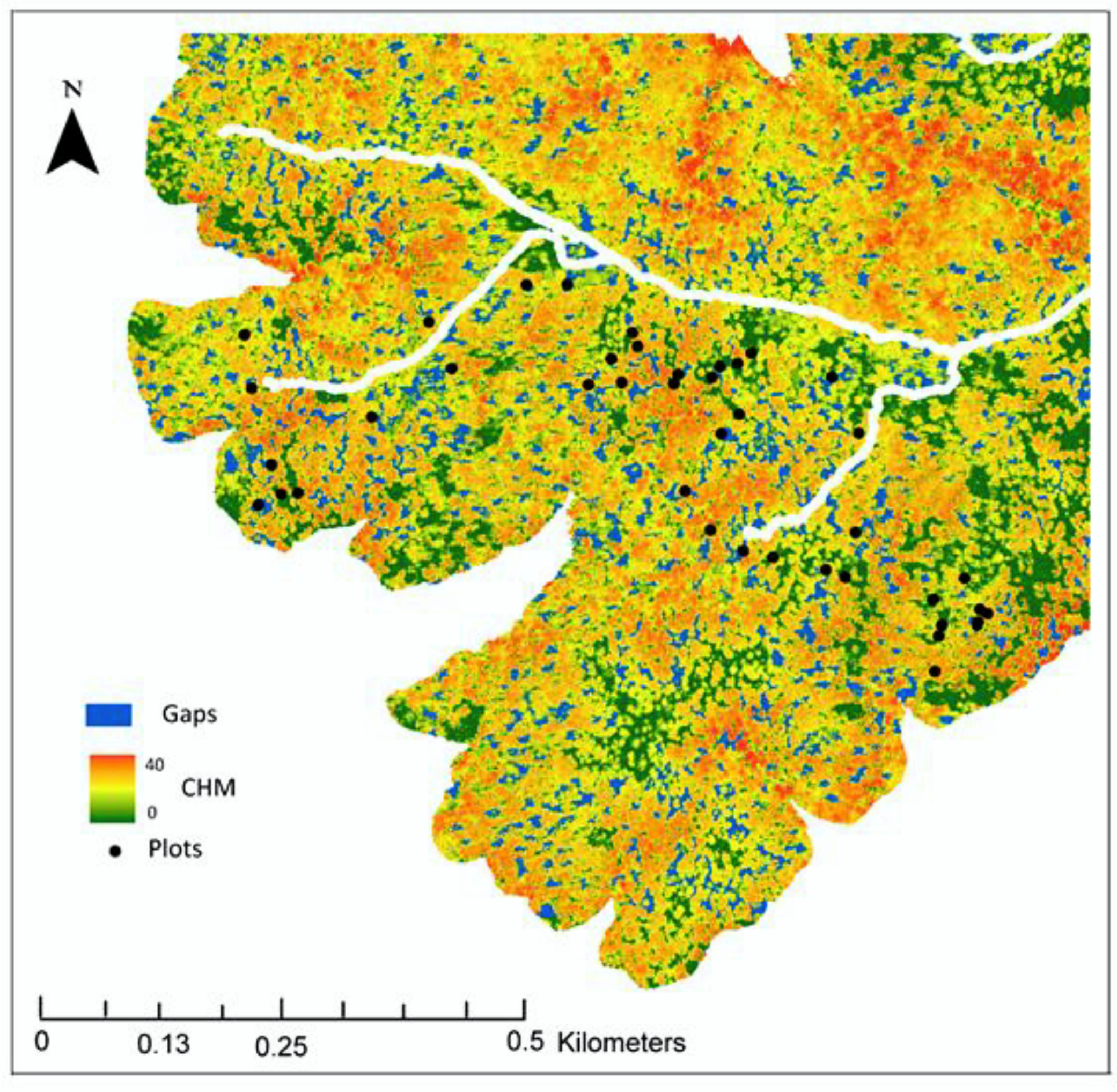
2.4. LiDAR Analysis
3. Results
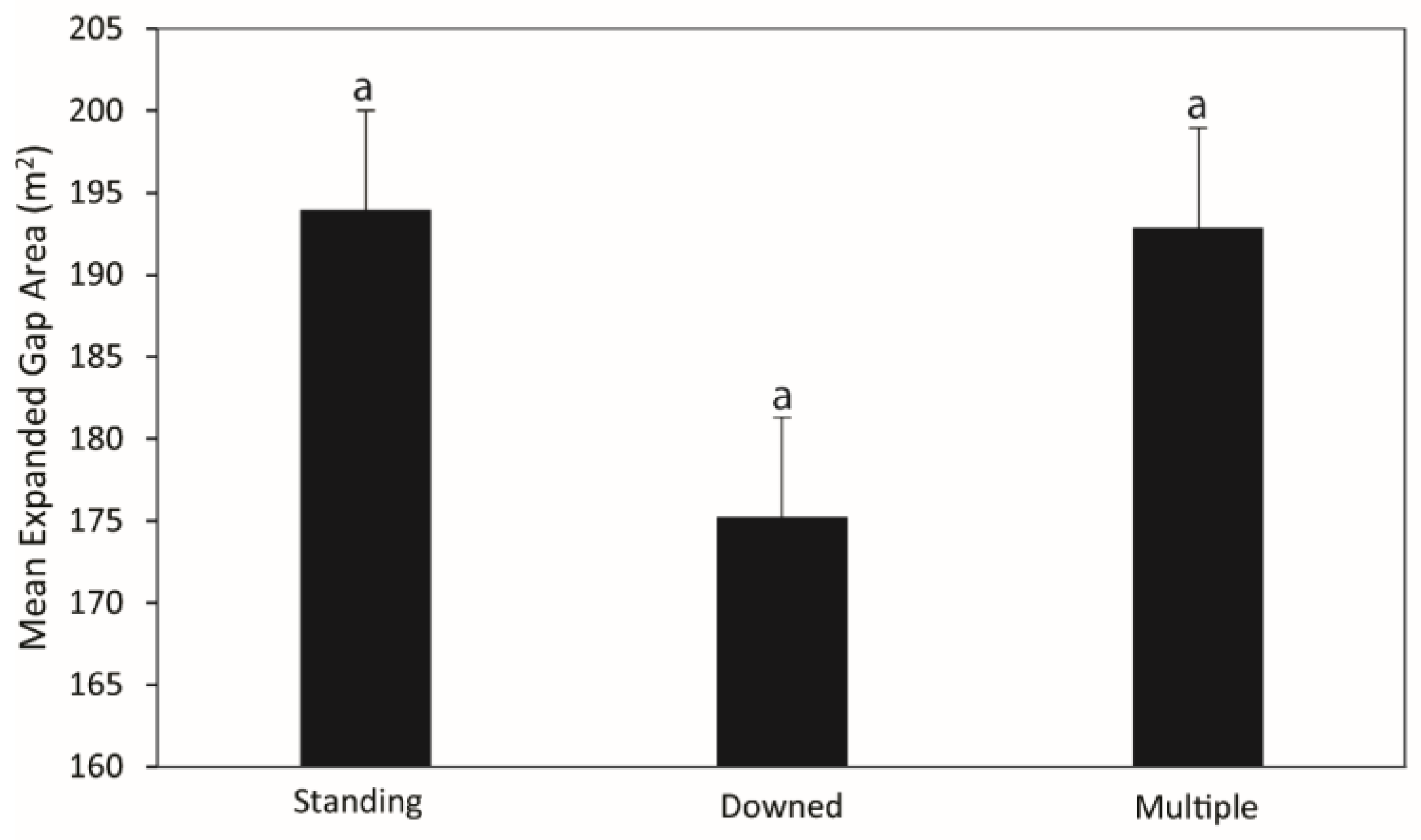
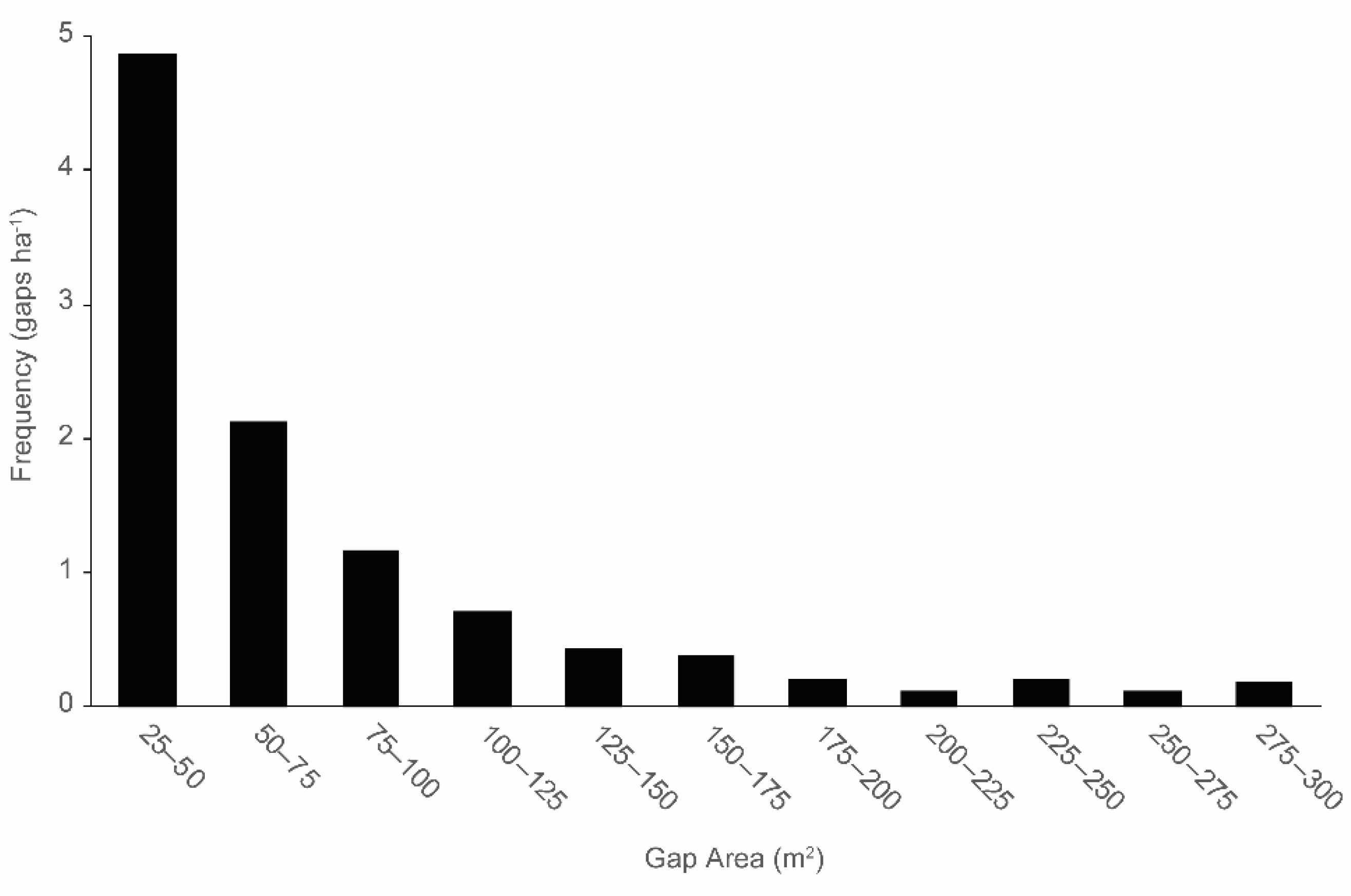
3.1. Canopy Gap and Gapmaker Characteristics
3.2. Forest Composition
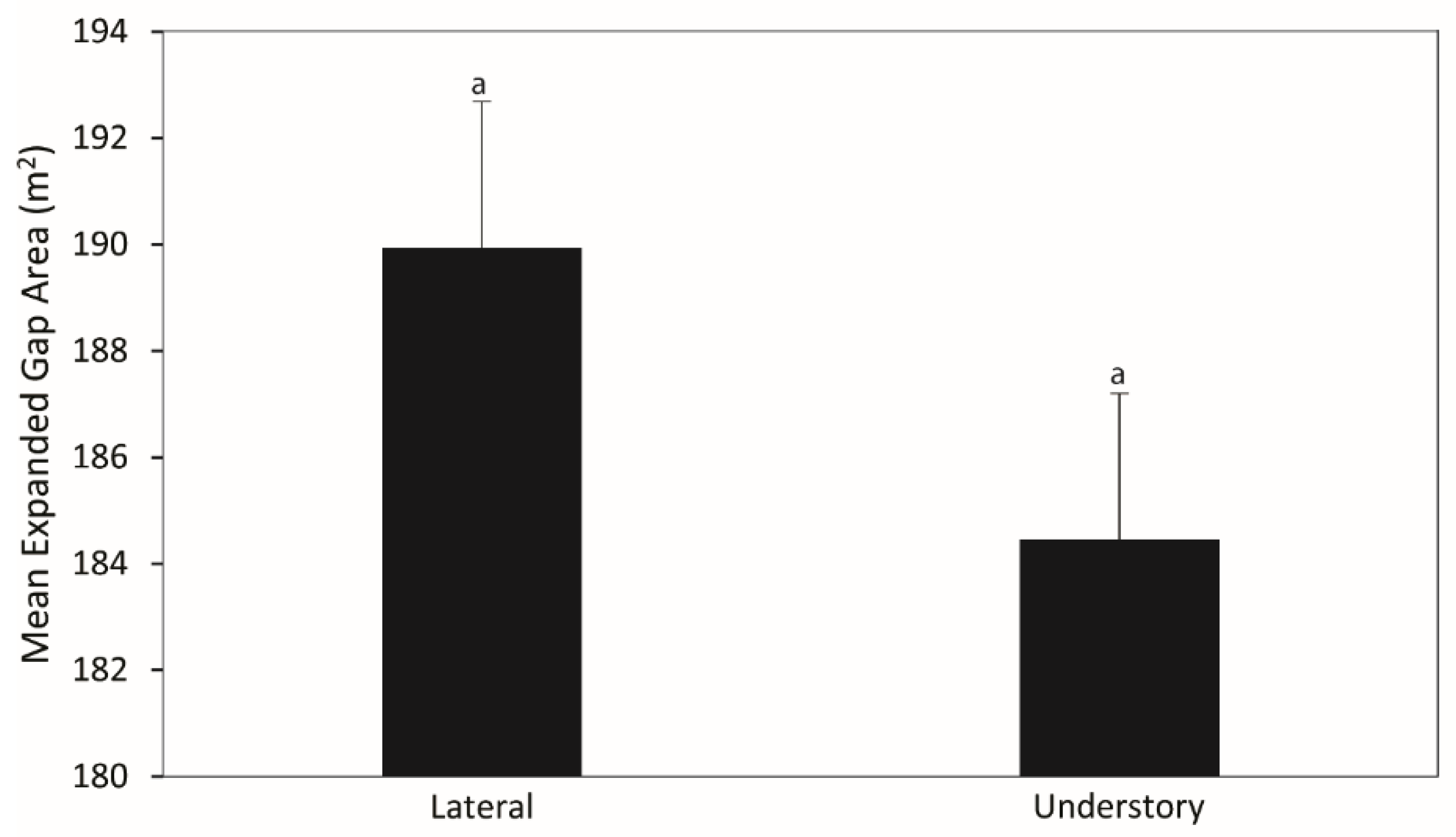
3.3. Gap Closure Mechanisms
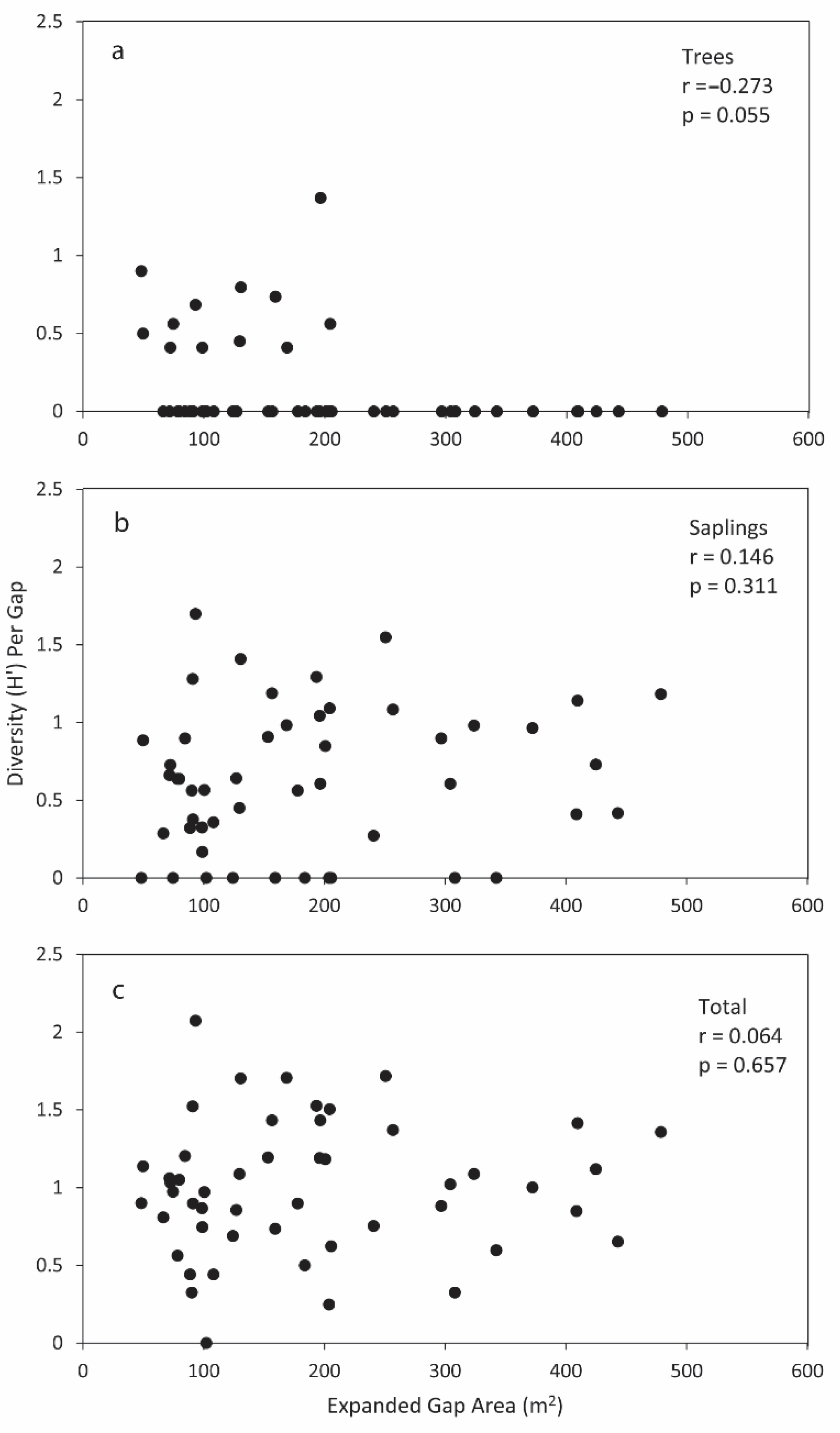
3.4. Gap Fraction and Spatial Distribution
4. Discussion
4.1. Canopy Gap and Gapmaker Characteristics
4.2. Forest Composition
4.3. Gap Closure Mechanisms
4.4. Gap Fraction and Spatial Distribution
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics; John Wiley and Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Hart, J.L.; Kleinman, J.S. What are intermediate-severity forest disturbances and why are they important? Forests 2018, 9, 579. [Google Scholar] [CrossRef] [Green Version]
- Runkle, J.R. Disturbance regimes in temperate forests. In The Ecology of Natural Disturbance and Patch Dynamics; Pickett, S.T.A., White, P.S., Eds.; Academic Press: San Diego, CA, USA, 1985; pp. 17–33. [Google Scholar]
- Yamamoto, S.I. Forest gap dynamics and tree regeneration. J. For. Res. 2000, 5, 223–229. [Google Scholar] [CrossRef]
- Frelich, L.E. Forest Dynamics and Disturbance Regimes: Studies from Temperate Evergreen-Deciduous Forests; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Hart, J.L. Gap-scale disturbances in central hardwood forests with implications for management. In Natural Disturbances and Historic Range of Variation; Greenberg, C.H., Collins, B.S., Eds.; Springer: New York, NY, USA, 2016; pp. 33–47. [Google Scholar]
- Brokaw, N.V. Gap-phase regeneration in a tropical forest. Ecology 1985, 66, 682–687. [Google Scholar] [CrossRef]
- Abe, S.; Masaki, T.; Nakashizuka, T. Factors influencing sapling composition in canopy gaps of a temperate deciduous forest. J. Veg. Sci. 1995, 120, 21–32. [Google Scholar] [CrossRef]
- Brokaw, N.; Busing, R.T. Niche versus chance and tree diversity in forest gaps. Trends. Ecol. Evol. 2000, 15, 183–188. [Google Scholar] [CrossRef]
- Hart, J.L.; Kupfer, J.A. Sapling richness and composition in canopy gaps of a southern Appalachian mixed Quercus forest. J. Torrey. Bot. Soc. 2011, 138, 207–219. [Google Scholar] [CrossRef]
- Canham, C.D.; Marks, P.L. The response of woody plants to disturbance patterns of establishment and growth. In The Ecology of Natural Disturbance and Patch Dynamics; Pickett, S.T.A., White, P.S., Eds.; Academic Press: San Diego, CA, USA, 1985; pp. 197–216. [Google Scholar]
- Putz, F.E. Treefall pits and mounds, buried seeds, and the importance of soil disturbance to pioneer trees on Barro Colorado Island, Panama. Ecology 1983, 64, 1069–1074. [Google Scholar] [CrossRef]
- Denslow, J.S. Tropical rainforest gaps and tree species diversity. Annu. Rev. Ecol. Evol. Syst. 1987, 18, 431–451. [Google Scholar] [CrossRef]
- McGuire, J.P.; Mitchell, R.J.; Moser, E.B.; Pecot, S.D.; Gjerstad, D.H.; Hedman, C.W. Gaps in a gappy forest: Plant resources, longleaf pine regeneration, and understory response to tree removal in longleaf pine savannas. Can. J. For. Res. 2001, 31, 765–778. [Google Scholar] [CrossRef]
- Dey, D.C.; Kabrick, J.M. Restoration of midwestern oak woodlands and savannas. In Restoration of Boreal and Temperate Forests, 2nd ed.; Stanturf, J.A., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 401–428. [Google Scholar]
- Palik, B.J.; Mitchell, R.J.; Houseal, G.; Pederson, N. Effects of canopy structure on resource availability and seedling responses in a longleaf pine ecosystem. Can. J. For. Res. 1997, 27, 1458–1464. [Google Scholar] [CrossRef]
- Battaglia, M.A.; Mitchell, R.J.; Mou, P.P.; Pecot, S.D. Light transmittance estimates in a longleaf pine woodland. For. Sci. 2003, 49, 752–762. [Google Scholar]
- Gagnon, J.L.; Jokela, E.J.; Moser, W.K.; Huber, D.A. Dynamics of artificial regeneration in gaps within a longleaf pine flatwoods ecosystem. For. Ecol. Manag. 2003, 172, 133–144. [Google Scholar] [CrossRef]
- Varner, J.M., III; Kush, J.S.; Meldahl, R.S. Structural characteristics of frequently-burned old-growth longleaf pine stands in the mountains of Alabama. Castanea 2003, 68, 211–221. [Google Scholar]
- Pecot, S.D.; Mitchell, R.J.; Palik, B.J.; Moser, E.B.; Hiers, J.K. Competitive responses of seedlings and understory plants in longleaf pine woodlands: Separating canopy influences above and below ground. Can. J. For. Res. 2007, 37, 634–648. [Google Scholar] [CrossRef]
- Platt, W.J.; Evans, G.W.; Rathbun, S.L. The population dynamics of a long-lived conifer (Pinus palustris). Am. Nat. 1988, 131, 491–525. [Google Scholar] [CrossRef]
- Brockway, D.G.; Outcalt, K.W. Gap-phase regeneration in longleaf pine wiregrass ecosystems. For. Ecol. Manag. 1998, 106, 125–139. [Google Scholar] [CrossRef]
- Gagnon, J.L.; Jokela, E.J.; Moser, W.K.; Huber, D.A. Characteristics of gaps and natural regeneration in mature longleaf pine flatwoods ecosystems. For. Ecol. Manag. 2004, 187, 373–380. [Google Scholar] [CrossRef]
- Jack, S.B.; Pecot, S.D. Regeneration Dynamics, Competition, and Seedling Response. In Ecological Restoration and Management of Longleaf Pine Forests; Kirkman, L.K., Jack, S.B., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2018; pp. 311–338. [Google Scholar]
- Wahlenberg, W.G. Longleaf Pine: Its Use, Ecology, Regeneration, Protection, Growth, and Management; United States Department of Agriculture, Forest Service: Washington, DC, USA, 1946. [Google Scholar]
- Boyer, W.D. Natural Regeneration of Longleaf Pine; United States Department of Agriculture, Forest Service, Technical Publication SA-TP3: Washington, DC, USA, 1979. [Google Scholar]
- Glitzenstein, J.S.; Streng, D.R.; Wade, D.D. Fire frequency effects on longleaf pine (Pinus palustris P. Miller) vegetation in South Carolina and northeast Florida, USA. Nat. Area. J. 2003, 23, 22–37. [Google Scholar]
- Brockway, D.G. Restoration of Longleaf Pine Ecosystems; United States Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2005; Volume 83. [Google Scholar]
- Kleinman, J.S.; Hart, J.L. Vascular flora of longleaf pine woodlands after wind disturbance and salvage harvesting in the Alabama Fall Line Hills. Castanea 2018, 83, 183–195. [Google Scholar] [CrossRef]
- Jin, S.; Moule, B.; Yu, D.; Wang, G.G. Fire survival of longleaf pine (Pinus palustris) grass stage seedlings: The role of seedling size, root collar position, and resprouting. Forests 2019, 10, 1070. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.J.; Hiers, J.K.; O’Brien, J.; Starr, G. Ecological forestry in the Southeast: Understanding the ecology of fuels. J. For. 2009, 107, 391–397. [Google Scholar]
- Dell, J.E.; Richards, L.A.; O’Brien, J.J.; Loudermilk, E.L.; Hudak, A.T.; Pokswinski, S.M.; Dyer, L.A. Overstory-derived surface fuels mediate plant species diversity in frequently burned longleaf pine forests. Ecosphere 2017, 8, e01964. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Hiers, J.K.; O’Brien, J.J.; Jack, S.B.; Engstrom, R.T. Silviculture that sustains: The nexus between silviculture, frequent prescribed fire, and conservation of biodiversity in longleaf pine forests of the southeastern United States. Can. J. For. Res. 2006, 36, 2724–2736. [Google Scholar] [CrossRef] [Green Version]
- Mugnani, M.P.; Robertson, K.M.; Miller, D.L.; Platt, W.J. Longleaf pine patch dynamics influence ground-layer vegetation in old-growth pine savanna. Forests 2019, 10, 389. [Google Scholar] [CrossRef] [Green Version]
- Beckett, S.; Golden, M.S. Forest vegetation and vascular flora of reed brake research natural area, Alabama. Castanea 1982, 47, 368–392. [Google Scholar]
- Hammond, D.H.; Varner, J.M.; Kush, J.S.; Fan, Z. Contrasting sapling bark allocation of five southeastern USA hardwood tree species in a fire prone ecosystem. Ecosphere 2015, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Klaus, N.A.; Rush, S.A.; Weitzel, S.L.; Holdrege, M.C. Changes in tree canopy, groundcover, and avian community following restoration of a Montane longleaf pine woodland. Am. Midl. Nat. 2020, 184, 163–176. [Google Scholar] [CrossRef]
- Hammond, D.H.; Varner, J.M.; Fan, Z.; Kush, J.S. Long-term stand dynamics of old-growth mountain longleaf pine (Pinus palustris) woodlands. For. Ecol. Manag. 2016, 364, 154–164. [Google Scholar] [CrossRef]
- Kressuk, J.M.; Goode, J.D.; Bhuta, A.A.; Hart, J.L.; Kleinman, J.S.; Phillips, D.L.; Willson, K.G. Composition and structure of a montane longleaf pine stand on the Alabama piedmont. Southeast. Nat. 2020, 19, 436–446. [Google Scholar] [CrossRef]
- US Department of Agriculture, Forest Service. Longleaf Ecosystem-Restoration Project: Final Environmental Impact Statement, National Forests in Alabama; Talladega National Forest, Oakmulgee District, USDA Forest Service: Brent, AL, USA, 2005; p. 15. [Google Scholar]
- Boyer, W.D.; Bledsoe, B.W. Establishment Report: Reed Brake Research Natural Area, National Forests in Alabama, Bibb County, Alabama; United States Department of Agriculture, Forest Service, Southern Forest Experiment Station: Asheville, NC, USA, 1975. [Google Scholar]
- Shankman, D.; Hart, J.L. The Fall Line: A Physiographic-Forest Vegetation Boundary. Geogr. Rev. 2007, 97, 502–519. [Google Scholar] [CrossRef]
- Cox, L.E.; Hart, J.L. Two centuries of forest compositional and structural changes in the Alabama Fall Line Hills. Am. Midl. Nat. 2015, 174, 218–237. [Google Scholar] [CrossRef]
- Kleinman, J.S.; Hart, J.L. Response by vertical strata to catastrophic wind in restored Pinus palustris stands. J. Torrey Bot. Soc. 2017, 144, 423–438. [Google Scholar] [CrossRef]
- Kleinman, J.S.; Ford, S.A.; Hart, J.L. Catastrophic wind and salvage harvesting effects on woodland plants. For. Ecol. Manag. 2017, 403, 112–125. [Google Scholar] [CrossRef]
- Harper, R.M. Forests of Alabama; Geological Survey of Alabama, Monograph 10; Wetumpka Printing Company: Wetumpka, AL, USA, 1943. [Google Scholar]
- Thornthwaite, C.W. An approach toward a rational classification of climate. Geogr. Rev. 1948, 38, 55–94. [Google Scholar] [CrossRef]
- PRISM Climate Group. Northwest Alliance for Computational Science and Engineering. Available online: http://www.prism.oregonstate.edu/ (accessed on 1 February 2022).
- USDA NRCS (United States Department of Agriculture, Natural Resources Conservation Service). Web Soil Survey. Available online: https://websoilsurvey.nrcs.usda.gov/ (accessed on 1 March 2022).
- Anderson, E.W. A guide for estimating cover. Rangelands 1986, 8, 236–238. [Google Scholar]
- Kleinman, J.S.; Goode, J.D.; Hart, J.L.; Dey, D.C. Prescribed fire effects on Pinus palustris woodland development after catastrophic wind disturbance and salvage logging. For. Ecol. Manag. 2020, 468, 118173. [Google Scholar] [CrossRef]
- Runkle, J.R. Guidelines and Sample Protocol for Sampling Forest Gaps; United States Department of Agriculture, Forest Service, Pacific Northwest Research Station: Asheville, NC, USA, 1992; Volume 283. [Google Scholar]
- Runkle, J.R. Patterns of disturbance in some old-growth mesic forests of eastern North America. Ecology 1982, 63, 1533–1546. [Google Scholar] [CrossRef] [Green Version]
- Ulyshen, M.D.; Horn, S.; Pokswinski, S.; McHugh, J.V.; Hiers, J.K. A comparison of coarse woody debris volume and variety between old-growth and secondary longleaf pine forests in the southeastern United States. For. Ecol. Manag. 2018, 429, 124–132. [Google Scholar] [CrossRef]
- Taylor, S.O.; Lorimer, C.G. Loss of oak dominance in dry-mesic deciduous forests predicted by gap capture methods. J. Plant. Ecol. 2003, 167, 71–88. [Google Scholar] [CrossRef]
- Hart, J.L.; Grissino-Mayer, H.D. Gap-scale disturbance processes in secondary hardwood stands on the Cumberland Plateau, Tennessee, USA. J. Plant. Ecol. 2009, 201, 131–146. [Google Scholar] [CrossRef]
- Richards, J.D.; Hart, J.L. Canopy gap dynamics and development patterns in secondary Quercus stands on the Cumberland Plateau, Alabama, USA. For. Ecol. Manag. 2011, 262, 2229–2239. [Google Scholar] [CrossRef]
- Weber, T.A.; Hart, J.L.; Schweitzer, C.J.; Dey, D.C. Influence of gap-scale disturbance on developmental and successional pathways in Quercus-Pinus stands. For. Ecol. Manag. 2014, 331, 60–70. [Google Scholar] [CrossRef]
- Barden, L.S. Tree replacement in small canopy gaps of a Tsuga canadensis forest in the southern Appalachians, Tennessee. Oecologia 1979, 44, 141–142. [Google Scholar] [CrossRef]
- Barden, L.S. Tree replacement in a cove hardwood forest of the southern Appalachians. Oikos 1980, 35, 16–19. [Google Scholar] [CrossRef]
- White, P.S.; MacKenzie, M.D.; Busing, R.T. Natural disturbance and gap-phase dynamics in southern Appalachian spruce-fir forests. Can. J. For. Res. 1985, 15, 233–240. [Google Scholar] [CrossRef]
- Yamamoto, S.; Nishimura, N. Canopy gap formation and replacement pattern of major tree species among developmental stages of beech (Fagus crenata) stands, Japan. J. Plant. Ecol. 1999, 140, 167–176. [Google Scholar] [CrossRef]
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; University of Arizona Press: Tucson, AZ, USA, 1996. [Google Scholar]
- Villalba, R.; Veblen, T.T. Improving estimates of total tree ages based on increment core samples. Ecoscience 1997, 4, 534–542. [Google Scholar] [CrossRef]
- Rubino, D.L.; McCarthyz, B.C. Comparative analysis of dendroecological methods used to assess disturbance events. Dendrochronologia 2004, 21, 97–115. [Google Scholar] [CrossRef]
- Hart, J.L.; van de Gevel, S.L.; Grissino-Mayer, H.D. Forest dynamics in a natural area of the southern Ridge and Valley, Tennessee. Nat. Areas J. 2008, 28, 275–289. [Google Scholar] [CrossRef] [Green Version]
- Beland, M.; Parker, G.; Sparrow, B.; Harding, D.; Chasmer, L.; Phinn, S.; Antonarakis, A.; Strahler, A. On promoting the use of lidar systems in forest ecosystem research. For. Ecol. Manag. 2019, 450, 117484. [Google Scholar] [CrossRef]
- NEON (National Ecological Observatory Network). Elevation-LiDAR, Release-2022 (DP3.30024.001). Available online: https://data.neonscience.org/data-products/DP3.30024.001 (accessed on 15 November 2021).
- NEON. Data Product. Available online: https://data.neonscience.org/data-products/explore (accessed on 15 November 2021).
- Silva, C.A.; Valbuena, R.; Pinagé, E.R.; Mohan, M.; de Almeida, D.R.; Broadbent, E.; Jaafar, W.S.W.M.; Papa, D.A.; Cardil, A.; Klauberg, C. ForestGapR: An r Package for forest gap analysis from canopy height models. Methods. Ecol. Evol. 2019, 10, 1347–1356. [Google Scholar] [CrossRef] [Green Version]
- Koukoulas, S.; Blackburn, G.A. Quantifying the spatial properties of forest canopy gaps using LiDAR imagery and GIS. Int. J. Remote. Sens. 2004, 25, 3049–3072. [Google Scholar] [CrossRef]
- Wiegand, T.; Kissling, W.D.; Cipriotti, P.A.; Aguiar, M.R. Extending point pattern analysis for objects of finite size and irregular shape. J. Ecol. 2006, 94, 825–837. [Google Scholar] [CrossRef]
- Wiegand, T.; Moloney, K.A. Handbook of Spatial Point Pattern Analysis in Ecology; Chapman and Hall/CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Rutledge, B.T.; Cannon, J.B.; McIntyre, R.K.; Holland, A.M.; Jack, S.B. Tree, stand, and landscape factors contributing to hurricane damage in a Coastal Plain forest: Post-hurricane assessment in a longleaf pine landscape. For. Ecol. Manag. 2021, 481, 118724. [Google Scholar] [CrossRef]
- Johnsen, K.H.; Butnor, J.R.; Kush, J.S.; Schmidtling, R.C.; Nelson, C.D. Hurricane Katrina winds damaged longleaf pine less than loblolly pine. South. J. Appl. For. 2009, 33, 178–181. [Google Scholar] [CrossRef] [Green Version]
- Landers, J.L.; Boyer, W.D. An Old-Growth Definition for Upland Longleaf and South Florida Slash Pine forests, Woodlands, and Savannas; United States Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 1999; Volume 29. [Google Scholar]
- Blanc, L.A.; Walters, J.R. Cavity-nesting community webs as predictive tools: Where do we go from here? J. Ornithol. 2007, 148, 417–423. [Google Scholar] [CrossRef]
- Outcalt, K.W. Lightning, fire and longleaf pine: Using natural disturbance to guide management. For. Ecol. Manag. 2008, 255, 3351–3359. [Google Scholar] [CrossRef] [Green Version]
- Runkle, J.R. Changes in southern Appalachian canopy tree gaps sampled thrice. Ecology 1998, 79, 1768–1780. [Google Scholar] [CrossRef]
- Himes, J.M.; Rentch, J.S. Canopy gap dynamics in a second-growth Appalachian hardwood forest in West Virginia. Castanea 2013, 78, 171–184. [Google Scholar] [CrossRef]
- Emery, R.K.; Kleinman, J.S.; Goode, J.D.; Hart, J.L. Effects of catastrophic wind disturbance, salvage logging, and prescribed fire on fuel loading and composition in a Pinus palustris woodland. For. Ecol. Manag. 2020, 478, 118515. [Google Scholar] [CrossRef]
- Canham, C.D. Suppression and release during canopy recruitment in Fagus grandifolia. Bull. Torrey Bot. Club. 1990, 117, 1–7. [Google Scholar] [CrossRef]
- De Lima, R.A.F.; Prado, P.I.; Martini, A.M.Z.; Fonseca, L.J.; Gandolfi, S.; Rodrigues, R.R. Improving methods in gap ecology: Revisiting size and shape distributions using a model selection approach. J. Veg. Sci. 2013, 24, 484–495. [Google Scholar] [CrossRef]
- Bigelow, S.W.; Whelan, A.W. Longleaf pine proximity effects on air temperatures and hardwood top-kill from prescribed fire. Fire. Ecol. 2019, 15, 1–14. [Google Scholar] [CrossRef]
- Outcalt, K.W.; Brockway, D.G. Structure and composition changes following restoration treatments of longleaf pine forests on the Gulf Coastal Plain of Alabama. For. Ecol. Manag. 2010, 259, 1615–1623. [Google Scholar] [CrossRef]
- Pedersen, B.S.; Howard, J.L. The influence of canopy gaps on overstory tree and forest growth rates in a mature mixed-age, mixed-species forest. For. Ecol. Manag. 2004, 196, 351–366. [Google Scholar] [CrossRef]
- Lertzman, K.P. Patterns of gap-phase replacement in a subalpine, old-growth forest. Ecology 1992, 73, 657–669. [Google Scholar] [CrossRef]
- Hobi, M.L.; Ginzler, C.; Commarmot, B.; Bugmann, H. Gap pattern of the largest primeval beech forest of Europe revealed by remote sensing. Ecosphere 2015, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Feldmann, E.; Drößler, L.; Hauck, M.; Kucbel, S.; Pichler, V.; Leuschner, C. Canopy gap dynamics and tree understory release in a virgin beech forest, Slovakian Carpathians. For. Ecol. Manag. 2018, 415, 38–46. [Google Scholar] [CrossRef]
- Goodbody, T.R.; Tompalski, P.; Coops, N.C.; White, J.C.; Wulder, M.A.; Sanelli, M. Uncovering spatial and ecological variability in gap size frequency distributions in the Canadian boreal forest. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Phillips, D.L.; Goode, J.D.; Hart, J.L. Spatial patterns of stand structure and canopy disturbance in a fire-maintained Pinus palustris woodland. Appl. Veg. Sci. 2022, 25, e12657. [Google Scholar] [CrossRef]
- Woods, K.D. Intermediate disturbance in a late-successional hemlock-northern hardwood forest. J. Ecol. 2004, 92, 464–476. [Google Scholar] [CrossRef]
- Worrall, J.J.; Lee, T.D.; Harrington, T.C. Forest dynamics and agents that initiate and expand canopy gaps in Picea–Abies forests of Crawford Notch, New Hampshire, USA. J. Ecol. 2005, 93, 178–190. [Google Scholar] [CrossRef]
- Curzon, M.T.; Keeton, W.S. Spatial characteristics of canopy disturbances in riparian old-growth hemlock–northern hardwood forests, Adirondack Mountains, New York, USA. Can. J. For. Res. 2010, 40, 13–25. [Google Scholar] [CrossRef]
- Koukoulas, S.; Blackburn, G.A. Spatial relationships between tree species and gap characteristics in broad-leaved deciduous woodland. J. Veg. Sci. 2005, 16, 587–596. [Google Scholar] [CrossRef]


| Canopy Gaps | Reference Plots | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Density/ha | Rel. den. | Dom. (m2/ha) | Rel. dom. | Rel. imp. | Density/ha | Rel. den. | Dom. (m2/ha) | Rel. dom. | Rel. imp. |
| Pinus palustris Mill. | 32.80 | 93.03 | 3.65 | 96.03 | 94.53 | 387.00 | 94.62 | 31.90 | 98.17 | 96.39 |
| Quercus falcata Michx. | 0.43 | 1.21 | 0.05 | 1.41 | 1.31 | 1.00 | 0.24 | 0.01 | 0.03 | 0.14 |
| Quercus marilandica Münchh. | 0.53 | 1.52 | 0.03 | 0.73 | 1.12 | 3.00 | 0.73 | 0.09 | 0.29 | 0.51 |
| Pinus taeda L. | 0.43 | 1.21 | 0.03 | 0.74 | 0.98 | 5.00 | 1.22 | 0.21 | 0.63 | 0.93 |
| Quercus stellata Wangenh. | 0.43 | 1.21 | 0.02 | 0.42 | 0.81 | 4.00 | 0.98 | 0.12 | 0.37 | 0.67 |
| Carya tomentosa (Lam.) Nutt. | 0.21 | 0.61 | 0.00 | 0.11 | 0.36 | 1.00 | 0.24 | 0.02 | 0.08 | 0.16 |
| Nyssa sylvatica Marshall. | 0.21 | 0.61 | 0.00 | 0.05 | 0.33 | - | - | - | - | - |
| Oxydendrum arboreum (L.) DC. | 0.11 | 0.30 | 0.01 | 0.27 | 0.29 | 2.00 | 0.49 | 0.02 | 0.06 | 0.27 |
| Quercus alba L. | 0.11 | 0.30 | 0.01 | 0.24 | 0.27 | - | - | - | - | - |
| Quercus laevis Walter | - | - | - | - | - | 4.00 | 0.98 | 0.03 | 0.08 | 0.53 |
| Pinus Virginiana Mill. | - | - | - | - | - | 2.00 | 0.49 | 0.10 | 0.30 | 0.40 |
| Total | 35.25 | 100 | 3.80 | 100 | 100 | 409 | 100 | 32.50 | 100 | 100 |
| Canopy Gaps | Reference Plots | |||
|---|---|---|---|---|
| Species | Density/ha | Rel. den. | Density/ha | Rel. den. |
| Vaccinium arboreum Marshall | 119 | 59.81 | 823 | 58 |
| Liquidambar styraciflua L. | 18 | 8.84 | 62 | 4.37 |
| Oxydendrum arboreum (L.) DC. | 16 | 8.3 | 305 | 21.49 |
| Symplocos tinctoria (L.) L’Hér. | 16 | 7.92 | 33 | 2.33 |
| Rhus copallinum L. | 14 | 6.9 | 18 | 1.27 |
| Diospyros virginiana L. | 5 | 2.69 | - | - |
| Acer rubrum L. | 2 | 1.24 | 40 | 2.82 |
| Pinus palustris Mill. | 2 | 1.02 | 50 | 3.52 |
| Quercus marilandica Münchh. | 1 | 0.7 | - | - |
| Ditrysinia fruticosa (W. Bartram) Govaerts & Frodin | 1 | 0.54 | - | - |
| Nyssa sylvatica Marshall. | 1 | 0.48 | 67 | 4.72 |
| Vaccinium stamineum L. | 1 | 0.27 | 3 | 0.21 |
| Cornus florida L. | 1 | 0.22 | 1 | 0.07 |
| Quercus falcata Michx. | 1 | 0.22 | 1 | 0.07 |
| Carya tomentosa (Lam.) Nutt. | 0 | 0.16 | - | - |
| Quercus laevis Walter | 0 | 0.16 | 8 | 0.56 |
| Quercus alba L. | 0 | 0.11 | - | - |
| Quercus nigra L. | 0 | 0.11 | - | - |
| Quercus margaretta (Ashe) Small | 0 | 0.11 | - | - |
| Amelanchier arborea (Michx. f.) Fernald | 0 | 0.05 | - | - |
| Callicarpa americana L. | 0 | 0.05 | 2 | 0.14 |
| Magnolia virginiana L. | 0 | 0.05 | - | - |
| Quercus coccinea Münchh. | 0 | 0.05 | - | - |
| Pinus taeda L. | - | - | 4 | 0.28 |
| Quercus hemisphaerica W. Bartram ex Willd. | - | - | 1 | 0.07 |
| Quercus stellata Wangenh. | - | - | 1 | 0.07 |
| Total | 198 | 100 | 1419 | 100 |
| Canopy Gaps | Reference Plots | |||
|---|---|---|---|---|
| Species | Density/ha | Rel. den. | Density/ha | Rel. den. |
| Vaccinium arboreum Marshall | 3183 | 61.84 | 2550 | 49.8 |
| Pinus palustris Mill. | 1098 | 21.33 | 1095 | 21.39 |
| Pinus taeda L. | 409 | 7.94 | 335 | 6.54 |
| Symplocos tinctoria (L.) L’Hér. | 104 | 2.03 | 30 | 0.59 |
| Rhus copallinum L. | 74 | 1.45 | 10 | 0.2 |
| Diospyros virginiana L. | 55 | 1.07 | - | - |
| Liquidambar styraciflua L. | 49 | 0.95 | - | - |
| Quercus falcata Michx. | 34 | 0.66 | 15 | 0.29 |
| Nyssa sylvatica Marshall. | 21 | 0.41 | 10 | 0.2 |
| Vaccinium stamineum L. | 19 | 0.37 | 10 | 0.2 |
| Quercus coccinea Münchh. | 17 | 0.33 | 5 | 0.1 |
| Quercus stellata Wangenh. | 17 | 0.33 | - | - |
| Carya glabra (Mill.) Sweet | 13 | 0.25 | - | - |
| Oxydendrum arboreum (L.) DC. | 13 | 0.25 | 20 | 0.39 |
| Quercus nigra L. | 13 | 0.25 | - | - |
| Acer rubrum L. | 11 | 0.21 | 5 | 0.1 |
| Quercus alba L. | 9 | 0.17 | - | - |
| Quercus marilandica Münchh. | 4 | 0.08 | 25 | 0.49 |
| Carya tomentosa (Lam.) Nutt. | 2 | 0.04 | 5 | 0.1 |
| Quercus laevis Walter | 2 | 0.04 | 20 | 0.39 |
| Gaylussacia dumosa (Andrews) Torr. & A. Gray | - | - | 965 | 18.85 |
| Asimina triloba (L.) Dunal | - | - | 10 | 0.2 |
| Ilex opaca Aiton | - | - | 10 | 0.2 |
| Total | 5147 | 100 | 5120 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller, H.L.; Goode, J.D.; Hart, J.L. Gap-Scale Disturbance Patterns and Processes in a Montane Pinus palustris Woodland. Forests 2022, 13, 1169. https://doi.org/10.3390/f13081169
Mueller HL, Goode JD, Hart JL. Gap-Scale Disturbance Patterns and Processes in a Montane Pinus palustris Woodland. Forests. 2022; 13(8):1169. https://doi.org/10.3390/f13081169
Chicago/Turabian StyleMueller, Helena L., J. Davis Goode, and Justin L. Hart. 2022. "Gap-Scale Disturbance Patterns and Processes in a Montane Pinus palustris Woodland" Forests 13, no. 8: 1169. https://doi.org/10.3390/f13081169
APA StyleMueller, H. L., Goode, J. D., & Hart, J. L. (2022). Gap-Scale Disturbance Patterns and Processes in a Montane Pinus palustris Woodland. Forests, 13(8), 1169. https://doi.org/10.3390/f13081169







