The Relationships of Habitat Conditions, Height Level, and Geographical Position with Fruit and Seed Traits in Populations of Invasive Vine Echinocystis lobata (Cucurbitaceae) in Central and Eastern Europe
Abstract
:1. Introduction
2. Materials and Methods
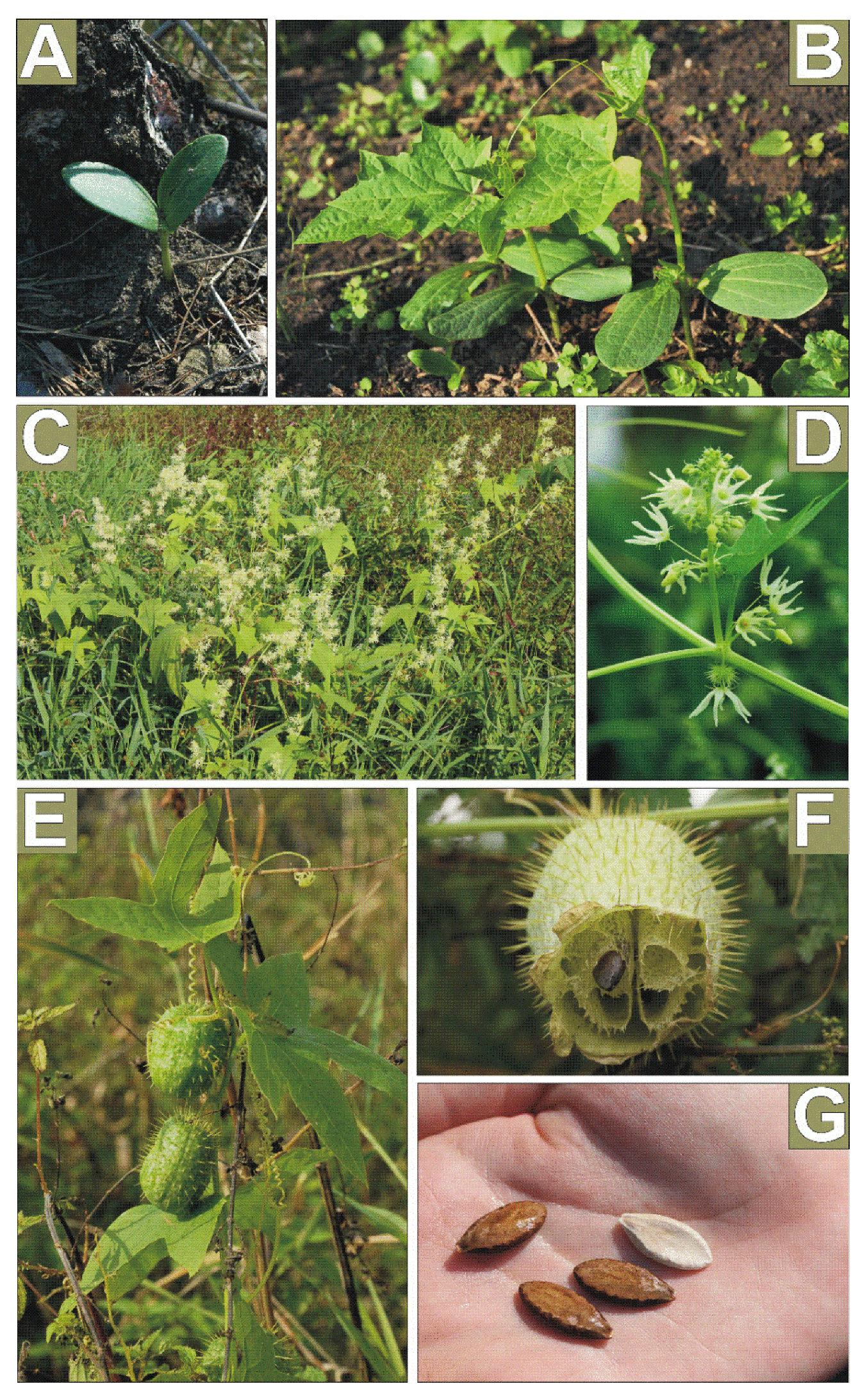
2.1. Study Species
2.2. Study Area
2.3. Fruit and Seed Sampling
2.4. Statistical Analyses
3. Results
3.1. The Effect of Habitat Origin on Fruit and Seed Traits
3.2. The Effect of Habitat Type on Fruit and Seed Traits
3.3. The Effect of Height Level on Fruit and Seed Traits
3.4. The Relationships of Habitat Origin, Habitat Type, and Height Level with Fruit and Seed Traits
3.5. The Interdependence between the Traits of Fruits and Seeds
3.6. The Effect of Geographical Position on Fruit and Seed Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Country Code | Study Site | GPS Coordinates | Altitude (m a.s.l.) | Habitat | Plant Cover * | Soil pH | Light Availability ** | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Latitude | Longitude | Code | Type | Origin | ||||||
| LV | Daugavpils, right bank of the Daugava river | N 55°51′53.4″ | E 26°31′13.9″ | 295 | F9.121 | Almond willow-osier scrub | N | B30% C-90% | 7.1 | 3 |
| LV | Daugavpils district, Tilti village, bank of the Tūkšna river | N 55°58′24.3″ | E 26°26′27.3″ | 289 | G1.11 | Riverine Salix woodland | N | A-20% B-40% C-80% | 6.9 | 2 |
| LV | Jekabpils, left bank of the Daugava River | N 56°29′43.0″ | E 25°53′52.1″ | 296 | F9.121 | Almond willow-osier scrub | N | B-40% C-90% | 7.1 | 3 |
| LV | Bauska, right bank of the Memele river | N 56°24′15.2″ | E 24°10′29.4″ | 40 | G1.11 | Riverine Salix woodland | N | A-20% B-20% C-95% | 7.3 | 3 |
| LV | Bauska district, Arce village, right bank of the Mūša river | N 56°23′3.7″ | E 24°15′03.6″ | 35 | C3.21 | Common reed (Phragmites) beds | N | C-100% | 7.1 | 4 |
| LT | Prienai district, Prienai, right bank of the Nemunas River | N 54°37′55.6″ | E 23°57′16.5″ | 51 | G1.11 | Riverine Salix woodland | N | A-20% B-30% C-95% | 7.1 | 3 |
| LT | Trakai district, Trakai | N 54°38′50.9″ | E 24°55′04.3″ | 165 | H3.1C | Disused siliceous quarries | A | C-60% | 7.6 | 5 |
| LT | Ukmergėdistr, Ukmergė, left bank of the Šventoji River | N 55°14′38.1″ | E 24°45′50.5″ | 56 | F9.121 | Almond willow-osier scrub | N | B-60% C-80% | 6.7 | 3 |
| LT | Panevėžys district, Berčiūnai, right bank of the Nevėžis River | N 55°44′19.3″ | E 24°14′24.0″ | 41 | F9.121 | Almond willow-osier scrub | N | A-10% B-50% C-90% | 6.9 | 3 |
| LT | Šiauliai district, Norkūnai village | N 56°01′12.9″ | E 23°16′55.6″ | 94 | J2.7 | Rural construction and demolition sites | A | B-10% C-70% | 7.3 | 4 |
| LT | Raseiniai district, Vandžiai village, left bank of the Dubysa River | N 55°18′01.9″ | E 23°25′46.0″ | 40 | F9.121 | Almond willow-osier scrub | N | B-40% C-70% | 6.8 | 4 |
| LT | Jurbarkas district, Skirsnemunė, right bank of the Nemunas River | N 55°05′25.9″ | E 22°52′50.16″ | 13 | F9.121 | Almond willow-osier scrub | N | B-50% C-80% | 6.8 | 4 |
| PL | Filipów Pierwszy | N 54°17′42″ | E 22°49′22″ | 166 | D5.2121 | Slender tufted sedge beds near sub-boreal swamp alder woods (G1.4114) | N | C-95% | 6.9 | 4 |
| PL | Bakałarzewo | N 54°19′29″ | E 22°43′00″ | 154 | G1.1112 | Eastern European poplar-willow forests | N | A-50% B-75% C-75% | 7.6 | 2 |
| PL | Grodzisk Wielkopolski | N 52°13′10.92″ | E 16°22′36.06″ | 80 | I2.2 | Small-scale ornamental and domestic garden areas | A | C-50% | 7.1 | 5 |
| PL | Kamieniec | N 52°09′53.46″ | E 16°28′20.52″ | 68 | C3.63 | Unvegetated river mud banks | N | C-80% | 7.6 | 4 |
| PL | Goździchowo | N 52°09′22.14″ | E 16°28′28.08″ | 53 | C3.63 | Unvegetated river mud banks | N | C-70% | 7.6 | 4 |
| PL | Bęczkowice | N 51°11′40.87″ | E 19°42′37.41″ | 208 | E3.4 | Moist or wet eutrophic and mesotrophic grassland | SN | A-5% B-5% C-90% | 6.4 | 4 |
| PL | Mileszki | N 51°46′15.58″ | E 19°34′26.79″ | 240 | I2.2 | Small-scale ornamental and domestic garden areas | A | A-10% B-90% C-70% | 5.4 | 3 |
| PL | Ługi | N 51°46′15.58″ | E 19°34′26.79″ | 177 | J4.2 | Road networks | A | A-10% B-10% C-95% | 7.3 | 4 |
| PL | Nowa Brzeźnica | E 19°10′52.46″ | E 19°10′52.46″ | 203 | G1.1 | Riparian and gallery woodland | N | A-40% B-70% C-70% | 6.3 | 2 |
| PL | Sieradz 1 | N 51°35′58.29″ | E 18°44′35.71″ | 129 | G1.1 | Riparian and gallery woodland | N | A-5% B-80% C-90% | 6.1 | 3 |
| PL | Sieradz 2 | N 51°36′27.10″ | E 18°43′47,42″ | 136 | G1.1 | Riparian and gallery woodland | N | A-5% B-80% C-90% | 6.2 | 3 |
| PL | Smolice | N 51°54′17.24″ | E 19°34′16.68″ | 153 | G1.1 | Riparian and gallery woodland | N | A-40% B-30% C-80% | 7.2 | 3 |
| PL | Smolice, Wczasowa Street | N 51°54′05.62″ | E 19°34′54.65″ | 158 | J2.5 | Constructed boundaries | A | A-10% B-20% C-20% | 6.4 | 5 |
| PL | Witkowice | N 51°46′21.94″ | E 19°44′53.78″ | 216 | J2.5 | Constructed boundaries | A | A-10% B-5% C-60% | 6.5 | 4 |
| PL | Łęczna | N 51°21′23″ | E 22°54′18″ | 150 | G1.11 | Riverine Salix woodland | N | A-40% B-30% C-100% | 6.5 | 3 |
| PL | Wólka | N 51°21′03″ | E 22°52′60″ | 177 | G1.11 | Riverine Salix woodland | N | A-60% B-30% C-90% | 6.5 | 2 |
| PL | Łysaków | N 51°31′50″ | E 22°38′60″ | 160 | X25 | Domestic gardens of villages and urban peripheries | A | B-70% C-90% | 6.0 | 3 |
| PL | Kępa Solecka | N 51°10′9″ | E 21°46′60″ | 123 | G1.22 | Mixed oak–elm–ash woodland of great rivers | N | A-20% B-40% C-100% | 6.5 | 3 |
| PL | Zastów Polanowski | N 51°30′31″ | E 22°05′09″ | 119 | G1.11 | Riverine Salix woodland | N | A-20% B-30% C-90% | 6.5 | 3 |
| PL | Niekłań 1 | N 51°16′38.43″ | E 20°61′79.85″ | 279 | E5.14 | Weed communities of recently abandoned extractive industrial sites | A | C-65% | 7.0 | 4 |
| PL | Niekłań 2 | N 51°16′89.82″ | E 20°62′23.55″ | 283 | F3.111 | Blackthorn-bramble scrub | N | A-5% B-60% C-90% | 7.0 | 3 |
| PL | Stąporków, Miła | N 51°13′74.93″ | N 20°58′96.74″ | 267 | E5.43 | Shady woodland edge fringes | N | A-35% B-60% C-90% | 7.0 | 2 |
| PL | Widawa rivervalley, between Paniowice and Szymanów | N 51°12′11″ | E 16°59′09.37″ | 134 | E5.41 | Screens or veils of perennial tall herbs lining watercourses | SN | C-90% | 7.5 | 4 |
| PL | Widawa rivervalley, between Paniowice and Szymanów | N 50°56′22.4″ | E 16°47′22.2″ | 148 | G1.11 | Riverine Salix woodland | N | A-10% C-80% | 8.0 | 4 |
| PL | Wrocław, Zgorzelisko, bank of Widawa river | N 51°07′49.2″ | E 17°07′44.0″ | 118 | E5.41 | Screens or veils of perennial tall herbs lining watercourses | N | A-10% B-50% C-70% | 6.5 | 3 |
| PL | Kamieniec Wrocławski, bank of the Oder | N 51°04′21.6″ | E 17°10′11.9″ | 119 | E5.41 | Screens or veils of perennial tall herbs lining watercourses | N | C-90% | 7.0 | 4 |
| PL | Wrocław, Pilczyce, bank of Ślęza river | N 51°08′54.6″ | E 16°57′22.6″ | 112 | E5.41 | Screens or veils of perennial tall herbs lining watercourses | N | B-40% C-50% | 6.5 | 4 |
| PL | Joachimówko, causeway of StawTrójkątny | N 51°32′20.9″ | E 17°29′33.8″ | 113 | C3.2111 | Freshwater (Phragmites) beds | N | C-100% | 5.0 | 4 |
| PL | Nowe Grodzisko | N 51°33′25.3″ | E 17°21′26.1″ | 107 | J6.4 | Solid agricultural and horticultural waste | A | C-100% | 7.5 | 4 |
| PL | Nowe Grodzisko, bank of Barycz river | N 51°33′10.9″ | E 17°21′39.7″ | 106 | E5.11 | Lowland habitats colonised by tall nitrophilous herbs | A | A-70% B-50% C-90% | 6.0 | 2 |
| PL | Nowe Brzesko | N 50°07′16.8″ | E 20°22′31.2″ | 186 | G1.111 | Middle European white willow forests | N | A-40% B-40% C-40% | 5.5 | 3 |
| PL | CzapleMałe | N 50°17′55.9″ | E 19°57′18.8″ | 304 | E5.11 | Lowland habitats colonised by tall nitrophilous herbs | A | C-100% | 5.2 | 4 |
| PL | Pławowice | N 50°10′25.6″ | E 20°24′50.5″ | 192 | F9.121 | Almond willow-osier scrub | N | A-40% B-50% C-40% | 5.5 | 4 |
| PL | Odwiśle | N 50°06′11.3″ | E 20°19′56.4″ | 181 | J4.2 | Road networks | A | C-100% | 5.8 | 4 |
| PL | Kraków, Dąbie | N 50°3′27″ | E 19°50′35″ | 195 | G1.1 | Riparian and gallery woodland | N | A-75% B-15% C-30% | 7.0 | 3 |
| PL | Modlniczka | N 50°11′46″ | E 19°48′50″ | 179 | I2.2 | Small-scale ornamental and domestic garden areas | A | A-10% B-40% C-70% | 7.0 | 3 |
| PL | Czajowice | N 50°11′18″ | E 19°48′18″ | 183 | J4.2 | Road networks | A | C-95% | 7.0 | 4 |
| PL | Kraków, Nowa Huta 1 | N 50°04′08.81″ | E 20°01′53.51″ | 198 | E2.7 | Unmanaged mesic grassland | A | B ≤ 5% C-100% | 6.5 | 4 |
| PL | Kraków, Nowa Huta 2 | N 50°04′04.0″ | E 20°02′24.4″ | 199 | E3.4 | Moist or wet eutrophic and mesotrophic grassland | SN | B ≤ 5% C-100% | 6.5 | 4 |
| PL | Strzyżów | N 49°51′52.3″ | E 21°46′45.4″ | 220 | G1.11 | Riverine Salix woodland | N | A-60% B-40% C-100% | 7.0 | 2 |
| PL | Dobrzechów | N 49°52′04.4″ | E 21°44′51.9″ | 219 | G1.2 | Mixed riparian floodplain and gallery woodland | N | B-60% C-100% | 7.0 | 3 |
| PL | Wiśniowa | N 49°51′40.4″ | E 21°39′33.7″ | 227 | C3.26 | Phalaris arundinacea beds | N | C-100% | 7.0 | 4 |
| PL | Wielopole Skrzyńskie | N 49°56′50.2″ | E 21°37′00.6″ | 254 | J4.2 | Road networks | A | C-80% | 6.5 | 4 |
| PL | Broniszów | N 49°59′18.6″ | E 21°33′41.3″ | 218 | J4.2 | Road networks | A | C-100% | 7.0 | 4 |
| PL | Okonin | N 50°01′49.52″ | E 21°32′56.03″ | 199 | G1.11 | Riverine Salix woodland | N | A-60% B-50% C-100% | 7.0 | 3 |
| PL | Rzeszów, the Rzeszów reservoir | N 49°59′54.2″ | E 21°58′44.6″ | 199 | G1.11 | Riverine Salix woodland | N | A-60% B-50% C-100% | 7.0 | 2 |
| PL | Rzeszów, boulevardsnearWisłokriver | N 50°01′24.9″ | E 21°00′01.6″ | 193 | J4.2 | Road networks | A | B-5% C-100% | 7.0 | 4 |
| SK | Budca, near Hron River 1 | N 48°33′44.7″ | E 19°02′36.2″ | 235 | G1.1 | Riparian and gallery woodland | N | B-90% C-60% | 7.0 | 3 |
| SK | Budca, near Hron River 2 | N 48°33′46.7″ | E 19°02′26.3″ | 233 | G1.1 | Riparian and gallery woodland | N | B-5% C-90% | 7.0 | 4 |
| SK | Budca, near Hron River 3 | N 48°33′47.7″ | E 19°02′22.8″ | 250 | G1.1 | Riparian and gallery woodland | N | A-10% B-5% C ≥ 95% | 6.0 | 4 |
| SK | Budca, near Hron River 4 | N 48°33′42.1″ | E 19°01′24.8″ | 255 | G1.1 | Riparian and gallery woodland | N | A-60% B-5% C ≥ 95% | 6.5 | 3 |
| SK | Slatina River1 | N 48°33′47.0″ | E 19°06′38.2″ | 271 | G1.1 | Riparian and gallery woodland | N | A ≥ 5% B-80% C95% | 7.0 | 2 |
| SK | Slatina River2 | N 48°33′46.3″ | E 19°06′36.6″ | 269 | G1.1 | Riparian and gallery woodland | N | A-70% B ≥ 5% C ≥ 95% | 7.0 | 3 |
References
- Gianoli, E. The behavioural ecology of climbing plants. AoB Plants 2015, 7, plv013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentry, A.H. The distribution and evolution of climbing plants. In The Biology of Vines; Putz, F.E., Mooney, H.A., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 3–49. [Google Scholar]
- Putz, F.E. Vine Ecology. 2012. Available online: http://www.ecology.info/vines.htm (accessed on 7 May 2021).
- Darwin, C.R. On the movements and habits of climbing plants. J. Linn. Soc. Bot. 1865, 9, 1–118. [Google Scholar] [CrossRef]
- Addo-Fordjour, P.; Anning, A.K.; Atakora, E.A.; Agyei, P.S. Diversity and distribution of climbing plants in a semi-deciduous rain forest, KNUST Botanic Garden, Ghana. Int. J. Bot. 2008, 4, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Bitomský, M.; Mládková, P.; Cimalová, Š.; Mládek, J. Herbaceous climbers in herbaceous systems are shade-tolerant and magnesium-demanding. J. Veg. Sci. 2019, 30, 799–808. [Google Scholar] [CrossRef]
- Stansbury, C.D.; Batchelor, K.L.; Morin, L.; Woodburn, T.L.; Scott, J.K. Standardized support to measure biomass and fruit production by the invasive climber (Asparagus asparagoides). Weed Technol. 2007, 21, 820–824. [Google Scholar] [CrossRef]
- Hu, L.; Li, M. Climbing capacity of the invasive vine Mikania micrantha Kunth on vertical artificial poles. Biol. Invasions 2014, 16, 295–302. [Google Scholar] [CrossRef]
- Grašič, M.; Piberčnik, M.; Zelnik, I.; Abram, D.; Gaberščik, A. Invasive alien vines affect leaf traits of riparian woody vegetation. Water 2019, 11, 2395. [Google Scholar] [CrossRef] [Green Version]
- Burnham, R.J.; Santanna, C.V. Distribution, diversity, and traits of native, exotic, and invasive climbing plants in Michigan. Brittonia 2015, 67, 350–370. [Google Scholar] [CrossRef]
- Yang, S.-I.; Walters, T.W. Ethnobotany and the economic role of the Cucurbitaceae of China. Econ. Bot. 1992, 46, 349–367. [Google Scholar] [CrossRef]
- Rahman, A.H.M.M.; Anisuzzaman, M.; Ferdous, A.; Rafiul Islam, A.K.M.; Naderuzzaman, A.T.M. Study of nutritive value and medicinal uses of cultivated cucurbits. J. Appl. Sci. Res. 2008, 4, 555–558. [Google Scholar]
- Kujawska, M.; Svanberg, I. From medicinal plant to noxious weed: Bryonia alba L. (Cucurbitaceae) in northern and eastern Europe. J. Ethnobiol. Ethnomed. 2019, 15, 22. [Google Scholar] [CrossRef]
- Zhao, F.X.; Yan, S.H.; Li, M.H.; Liu, X.Y.; Zhang, X.W.; Cao, Y.; Zhao, H. Adaptive strategies of structures that enhance invasion in Sicyos angulatus. Not. Bot. HortiAgrobo. 2019, 47, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Larché, J.F. Sicyos angulatus, a new weed for corn crops in SW France. Phytoma 2004, 571, 19–22. [Google Scholar]
- Kil, J.H.; Kong, H.Y.; Koh, K.S.; Kim, J.M. Management of Sicyos angulata spread in Korea. In: Neobiota. From Ecology to Conservation. In Proceedings of the 4th European Conference on Biological Invasions, Vienna, Austria, 27–29 September 2006. BfN-Skripten 184:170. [Google Scholar]
- EPPO. Sicyos angulatus. 2010. Available online: https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2338.2010.02415.x (accessed on 15 November 2010). [CrossRef]
- Starr, F.; Starr, K.; Loope, L. Coccinia grandis—Hawaiian Ecosystems at Risk Project (HEAR). Available online: http://www.hear.org/starr/hiplants/reports/pdf/coccinia_grandis.pdf (accessed on 8 October 2020).
- Muniappan, R.; Reddy, G.V.P.; Raman, A. Coccinia grandis (L.) Voigt (Cucurbitaceae). In Biological Control of Tropical Weeds Using Arthropods; Muniappan, R., Ed.; Cambridge University Press: Cambridge, UK, 2009; pp. 175–182. [Google Scholar]
- Chun, M.E. Biology and host specificity of Melittia oedipus (Lepidoptera: Sesiidae), a biological control agent of Coccinia grandis (Cucurbitaceae). Proc. Hawaii Entomol. Soc. 2001, 35, 85–93. [Google Scholar]
- Kuluev, B.R.; Shvets, D.Y.; Golovanov, Y.M.; Probatova, N.S. Thladiantha dubia (Cucurbitaceae) in the Republic of Bashkortostan as a dangerous weed with high invasive potential. Russ. J. Biol. Invasions 2019, 10, 160–170. [Google Scholar] [CrossRef]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef]
- Celesti-Grapow, L.; Ricottam, C. Plant invasion as an emerging challenge for the conservation of heritage sites: The spread of ornamental trees on ancient monuments in Rome, Italy. Biol. Invasions 2021, 23, 1191–1206. [Google Scholar] [CrossRef]
- Oh, M.; Heo, Y.; Lee, E.J.; Lee, H. Major environmental factors and traits of invasive alien plants determine their spatial distribution: A case study in Korea. J. Ecol. Environ. 2021, 45, 18. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; Rossi, E.; Milbau, A.; Nijs, I. Habitat properties and plant traits interact as drivers of non- native plant species’ seed production at the local scale. Ecol. Evol. 2018, 8, 4209–4223. [Google Scholar] [CrossRef]
- Johnson, J.S.; Cantrell, R.S.; Cosner, C.; Hartig, F.; Hastings, A.; Rogers, H.S.; Schupp, E.W.; Shea, K.; Teller, B.J.; Yu, X.; et al. Rapid changes in seed dispersal traits may modify plant responses to global change. AoB PLANTS 2019, 11, plz020. [Google Scholar] [CrossRef] [Green Version]
- DiTommaso, A.; Stokes, C.A.; Cordeau, S.; Milbrath, L.R.; Whitlow, T.H. Seed-dispersal ability of the invasive perennial vines Vincetoxicum nigrum and Vincetoxicum rossicum. Invasive Plant Sci. Manag. 2018, 11, 10–19. [Google Scholar] [CrossRef]
- Tokarska-Guzik, B. The Establishment and Spread of Alien Plant Species (Kenophytes) in the Flora of Poland; Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2005. [Google Scholar]
- Klotz, S. Echinocystis lobata (Michx.) Torr. & Gray., wild cucumber (Cucurbitaceae, Magnoliophyta). In Handbook of Alien Species in Europe; Hulme, P.E., Nentwig, W., Pyšek, P., Vilà, M., Eds.; Invading Nature—Springer Series in Invasion Ecology; Springer: Dordrecht, The Netherlands, 2009; p. 347. [Google Scholar]
- Priede, A. Distribution of some invasive alien plant species in riparian habitats in Latvia. Bot. Lith. 2008, 14, 137–150. [Google Scholar]
- Rutkovska, S.; Pučka, I.; Novicka, I.; Evarts-Bunders, P. Relationship of geographic distribution of the most characteristical invasive plant species in habitats adjacent to the river Daugava within the territory of Daugavpils city. Acta Biol. Univ. Daugavp. 2011, 11, 163–175. [Google Scholar]
- Dylewski, Ł.; Myczko, Ł.; Pearson, D.E. Native generalist consumers interact strongly with seeds of the invasive wild cucumber (Echinocystis lobata). NeoBiota 2019, 53, 25–39. [Google Scholar] [CrossRef]
- European Environmental Agency. The EUNIS Habitat Classification. Available online: https://www.eea.europa.eu/data-and-maps/data/eunis-habitat-classification (accessed on 25 February 2020).
- Choate, H.A. Dormancy and germination in seeds of Echinocystis lobata. Am. J. Bot. 1940, 27, 156–160. [Google Scholar] [CrossRef]
- Silvertown, J. Survival, fecundity and growth of wild cucumber, Echinocystis lobata. J. Ecol. 1985, 73, 841–849. [Google Scholar] [CrossRef]
- Stocking, K.M. Some considerations of the genera Echinocystis and Echinopepon in the United States and northern Mexico. Madroño 1955, 13, 84–100. [Google Scholar]
- Nature Manitoba; Reaume, T. Wild Cucumber Echinocystis lobata. 2010. Available online: https://www.naturemanitoba.ca/sites/default/files/WildCucumber.pdf (accessed on 7 May 2021).
- Bagi, I.; Böszörményi, A. Wild cucumber (Echinocystis lobata Torr. et Gray). In The Most Important Invasive Plants in Hungary; Botta-Dukát, Z., Balogh, L., Eds.; Institute of Ecology and Botany, Hungarian Academy of Sciences: Vácrátót, Hungary, 2008; pp. 103–114. [Google Scholar]
- Randall, R.P. A Global Compendium of Weeds, 3rd ed.; Randall RP: Perth, WE, Australia, 2017. [Google Scholar]
- Vasić, O. Echinocystis lobata (Michx) Torrey et A. Gray in Serbia. Acta Bot. Croat. 2005, 64, 369–373. [Google Scholar]
- Botta-Dukát, Z. Invasion of alien species to Hungarian (semi-) natural habitats. Acta Bot. Hung. 2008, 50, 219–227. [Google Scholar] [CrossRef]
- Towpasz, K.; Stachurska-Swakoń, A. Alder-ash and willow communities and their diversity in the PogórzeStrzyżowskie foothills (Western Carpathians). Acta Soc. Bot. Pol. 2008, 77, 327–338. [Google Scholar]
- Towpasz, K.; Stachurska-Swakoń, A. The analysis of the forest flora of the Strzyżowskie Foothills from the perspective of presence of anthropogenic species. Acta Univ. Lodz. Folia Biol. Oecol. 2011, 7, 99–110. [Google Scholar] [CrossRef]
- Towpasz, K.; Stachurska-Swakoń, A. Occurrence of alien species in the agriculture landscape: A case of Proszowice Plateau (Southern Poland). Ann. Univ. Paedagog. Crac. Stud. Nat. 2018, 3, 7–21. [Google Scholar] [CrossRef]
- Zelnik, I. The presence of invasive alien plant species in different habitats: Case study from Slovenia. Acta Biol. Slov. 2012, 55, 25–38. [Google Scholar]
- Kołaczkowska, E. Kolczurka klapowana Echinocystis lobata (F. Michx.) Torrey& A. Gray. In Inwazyjne gatunki roślin w Kampinoskim Parku Narodowym i jego sąsiedztwie. Kampinoski Park Narodowy; Otręba, A., Michalska-Hejduk, D., Eds.; Kampinoski Park Narodowy: Izabelin, Poland, 2014; pp. 37–40. [Google Scholar]
- Gerrath, J.M.; Guthrie, T.B.; Zitnak, T.A.; Posluszny, U. Development of the axillary bud complex in Echinocystislobata (Cucurbitaceae): Interpreting the Cucurbitaceoustendril. Am. J. Bot. 2008, 95, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Kovach, W.L. MVSP—A MultiVariate Statistical Package for Windows ver. 3; Kovach Computing Services: Pentraeth, UK, 2010. [Google Scholar]
- Van Emden, H. Statistics for Terrified Biologists; Blackwell Publishing: Hoboken, NJ, USA, 2008. [Google Scholar]
- Rolbiecki, R.; Rolbiecki, S.; Figas, A.; Wichrowska, D.; Jagosz, B.; Ptach, W. The efficiency of drip fertigation in cultivation of winter squash ‘Gomez’ on the very light soil. Infrastruct. Ecol. Rural. Areas 2017, 3, 1201–1211. [Google Scholar]
- Spjut, R.W. A Systematic Treatment of the Fruit Types. 2015. Available online: http://www.worldbotanical.com/fruit_types.htm (accessed on 7 May 2021).
- Nerson, H. Seed production and germinability of cucurbit crops. Seed Sci. Biotech. 2007, 1, 1–10. [Google Scholar]
- Oliveira, E.C.; de Carvalho, J.A.; da Silva, W.G.; Rezende, F.C.; de Almeida, W.F. Effects of water deficit in two phenological stages on production of japanese cucumber cultived in greenhouse. Eng. Agríc. 2011, 31, 676–686. [Google Scholar] [CrossRef] [Green Version]
- Maynard, L. Cucurbit crop growth and development. In Indiana CCA Conference Proceedings; Purdue University: West Lafayette, IN, USA, 2007. [Google Scholar]
- Esbenshade, W.R.; Curran, W.S.; Roth, G.W.; Hartwig, N.L.; Orzolek, M.D. Effect of establishment date and crop competition on burcucumber fecundity. Weed Sci. 2001, 49, 524–527. [Google Scholar] [CrossRef]
- Zelnik, I.; Klenovsek, V.M.; Gaberscik, A. Complex undisturbed riparian zones are resistant to colonisation by invasive alien plant species. Water 2020, 12, 345. [Google Scholar] [CrossRef] [Green Version]
- Obidziński, A.; Mędrzycki, P.; Kołaczkowska, E.; Ciurzycki, W.; Marciszewska, K. Do David and Goliathplay the same game? Explanation of the abundance of rare and frequent invasive alien plants in urban woodlands in Warsaw, Poland. PLoS ONE 2016, 11, e0168365. [Google Scholar] [CrossRef] [Green Version]
- Kazinczi, G.; Horváth, J.; Hunyadi, K. Germination biology and virus susceptibility of wild cucumber (Echinocystis lobata Torr. et Gray). Növénytermelés 1998, 47, 645–654. [Google Scholar]
- Lukatkin, A.S.; Tyutyaev, E.V.; Sharkaeva, E.S.; Lukatkin, A.A.; Teixeira da Silva, J.A. Mild abiotic stresses have different effects on chlorophyll fluorescence parameters in leaves of young woody and herbaceous invasive plants. Acta Physiol. Plant 2017, 39, 20. [Google Scholar] [CrossRef]
- Önen, H.; Farooq, S.; Tad, S.; Özaslan, C.; Gunal, H.; Chauhan, B.S. The influence of environmental factors on germination of burcucumber (Sicyos angulatus) seeds: Implications for range expansion and management. Weed Sci. 2018, 66, 494–501. [Google Scholar] [CrossRef]
- Wyka, T.P.; Zadworny, M.; Mucha, J.; Żytkowiak, R.; Nowak, K.; Oleksyn, J. Species-specific responses of growth and biomass distribution to trellis availability in three temperate lianas. Trees 2019, 33, 921–932. [Google Scholar] [CrossRef] [Green Version]
- Paul, G.S.; Yavitt, J.B. Tropical vine growth and the effects on forest succession: A review of the ecology and management of tropical climbing plants. Bot. Rev. 2011, 77, 11–30. [Google Scholar] [CrossRef]
- Driedonks, N.; Rieu, I.; Vriezen, W.H. Breeding for plant heat tolerance at vegetative and reproductive stages. Plant Reprod. 2016, 29, 67–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushal, N.; Bhandarik, K.; Siddique, K.H.; Nayyar, H. Food crops face rising temperatures: An overview of responses, adaptive mechanisms, and approaches to improve heat tolerance. Cogent Food Agric. 2016, 2, 1134380. [Google Scholar] [CrossRef]
- Janni, M.; Gullì, M.; Maestri, E.; Marmiroli, M.; Valliyodan, B.; Nguyen, H.T.; Marmiroli, N. Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. J. Exp. Bot. 2020, 71, 3780–3802. [Google Scholar] [CrossRef]
- Jing, H.-C.; Bergervoet, J.H.W.; Jalink, H.; Klooster, M.; Du, S.-L.; Bino, R.J.; Hilhorst, H.W.M.; Groot, S.P.C. Cucumber (Cucumis sativus L.) seed performance as influenced by ovary and ovule position. Seed Sci. Res. 2000, 10, 435–445. [Google Scholar] [CrossRef]
- Nerson, H. Fruit-set order affects seed yield and germinability in melon (Cucumis melo L.). J. Hortic. Sci. Biotechnol. 2004, 79, 985–990. [Google Scholar] [CrossRef]
- Nerson, H. Does fruit number per plant, or fruit-set order affect seed yield and quality in cucumber? J. Hortic. Sci. Biotechnol. 2008, 83, 160–164. [Google Scholar] [CrossRef]
- Nerson, H.; Paris, H.S. Effects of fruit age, fermentation and storage on germination of cucurbit seeds. Sci. Hortic. 1988, 35, 15–26. [Google Scholar] [CrossRef]
- Khan, A.S.M.M.R.; Kabir, M.Y.; Alam, M.M. Variability, correlation path analysis of yield and yield components of pointed gourd. J. Agric. Rural. Dev. 2009, 7, 93–98. [Google Scholar] [CrossRef]
- Tanaka, K.; Duong, T.-T.; Yamashita, H.; Heng, S.L.; Sophany, S.; Kato, K. Collection of cucurbit crops (Cucurbitaceae) from Eastern Cambodia. AREIPGR 2017, 32, 109–137. [Google Scholar]
- Sari, N.; Solmaz, I.; Pamuk, S.; Cetin, B.; Gocmen, M.; Simsek, I. Fruit and seed size in some mini watermelon lines. Acta Hortic. 2017, 1151, 109–114. [Google Scholar] [CrossRef]
- Nerson, H. Plant density, fruit length and fruit type affect seed yield and quality in cucumber. Adv. Hortic. Sci. 2005, 19, 206–212. [Google Scholar]
- Barzegar, R.; Houshmand, S.; Peyvast, G.H. Relationship between seed yield and some of fruit traits in Iranian squash (Cucurbita pepo L.) accissions. J. Hortic. Sci. 2015, 29, 142–149. [Google Scholar] [CrossRef]
- Dylewski, Ł.; Maćkowiak, Ł.; Myczko, Ł. Physical defence of the wild cucumber Echinocystis lobata in an invasive range changing seed removal by rodents. Plant Ecol. 2018, 219, 863–873. [Google Scholar] [CrossRef] [Green Version]
- Golivets, M. Variation in quantitative seed traits of Echinocystis lobata (Michx.) Torr. et A. Gray (Cucurbitaceae). Mod. Phytomorphol. 2014, 6, 43–44. [Google Scholar]
- Louda, S.M. Distribution ecology: Variation in plant recruitment over a gradient in relation to insect seed predation. Ecol. Monogr. 1982, 52, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Maron, J.L.; Gardner, S.N. Consumer pressure, seed versus safe-site limitation, and plant population dynamics. Oecologia 2000, 124, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Maron, J.L.; Crone, E. Herbivory: Effects on plant abundance, distribution and population growth. Proc. Royal Soc. B 2006, 273, 2575–2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stachurska-Swakoń, A.; Barabasz-Krasny, B.; Klasa, A.; Palaczyk, A. Reduced plant fitness by pre-dispersal seed predation in the threatened plant species Cirsium decussatum. Seed Sci. Res. 2018, 28, 123–130. [Google Scholar] [CrossRef]
- Sinnott-Armstrong, M.A.; Downie, A.E.; Federman, S.; Valido, A.; Jordano, P.; Donoghue, M.J. Global geographic patterns in the colours and sizes of animal-dispersed fruits. Glob. Ecol. Biogeogr. 2018, 27, 1–13. [Google Scholar] [CrossRef]
- Richardson, D.M.; Allsopp, N.; D’Antonio, C.M.; Milton, S.J.; Rejmánek, M. Plant invasions—The role of mutualisms. Biol. Rev. 2000, 75, 65–93. [Google Scholar] [CrossRef]
- Zając, A.; Tokarska-Guzik, B.; Zając, M. The role of rivers and streams in the migration of alien plants into the Polish Carpathians. Biodiv. Res.Conserv. 2011, 23, 43–56. [Google Scholar] [CrossRef]
- Moles, A.T.; Westoby, M. Latitude, seed predation and seed mass. J. Biogeogr. 2003, 30, 105–128. [Google Scholar] [CrossRef]
- Moles, A.T.; Ackerly, D.D.; Tweddle, J.C.; Dickie, J.B.; Smith, R.; Leishman, M.R.; Mayfield, M.M.; Pitman, A.; Wood, J.T.; Westoby, M. Global patterns in seed size. Glob. Ecol. Biogeogr. 2007, 16, 109–116. [Google Scholar] [CrossRef]
- Pluess, A.R.; Schutz, W.; Stocklin, J. Seed weight increases with altitude in the Swiss Alps between related species but not among populations of individual species. Oecologia 2005, 144, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Mazer, S.J.; Du, G. Geographic variation in seed mass within and among nine species of Pedicularis (Orobanchaceae): Effects of elevation, plant size and seed number per fruit. J. Ecol. 2010, 98, 1232–1242. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Lai, L.; Jiang, L.; Zhuang, P.; Zhang, L. Geographic variation in seed traits within and among forty-two species of Rhododendron (Ericaceae) on the Tibetan plateau: Relationships with altitude, habitat, and phylogeny. Ecol. Evol. 2014, 4, 1913–1923. [Google Scholar] [CrossRef]
- Olejniczak, P.; Czarnoleski, M.; Delimat, A.; Majcher, B.M.; Szczepka, K. Seed size in mountain herbaceous plants changes with elevation in a species-specific manner. PLoS ONE 2018, 13, e0199224. [Google Scholar] [CrossRef] [PubMed]
- Fenner, M.; Thompson, K. Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Guo, Y.; Gao, M.; Liang, X.; Xu, M.; Liu, X.; Zhang, Y.; Liu, X.; Liu, J.; Gao, Y.; Qu, S.; et al. Quantitative trait loci for seed size variation in cucurbits—A review. Front. Plant. Sci. 2020, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Paris, H.S.; Nerson, H. Seed dimensions in the subspecies and cultivar-groups of Cucurbita pepo. Gen. Resour. Crop Evol. 2003, 50, 615–625. [Google Scholar] [CrossRef]
- Rindyastuti, R.; Hapsari, L.; Byun, C. Comparison of ecophysiological and leaf anatomical traits of native and invasive plant species. J. Ecol. Environ. 2021, 45, 4. [Google Scholar] [CrossRef]
- Molina-Montenegro, M.A.; Acuña-Rodríguez, I.S.; Flores, T.S.M.; Hereme, R.; Lafon, A.; Atala, C.; Torres-Díaz, C. Is the Success of Plant Invasions the Result of Rapid Adaptive Evolution in Seed Traits? Evidence from a Latitudinal Rainfall Gradient. Front. Plant. Sci. 2018, 9, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Habitat Origin | Number of Records | Fruit Length (cm) | Fruit Width (cm) | Fruit Fresh Weight (g) | Total Number of Seeds per Fruit | Number of Undeveloped Seeds per Fruit |
|---|---|---|---|---|---|---|
| A | 566 | 4.17 (±0.59) a | 3.18 (±0.41) a | 12.95 (±4.92) a | 3.86 (±1.04) a | 0.56 (±1.00) a |
| N | 1758 | 4.70 (±0.63) b | 3.43 (±0.84) b | 15.92 (±4.79) b | 4.04 (±0.89) b | 0.55 (±1.16) a |
| SN | 103 | 4.45 (±0.82) c | 3.08 (±0.46) a | 14.17 (±6.19) b | 4.33 (±0.99) c | 0.45 (±1.02) a |
| F value (df = 2) | 151.85 | 30.91 | 80.79 | 14.59 | 0.38 | |
| p value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.67 | |
| Habitat Type | Number of Records | Fruit Length (cm) | Fruit Width (cm) | Fruit Fresh Weight (g) | Total Number of Seeds per Fruit | Number of Undeveloped Seeds per Fruit |
|---|---|---|---|---|---|---|
| C | 100 | 4.51 (±0.94) | 3.24 (±0.57) | 15.77 (±6.01) | 4.50 (±0.85) | 0.64 (±1.15) |
| D | 30 | 5.16 (±0.28) | 3.97 (±0.28) | 21.69 (±4.47) | 4.33 (±0.54) | 0.36 (±0.76) |
| E | 430 | 4.42 (±0.70) | 3.33 (±0.40) | 14.48 (±5.28) | 4.19 (±0.87) | 0.48 (±0.98) |
| F | 474 | 4.92 (±0.53) | 3.42 (±0.33) | 16.28 (±4.49) | 4.17 (±0.54) | 0.35 (±0.71) |
| G | 919 | 4.67 (±0.60) | 3.45 (±1.11) | 15.96 (±4.64) | 3.88 (±1.03) | 0.70 (±1.38) |
| H | 29 | 4.72 (±0.35) | 3.15 (±0.23) | 16.56 (±3.55) | 4.38 (±0.37) | 0.65 (±0.67) |
| I | 96 | 4.02 (±0.55) | 3.15 (±0.44) | 12.59 (±4.27) | 3.91 (±1.06) | 0.30 (±0.63) |
| J | 289 | 4.09 (±0.53) | 3.15 (±0.41) | 12.05 (±4.42) | 3.83 (±1.07) | 0.62 (±1.24) |
| X | 60 | 4.09 (±0.61) | 3.26 (±0.47) | 12.77 (±5.99) | 3.43 (±0.92) | 0.18 (±0.53) |
| Height Level (m) | Number of Records | Fruit Length (cm) | Fruit Width (cm) | Fruit Fresh Weight (g) | Total Number of Seeds per Fruit | Number of Undeveloped Seeds per Fruit |
|---|---|---|---|---|---|---|
| 0.00–0.50 | 469 | 4.52 (±0.69) | 3.32 (±0.45) | 15.49 (±5.33) | 4.08 (±0.84) | 0.89 (±1.42) |
| 0.51–1.00 | 387 | 4.61 (±0.73) | 3.35 (±0.41) | 15.65 (±5.62) | 4.12 (±0.97) | 0.43 (±1.00) |
| 1.01–1.50 | 517 | 4.53 (±0.64) | 3.38 (±1.44) | 14.40 (±5.15) | 3.88 (±1.10) | 0.60 (±1.21) |
| 1.51–2.00 | 397 | 4.53 (±0.64) | 3.40 (±0.45) | 14.94 (±4.86) | 4.03 (±0.80) | 0.42 (±1.02) |
| 2.01–2.50 | 296 | 4.55 (±0.66) | 3.36 (±0.36) | 15.13 (±4.04) | 4.11 (±0.79) | 0.45 (±1.02) |
| 2.51–3.00 | 216 | 4.60 (±0.66) | 3.36 (±0.34) | 15.69 (±4.02) | 4.05 (±0.75) | 0.29 (±0.59) |
| 3.01–3.50 | 82 | 4.74 (±0.67) | 3.33 (±0.39) | 15.23 (±4.72) | 3.97 (±0.81) | 0.21 (±0.56) |
| 3.51–4.00 | 63 | 4.77 (±0.55) | 3.35 (±0.33) | 15.25 (±3.55) | 3.19 (±1.51) | 0.73 (±1.43) |
| Height Level (m) | Number of Seeds | Fresh Mass of Seeds (mg) |
|---|---|---|
| 0.00–0.50 | 30 | 1.53 (±0.42) |
| 0.51–1.00 | 100 | 1.55 (±0.45) |
| 1.01–1.50 | 117 | 1.59 (±0.57) |
| 1.51–2.00 | 77 | 1.65 (±0.44) |
| 2.01–2.50 | 60 | 1.82 (±0.39) |
| 2.51–3.00 | 47 | 1.61 (±0.42) |
| 3.01–3.50 | 30 | 1.55 (±0.53) |
| 3.51–4.00 | 30 | 1.50 (±0.42) |
| Height Level (m) | Number of Records | Dry Mass of Seeds (g) |
|---|---|---|
| 0.00–0.50 | 42 | 0.27 (±0.03) |
| 0.51–1.00 | 37 | 0.27 (±0.03) |
| 1.01–1.51 | 38 | 0.29 (±0.04) |
| 1.51–2.00 | 29 | 0.28 (±0.04) |
| 2.01–2.50 | 23 | 0.28 (±0.04) |
| 2.51–3.00 | 20 | 0.29 (±0.04) |
| 3.01–3.50 | 6 | 0.30 (±0.03) |
| 3.51–4.00 | 4 | 0.31 (±0.03) |
| Height Level (m) | Habitat Type | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | D | E | F | G | H | I | J | X | |
| 0.00–0.50 | 4.38 (±0.79) | 5.22 (±0.28) | 4.53 (±0.74) | 4.75 (±0.52) | 4.70 (±0.73) | 4.56 (±0.38) | 3.96 (±0.67) | 4.21 (±0.40) | |
| 0.51–1.00 | 4.79 (±1.34) | 4.58 (±0.58) | 5.05 (±0.52) | 4.68 (±0.66) | 4.91 (±0.33) | 4.03 (±0.53) | 4.01 (±0.62) | 3.76 (±0.46) | |
| 1.01–1.51 | 4.56 (±0.25) | 5.20 (±0.25) | 4.52 (±0.61) | 4.92 (±0.55) | 4.57 (±0.61) | 4.68 (±0.26) | 4.04 (±0.43) | 4.08 (±0.50) | 4.08 (±0.32) |
| 1.51–2.00 | 4.50 (±0.29) | 5.07 (±0.30) | 4.19 (±0.56) | 4.95 (±0.51) | 4.64 (±0.57) | 3.95 (±0.68) | 4.10 (±0.64) | 4.51 (±0.59) | |
| 2.01–2.50 | 4.20 (±0.80) | 4.84 (±0.54) | 4.70 (±0.49) | 4.13 (±0.48) | 3.63 (±0.52) | 4.54 (±0.78) | |||
| 2.51–3.00 | 4.25 (±0.86) | 4.93 (±0.51) | 4.74 (±0.50) | 4.05 (±0.37) | 4.17 (±0.37) | 4.09 (±0.33) | |||
| 3.01–3.50 | 5.22 (±0.61) | 4.77 (±0.52) | 3.60 (±0.44) | ||||||
| 3.51–4.00 | 5.17 (±0.22) | 4.68 (±0.49) | |||||||
| Height Level (m) | Habitat Type | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | D | E | F | G | H | I | J | X | |
| 0.00–0.50 | 3.03 (±0.63) | 3.92 (±0.26) | 3.43 (±0.42) | 3.40 (±0.32) | 3.40 (±0.42) | 3.15 (±0.27) | 2.91 (±0.49) | 3.28 (±0.37) | |
| 0.51–1.00 | 3.57 (±0.53) | 3.35 (±0.37) | 3.49 (±0.34) | 3.41 (±0.39) | 3.27 (±0.20) | 3.45 (±0.52) | 3.04 (±0.40) | 2.93 (±0.34) | |
| 1.01–1.51 | 3.28 (±0.35) | 3.92 (±0.36) | 3.33 (±0.33) | 3.38 (±0.35) | 3.53 (± 2.29) | 3.03 (±0.15) | 3.15 (±0.35) | 3.09 (±0.37) | 3.32 (±0.24) |
| 1.51–2.00 | 3.59 (±0.13) | 4.07 (±0.18) | 3.26 (±0.40) | 3.47 (±0.32) | 3.46 (±0.49) | 3.11 (±0.48) | 3.20 (±0.41) | 3.65 (±0.50) | |
| 2.01–2.50 | 3.34 (±0.42) | 3.38 (±0.36) | 3.41 (±0.25) | 3.32 (±0.33) | 2.88 (±0.59) | 3.58 (±0.32) | |||
| 2.51–3.00 | 3.26 (±0.46) | 3.43 (±0.29) | 3.39 (±0.29) | 3.26 (±0.26) | 3.29 (±0.27) | 3.28 (±0.33) | |||
| 3.01–3.50 | 3.37 (±0.27) | 3.42 (±0.25) | 2.79 (±0.35) | ||||||
| 3.51–4.00 | 3.43 (±0.27) | 3.34 (±0.34) | |||||||
| Height Level (m) | Habitat Type | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | D | E | F | G | H | I | J | X | |
| 0.00–0.50 | 15.44 (±5.60) | 23.60 (±6.25) | 15.33 (±5.51) | 15.98 (±4.63) | 16.59 (±4.92) | 15.43 (±3.05) | 13.59 (±6.09) | 13.20 (±4.72) | |
| 0.51–1.00 | 18.17 (±7.71) | 16.54 (±5.99) | 17.40 (±4.71) | 15.67 (±5.32) | 18.77 (±3.85) | 13.03 (±3.02) | 11.37 (±3.85) | 10.88 (±3.27) | |
| 1.01–1.51 | 15.12 (±6.07) | 21.94 (±3.07) | 13.62 (±5.01) | 16.22 (±5.09) | 14.97 (±4.95) | 15.36 (±2.78) | 12.30 (±3.48) | 11.30 (±4.53) | 12.06 (±2.24) |
| 1.51–2.00 | 13.93 (±2.41) | 19.53 (±2.60) | 13.22 (±4.31) | 15.63 (±3.44) | 16.29 (±5.01) | 11.14 (±4.45) | 12.12 (±4.23) | 17.51 (±8.11) | |
| 2.01–2.50 | 13.62 (±4.63) | 15.78 (±5.52) | 16.49 (±3.28) | 12.95 (±2.87) | 8.85 (±2.82) | 14.91 (±7.98) | |||
| 2.51–3.00 | 13.54 (±4.60) | 17.36 (±3.10) | 16.51 (±3.54) | 12.98 (±3.38) | 14.50 (±2.37) | 12.87 (±4.57) | |||
| 3.01–3.50 | 15.00 (±3.56) | 16.63 (±4.13) | 8.41 (±3.72) | ||||||
| 3.51–4.00 | 16.59 (±1.73) | 14.96 (±3.78) | |||||||
| Height Level (m) | Habitat Type | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | D | E | F | G | H | I | J | X | |
| 0.00–0.50 | 4.42 (±0.99) | 4.60 (±0.51) | 4.00 (±0.71) | 4.30 (±0.59) | 4.00 (±0.77) | 4.22 (±0.46) | 3.91 (±1.26) | 3.92 (±0.99) | |
| 0.51–1.00 | 4.65 (±0.81) | 4.30 (±0.98) | 4.28 (±0.68) | 3.96 (±1.04) | 4.70 (±0.44) | 3.75 (±1.28) | 3.96 (±0.87) | 3.10 (±1.10) | |
| 1.01–1.51 | 4.70 (±0.65) | 4.40 (±0.69) | 4.25 (±0.88) | 4.05 (±0.46) | 3.62 (±1.30) | 4.20 (±0.94) | 3.60 (±0.50) | 3.70 (±1.36) | 3.70 (±0.67) |
| 1.51–2.00 | 4.20 (±0.42) | 4.00 (±0.00) | 4.08 (±0.89) | 4.06 (±0.55) | 4.04 (±0.74) | 4.20 (±1.47) | 3.86 (±0.95) | 4.00 (±0.81) | |
| 2.01–2.50 | 4.16 (±0.97) | 4.26 (±0.57) | 4.12 (±0.84) | 3.90 (±0.64) | 3.66 (±0.89) | 3.80 (±0.42) | |||
| 2.51–3.00 | 4.40 (±0.90) | 4.11 (±0.37) | 3.92 (±0.67) | 4.33 (±0.81) | 3.54 (±1.12) | 3.30 (±0.82) | |||
| 3.01–3.50 | 4.05 (±0.22) | 4.19 (±0.68) | 2.70 (±1.05) | ||||||
| 3.51–4.00 | 4.45 (±0.52) | 2.92 (±1.51) | |||||||
| Height Level (m) | Habitat Type | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | D | E | F | G | H | I | J | X | |
| 0.00–0.50 | 1.04 (±1.42) | 0.40 (±0.96) | 0.50 (±1.04) | 0.33 (±0.63) | 1.04 (±1.61) | 0.55 (±0.72) | 0.41 (±0.59) | 1.58 (±1.73) | |
| 0.51–1.00 | 0.35 (±0.58) | 0.47 (±0.95) | 0.28 (±0.56) | 0.63 (±1.37) | 0.80 (±0.63) | 0.25 (±0.46) | 0.19 (±0.56) | 0.20 (±0.63) | |
| 1.01–1.51 | 0.25 (±0.71) | 0.30 (±0.67) | 0.58 (±1.11) | 0.43 (±0.83) | 0.99 (±1.38) | 0.60 (±0.69) | 0.15 (±0.48) | 0.10 (±0.30) | 0.00 (±0.00) |
| 1.51–2.00 | 0.00 (±0.00) | 0.30 (±0.69) | 0.41 (±0.80) | 0.19 (±0.48) | 0.65 (±1.38) | 0.40 (±0.94) | 0.29 (±0.75) | 0.00 (±0.00) | |
| 2.01–2.50 | 0.52 (±1.05) | 0.54 (±0.99) | 0.48 (±1.16) | 0.20 (±0.41) | 0.26 (±0.59) | 0.10 (±0.31) | |||
| 2.51–3.00 | 0.32 (±0.76) | 0.32 (±0.53) | 0.29 (±0.55) | 0.50 (±0.83) | 0.09 (±0.30) | 0.10 (±0.31) | |||
| 3.01–3.50 | 0.50 (±0.76) | 0.02 (±0.13) | 0.70 (±0.94) | ||||||
| 3.51–4.00 | 0.09 (±0.30) | 0.87 (±1.54) | |||||||
| Length of Fruits | Width of Fruits | Fresh Weight of Fruits | Total Number of Seeds | Number of Undeveloped Seeds | |
|---|---|---|---|---|---|
| Length of fruits | 1.00 | 0.29 | 0.66 | 0.28 | −0.07 |
| Width of fruits | 1.00 | 0.35 | 0.10 | 0.01 | |
| Fresh weight of fruits | 1.00 | 0.31 | −0.10 | ||
| Total number of seeds | 1.00 | −0.08 | |||
| Number of undeveloped seeds | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostrakiewicz-Gierałt, K.; Pliszko, A.; Barabasz-Krasny, B.; Bomanowska, A.; Dajdok, Z.; Gudžinskas, Z.; Kucharczyk, M.; Maćkowiak, Ł.; Majk, J.; Możdżeń, K.; et al. The Relationships of Habitat Conditions, Height Level, and Geographical Position with Fruit and Seed Traits in Populations of Invasive Vine Echinocystis lobata (Cucurbitaceae) in Central and Eastern Europe. Forests 2022, 13, 256. https://doi.org/10.3390/f13020256
Kostrakiewicz-Gierałt K, Pliszko A, Barabasz-Krasny B, Bomanowska A, Dajdok Z, Gudžinskas Z, Kucharczyk M, Maćkowiak Ł, Majk J, Możdżeń K, et al. The Relationships of Habitat Conditions, Height Level, and Geographical Position with Fruit and Seed Traits in Populations of Invasive Vine Echinocystis lobata (Cucurbitaceae) in Central and Eastern Europe. Forests. 2022; 13(2):256. https://doi.org/10.3390/f13020256
Chicago/Turabian StyleKostrakiewicz-Gierałt, Kinga, Artur Pliszko, Beata Barabasz-Krasny, Anna Bomanowska, Zygmunt Dajdok, Zigmantas Gudžinskas, Marek Kucharczyk, Łukasz Maćkowiak, Jakub Majk, Katarzyna Możdżeń, and et al. 2022. "The Relationships of Habitat Conditions, Height Level, and Geographical Position with Fruit and Seed Traits in Populations of Invasive Vine Echinocystis lobata (Cucurbitaceae) in Central and Eastern Europe" Forests 13, no. 2: 256. https://doi.org/10.3390/f13020256
APA StyleKostrakiewicz-Gierałt, K., Pliszko, A., Barabasz-Krasny, B., Bomanowska, A., Dajdok, Z., Gudžinskas, Z., Kucharczyk, M., Maćkowiak, Ł., Majk, J., Możdżeń, K., Podgórska, M., Rasimavičius, M., Rewicz, A., Szczęśniak, E., Wójcik, T., & Stachurska-Swakoń, A. (2022). The Relationships of Habitat Conditions, Height Level, and Geographical Position with Fruit and Seed Traits in Populations of Invasive Vine Echinocystis lobata (Cucurbitaceae) in Central and Eastern Europe. Forests, 13(2), 256. https://doi.org/10.3390/f13020256







