Impacts of Site Conditions and Stand Structure on the Biomass Allocation of Single Trees in Larch Plantations of Liupan Mountains of Northwest China
Abstract
:1. Introduction
2. Materials and Methods
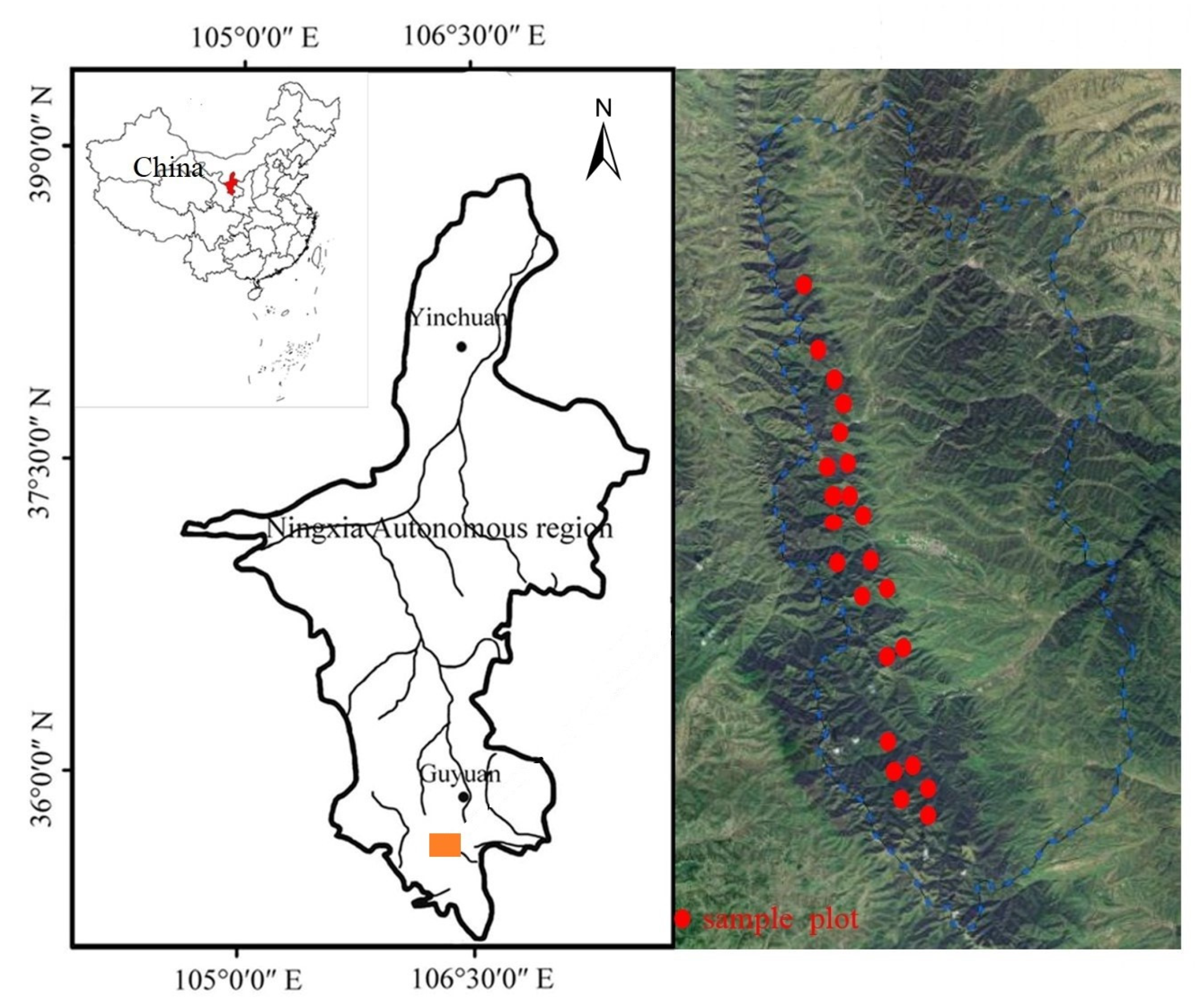
2.1. Study Area
2.2. Data Collection
2.2.1. Study Plots and Sample Trees
2.2.2. Biomass Assessment
2.3. Statistical Analyses
2.3.1. Univariate Response of Biomass Allocation to Site Conditions and Stand Structure
2.3.2. Structural Equation Models of Organ Biomass
2.3.3. Statistics and Figures
3. Results
3.1. The Variation of Biomass Allocation with Site Conditions and Stand Structure
3.2. The R/S Responses to Stand Structure and Site Conditions
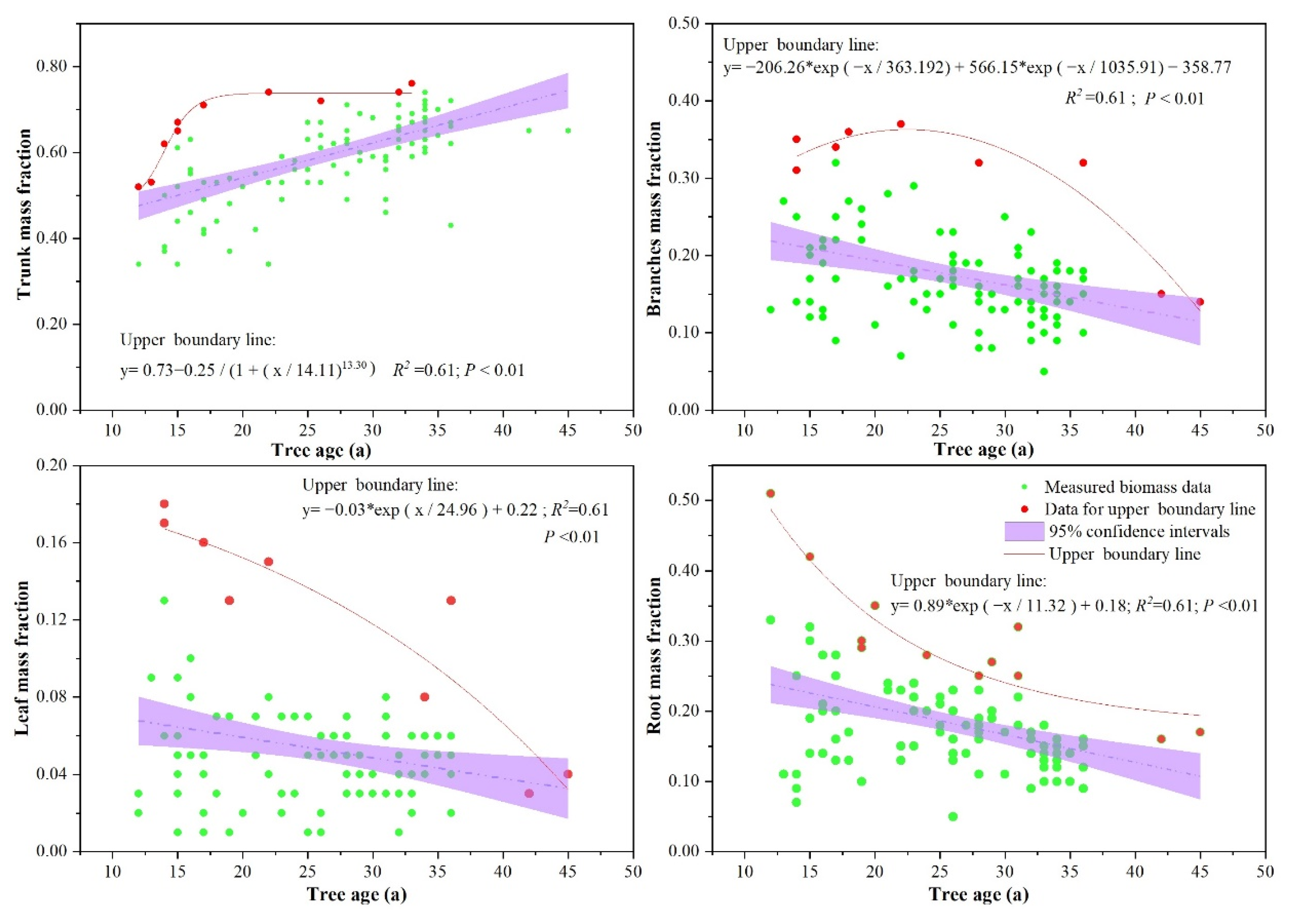
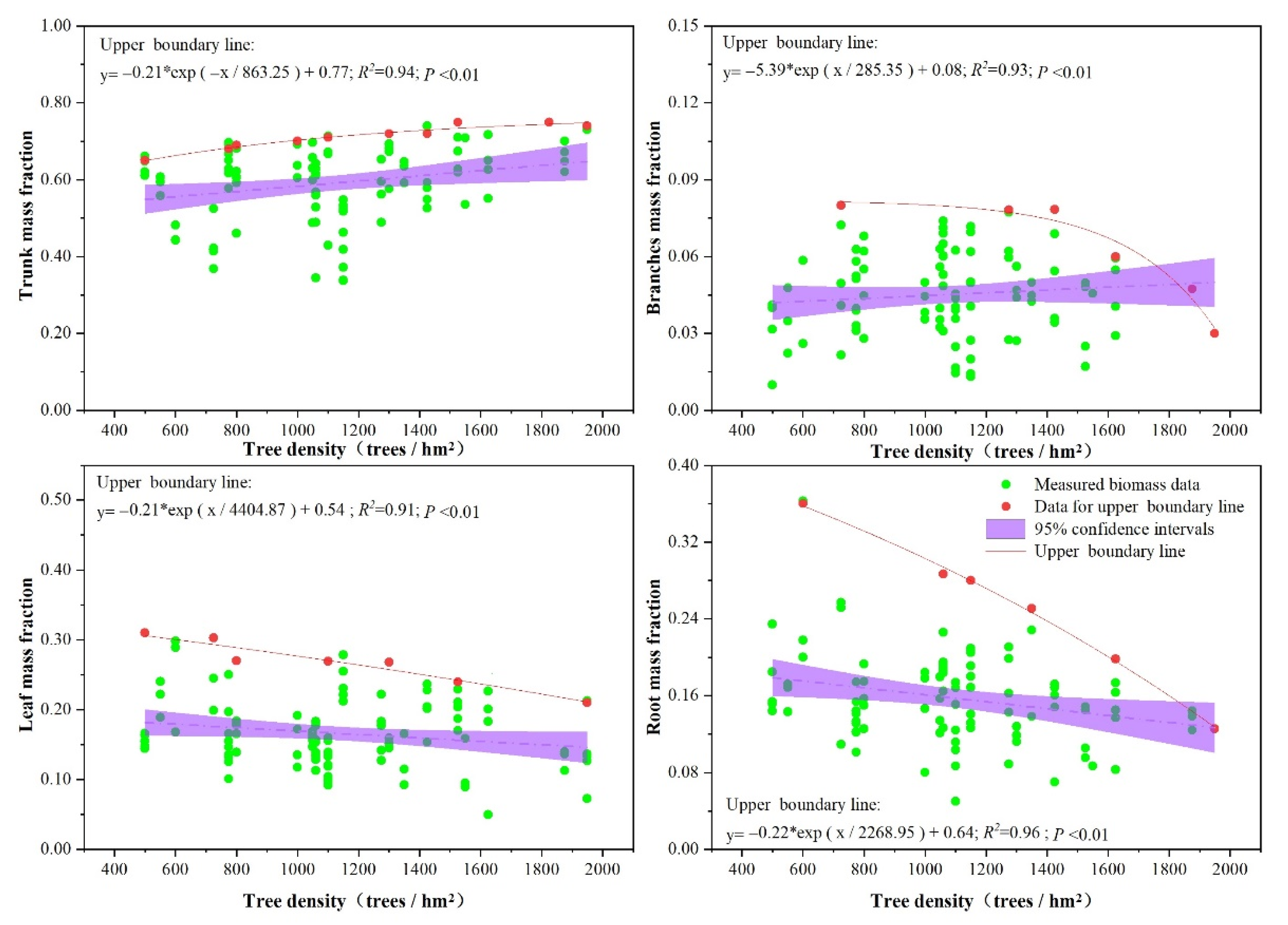
3.3. Effects of Stand Structure (Tree Age, Tree Dominance, and Tree Density) on Biomass Allocation
3.4. Effects of Elevation on Biomass Allocation
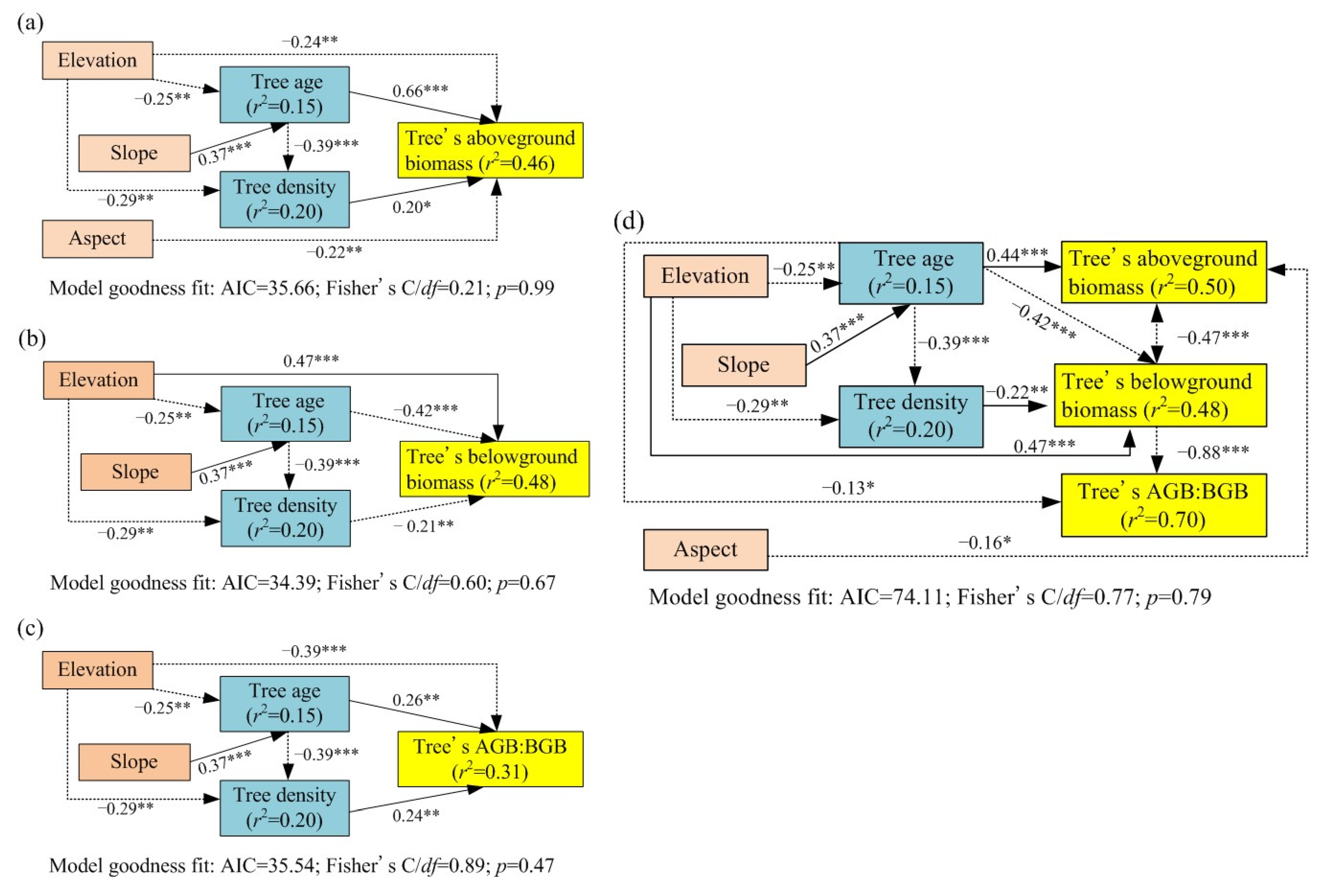
3.5. Establishment of Tree Biomass Allocation Multifactor Model
4. Discussion
4.1. Effects of Stand Structure on Biomass Allocation
4.2. Effects of Site Conditions on Biomass Allocation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schimel, D.S. Terrestrial ecosystems and the carbon cycle. Glob. Chang. Biol. 1995, 1, 77–91. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, H.; Zhou, G.; Zhou, X.; Hong, Y.; Li, C.; Lu, C.; He, Y.; Shao, J.; Sun, X.; et al. Responses of biomass allocation to multi-factor global change: A global synthesis. Agr. Ecosyst. Environ. 2020, 304, 107115. [Google Scholar] [CrossRef]
- Aguirre, A.; Río, M.D.; Ruiz-Peinado, R.; Condés, S. Stand-level biomass models for predicting c stock for the main spanish pine species. For. Ecosyst. 2021, 8, 29. [Google Scholar] [CrossRef]
- Agathokleous, E.; Belz, R.G.; Kitao, M.; Koike, T.; Calabrese, E.J. Does the root to shoot ratio show a hormetic response to stress? An ecological and environmental perspective. J. For. Res. 2019, 30, 1569–1580. [Google Scholar] [CrossRef] [Green Version]
- Mokany, K.; Raison, R.J.; Prokushkin, A.S. Critical analysis of root: Shoot ratios in terrestrial biomes. Glob. Chang. Biol. 2006, 12, 84–96. [Google Scholar] [CrossRef]
- Litton, C.M.; Raich, J.W.; Ryan, M.G. Carbon allocation in forest ecosystems. Glob. Chang. Biol. 2007, 13, 2089–2109. [Google Scholar] [CrossRef] [Green Version]
- Yin, Q.; Tian, T.; Han, X.; Xu, J.; Chai, Y.; Mo, J. The relationships between biomass allocation and plant functional trait. Ecol. Indic. 2019, 102, 302–308. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Funct. Plant Biol. 2000, 27, 595–607. [Google Scholar] [CrossRef]
- Toledo, M.; Poorter, L.; Pea-Claros, M.; Alarcón, A.; Bongers, F. Climate is a stronger driver of tree and forest growth rates than soil and disturbance. J. Ecol. 2011, 99, 254–264. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Rao, X.; Wang, X.; Liang, C.; Lin, Y.; Zhou, L.; Cai, X.-A.; Fu, S. Carbon storage and allocation pattern in plant biomass among di_erent forest plantation stands in Guangdong, China. Forests 2015, 6, 794–808. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Cai, Q.; Xiong, X.; Fang, W.; Zhu, J.; Zhu, J.; Ji, C. Ecosystem carbon stock and within-system distribution in successional Fagus lucida forests in Mt. Yueliang, Guizhou, China. Chin. J. Plant Ecol. 2018, 42, 703–712. [Google Scholar]
- Ouyang, S.; Xiang, W.; Wang, X.; Xiao, W.; Chen, L.; Li, S.; Sun, H.; Deng, X.; Forrester, D.I.; Zeng, L. Effects of stand age, richness and density on productivity in subtropical forests in China. J. Ecol. 2019, 107, 2266–2277. [Google Scholar] [CrossRef]
- Wertz, B.; Bembenek, M.; Karaszewski, Z.; Ocha, W.; Mederski, P.S. Impact of stand density and tree dominance on aboveground biomass allocation of scots pine Pinus sylvestris L. Forests 2020, 11, 765. [Google Scholar] [CrossRef]
- Trugman, A.T.; Anderegg, L.D.L.; Wolfe, B.T.; Birami, B.; Anderegg, W.R.L. Climate and plant trait strategies determine tree carbon allocation to leaves and mediate future forest productivity. Glob. Chang. Biol. 2019, 25, 3395–3405. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Liu, Z.; Zander, P.; Hermanns, T.; Wang, J. Does farmland conversion improve or impair household livelihood in small holder agriculture system? A case study of Grain for Green project impacts in China’s Loess Plateau. World Dev. Pers. 2016, 2, 43–54. [Google Scholar] [CrossRef]
- Wang, S.; Fu, B.; Chen, H.; Liu, Y. Regional development boundary of China’s Loess Plateau: Water limit and land shortage. Land Use Policy 2018, 74, 130–136. [Google Scholar] [CrossRef]
- Wang, G.; Innes, J.L.; Lei, J.; Dai, S.; Wu, S.W. China’s forestry reforms. Science 2007, 318, 1556–1557. [Google Scholar] [CrossRef] [Green Version]
- Liu, H. It is difficult for Chinas greening through large-scale afforestation to cross the Hu Line. Sci. China Earth Sci. 2019, 62, 194–196. [Google Scholar] [CrossRef]
- Wang, Y.; Bonell, M.; Feger, K.H.; Yu, P.; Xiong, W.; Xu, L. Changing forestry policy by integrating water aspects into forest/vegetation restoration in dryland areas in China. Bull. Chin. Acad. Sci. 2012, 26, 59–67. [Google Scholar]
- Tian, A.; Wang, Y.; Webb, A.A.; Yu, P.; Liu, Z.; Wang, X. Water yield variation with elevation, tree age and density of larch plantation in the liupan mountains of the loess plateau and its forest management implications. Sci. Total Environ. 2020, 752, 141752. [Google Scholar] [CrossRef]
- Schädelin, W. Die Durchforstung als Auslese-und Veredelungsbetrieb Höchster Wertleistung; Verlag Paul Haupt, Bern-Leipzig: Bern, Switzerland, 1936; pp. 23–26. [Google Scholar]
- Struhar, M.; Tomsik, K.; Mimra, K. Application of the “boundary line analysis method” for the optimisation of the number of tractors used in an agricultural company. Agr. Econ. 2014, 38, 303–319. [Google Scholar]
- Da, R.; Zhang, Y.; Guo, J.; Wang, J.; Chen, S. Effect of thinning intensity on growth of Larix principis-rupprechtii by using survey method of circular sample of target tree. Chin. Agr. Sci. Bull. 2016, 32, 11–15. [Google Scholar]
- Ali, A.; Sanaei, A.; Li, M.; Asadi Nalivan, O.; Pour, M.J.; Valipour, A.; Karami, J.; Aminpour, M.; Kaboli, H.; Askari, Y. Big-trees—Energy mechanism underlies forest diversity and aboveground biomass. For. Ecol. Manag. 2020, 461, 117968. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Landsberg, J.; Sands, P. Physiological Ecology of Forest Production; Academic Press: London, UK, 2011; pp. 30–35. [Google Scholar]
- Xiang, W.; Li, L.; Ouyang, S.; Xiao, W.; Zeng, L.; Chen, L. Effects of stand age on tree biomass partitioning and allometric equations in chinese fir (cunninghamia lanceolata) plantations. Eur. J. For. Res. 2021, 140, 317–332. [Google Scholar] [CrossRef]
- Saint-André, L.; M’Bou, A.T.; Mabiala, A.; Mouvondy, W.; Jourdan, C.; Roupsard, O.; Deleporte, P.; Hamel, O.; Nouvellon, Y. Age related equations for above- and below-ground biomass of a Eucalyptus hybrid in Congo. For. Ecol. Manag. 2005, 205, 199–214. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M.A. Allometry and partitioning of above- and belowground tree biomass in an age-sequence of white pine forests. For. Ecol. Manag. 2007, 253, 68–80. [Google Scholar] [CrossRef]
- Seo, Y.O.; Lee, Y.J.; Lumbres, R.I.C.; Pyo, J.K.; Kim, R.H.; Son, Y.M.; Lee, K.H. Influence of stand age class on biomass expansion factor and allometric equations for Pinus rigida plantations in South Korea. Scand. J. For. Res. 2013, 28, 566–573. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Zha, T.; Liu, J.; Jia, X.; Wang, X.; Chen, W.; He, G. Patterns of biomass allocation in an age-sequence of secondary Pinus bungeana forests in China. For. Chron. 2014, 90, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.; Lee, K.H.; Lee, K.H.; Park, I.H. Biomass expansion factors and allometric equations in an age sequence for Japanese cedar (Cryptomeria japonica) in southern Korea. J. For. Res. 2014, 18, 316–322. [Google Scholar] [CrossRef]
- Fatemi, F.R.; Yanai, R.D.; Hamburg, S.P.; Vadeboncoeur, M.A.; Arthur, M.A.; Briggs, R.D.; Levine, C.R. Allometric equations for young northern hardwoods: The importance of age-specific equations for estimating aboveground biomass. Can. J. For. Res. 2011, 41, 881–891. [Google Scholar] [CrossRef]
- Mensah, S.; Kaka, R.G.; Seifert, T. Patterns of biomass allocation between foliage and woody structure: The effects of tree size and specific functional traits. Ann. For. Res. 2016, 59, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Naidu, S.L.; DeLucia, E.H.; Thomas, R.B. Contrasting patterns of biomass allocation in dominant and suppressed loblolly pine. Can. J. For. Res. 1998, 128, 1116–1124. [Google Scholar] [CrossRef]
- Peri, P.L.; Gargaglione, V.; Pastur, G.M. Dynamics of above- and below-ground biomass and nutrient accumulation in an age sequence of Nothofagus antartica forest of southern Patagonia. For. Ecol. Manag. 2006, 233, 85–99. [Google Scholar] [CrossRef]
- Zeide, B. Optimal stand density: A solution. Can. J. For. Res. 2004, 34, 846–854. [Google Scholar] [CrossRef]
- Zhang, J.; Oliver, W.W.; Ritchie, M.W. Effect of stand densities on stand dynamics in white fir (Abies concolor) forests in northeast California, USA. For. Ecol. Manag. 2007, 244, 50–59. [Google Scholar] [CrossRef]
- MacDonald, E.; Hubert, J. A review of the effects of silviculture on timber quality of Sitka spruce. For. Inst. For. Great Br. 2002, 75, 107–138. [Google Scholar] [CrossRef] [Green Version]
- Bembenek, M.; Karaszewski, Z.; Kondracki, K.; Łacka, A.; Mederski, P.S.; Skorupski, M.; Strzeli’nski, P.; Sułkowski, S.; Wegiel, A. Value of merchantable timber in Scots pine stands of different densities. Drewno 2014, 57, 133–142. [Google Scholar]
- Gizachew, B.; Brunner, A. Density-growth relationships in thinned and unthinned Norway spruce and Scots pine stands in Norway. Scand. J. For. Res. 2011, 26, 543–554. [Google Scholar]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. N. Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Baskaran, P.; Hyvönen, R.; Berglund, S.L.; Clemmensen, K.E.; Ågren, G.I.; Lindahl, B.D.; Manzoni, S. Modelling the influence of ectomycorrhizal decomposition on plant nutrition and soil carbon sequestration in boreal forest ecosystems. N. Phytol. 2011, 213, 1452–1465. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, L.; Wu, G.; Wang, K.; Shangguan, Z. Large-scale soil organic carbon mapping based on multivariate modelling: The case of grasslands on the Loess Plateau. Land Degrad. Dev. 2018, 29, 26–37. [Google Scholar] [CrossRef]
- Wang, J.; Fan, X.; Fan, J.; Zhang, C.; Xia, F. Effects of tree competition on the biomass partitioning of Abies nephrolepis. Sci. Silvae Sin. 2012, 48, 14–20. [Google Scholar]
- Landuyt, D.; Maes, S.L.; Depauw, L.; Ampoorter, E.; Blondeel, H.; Perring, M.P.; Brūmelis, G.; Brunet, J.; Decocq, G.; den Ouden, J.; et al. Drivers of above-ground understorey biomass and nutrient stocks in temperate deciduous forests. J. Ecol. 2020, 108, 982–997. [Google Scholar] [CrossRef]
- Don, A.; Janssens, I.A.; Marin, G.; Schulze, E.D. Effects of forest management on biomass stocks in romanian beech forests. For. Ecosyst. 2019, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Zhong, Q.; Ma, Y.; Lu, H.; Jin, B.; Li, M.; Zheng, Y.; Cheng, D. The effect of stand and climatic factors on the root–shoot allocation in Chinese natural forest. Chin. J. Appl. Environ. Biol. 2016, 22, 326–331. [Google Scholar]
- Tobin, B.; Nieuwenhuis, M. Biomass expansion factors for Sitka spruce (Picea sitchensis (Bong). Carr.) in Ireland. Eur. J. For. Res. 2005, 126, 189–196. [Google Scholar] [CrossRef]
- Li, B.; Suzuki, J.I.; Hara, T. Latitudinal variation in plant size and relative growth rate in Arabidopsis thaliana. Oecologia 1998, 115, 293–301. [Google Scholar] [CrossRef]
- Primiciaa, I.; Camarerob, J.J.; Jandaa, P. Age, competition, disturbance and elevation effects on tree and stand growth response of primary Picea abies forest to climate. For. Ecol. Manag. 2015, 354, 77–86. [Google Scholar] [CrossRef]
- Tom, E. Growth and shoot: Root ratio of seedlings in relation to nutrient availability. Plant Soil 1995, 18, 259–274. [Google Scholar]
- de Castilho, C.V.; Magnusson, W.E.; de Araújo, R.N.O. Variation in aboveground tree live biomass in a central Amazonian Forest: Effects of soil and topography. For. Ecol. Manag. 2006, 234, 85–96. [Google Scholar] [CrossRef]
- Girardin, C.A.J.; Farfan-Rios, W.; Garcia, K.; Feeley, K.J.; Jørgensen, P.M.; Murakami, A.A. Spatial patterns of above-ground structure, biomass and composition in a network of six Andean elevation transects. Plant Ecol. Divers. 2013, 7, 161–171. [Google Scholar] [CrossRef]
- Pastorella, F.; Paletto, A. Biomass allocation in natural regeneration of Fagus sylvatica and Picea abies trees in Italian alps. For. Stud. 2014, 61, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Amo, L.D.L.; Baares-De-Dios, G.; Cala, V.; Cerda, G.D.L.; Cayuela, L. Trade-offs among aboveground, belowground, and soil organic carbon stocks along altitudinal gradients in Andean tropical montane forests. Front. Plant Sci. 2020, 11, 106. [Google Scholar] [CrossRef] [Green Version]
- Tashi, S.; Singh, B.; Keitel, C.; Adams, M. Soil carbon and nitrogen stocks in forests along an altitudinal gradient in the eastern Himalayas and a meta-analysis of global data. Glob. Chang. Biol. 2016, 22, 2255–2268. [Google Scholar] [CrossRef]
- Ensslin, A.; Rutten, G.; Pommer, U.; Zimmermann, R.; Hemp, A.; Fischer, M. Effects of elevation and land use on the biomass of trees, shrubs and herbs at Mount Kilimanjaro. Ecosphere 2015, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Venter, M.; Dwyer, J.; Dieleman, W.; Ramachandra, A.; Gillieson, D.; Laurance, S. Optimal climate for large trees at high elevations drives patterns of biomass in remote forests of Papua New Guinea. Glob. Chang. Biol. 2017, 23, 4873–4883. [Google Scholar] [CrossRef]
- Peña, M.A.; Feeley, K.J.; Duque, A. Effects of endogenous and exogenous processes on aboveground biomass stocks and dynamics in Andean forests. Plant Ecol. 2018, 219, 1481–1492. [Google Scholar] [CrossRef]
- Fu, B. Simulation of the spatial distribution of climatic element in mountain regions. Acta Meteorol. Sin. 1989, 3, 669–676. [Google Scholar]



| Sample Plot No. | Elevation (m) | Slope Aspect (°) | Slope Gradient (°) | Tree Age (a) | Tree Density (tree/hm2) | Mean DBH * (cm) | Mean Tree Height (m) |
|---|---|---|---|---|---|---|---|
| 1 | 2033 | N15 | 10 | 14 | 1875 | 10.3 | 8.5 |
| 2 | 2042 | E68 | 15 | 17 | 1550 | 11.1 | 9.7 |
| 3 | 2086 | SE115 | 20 | 16 | 1950 | 10.1 | 8.7 |
| 4 | 2150 | SE140 | 25 | 37 | 500 | 22 | 18.6 |
| 5 | 2285 | SE206 | 35 | 34 | 1100 | 17.5 | 17.2 |
| 6 | 2333 | NE66 | 18 | 35 | 1100 | 18.6 | 19.1 |
| 7 | 2355 | W260 | 19 | 32 | 775 | 20.1 | 19 |
| 8 | 2375 | SE121 | 20 | 34 | 775 | 20.1 | 19 |
| 9 | 2560 | SE156 | 33 | 32 | 1275 | 15.2 | 13 |
| 10 | 2570 | SE238 | 25 | 32 | 1050 | 18 | 15.9 |
| 11 | 2624 | W256 | 16 | 32 | 800 | 18.8 | 14 |
| 12 | 2626 | S200 | 23 | 32 | 1000 | 19.1 | 16.1 |
| 13 | 2631 | E86 | 30 | 32 | 1300 | 16.8 | 15.9 |
| 14 | 2693 | W296 | 23 | 22 | 1425 | 16.8 | 11.6 |
| 15 | 2716 | E96 | 36 | 31 | 1350 | 17.8 | 10.7 |
| 16 | 2778 | SW229 | 30 | 28 | 1625 | 15.7 | 12.5 |
| 17 | 2784 | S188 | 12 | 27 | 1525 | 16.6 | 11.7 |
| 18 | 2789 | W269 | 19 | 21 | 775 | 15.8 | 11.5 |
| 19 | 2811 | SE248 | 21 | 19 | 1150 | 12.4 | 7.4 |
| 20 | 2813 | SW239 | 22 | 21 | 550 | 19.1 | 9.8 |
| 21 | 2823 | NW359 | 17 | 14 | 1150 | 7.6 | 4.4 |
| 22 | 2886 | W266 | 30 | 17 | 1066 | 11.7 | 6.7 |
| 23 | 2920 | W281 | 31 | 18 | 725 | 9 | 5.2 |
| Trunk Mass Fraction | Branch Mass Fraction | Leaf Mass Fraction | Root Mass Fraction | |||||
|---|---|---|---|---|---|---|---|---|
| R2 | p | R2 | p | R2 | p | R2 | p | |
| Tree age | +0.61 ** | <0.01 | −0.37 ** | <0.01 | −0.26 ** | 0.008 | −0.24 ** | <0.01 |
| Tree dominance | +0.21 ** | <0.01 | −0.12 | 0.18 | +0.03 | 0.73 | −0.20 | 0.37 |
| Tree density | +0.35 ** | <0.01 | −0.23 ** | <0.01 | −0.38 ** | <0.01 | −0.27 ** | <0.01 |
| Elevation | −0.28 ** | <0.01 | −0.03 | 0.79 | −0.45 ** | <0.01 | +0.59 ** | <0.01 |
| Slope aspect | −0.21 * | 0.04 | −0.08 | 0.42 | −0.39 ** | <0.01 | +0.54 ** | <0.01 |
| Slope gradient | 0.09 | 0.35 | −0.01 | 0.97 | −0.21 * | 0.03 | −0.04 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Huang, X.; Wang, Y.; Yu, P.; Guo, J. Impacts of Site Conditions and Stand Structure on the Biomass Allocation of Single Trees in Larch Plantations of Liupan Mountains of Northwest China. Forests 2022, 13, 177. https://doi.org/10.3390/f13020177
Wang X, Huang X, Wang Y, Yu P, Guo J. Impacts of Site Conditions and Stand Structure on the Biomass Allocation of Single Trees in Larch Plantations of Liupan Mountains of Northwest China. Forests. 2022; 13(2):177. https://doi.org/10.3390/f13020177
Chicago/Turabian StyleWang, Xiao, Xiaonan Huang, Yanhui Wang, Pengtao Yu, and Jianbin Guo. 2022. "Impacts of Site Conditions and Stand Structure on the Biomass Allocation of Single Trees in Larch Plantations of Liupan Mountains of Northwest China" Forests 13, no. 2: 177. https://doi.org/10.3390/f13020177
APA StyleWang, X., Huang, X., Wang, Y., Yu, P., & Guo, J. (2022). Impacts of Site Conditions and Stand Structure on the Biomass Allocation of Single Trees in Larch Plantations of Liupan Mountains of Northwest China. Forests, 13(2), 177. https://doi.org/10.3390/f13020177






