Alpine Litter Humification and Its Response to Reduced Snow Cover: Can More Carbon Be Sequestered in Soils?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Snow Manipulation
2.3. Litterbag Experiment
2.4. Chemical Analysis
2.5. Data Analysis
3. Results
3.1. Humus
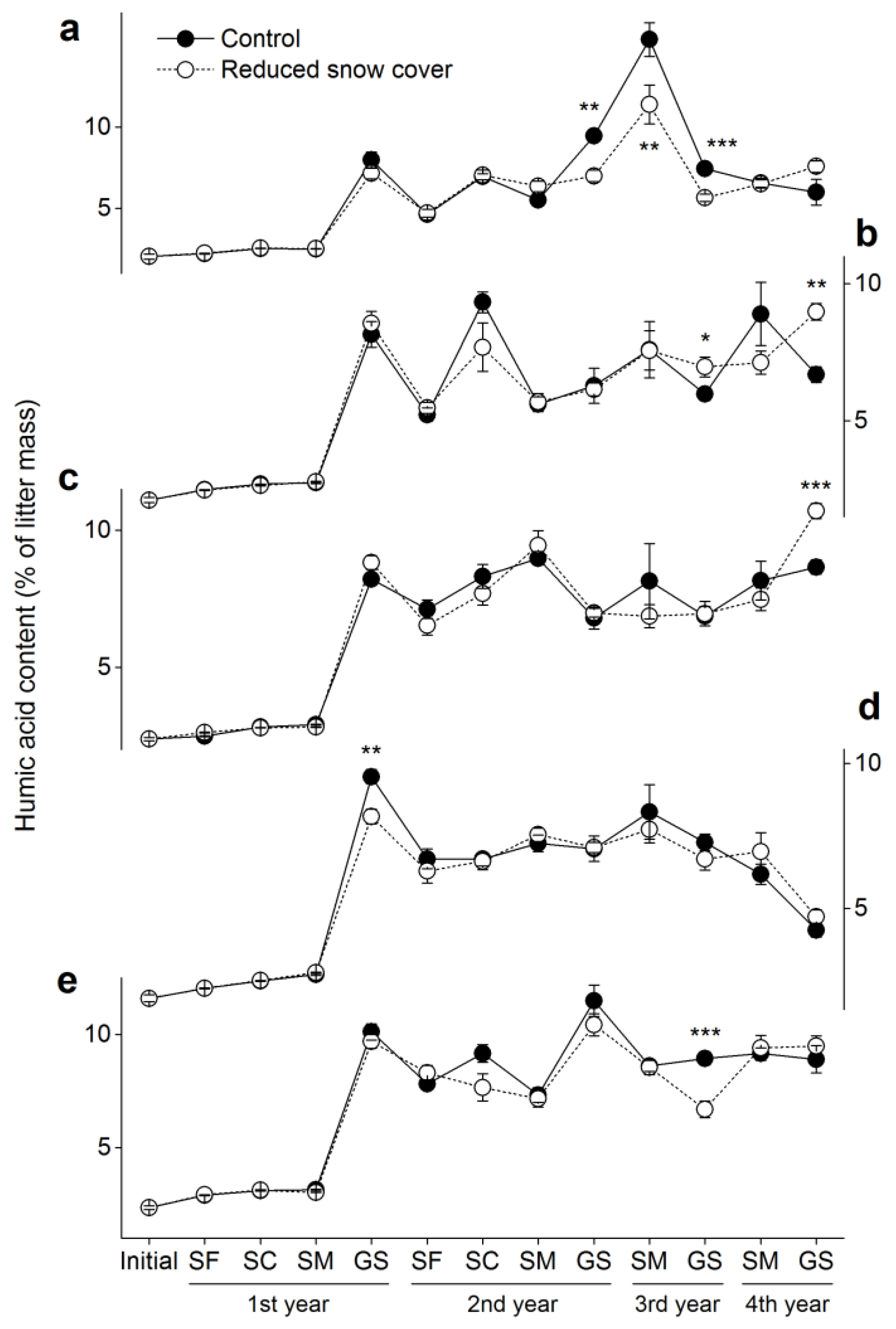
3.2. Humic Acid
3.3. Fulvic Acid
4. Discussion
4.1. Is Humus Really Formed at a Very Late Stage of Litter Decomposition?
4.2. Will Reduced Snow Cover Alter This Carbon Sequestration Process in High-Altitudinal Ecosystems?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration, 4th ed.; Springer: New York, NY, USA, 2020; pp. 13–37. [Google Scholar]
- Bailey, V.L.; Pries, C.H.; Lajtha, K. What do we know about soil carbon destabilization? Environ. Res. Lett. 2019, 14, 083004. [Google Scholar] [CrossRef]
- Tie, L.; Hu, J.; Penuelas, J.; Sardans, J.; Wei, S.; Liu, X.; Zhou, S.; Huang, C. The amounts and ratio of nitrogen and phosphorus addition drive the rate of litter decomposition in a subtropical forest. Sci. Total Environ. 2022, 833, 155163. [Google Scholar] [CrossRef]
- Moore, C.J.; Mihaylov, D.P.; Lasenby, A.; Gilmore, G. Astrometric search method for individually resolvable gravitational wave sources with Gaia. Phys. Rev. Lett. 2017, 119, 261102. [Google Scholar] [CrossRef]
- Prescott, C.E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kogel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Rubino, M.; Dungait, J.A.J.; Evershed, R.P.; Bertolini, T.; De Angelis, P.; D’Onofrio, A.; Lagomarsino, A.; Lubritto, C.; Merola, A.; Terrasi, F.; et al. Carbon input belowground is the major C flux contributing to leaf litter mass loss: Evidences from a C-13 labelled-leaf litter experiment. Soil Biol. Biochem. 2010, 42, 1009–1016. [Google Scholar] [CrossRef]
- Haddix, M.L.; Paul, E.A.; Cotrufo, M.F. Dual, differential isotope labeling shows the preferential movement of labile plant constituents into mineral-bonded soil organic matter. Glob. Chang. Biol. 2016, 22, 2301–2312. [Google Scholar] [CrossRef]
- Olk, D.C.; Bloom, P.R.; Perdue, E.M.; McKnight, D.M.; Chen, Y.; Farenhorst, A.; Senesi, N.; Chin, Y.-P.; Schmitt-Kopplin, P.; Hertkorn, N.; et al. Environmental and agricultural relevance of humic fractions extracted by alkali from soils and natural waters. J. Environ. Qual. 2019, 48, 217–232. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, A.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776. [Google Scholar] [CrossRef]
- Pries, C.E.H.; Bird, J.A.; Castanha, C.; Hatton, P.J.; Torn, M.S. Long term decomposition: The influence of litter type and soil horizon on retention of plant carbon and nitrogen in soils. Biogeochemistry 2017, 134, 5–16. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Luojus, K.; Cohen, J.; Ikonen, J.; Pulliainen, J.; Takala, M.; Veijola, K.; Lemmetyinen, J.; Nagler, T.; Derksen, C.; Brown, R.; et al. Assessment of seasonal snow cover mass in northern hemisohere during the satellite-era. In Proceedings of the 38th IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Valencia, Spain, 22–27 July 2018. [Google Scholar]
- Li, Z.; Yang, W.; Yue, K.; Justine, M.F.; He, R.; Yang, K.; Zhuang, L.; Wu, F.; Tan, B.; Zhang, L.; et al. Effects of snow absence on winter soil nitrogen dynamics in a subalpine spruce forest of southwestern China. Geoderma 2017, 307, 107–113. [Google Scholar] [CrossRef]
- Cooper, E.J. Warmer Shorter Winters Disrupt Arctic Terrestrial Ecosystems. In Annual Review of Ecology, Evolution, and Systematics; Futuyma, D.J., Ed.; Annual Reviews: Palo Alto, CA, USA, 2014; Volume 45, p. 271. [Google Scholar]
- Marco-Barba, J.; Holmes, J.A.; Mesquita-Joanes, F.; Miracle, M.R. The influence of climate and sea-level change on the Holocene evolution of a Mediterranean coastal lagoon: Evidence from ostracod palaeoecology and geochemistry. Geobios 2013, 46, 409–421. [Google Scholar] [CrossRef]
- Kreyling, J.; Haei, M.; Laudon, H. Erratum to: Absence of snow cover reduces understory plant cover and alters plant community composition in boreal forests. Oecologia 2013, 173, 1157. [Google Scholar] [CrossRef][Green Version]
- Saccone, P.; Morin, S.; Baptist, F.; Bonneville, J.M.; Colace, M.P.; Domine, F.; Faure, M.; Geremia, R.; Lochet, J.; Poly, F.; et al. The effects of snowpack properties and plant strategies on litter decomposition during winter in subalpine meadows. Plant Soil 2013, 363, 215–229. [Google Scholar] [CrossRef]
- Bastida, F.; Eldridge, D.J.; Garcia, C.; Kenny Png, G.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil microbial diversity-biomass relationships are driven by soil carbon content across global biomes. Isme J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef]
- Gavazov, K.S. Dynamics of alpine plant litter decomposition in a changing climate. Plant Soil 2010, 337, 19–32. [Google Scholar] [CrossRef]
- Ni, X.Y.; Yang, W.Q.; Li, H.; Xu, L.Y.; He, J.; Tan, B.; Wu, F.Z. The responses of early foliar litter humification to reduced snow cover during winter in an alpine forest. Can. J. Soil Sci. 2014, 94, 453–461. [Google Scholar] [CrossRef]
- Bradford, M.A.; Keiser, A.D.; Davies, C.A.; Mersmann, C.A.; Strickland, M.S. Empirical evidence that soil carbon formation from plant inputs is positively related to microbial growth. Biogeochemistry 2013, 113, 271–281. [Google Scholar] [CrossRef]
- Xiang, B.; Liu, E.; Yang, L. Influences of freezing-thawing actions on mechanical properties of soils and stress and deformation of soil slope in cold regions. Sci. Rep. 2022, 12, 5387. [Google Scholar] [CrossRef] [PubMed]
- Aanderud, Z.T.; Jones, S.E.; Schoolmaster, D.R., Jr.; Fierer, N.; Lennon, J.T. Sensitivity of soil respiration and microbial communities to altered snowfall. Soil Biol. Biochem. 2013, 57, 217–227. [Google Scholar] [CrossRef]
- Hui, R.; Liu, L.; Xie, M.; Yang, H. Variation in snow cover drives differences in soil properties and microbial biomass of BSCs in the Gurbantunggut Desert-3 years of snow manipulations. Ecohydrology 2019, 12, e2118. [Google Scholar] [CrossRef]
- Castellano, M.J.; Mueller, K.E.; Olk, D.C.; Sawyer, J.E.; Six, J. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob. Chang. Biol. 2015, 21, 3200–3209. [Google Scholar] [CrossRef]
- Aerts, R.; Callaghan, T.V.; Dorrepaal, E.; van Logtestijn, R.S.P.; Cornelissen, J.H.C. Seasonal climate manipulations have only minor effects on litter decomposition rates and N dynamics but strong effects on litter P dynamics of sub-arctic bog species. Oecologia 2012, 170, 809–819. [Google Scholar] [CrossRef]
- Li, H.; Wu, F.Z.; Yang, W.Q.; Xu, L.Y.; Ni, X.Y.; He, J.; Tan, B.; Hu, Y. Effects of forest gaps on litter lignin and cellulose dynamics vary seasonally in an alpine forest. Forests 2016, 7, 27. [Google Scholar] [CrossRef]
- He, J.; Yang, W.Q.; Xu, L.Y.; Ni, X.Y.; Li, H.; Wu, F.Z. Copper and zinc dynamics in foliar litter during decomposition from gap center to closed canopy in an alpine forest. Scand. J. Forest Res. 2016, 31, 355–367. [Google Scholar] [CrossRef]
- Ni, X.Y.; Yang, W.Q.; Tan, B.; He, J.; Xu, L.Y.; Li, H.; Wu, F.Z. Accelerated foliar litter humification in forest gaps: Dual feedbacks of carbon sequestration during winter and the growing season in an alpine forest. Geoderma 2015, 241, 136–144. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, F.; Yang, W.; He, W.; Tan, B.; Xu, Z. Bacterial community changes during fir needle litter decomposition in an alpine forest in eastern Tibetan Plateau. Russ. J. Ecol. 2016, 47, 145–157. [Google Scholar] [CrossRef]
- Wu, F.; Peng, C.; Zhu, J.; Zhang, J.; Tan, B.; Yang, W. Impacts of freezing and thawing dynamics on foliar litter carbon release in alpine/subalpine forests along an altitudinal gradient in the eastern Tibetan Plateau. Biogeosciences 2014, 11, 6871. [Google Scholar] [CrossRef]
- Wu, F.Z.; Yang, W.Q.; Zhang, J.; Deng, R.J. Litter decomposition in two subalpine forests during the freeze-thaw season. Acta Oecol. 2010, 36, 135–140. [Google Scholar] [CrossRef]
- Ladwig, L.M.; Ratajczak, Z.R.; Ocheltree, T.W.; Hafich, K.A.; Churchill, A.C.; Frey, S.J.K.; Fuss, C.B.; Kazanski, C.E.; Munoz, J.D.; Petrie, M.D.; et al. Beyond arctic and alpine: The influence of winter climate on temperate ecosystems. Ecology 2016, 97, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.Y.; Yang, W.Q.; Tan, B.; Li, H.; He, J.; Xu, L.Y.; Wu, F.Z. Forest gaps slow the sequestration of soil organic matter: A humification experiment with six foliar litters in an alpine forest. Sci. Rep. 2016, 6, 19744. [Google Scholar] [CrossRef] [PubMed]
- Konestabo, H.S.; Michelsen, A.; Holmstrup, M. Responses of springtail and mite populations to prolonged periods of soil freeze-thaw cycles in a sub-arctic ecosystem. Appl. Soil Ecol. 2007, 36, 136–146. [Google Scholar] [CrossRef]
- Lan, L.Y.; Yang, W.Q.; Wu, F.Z.; Liu, Y.W.; Yang, F.; Guo, C.H.; Chen, Y.; Tan, B. Effects of soil fauna on microbial community during litter decomposition of Populus simonii and Fargesia spathacea in the subalpine forest of western Sichuan, China. J. Appl. Ecol. 2019, 30, 2983–2991. [Google Scholar]
- Mehmood, T.; Liland, K.H.; Snipen, L.; Saebo, S. A review of variable selection methods in Partial Least Squares Regression. Chemometr. Intell. Lab. 2012, 118, 62–69. [Google Scholar] [CrossRef]
- Trap, J.; Akpa-Vinceslas, M.; Margerie, P.; Boudsocq, S.; Richard, F.; Decaens, T.; Aubert, M. Slow decomposition of leaf litter from mature Fagus sylvatica trees promotes offspring nitrogen acquisition by interacting with ectomycorrhizal fungi. J. Ecol. 2017, 105, 528–539. [Google Scholar] [CrossRef]
- Oldfield, E.E.; Crowther, T.W.; Bradford, M.A. Substrate identity and amount overwhelm temperature effects on soil carbon formation. Soil Biol. Biochem. 2018, 124, 218–226. [Google Scholar] [CrossRef]
- Hall, S.J.; Huang, W.; Timokhin, V.I.; Hammel, K.E. Lignin lags, leads, or limits the decomposition of litter and soil organic carbon. Ecology 2020, 101, e03113. [Google Scholar] [CrossRef]
- Berg, B.; Sun, T.; Johansson, M.B.; Sanborn, P.; Ni, X.Y.; Akerblom, S.; Lonn, M. Magnesium dynamics in decomposing foliar litter-A synthesis. Geoderma 2021, 382, 114756. [Google Scholar] [CrossRef]
- Berg, B.; Erhagen, B.; Johansson, M.B.; Nilsson, M.; Stendahl, J.; Trum, F.; Vesterdal, L. Manganese in the litter fall-forest floor continuum of boreal and temperate pine and spruce forest ecosystems-A review. Forest Ecol. Manag. 2015, 358, 248–260. [Google Scholar] [CrossRef]
- Qualls, R.G.; Takiyama, A.; Wershaw, R.L. Formation and loss of humic substances during decomposition in a pine forest floor. Soil Sci. Soc. Am. J. 2003, 67, 899–909. [Google Scholar] [CrossRef]
- Christenson, L.M.; Mitchell, M.J.; Groffman, P.M.; Lovett, G.M. Winter climate change implications for decomposition in northeastern forests: Comparisons of sugar maple litter with herbivore fecal inputs. Glob. Chang. Biol. 2010, 16, 2589–2601. [Google Scholar] [CrossRef]
- Blok, D.; Elberling, B.; Michelsen, A. Initial stages of tundra shrub litter decomposition may be accelerated by deeper winter snow but slowed down by spring warming. Ecosystems 2016, 19, 155–169. [Google Scholar] [CrossRef]
- Baptist, F.; Yoccoz, N.G.; Choler, P. Direct and indirect control by snow cover over decomposition in alpine tundra along a snowmelt gradient. Plant Soil 2010, 328, 397–410. [Google Scholar] [CrossRef]
- Bokhorst, S.; Bjerke, J.W.; Melillo, J.; Callaghan, T.V.; Phoenix, G.K. Impacts of extreme winter warming events on litter decomposition in a sub-Arctic heathland. Soil Biol. Biochem. 2010, 42, 611–617. [Google Scholar] [CrossRef]
- Bokhorst, S.; Metcalfe, D.B.; Wardle, D.A. Reduction in snow depth negatively affects decomposers but impact on decomposition rates is substrate dependent. Soil Biol. Biochem. 2013, 62, 157–164. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Chang. Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
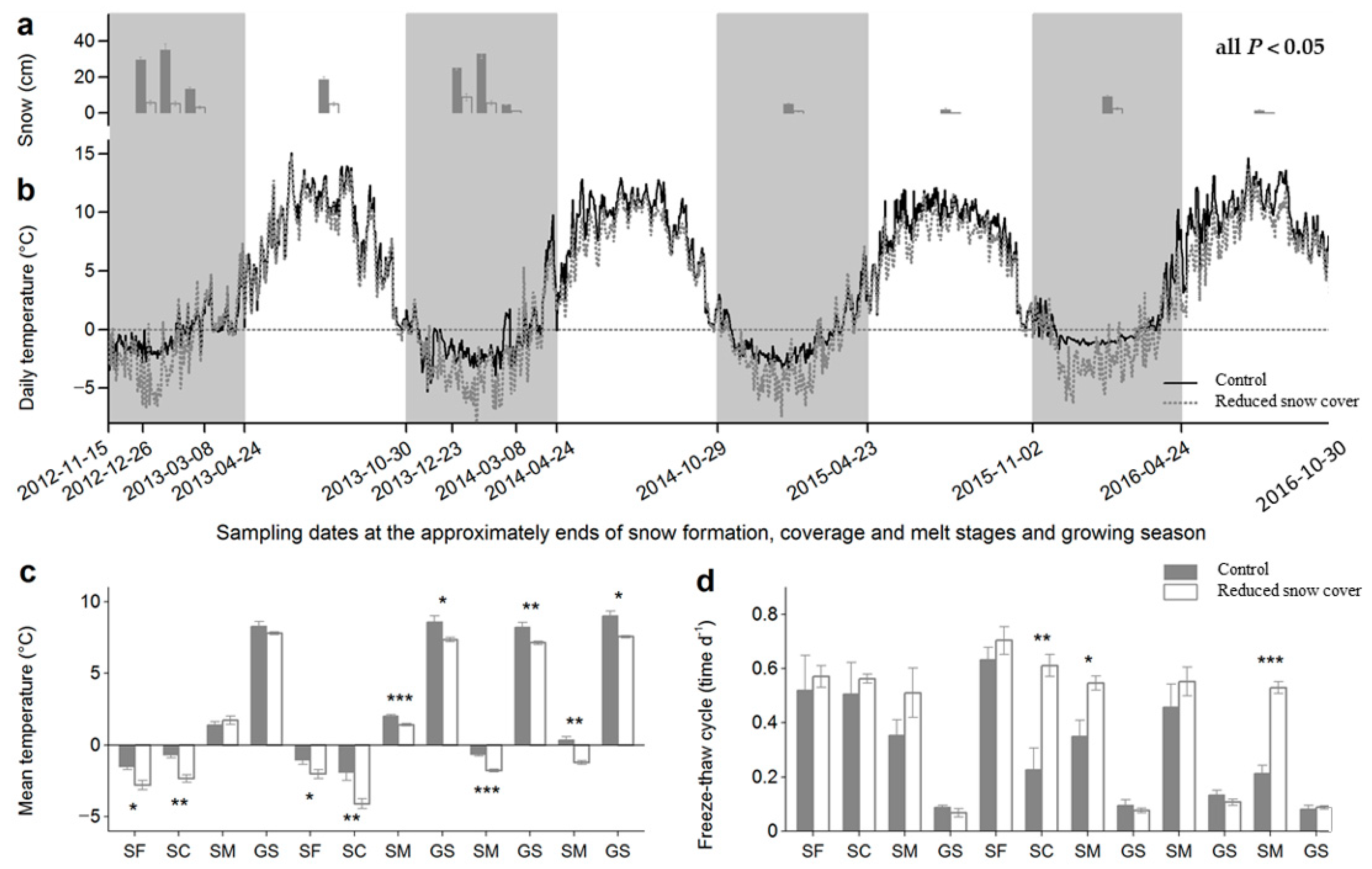
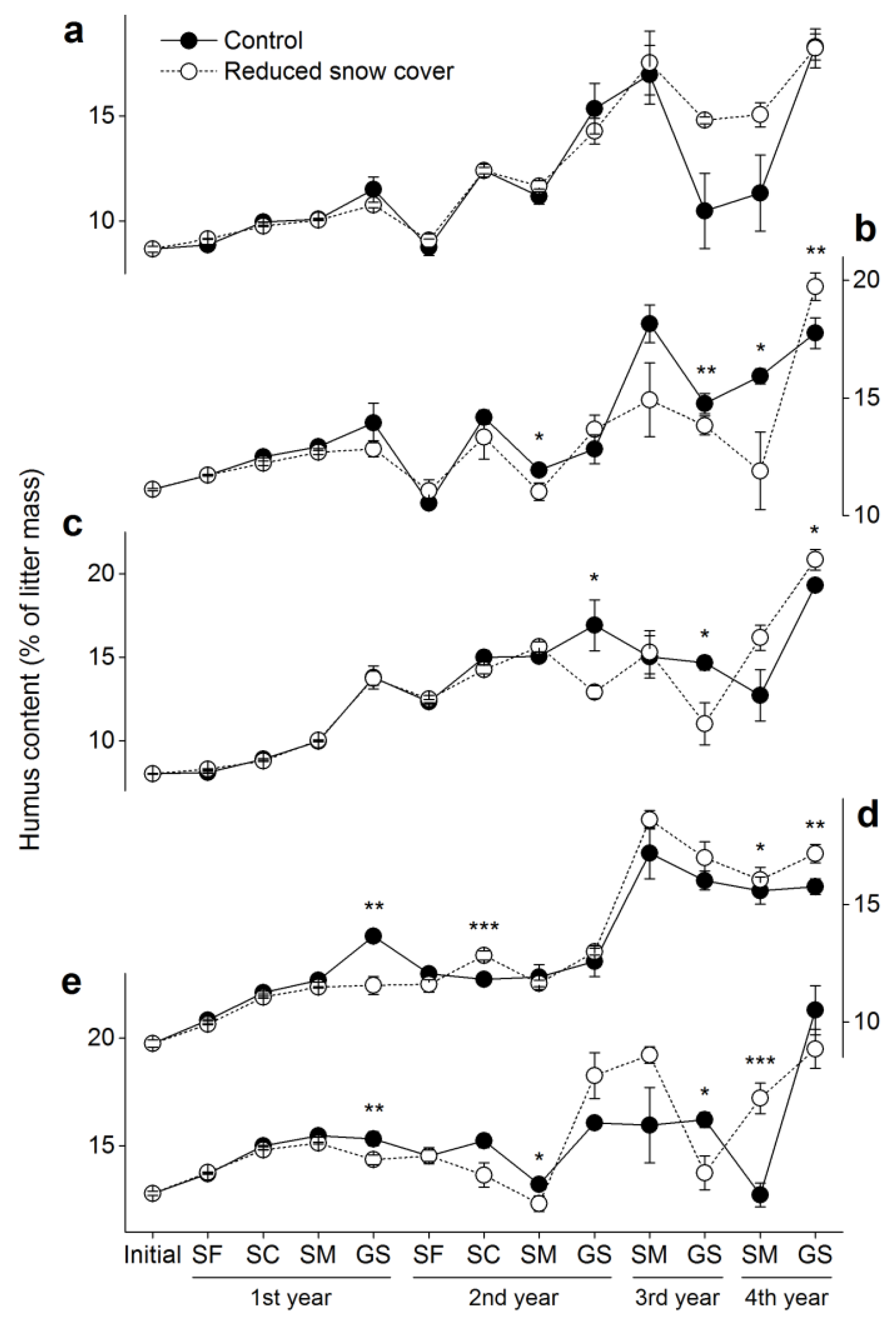
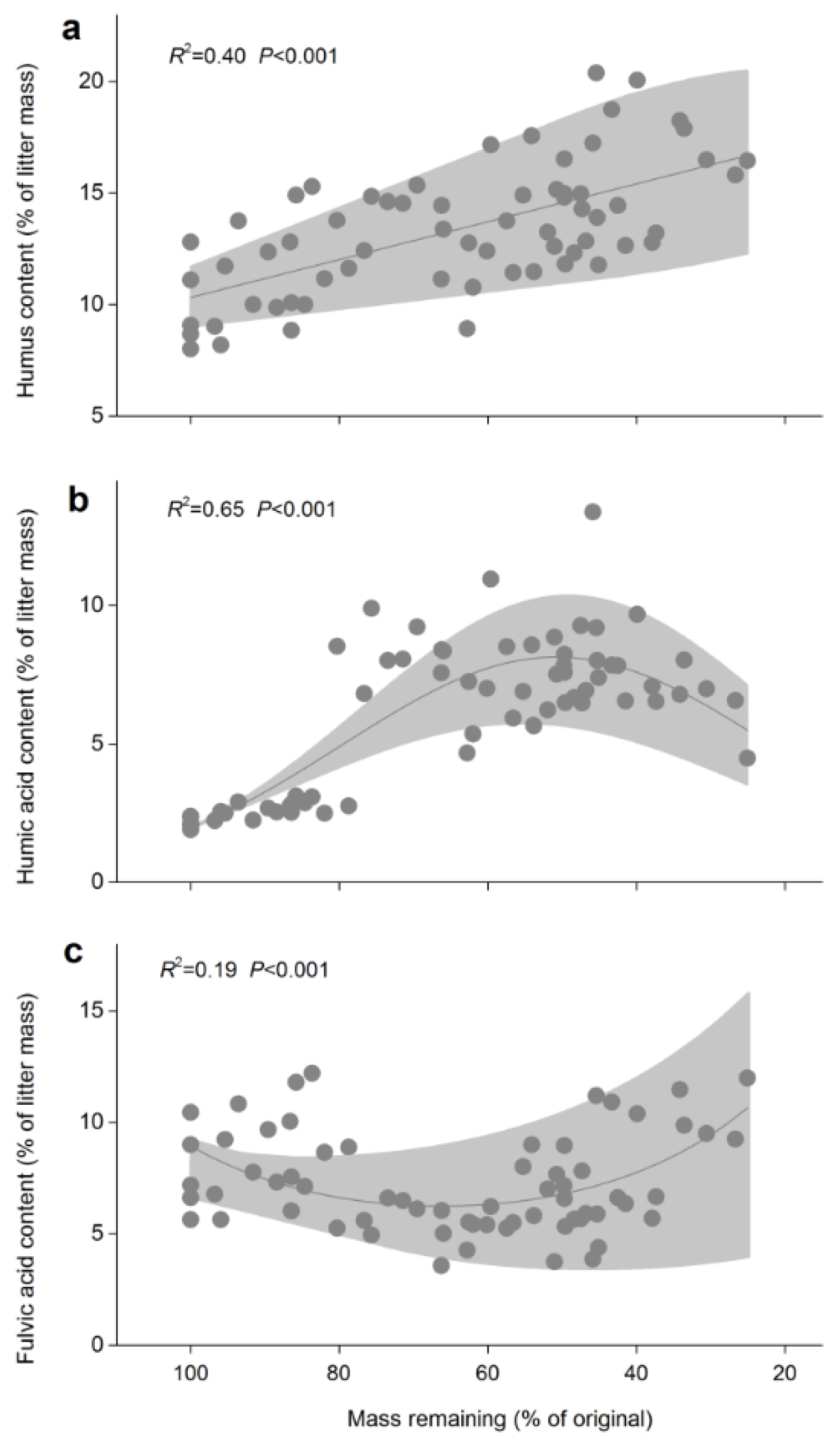
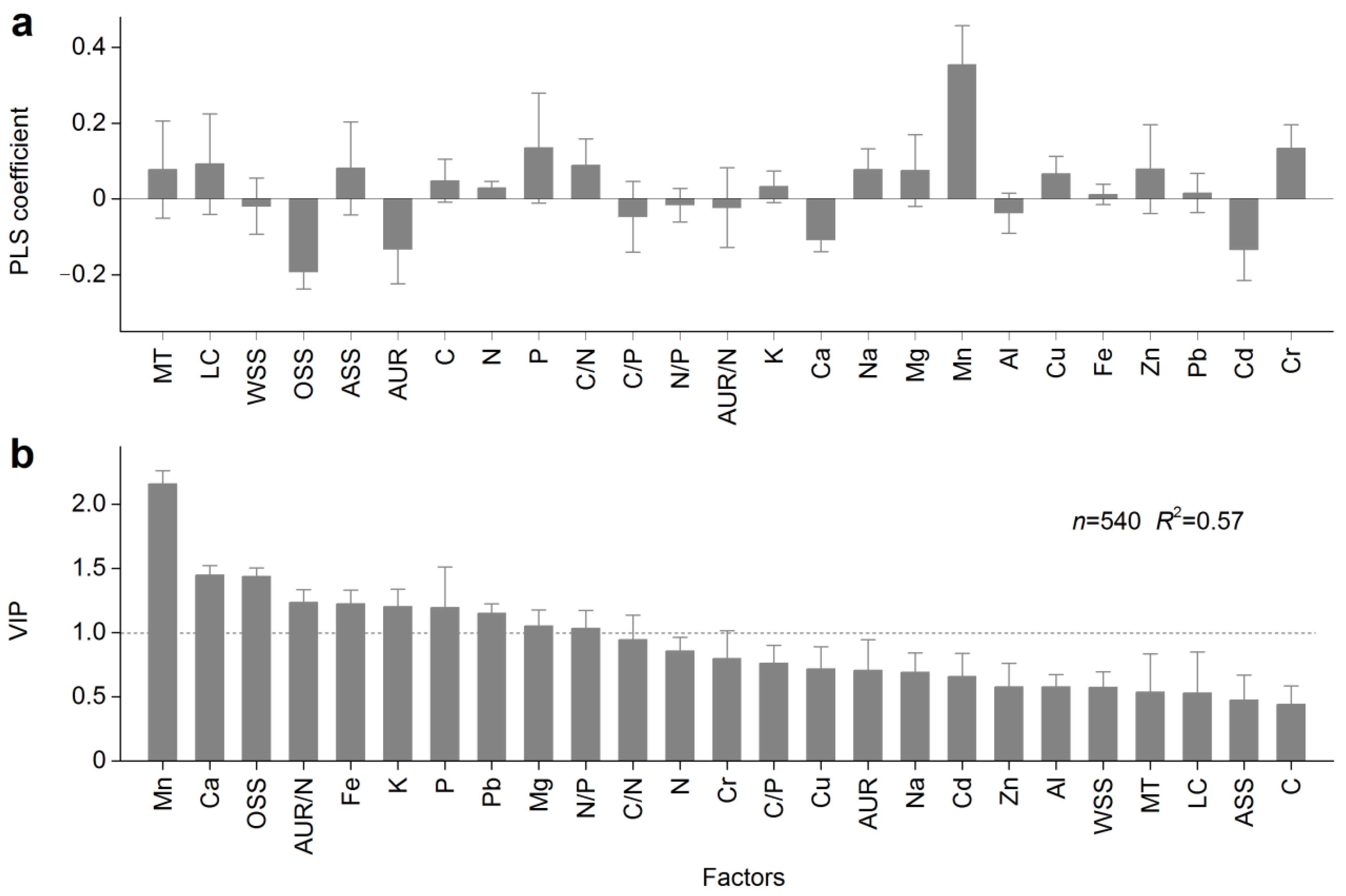

| Winter | Timing of Winter | Length of Winter (Days) | Sampling Date | ||
|---|---|---|---|---|---|
| Beginning | End | End of Growing Season | End of Snowmelt | ||
| 2012/2013 winter | 30 October 2012 | 24 April 2013 | 176 | Experiment began | 24 April 2013 |
| 2013/2014 winter | 31 October 2013 | 30 April 2014 | 181 | 30 October 2013 | 24 April 2014 |
| 2014/2015 winter | 29 October 2014 | 26 April 2015 | 179 | 29 October 2014 | 23 April 2015 |
| 2015/2016 winter | 30 October 2015 | 26 April 2016 | 179 | 2 November 2015 | 24 April 2016 |
| Litter | C (%) | N (%) | P (%) | WSS (%) | OSS (%) | ASS (%) | AUR (%) | C/N Ratio | AUR/N Ratio |
|---|---|---|---|---|---|---|---|---|---|
| Cypress | 51.64 (1.77) a | 0.88 (0.010) c | 0.12 (0.006) ab | 35.74 (0.69) c | 33.16 (3.43) a | 32.43 (1.29) a | 20.60 (3.41) b | 58.86 (2.21) b | 23.48 (3.89) b |
| Larch | 54.35 (0.63) a | 0.86 (0.041) c | 0.13 (0.002) a | 40.08 (1.08) b | 19.11 (0.68) c | 29.24 (0.87) ab | 21.46 (0.94) b | 63.32 (3.49) b | 25.01 (2.04) b |
| Birch | 49.69 (1.45) ab | 1.33 (0.022) a | 0.09 (0.004) c | 25.06 (1.96) d | 11.43 (0.75) d | 27.74 (0.94) b | 50.96 (0.96) a | 37.24 (1.35) c | 38.19 (1.01) a |
| Willow | 45.23 (1.65) b | 1.15 (0.028) b | 0.11 (0.002) b | 41.71 (0.32) ab | 18.48 (1.57) c | 28.56 (1.88) b | 26.15 (3.29) b | 39.49 (2.18) c | 22.79 (2.45) b |
| Azalea | 50.29 (1.60) a | 0.67 (0.020) d | 0.11 (0.009) bc | 43.14 (1.16) a | 25.84 (2.29) b | 27.00 (0.59) b | 21.84 (3.42) b | 75.54 (4.47) a | 32.90 (6.13) ab |
| Source of Variation | df | Humus | Humic Acid | Fulvic Acid | |||
|---|---|---|---|---|---|---|---|
| F Value | p-Value | F Value | p-Value | F Value | p-Value | ||
| Time | 11 | 112.0 | <0.001 | 300.7 | <0.001 | 63.1 | <0.001 |
| Litter | 4 | 59.6 | <0.001 | 50.0 | <0.001 | 22.6 | <0.001 |
| Snow | 1 | 0.02 | 0.90 | 6.0 | 0.015 | 2.1 | 0.14 |
| Time × Litter | 44 | 7.5 | <0.001 | 15.9 | <0.001 | 6.4 | <0.001 |
| Time × Snow | 11 | 2.2 | 0.015 | 5.8 | <0.001 | 3.1 | <0.001 |
| Litter × Snow | 4 | 2.9 | 0.022 | 2.0 | 0.097 | 5.3 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Ni, X.; Guo, H.; Dai, W. Alpine Litter Humification and Its Response to Reduced Snow Cover: Can More Carbon Be Sequestered in Soils? Forests 2022, 13, 897. https://doi.org/10.3390/f13060897
Wang D, Ni X, Guo H, Dai W. Alpine Litter Humification and Its Response to Reduced Snow Cover: Can More Carbon Be Sequestered in Soils? Forests. 2022; 13(6):897. https://doi.org/10.3390/f13060897
Chicago/Turabian StyleWang, Dingyi, Xiangyin Ni, Hongrong Guo, and Wenyuan Dai. 2022. "Alpine Litter Humification and Its Response to Reduced Snow Cover: Can More Carbon Be Sequestered in Soils?" Forests 13, no. 6: 897. https://doi.org/10.3390/f13060897
APA StyleWang, D., Ni, X., Guo, H., & Dai, W. (2022). Alpine Litter Humification and Its Response to Reduced Snow Cover: Can More Carbon Be Sequestered in Soils? Forests, 13(6), 897. https://doi.org/10.3390/f13060897






