Abstract
There are various studies on annual ring structural variations in plants grown in the field under varying meteorological statistics. However, related experimental approach is limited, hitherto. In this study, complete artificial conditions with growth chambers were adopted to evaluate the influence of day length and temperature on intra-ring structure formation. The basic artificial growing conditions have been previously reported as “shortened annual cycle system”, which consisted of the following three stages mimicking seasons approximately: Stage 1, spring/summer; Stage 2, autumn; and Stage 3, winter. This system shortens an annual cycle of Populus alba to 5 months. In this study, Stage 2 was modified in two ways: one was a condition in which the temperature was fixed and the day length was gradually shortened, and the other was a condition with a fixed day length and gradually lowered temperature. In the former condition, the cell wall of fibers thickened from the middle of the ring, and the vessel diameter became smaller from the same position. The wood in the latter condition appeared more natural in terms of wall thickness and vessel shape; however, the thickness of the wall reduced in the starting position of Stage 2. It may have been caused by the shortage of material for cell production under a high temperature but a short day length.
1. Introduction
Trees usually form growth rings in the wood. Growth rings are recognized by different tissue structures synchronized according to the seasons. A typical growth ring is an annual ring formed in the temperate regions. The tissue structure variation with seasons is considered to be due to a combination of genetic factors and climatic environment. In dendrochronology, dendroclimatology, and similar fields, the ring width was first observed as a proxy of climate variations [1]; then, studies focused on intra-ring tissue fluctuations as a more detailed indicator of climate change, for example [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. The relationship between intra-ring structure and climate variations has been shown in not only gymnosperms [4,5] (in which the wood structure is relatively simple) but also angiosperms [6,7]. Such studies are useful to predict the relationship between wood tissue and climate variations. In these studies, data on environmental factors such as temperature, day length, and precipitation were obtained primarily from meteorological statistics. Although an accurate record of events that occurred can be retrieved from these statistics, the meteorological datasets are inseparable from each other (for example, summer has long days and high temperatures, and rainy days have precipitation and lower temperature and light intensity than sunny days). Undeniably, some aspects must eventually be speculated (e.g., how individual parameters affect xylem formation) because it is not always possible to anticipate their presence and effect, and exact prevailing conditions cannot be reexamined. To overcome these limitations, several experiments with varying approaches have been performed, such as placing a heater on the stem for partial heating [18,19,20] and wrapping a cooling pipe around a part of a stem to lower its temperature [20,21]. In these semi-artificial heating and cooling experiments, conditions other than the temperature were natural (the temperature conditions were also natural before the start of the experiments). However, under completely artificial environment in a laboratory, the conditions that a tree is exposed to can be designed and regulated. Research on forest trees grown under field conditions reflects the original growth of trees, but by controlling environmental conditions in the laboratory, novel findings and proposals for some hypotheses could be obtained.
It requires 1 year for a tree to form an annual ring with the usual xylem formation schedule. When conducting experiments under different artificial weather conditions on the same tree, it takes as many years as the different conditions, and the experiment time is prolonged. We established a shortened annual cycle system using Populus alba under completely artificial conditions using growth chambers [22]. In the shortened annual cycle system, we mimicked leaf color change, defoliation, dormancy, bud breaking, and growth within a period of 5 months, and the cycle can repeat several times. Populus species are used as model woody plants [23]. The wood of P. alba grown under natural conditions is diffuse-porous with a slight gradual decrease in vessel size and a slight gradual increase in fiber wall thickness [24]. When grown in this shortened annual cycle system for multiple cycles, saplings formed multi-branched architecture naturally [25] and growth rings with boundary structures similar to those in field-grown trees [24]. In this study, we used this shortened annual cycle system and studied intra-ring variations in wood tissue structure formed under the combination of different temperatures and day lengths by fixing one parameter and changing the other gradually. This study might clarify the effects of temperature and photoperiod individually on the xylem structure.
2. Materials and Methods
2.1. Plant Material
Sapling cuttings of Populus alba L. were prepared as previously reported [24]. Briefly, cuttings of length 3–5 cm were placed in pots with soil and grown in a growth chamber. They were placed on trays containing water with fertilizer to a depth of 5–20 mm. The saplings, which were the ramets of the same clone (grown to 8–11 cm), were placed in Stage 2 of the shortened annual cycle system (described below) and allowed to grow under this system. Four individuals were placed under each condition.
2.2. Shortened Annual Cycle System and Its Modification
The original culture conditions of the shortened annual cycle system are shown in Table 1 [24]. This system consists of the following three stages: Stage 1, long day and high temperature and carried out; Stage 2, short day and moderate temperature; and Stage 3, short day and low temperature. Stages 1 and 2 were set for 1 month each, and Stage 3 was set for 3 months. Both Stages 2 and 3 were set within plant growth cabinets (LH-241S and LH-410PFD-SP, respectively; NK System, Osaka, Japan). These stages mimic spring/summer, autumn, and winter, respectively.

Table 1.
Culture conditions.
The conditions in Stage 2 were modified in two ways as shown in Table 1. One modification involved the temperature; it was decreased by 2 °C, each time, twice a week, every 3 or 4 days (from 23 to 5 °C) (T-grad in Table 1). It had nine steps and lasted 4.5 weeks. The other modification involved day length; it was decreased by 40 min each time, twice a week, every 3 or 4 days (from 14 to 8 h). It also had nine steps and lasted 4.5 weeks (D-grad in Table 1). In both modified culture systems, the conditions of Stages 1 and 3 were exactly same as those of the original shortened annual cycle system: fixed temperature and day length.
The saplings, after exposure to the first cycle of the original annual cycle system, were placed under T-grad or D-grad conditions for one cycle. Thereafter, their culture conditions were exchanged; that is, the saplings grown under D-grad were then placed under T-grad, and vice versa. They were grown in three cycles.
2.3. Microscopy and Measurement
After culturing three cycles of the shortened system described above, stem samples of approximately 2 cm were cut and preserved in FAA fixative (5% formalin, 5% acetic acid, and 40% ethanol) until further analyses. Transverse stem sections of 25-μm thickness were prepared using a sliding microtome and stained with 1% safranin aqueous solution. After dehydrating the sections with an ethanol series, the sections were soaked in xylene twice before mounting on glass slides with Biolite (Okenshoji, Tokyo, Japan). These sections were observed under a light microscope (BX50; Olympus, Tokyo, Japan) and digital micrographs were obtained using a camera (DP73; Olympus) and its software (Cell Sens; Olympus).
Three sizes were measured here: the cell diameter, wall thickness, and their distance from the beginning of the ring. They were measured manually avoiding tension wood from the micrographs of an individual for each condition using ImageJ 1.53i (NIH, Stapleton, NY, USA). Resulting scatter plots were prepared on Excel. The relative position in annual ring was calculated by dividing the distance from the beginning of the ring by the width of the annual ring.
2.4. Determination of the Starting Position of Stages 2 and 3
The knife marking method [26] was adopted to determine the starting position of Stages 2 and 3. The saplings that completed one cycle of the original shortened annual culture were used. The stems were injured using a small razor blade (2–3 mm in length) instead of a knife when they were transferred to Stages 2 and 3 because the samples were small saplings. The blade along a stem axis was gently pushed from the outside into the xylem. After Stage 3 culture, the stem samples were treated and observed in the same manner as described in Section 2.3. The starting position of each stage was determined by the shape of the wounded tissue, according to Kuroda’s observation [27].
3. Results and Discussion
Here, Populus saplings were grown under artificial environment using growth chambers, with a shortened annual cycle of 5 months. We adopted two different conditions in transitional Stage 2 of the shortened annual cycle system (Table 1, T-grad and D-grad). The “T-grad” condition involves a gradual decrease in temperature with a fixed day length. The “D-grad” condition involves a gradual decrease in day length with a fixed temperature. One group of saplings was grown in the “D-grad” cycle after the “T-grad” cycle, and the other group was grown vice versa. The wood structures with rings of plants grown in “D-grad” after “T-grad” and those grown in “T-grad” after “D-grad” are shown in Figure 1. The area consisting of fibers with a thicker wall (after the arrows in Figure 1) under D-grad was wider than that under T-grad regardless of the growing order of D-grad and T-grad.
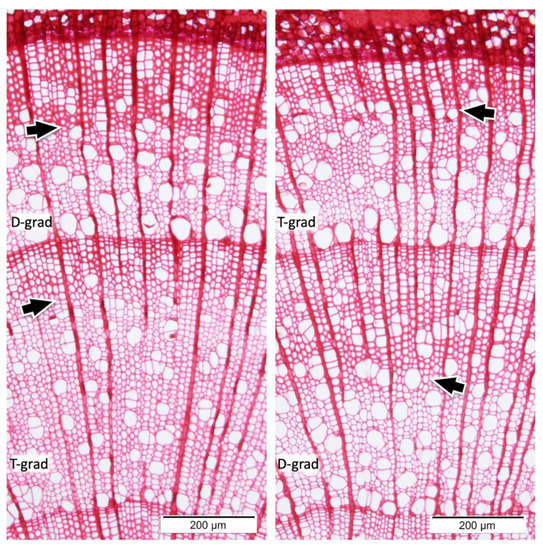
Figure 1.
Transverse micrographs of the stems of Populus alba L. grown under the artificial shortened annual cycle with two modifications in Stage 2. “T-grad” shows a growth ring formed under the condition in which the temperature was gradually decreased with a fixed day length in Stage 2. “D-grad” shows a growth ring formed under the condition in which the day length gradually decreased with a fixed temperature in Stage 2. Arrows show the changing line of red intensity, the later tissue of the arrow’s colors deeper than the earlier.
Double wall thickness of the fibers was measured and plotted with the relative position within the ring (Figure 2). A regression line has been drawn for all data (Figure 2, full span) and a regression line has been drawn for a part near the latter half of the center of the annual ring (Figure 2, 0.4–0.7). As an overall trend, the fiber wall in both T-grad and D-grad gradually thickened from the beginning to final ends of the ring, where the inclination was higher in D-grad (coefficient = 4.6729) than in T-grad (coefficient = 2.9038) as shown in Figure 2 (full span). However, in detail, the wall thickness in T-grad reduced once between 0.4 and 0.7 relative positions of annual ring with a negative regression line (Figure 2, 0.4–0.7: green marker and line), whereas that in D-grad linearly thickened in the same area with a higher coefficient of inclination than that for full span of the annual ring. Considering the micrographs (Figure 1) and the scatter plot (Figure 2), the border for the deep red appearance (Figure 1, arrows) would be approximately 3.0 μm higher than the range of the double wall. In T-grad, the thick wall area (Figure 1, after the arrows) would be more than 3.7 μm double-wall thickness (Figure 2, T-grad), but in D-grad, the thick wall area (Figure 1, after the arrows) would not consist of fibers less than approximately 3 μm double-wall thickness (Figure 2, D-grad).
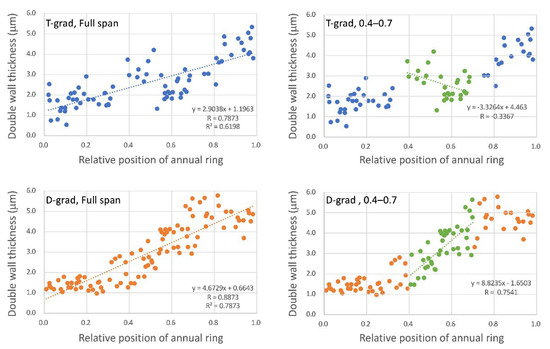
Figure 2.
Double-wall thickness of fibers at different distances from the ring boundary. Relative position of annual ring on the horizontal axis; 0 and 1 indicate the start and end of the annual ring, respectively. “T-grad” shows a growth ring formed under the condition in which the temperature gradually decreased with a fixed day length in Stage 2. “D-grad” shows a growth ring formed under the condition in which the day length gradually decreased with a fixed temperature in Stage 2. “Full span” means a regression line drawn using all measurement points. Similarly, “0.4–0.7” means a regression line drawn using measurement points between approximately 0.4 and 0.7 relative positions (green symbols). The equation shows each regression line, R = correlation coefficient, R2 = coefficient of determination.
In order to determine the relationship between the stages of the shorten system and the valley of wall thickness in T-grad or deep red area in D-grad, a knife mark was made at the stage transfer moment (Figure 3). The arrows in Figure 3 show cambial position at stage 2 transition by the shape of the wound tissue according to Kuroda [27]. In T-grad wood, the wall (depicted after the arrow) was once thinner than that before the arrow and became thicker toward the end of the ring. In contrast, the wall after the arrow was thicker than that observed earlier in D-grad wood. These results suggest that both valley of wall thickness in T-grad and deep red area in D-grad occurred just after the transition to Stage 2. Regarding Stage 3, no cells were newly produced just after the stage transition because there was no wound tissue within the grooves by cutting arrowheads, neither in T-grad nor in D-grad (Figure 3, Stage 3). They were injured on the day of transition to Stage 3. The valley of cell wall thickness observed under T-grad condition might have been caused by a shortage of material for cell production. The condition of T-grad during the first half of Stage 2 comprised a high temperature but a suddenly shortened day. Cell division would be maintained at a relatively high rate because of the high temperature [28]; however, cell wall components would be produced at a small amount by photosynthesis because of the sudden shortening of day in the beginning of Stage 2 of T-grad condition.
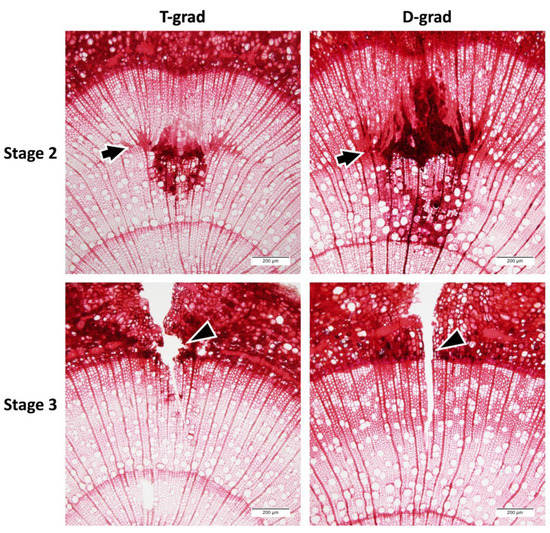
Figure 3.
Transverse micrographs of the stems of Populus alba L. injured using a razor blade to determine the cambial position at the stage transition. The column “T-grad” shows a growth ring formed under the condition in which the temperature gradually decreased with a fixed day length in Stage 2. The column “D-grad” shows a growth ring formed under the condition in which the day length gradually decreased with a fixed temperature in Stage 2. The row “Stage 2” shows what is marked at Stage 2 transition. The row “Stage 3” shows what is marked at Stage 3 transition. Arrows show the estimated cambium position at Stage 2 transition. Arrowheads show the knife groove filled without any injured tissue.
Regarding the vessels in Figure 1, small and nearly square vessels occupied a radially wider area just before the end of the ring in D-grad than those in T-grad. Subsequently, the vessel size (radial and tangential diameters) was measured and plotted with the relative position within the ring (Figure 4). Although it was difficult to find the difference between T-grad and D-grad by comparing only the scatter plots, regression lines might provide some explanation of the difference between them. The coefficients of the lines of D-grad were −19.94 for radial and −24.488 for tangential. Both were larger than those for T-grad. This finding showed that the vessel size in T-glad slightly decreased during ring growth. In D-grad, the slope of tangential was steeper than that of radial. On the contrary, the coefficients of the inclination for T-grad were in the latter half of the −14 range and were similar. This observation indicates that the size of T-grad vessel changes in the radial and tangential directions proceed in the same way. This may explain that the shape of T-grad vessel looks round until near the end of ring boundary.
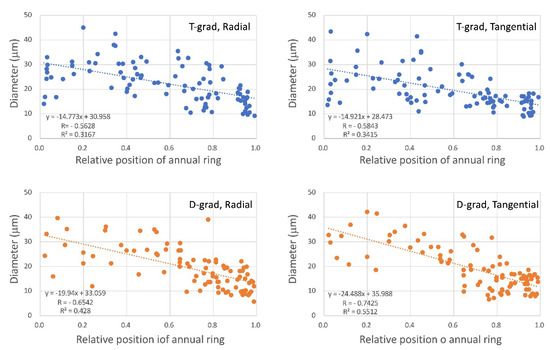
Figure 4.
Vessel diameters at different distances from the ring boundary. Relative position in the annual ring on the horizontal axis; 0 and 1 indicate the start and end of the annual ring, respectively. “T-grad” shows a growth ring formed under the condition in which the temperature gradually decreased with a fixed day length in Stage 2. “D-grad” shows a growth ring formed under the condition in which the day length gradually decreased with a fixed temperature in Stage 2. “Radial” shows the radial diameter and “Tangential” shows the tangential diameter. The equation shows each regression line, R = correlation coefficient, R2 = coefficient of determination.
The wood structure in the original shortened annual cycle system (both temperature and day length were fixed) were just in the middle of D-grad and T-grad in terms of the position where cell wall thickened or vessel size decreased [24]. The most effective conditions for the fluctuation in each structural element within the annual ring, including, temperature, day length, precipitation, and genetic control, will be revealed in the future by developing conditions which the temperature, day length, and other factors are not linked. In our previous research, using this shortened annual cycle system, we demonstrated several phenomena related to the annual cycle. Dormancy affected the development of bast fibers and their cluster structure in P. alba [29]. Phosphate re-translocation from the leaves to stem during autumn to winter transition [30] and storage form in parenchyma cells as globoidal form [31] have been shown. These phenomena were similar to those observed in the field-grown poplar trees. These studies could be conducted under artificial conditions.
4. Conclusions
In this study, we experimentally demonstrated the variations in tissue structure within annual rings at different temperatures and photoperiods individually under completely artificial conditions on Populus alba. The wood formed under the same conditions showed similar characteristics regardless of their culture order. The ring formed under the condition in which the day length gradually reduced showed a wider thick wall region in the latter half of the ring. In the ring formed under the condition in which the temperature gradually reduced, the wall thickness reduced once in the middle area and thickened walled area was small. These results indicate that this shortened annual cycle system has high reproducibility with respect to xylem structure variations. In future research, the reason of intra-ring variations could be experimentally confirmed using such a fully artificial repeatable annual cycle system customized for target species.
Author Contributions
Conceptualization, T.M.; data curation, K.B.; funding acquisition, K.B.; investigation, K.B.; methodology, Y.K.; project administration, K.B.; writing—original draft, K.B.; writing—review and editing, Y.K. and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Number JP18K05761 and the Research Institute for Sustainable Humanosphere, Kyoto University (Mission 1-1).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fritts, H.C. Tree Rings and Climate; Academic Press Inc.: London, UK, 1976. [Google Scholar]
- Fonti, P.; Garcia-Gonzalez, I. Suitability of chestnut earlywood vessel chronologies for ecological studies. New Phytol. 2004, 163, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, R. Wood anatomical features in tree-rings as indicators of environmental change. Dendrochronologia 2002, 20, 21–36. [Google Scholar] [CrossRef]
- Nabais, C.; Campelo, F.; Vieira, J.; Cherubini, P. Climatic signals of tree-ring width and intra-annual density fluctuations in Pinus pinaster and Pinus pinea along a latitudinal gradient in Portugal. Forestry 2014, 87, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Castagneri, D.; Fonti, P.; von Arx, G.; Carrer, M. How does climate influence xylem morphogenesis over the growing season? Insights from long-term intra-ring anatomy in Picea abies. Ann. Bot. 2017, 119, 1011–1020. [Google Scholar]
- Corcuera, L.; Camarero, J.J.; Gil-Pelegrin, E. Effects of a severe drought on growth and wood anatomical properties of Quercus faginea. IAWA J. 2004, 25, 185–204. [Google Scholar] [CrossRef]
- Fonti, P.; Heller, O.; Cherubini, P.; Rigling, A.; Arend, M. Wood anatomical responses of oak saplings exposed to air warming and soil drought. Plant Biol. 2013, 15, 210–219. [Google Scholar] [CrossRef]
- Bouriaud, O.; Leban, J.-M.; Bert, D.; Deleuze, C. Intra-annual variations in climate influence growth and wood density of Norway spruce. Tree Physiol. 2005, 25, 651–660. [Google Scholar] [CrossRef] [Green Version]
- Campelo, F.; Vieria, J.; Nabais, C. Tree-ring growth and intra-annual density fluctuations of Pinus pinaster responses to climate: Does size matter? Trees 2013, 27, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Carrer, M.; Unterholzner, L.; Castagneri, D. Wood anatomical traits highlight complex temperature influence on Pinus cembra at high elevation in the Eastern Alps. Int. J. Biometeorol. 2018, 62, 1745–1753. [Google Scholar] [CrossRef]
- Fonti, P.; García-González, I. Earlywood vessel size of oak as a potential proxy for spring precipitation in mesic sites. J. Biogeogr. 2008, 35, 2249–2257. [Google Scholar] [CrossRef]
- Li, Z.-H.; Labbé, N.; Driese, S.G.; Grissino-Mayer, H.D. Micro-scale analysis of tree-ring δ18O and δ13C on α-cellulose spline reveals high-resolution intra-annual climate variability and tropical cyclone activity. Chem. Geol. 2011, 284, 138–147. [Google Scholar] [CrossRef]
- Michelot, A.; Simard, S.; Rathgeber, C.; Dufrêne, E.; Damesin, C. Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol. 2012, 32, 1033–1043. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Ramírez, E.C.; Vázquez-García, J.A.; García-González, I.; Alcántara-Ayala, O.; Luna-Vega, I. Drought effects on the plasticity in vessel traits of two endemic Magnolia species in the tropical montane cloud forests of eastern Mexico. J. Plant Ecol. 2020, 13, 331–340. [Google Scholar] [CrossRef]
- Rozas, V.; García-González, I.; Zas, R. Climatic control of intra-annual wood density fluctuations of Pinus pinaster in NW Spain. Trees 2011, 25, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Fukatsu, E.; Muraoka, H.; Saitoh, T.M.; Hirano, Y.; Yasue, K. Climate responses of ring widths and radial growth phenology of Betula ermanii, Fagus crenata and Quercus crispula in a cool temperate forest in central Japan. Trees 2020, 34, 679–692. [Google Scholar] [CrossRef]
- Wilkinson, S.; Ogée, J.; Domec, J.-C.; Rayment, M.; Wingate, L. Biophysical modelling of intra-ring variations in tracheid features and wood density of Pinus pinaster trees exposed to seasonal droughts. Tree Physiol. 2015, 35, 305–318. [Google Scholar] [CrossRef] [Green Version]
- Kudo, K.; Nabeshima, E.; Begum, S.; Yamagishi, Y.; Nakaba, S.; Oribe, Y.; Yasue, K.; Funada, R. The effects of localized heating and disbudding on cambial reactivation and formation of earlywood vessels in seedlings of the deciduous ring-porous hardwood, Quercus serrata. Ann. Bot. 2014, 113, 1021–1027. [Google Scholar] [CrossRef] [Green Version]
- Oribe, Y.; Kubo, T. Effect of heat on cambial reactivation during winter dormancy in evergreen and deciduous conifers. Tree Physiol. 1997, 17, 81–87. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiol. Plant. 2013, 147, 46–54. [Google Scholar] [CrossRef]
- Begum, S.; Kudo, K.; Matsuoka, Y.; Nakaba, S.; Yamagishi, Y.; Nabeshima, E.; Rahman, M.H.; Nugroho, W.D.; Oribe, Y.; Jin, H.-O.; et al. Localized cooling of stems induces latewood formation and cambial dormancy during seasons of active cambium in conifers. Ann. Bot. 2016, 117, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Kurita, Y.; Baba, K.; Ohnishi, M.; Anegawa, A.; Shichijo, C.; Kosuge, K.; Fukaki, H.; Mimura, T. Establishment of a shortened annual cycle system; a tool for the analysis of annual re-translocation of phosphorus in the deciduous woody plant (Populus alba L.). J. Plant Res. 2014, 127, 545–551. [Google Scholar] [CrossRef]
- Chaffey, N. Why is there so little research into the cell biology of the secondary vascular system of trees? New Phytol. 2002, 153, 213–223. [Google Scholar] [CrossRef]
- Baba, K.; Kurita, Y.; Mimura, T. Wood structure of Populus alba formed in a shortened annual cycle system. J. Wood Sci. 2018, 64, 1–5. [Google Scholar] [CrossRef]
- Baba, K.; Kurita, Y.; Mimura, T. Architectural morphogenesis of poplar grown in a shortened annual cycle system. Sustain. Hum. 2017, 13, 1–4. [Google Scholar]
- Kuroda, K.; Kiyono, Y. Precise measuring of the radial growth of hinoki (Chamaecyparis obtusa) trunks with an artificial wound tissue analysis—Comparison with the dendrometer method. J. Jpn. For. Sci. 1996, 78, 183–189. [Google Scholar]
- Kuroda, K.; Shimaji, K. The Pinning Method for Marking Xylem Growth in Hardwood Species. Forest Sci. 1984, 30, 548–554. [Google Scholar]
- Ben-Haj-Salah, H.; Tardieu, F. Temperature Affects Expansion Rate of Maize Leaves without Change in Spatial Distribution of Cell Length. Plant Physiol. 1995, 109, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Baba, K.; Kurita, Y.; Mimura, T. Effect of dormancy on the development of phloem fiber clusters. J. Wood Sci. 2019, 65, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Kurita, Y.; Kanno, S.; Sugita, R.; Hirose, A.; Ohnishi, M.; Tezuka, A.; Deguchi, A.; Ishizaki, K.; Fukaki, H.; Baba, K.; et al. Visualization of phosphorus re-translocation and phosphate transporter expression profiles in a shortened annual cycle system of poplar. Plant Cell Environ. 2022, 45, 1749–1764. [Google Scholar] [CrossRef]
- Kurita, Y.; Baba, K.; Ohnishi, M.; Matsubara, R.; Kosuge, K.; Anegawa, A.; Shichijo, C.; Ishizaki, K.; Kaneko, Y.; Hayashi, M.; et al. Inositol hexakis phosphate is the seasonal phosphorus reservoir in the deciduous woody plant Populus alba L. Plant Cell Physiol. 2017, 58, 1477–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).