Abstract
Phylogenetic relationships of the orchid genus Spathoglottis (Orchidaceae: Collabieae) in Peninsular Malaysia and Borneo were inferred using the internal transcribed spacer of a nuclear ribosomal DNA (nrITS), a plastid gene maturaseK (matK) and the plastid region trnL-F. Eleven species and three infraspecific taxa of Spathoglottis were examined, with two outgroup species, were included in the phylogenetic analysis. The combined plastid and nuclear data revealed Spathoglottis as monophyletic. From the maximum likelihood, maximum parsimony and Bayesian analyses, Spathoglottis is divided into four major groups which are, (1) the Dwarf Purple Spathoglottis, (2) the Dwarf Yellow Spathoglottis, (3) the Large Purple Spathoglottis, and (4) the Large Yellow Spathoglottis. The split in the Dwarf and Large Spathoglottis groups might reflect an early differentiation of plant size, flower colours and flower size. Phylogeny reconstruction of the orchid genus Spathoglottis also exhibited strong support towards the taxonomic delimitation of the two mostly debated taxa in the genus, S. aurea and S. microchilina.
1. Introduction
The genus Spathoglottis Blume (subfamily Epidendroideae, tribe Collabieae) is a well-known genus with a total of 49 terrestrial geophyte species and is widely distributed in tropical and subtropical Asia and the Pacific Islands, where 44 species were recorded in the Malesian region and are concentrated particularly in New Guinea [1]. Eight species and three infraspecific taxa of Spathoglottis are recognized as native to Peninsular Malaysia and Borneo, namely Spathoglottis affinis de Vriese, S. aurea Lindl., S. confusa J.J.Sm., S. gracilis Rolfe ex Hook.f., S. hardingiana C.S.P.Parish & Rchb.f., S. kimballiana Hook.f., S. kimballiana var. angustifolia Ames, S. kimballiana var. kimballiana, S. microchilina Kraenzl., S. plicata Blume and S. plicata var. alba.
Despite being popular in horticulture, Spathoglottis is a taxonomically confused genus in Orchidaceae [2,3,4,5]. Early revision work on this genus was first discussed for the Australian Spathoglottis, later followed by revisions for the Pacific Islands and the New Caledonian species [2,6,7]. However, a comprehensive revision on the genus has been lacking until now, and no molecular phylogenetic examination has ever been attempted before. Investigations on the morphology and anatomy of Spathoglottis have been reported by several authors [8,9,10,11,12,13]. On the cytogenetics part, dysploidy was observed in Spathoglottis (chromosome count of 2n = 18, 38, 40, 42, and 60) [14,15]. Updates on the palynology of Spathoglottis are very scarce, although pollen characters were suggested to be taxonomically informative.
Recently, a study on the chloroplast DNA barcoding of Spathoglottis in Malaysia for genetic conservation has been carried out using four chloroplast regions. The chloroplast regions which are matK, rbcL-a, rpoB and rpoC1 were successfully amplified from four species and one infraspecies namely S. aurea, S. gracilis, S. kimballiana, S. plicata and S. plicata var. alba [16]. However, the findings do not provide a deep phylogenetic inference, as the main goal was not to determine patterns of relationship but to identify an unknown sample in terms of a preexisting Spathoglottis classification.
Among others, the most frequently misidentified species within Spathoglottis are the two yellow-flower species with a distinctive narrow lip, S. aurea and S. microchilina. Over the years, taxonomic status between these two species has been repeatedly questioned. Spathoglottis aurea and S. microchilina are among the most debated taxa, with checkered histories of species identification [9,17,18,19,20]. Morphologically, the two species are distinguished based on the width of the lip (1.5 mm in S. microchilina; 4 mm wide in S. aurea) and the ability of the flower of S. microchilina to self-pollinate (cleistogamy). In the wild, populations of both S. aurea and S. microchilina show great phenotypic variations and plasticity. Thus, further examination is required to delimit S. aurea and S. microchilina as two distinct species or just one highly variable form of S. aurea.
On a biogeographical note, it is very informative to look at the distribution patterns among the species of this genus. Certain species of Spathoglottis are observed to confine to only particular biogeographical regions, with almost a complete no crossing-over between the area of their occurrences, except for the most widespread and weedy species, S. plicata. Vicariance hypothesis is assumed for the dispersal pattern of Spathoglottis and intercontinental long-dispersal is relatively uncommon or impossible.
It is a pressing need, since establishing species relationships will address issues such as evolutionary relationships between species as well as determination of identity or taxonomic status of certain species. Thus, the present work aimed to elucidate species relationships between members in genus Spathoglottis from Peninsular Malaysia and Borneo; and to resolve the taxonomic questions between the controversial yellow-flower S. aurea and S. microchilina.
2. Materials and Methods
2.1. Taxon Sampling
A total of 32 accessions from 11 species and three infraspesific taxa of Spathoglottis were analyzed in this present work (Figure 1). Two species from tribe Collabieae namely Tainia paucifolia (Breda) J.J.Sm. and Calanthe tankervilleae (Banks) M.W.Chase, Christenh. & Schuit. were selected as the outgroups. The fresh DNA samples were obtained from different localities in Peninsular Malaysia and Borneo (Sabah and Sarawak). Four Spathoglottis species from Thailand, Irian Jaya and New Caledonia; which some of the plant materials were provided by our collaborators were also included in this phylogenetic analysis to examine discreet evolutionary relationships between members of the genus.

Figure 1.
The species and infraspecific taxa of Spathoglottis from Peninsular Malaysia, Borneo, Thailand, Irian Jaya and the New Caledonia used in this present work: (A) S. plicata Blume; (B) S. plicata var. alba; (C) S. unguiculata (Labill.) Rchb.f.; (D) S. affinis de Vriese; (E) S. hardingiana C.S.P.Parish & Rchb.f.; (F) S. pubescens Lindl.; (G) S. eburnea Gagnep.; (H) S. parviflora Kraenzl.; (I) S. gracilis Rolfe ex Hook.f.; (J) S. kimballiana Hook.f.; (K) S. kimballiana var. kimballiana; (L) S. kimballiana var. angustifolia Ames; (M) S. aurea Lindl.; (N) S. microchilina Kraenzl.; and (O) cleistogamous flowers of S. aurea. Photos: Farah Alia Nordin and Peter O’ Byrne (I, K and L: KIP1266f and THH13-6-99).
The localities for all Spathoglottis species and the outgroups used, with voucher information and GenBank accession numbers, are listed in Table 1. All voucher specimens were deposited in the Herbarium, School of Biological Sciences, Universiti Sains Malaysia, Penang, Malaysia (USMP) [21].

Table 1.
Species list, herbarium voucher numbers, localities and GenBank accession numbers of the Spathoglottis species and outgroups used in this present work.
2.2. Morphological Observations
Morphological examinations were conducted on all 11 species and three infraspesific taxa of Spathoglottis analyzed in this present work. Taxa with the plant size below 30 cm tall was categorized as Dwarf Spathoglottis, while taxa with the plant size above 30 cm tall; and up to 2 m tall was categorized as Large Spathoglottis.
The flower was categorized as small with 3.0 cm cross, medium–sized with 3.0–4.0 cm cross, and large–sized if the flower is >5.0 cm cross when measured while opening. The taxa are also further distinguished based on the shape of the labellum, as either with narrow lip or broad/bilobulate lip.
2.3. DNA Extraction
Total genomic DNA was extracted from fresh or silica gel dried leaf materials following the 2 × Cetyl Trimethylammonium Bromide (CTAB) method with some modifications [22]. The modifications were applied especially when to treat the over-dried leaves. The modifications are: (1) the amount of polyvinyl-pyrrolidone (PVP-40T) was increased to 2% (w/v); equal to the amount of CTAB used, (2) the chloroform: isoamyl alcohol (24:1) were added three times instead of two, and (3) after adding the ice-cold propan-2-ol (isopropanol), the samples were kept in the freezer at −20 °C for two days. For Spathoglottis, DNA with good quality and quantity was obtained based on this modified method.
2.4. Amplification
The entire nrITS region was amplified using 17SE and 26SE primer set [23]. This region is approximately 800 base pair (bp) and includes ITS1, ITS2 and the 5.8S ribosomal gene. Partial matK, approximately 930 bp in length, was amplified using primer pairs 360F and 1326R [24]. This set of primers were chosen as they give more than 80% sequencing success; and show better sequence quality and high discriminatory. Meanwhile for trnL-F, the region was amplified using the universal forward and reverse primers set (c and f) [25]. This region is approximately 1000 bp long and includes the trnL intron and trnL-trnF spacer (Table 2).

Table 2.
Primers information for polymerase chain reaction amplification of nrITS, matK, and trnL-F regions.
The polymerase chain reaction (PCR) protocol used to amplify ITS, matK and trnL-F was as follows: initial denaturation at 94 °C for 5 min (2 min for ITS), denaturation at 94 °C for 1 min (30 cycles for ITS, 35 cycles for matK, 32 cycles for trnL-F), 1–1.5 min annealing (58 °C for ITS, 48 °C for matK, 56 °C for trnL-F), 1 min elongation at 72 °C (3 min for ITS), and followed by a final elongation period of 7 min at 72 °C. PCR was performed on MyCycler Thermal Cycler (Bio-Rad, Hercules, CA, USA). The products were purified using Wizard® SV Gel and PCR Clean-Up System Kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
For Spathoglottis, amplifications of matK were rather straight forward and easy. However, the process was somewhat complex and deliberated for the ITS and trnL-F regions. Bovine serum albumin (BSA) was added into reaction volumes of difficult templates (GC-rich or containing secondary structures) or samples with low PCR yields. BSA helps to increase PCR products from low purity templates and improves its availability for hybridization. For ITS, BSA is required in preventing the misamplification of ITS regions of other organisms (exp. Fungi, thus producing the multiple bands).
2.5. Sequence Editing and Alignment
All the sequences obtained from both sense and antisense strands of ITS, matK, and trnL-F sequences were assembled to produce contig sequences using BioEdit ver. 7.2.5 [26]. Sequences with high noise were checked through for a second time by eye using the chromatogram of the sequences. Multiple alignments of all sequences from each gene region were performed using CLUSTAL W in MEGA ver. 6.06 by default settings [27]. Again, the alignments were checked by eye and manually adjusted. All Spathoglottis nucleotide sequences obtained in this study were submitted and deposited in GenBank Nucleotide database (NCBI).
2.6. Database Search—BLAST
To ensure sequence similarities and all are Spathoglottis species, the sequences obtained were assessed using BLAST (Basic Local Alignment Search) through the National Center for Biotechnology Information (NCBI) databases (https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 1 July 2018)). When compared to the available Spathoglottis sequences in NCBI, most of the sequences from this study showed query coverage of more than 90% with E-value = zero. Therefore, sequences with less than 90% query coverage were not used for phylogenetic data analysis.
2.7. Maximum Likelihood Analysis
The maximum likelihood (ML) analysis was performed in MEGA ver. 6.06 [27]. The best substitution model was determined using the same software, implemented as the Find Best DNA/Protein Models (ML) Test [28]. The ML method was performed to find the optimal tree using a heuristic search strategy with 1000 replicates of random taxon addition, subtree-pruning-regrafting (SPR) branch swapping, and gaps treated as missing data. Starting tree was generated automatically by applying NJ/BioNJ algorithms. Levels of group support were estimated with 1000 bootstrap replicates. Groups were retained when bootstrap percentages (BS) ≥50%. Bootstrap percentages (BS) of 50–70% considered as weak; 71–85% as moderate and >85% as strong. Likelihood analyses for each of the gene region and combinations of gene regions were run separately.
2.8. Maximum Parsimony Analysis
Parsimony analysis for each of the gene region and combinations of gene regions were run separately in MEGA ver. 6.06 [27]. To find the most parsimonious trees, Maximum Parsimony (MP) analysis was performed using a heuristic search with 1000 replicates of random taxon addition, tree rearrangements using Tree-Bisection-Reconnection (TBR) branch swapping, holding ten trees per replicate (to minimize time spent on searching for large numbers of trees), and gaps treated as missing data. All characters were treated as unordered and equally weighted [29]. Internal support for clades/groups was assessed by bootstrapping with 1000 replicates and groups were retained when bootstrap percentages (BS) ≥50%.
2.9. Bayesian Inference Analysis
The Bayesian inference (BI) of phylogeny was generated using MrBayes 3.1.1 in TOPALi ver. 2.5 software [30,31]. Each of the gene regions and combinations of gene regions were treated as a separate partition. Model selection was made according to the Bayesian inference criterion (BIC), Akaike information criterion (AIC), and the likelihood ratio tests (LRTs) with 1000 bootstrapping threshold run [32]. The model with the lowest BIC score was chosen as the optimal model of sequence evolution. Each analysis consisted of two independent runs with four chains for 1,000,000 generations, sampling one tree in every 100th generation. The first 25% of the trees was discarded as the burn-in period, and the remaining trees were used to assess the posterior probabilities (PP) in a majority-consensus tree. Because posterior probabilities in Bayesian analysis are not equivalent to bootstrap percentages (as PP are generally much higher), criteria equivalent to the standard statistical test was used [33]. Groups with PP > 95% were considered as strongly supported, PP 90–95% as moderately supported, and PP < 90% as weakly supported.
2.10. Test of Incongruence
To evaluate congruence between different DNA regions (ITS, matK, trnL-F), each dataset was analyzed independently using the Likelihood Ratio Tests (LRTs) to see if they produce similar topology [32]. The ILD (Incongruence Length Difference) test was also conducted using PAUP* 4.0b10 to assess heterogeneity of the different gene regions (nuclear and plastid), and to determine whether the gene regions can be combined [34,35]. If the ILD p-value is ≥0.05, it indicates that the two regions are congruent thus can be combined. The combination of nuclear and plastid gene regions enhanced the phylogenetic signal in the data and increased cladogram resolution.
3. Results
A total of 34 individual sequences representing 11 species and three infraspecific taxa of Spathoglottis, including two outgroup species were analyzed in this present work; of which two species were of Thailand (Indochina) origin (S. eburnea and S. pubescens), four species and an infraspecies from Peninsular Malaysia (S. affinis, S. hardingiana, S. aurea, S. plicata and S. plicata var. alba), three species and two infraspecific taxa from Borneo (S. gracilis, S. kimballiana, S. kimballiana var. angustifolia, S. kimballiana var. kimballiana and S. microchilina), S. parviflora from Irian Jaya (East Malesia) and the New Caledonian species, S. unguiculata. Tainia paucifolia and Calanthe tankervilleae were used as outgroup species to root the phylogenies of each gene region.
3.1. Groupings of Taxa Based on Selected Morphological Characters
Table 3 shows the groupings of 11 species and three infraspecific taxa of Spathoglottis based on their plant size, flower size and shape of labellum.

Table 3.
The 11 species and three infraspecific taxa of Spathoglottis grouped based on the plant sizes (Dwarf Spathoglottis = ≤30 cm tall and Large Spathoglottis = ≥30 cm up to 2 m tall), flower sizes (small–sized = 3.0 cm cross, medium–sized = 3.0–4.0 cm cross and large–sized = >5.0 cm cross) and shape of labellum (narrow or broad/bilobulate).
3.2. Phylogenetic Analysis Based on ITS Data Matrix
The ITS data matrix of 34 taxa, two of which were the outgroups, comprised 872 nucleotide characters (including gaps); of which 594 characters (68.1%) were conserved among all taxa, 186 characters (21.3%) were parsimony informative, and 92 characters (10.6%) were non-informative. The ITS1 and ITS2 regions showed variable sequence lengths and G+C content (%). Boundaries of ITS1, ITS2 and 5.8S were determined from the published ITS sequences of S. pubescens in NCBI (Accession No: KM25162.1, KP51403.1, and KP51405.1); and start codons for translation initiation for 5.8S gene were assessed by referring to a validly published report [36].
The sequence lengths of ITS1 for all 11 species and three infraspecific taxa of Spathoglottis ranged from 244–260 bp, while ITS2 sequence lengths ranged from 318–345 bp (Table 4). In contrast, the length of 5.8S was uniform for all Spathoglottis species (157 bp). The G+C content (%) of ITS2 was found to be slightly higher compared to ITS1; with average G+C content of 64.4% and 57.6% were recorded for ITS2 and ITS1, respectively. The 5.8S region has been found to be more conserved as evidenced from the number of conserved sites (140/157 = 89.2%), followed by ITS2 (60.3%), and ITS1 (53.7%).

Table 4.
Sequence data analysis of ITS1, 5.8S and ITS2 regions in 11 species and three infraspecific taxa of Spathoglottis.
The average percentage of sequence divergence within Spathoglottis was 6.8% with highest percent divergence between species was shown to be between S. affinis and S. unguiculata (13.8%). The average base compositions among all taxa analyzed were A = 22.8%, C = 26.6%, T = 18.7%, and G = 20.21%. The estimated transition/transversion bias (R) was 3.28.
Figure 2 shows a combined tree of ML and Bayesian analysis as both trees shared similar tree topology. For the ML analysis, there was a total of 780 positions in the final dataset with highest log likelihood = (−2833.908).
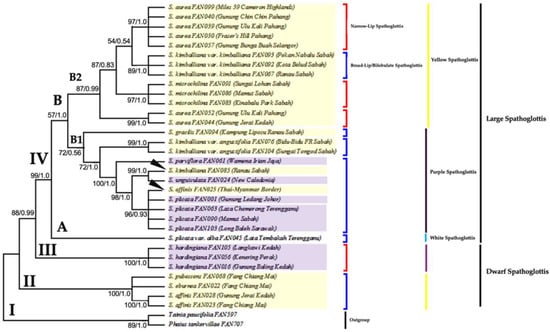
Figure 2.
The combined majority-rule consensus tree results from ML analysis and optimal tree results from Bayesian analysis generated from the ITS sequence dataset. Number below branches are bootstrap percentages ≥ 50 and probability values ≥ 0.5 (BS/PP). Clade II–IV shows the ingroups of genus Spathoglottis; Clade I indicates outgroup. Clade II and III holds the Dwarf Spathoglottis species, while Clade IV (A and B) consists of the Large Spathoglottis species. Clade B is further separated into subclades B1 and B2; each holds the Large Purple and Large Yellow Spathoglottis species group, respectively. Taxa highlighted in yellow box are the yellow flower Spathoglottis while purple box comprises of flowers in different shades of purple. Groupings of taxa were also based on the shape of the labellum; either narrow or broad/bilobulate.
The unweighted parsimony analysis resulted in seven most parsimonious trees. The strict-consensus tree is shown in Figure 3. Bootstrap percentages (BS) of parsimonious trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches.
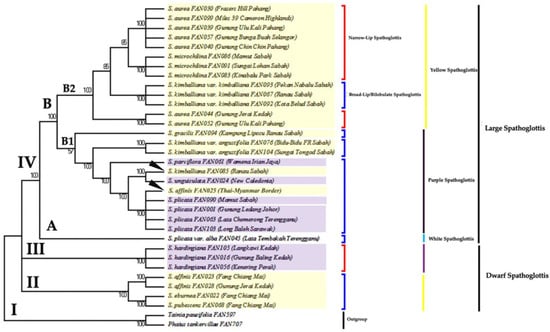
Figure 3.
The consensus tree inferred from seven equally parsimonious trees via MP analysis of genus Spathoglottis based on ITS sequence. Number below branches are bootstrap percentages ≥ 50. Branches corresponding to partitions reproduced in less than 50% trees are collapsed. Tree length = 346; consistency index, CI = 0.712; retention index, RI = 0.909; and composite index, RC = 0.699 (RC = 0.647) for all sites and parsimony-informative sites (in parentheses). Clade II–IV shows the ingroups of genus Spathoglottis; Clade I indicates outgroup. Clade II and III holds the Dwarf Spathoglottis species, while Clade IV (A and B) consists of the Large Spathoglottis species. Clade B is further separated into subclades B1 and B2; each holds the Large Purple and Large Yellow Spathoglottis species group, respectively.
The topology of the ML, MP and Bayesian trees inferred from the ITS region were significantly congruent together. Four major clades (I–IV) were identified (Figure 2 and Figure 3) based on the following criteria: (1) that they are well-supported and monophyletic, and (2) that they are morphologically distinguishable. Based on the ITS trees, species under a clade were grouped together according to the: (1) plant size (dwarf or large-sized); (2) flower size (small, medium, or large); (3) flower colour (purple or yellow); and 4) lip/midlobe shape (narrow or broad/bilobulate). The monophyly of the clades was supported by strong (BS) and (PP) values.
3.3. Phylogenetic Analysis Based on Combined Plastid Sequence Data
The ILD test between combined plastid regions gave a p-value of 0.10; showing significant congruence among the partitions of the dataset, hence was subjected to a combined analysis. The aligned sequences of the combined plastid dataset consisted of 2373 nucleotide characters (with gaps), of which 1870 (78.8%) were conserved characters, 337 characters (14.2%) were parsimony informative, and 166 characters were non-informative (7.0%). The average sequence divergence for Spathoglottis species from the combined plastid data matrix was 2.8%. Species with the highest percent of divergence was shown between S. affinis and S. unguiculata (5.4%).
Figure 4 shows a combined tree of ML and Bayesian analysis as both trees shared similar tree topology. The ML tree has 1000 replicates with highest log likelihood (−4503.460).
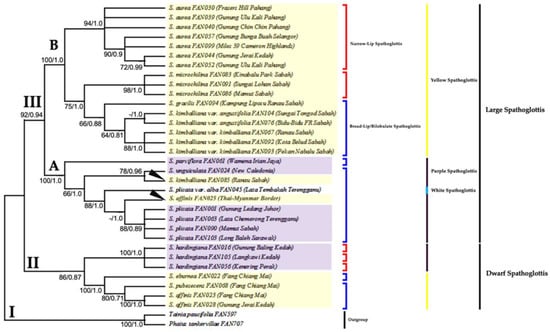
Figure 4.
The combined majority-rule consensus tree results from ML analysis and optimal tree results from Bayesian analysis generated from the combined plastid gene dataset. Number below branches are bootstrap percentages ≥ 50 and probability values ≥ 0.5 (BS/PP). Clade II and III holds all 11 species and three infraspecific taxa (ingroups) of Spathoglottis; Clade I indicates outgroup. Clade II comprises of the Dwarf Spathoglottis species, while Clade III consists of the Large Spathoglottis species. Clade III is further separated into subclades A and B; the Large Purple and Large Yellow Spathoglottis groups, respectively. Groupings of taxa were also based on the shape of the labellum; either narrow or broad/bilobulate. Both clades of Dwarf and Large Spathoglottis (Clade II and Clade III) are monophyletic.
Meanwhile, the unweighted parsimony analysis resulted in four most parsimonious trees with tree length = 337, consistency index (CI) = 0.780, retention index (RI) = 0.935, and composite index, RC = 0.799 (0.729) for all sites and parsimony-informative sites (in parentheses). The strict-consensus tree is shown in Figure 5.
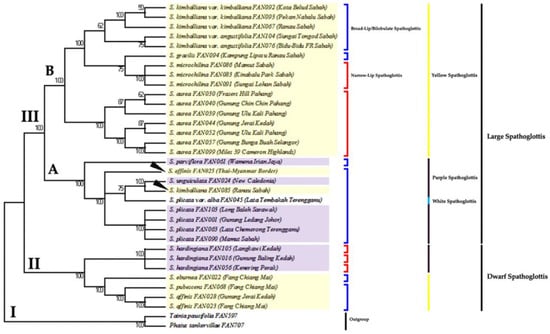
Figure 5.
The consensus tree inferred from eight equally parsimonious trees via MP analysis of genus Spathoglottis based on combined plastid gene dataset. Number below branches are bootstrap percentages ≥ 50. Branches corresponding to partitions reproduced in less than 50% trees are collapsed. Clade II and III holds all the ingroups; Clade I indicates outgroup. Clade II comprises of the Dwarf Spathoglottis species, while Clade III (A and B) consists of the Large Spathoglottis species.
This combined plastid data analysis has produced a better result with improved tree resolutions, than analyses with each plastid marker individually. The trees were topologically similar to the matK trees but with better branch support, and show no contradiction to the trnL-F trees. All the ML, MP and Bayesian trees of the combined plastid markers were congruent to each other with high (BS) and (PP) support values.
There were three major clades in the combined plastid trees (Figure 4 and Figure 5). Clade II-III holds all the 11 species and three infraspecific taxa of Spathoglottis, while Clade I indicates outgroup. Clade II consists of the two Dwarf Spathoglottis groups, the Dwarf Yellow Spathoglottis and the Dwarf Purple Spathoglottis. This clade is strongly supported in both ML and MP trees with BSML = 86%, BSMP = 100%; but show moderate branch support in the Bayesian tree (PPB = 0.87). The Dwarf Spathoglottis species formed the basal groupings. Species within Clade II were also finely separated according to the colour of the flowers and shape of the lip. The Dwarf Yellow Spathoglottis clade (BSML = 100%, BSMP = 100%, PPB = 1.0) that housed together all the broad-lip/bilobulate species formed a distinct clade from its narrow-lip sister, S. hardingiana in the Dwarf Purple clade (BSML = 100%, BSMP = 100%, PPB = 1.0). Clade II is monophyletic.
All the large species of Spathoglottis were nested within Clade III (Large Spathoglottis group). This clade is monophyletic and is well-supported in all trees with (BSML= 92%, BSMP = 100%, PPB = 0.94). Clade III is further divided into Subclade III-A and Subclade III-B, the Large Purple Spathoglottis group and the Large Yellow Spathoglottis group, respectively. Both subclades obtained maximum branch supports with BSML = 100%, BSMP = 100%, and PPB = 1.0. Positions of the purple-flower Spathoglottis within Subclade III-A were also much resolved. However, from this combined analysis, the Large Purple Spathoglottis group (Subclade III-A) revealed to be polyphyletic due to the unsolved placements of the two yellow-flower species (S. affinis_FAN 025 and S. kimballiana_FAN085, marked in figures) embedded within the group.
Meanwhile, subclade III-B (Large Yellow Spathoglottis group) is monophyletic, and the clade is fully supported by strong (BS) and (PP) values (BSML = 100%, BSMP = 100%, PPB = 1.0). The yellow-flower species within this clade is further divided into two subgroups based on their geographical distribution and geological history. The S. aurea clade is strongly-supported with BSML = 94%, BSMP = 100%, and PPB = 1.0. Spathoglottis aurea is then further grouped according to their pollination strategies; between the cleistogamous flower group (BSML = 93%, BSMP = 87%, PPB = 1.0) and the geitonogamous flower group (BSML = 90%, BSMP = 100%, PPB = 0.9). For the Bornean Spathoglottis clade (BSML = 75%, BSMP = 100%, PPB = 1.0), it is very obvious that S. gracilis and S. microchilina that possessed broad-plicate leaf were separated from the two narrow-grassy leaf infraspecies, S. kimballiana var. angustifolia and S. kimballiana var. kimballiana. In the MP tree, S. gracilis was observed to be genetically close to the narrow-lip S. microchilina (BSMP = 75%), but is sister to S. kimballiana var. kimballiana and S. kimballiana var. angustifolia in the ML and Bayesian tree (BSMP = 66%), PPB = 0.8).
This combined plastid data analysis has provided a better insight into the relationship among species of the main four groups of Spathoglottis. The cladograms were well-resolved with better resolution and phylogenetic signals. However, the positions of S. affinis (FAN025) and S. kimballiana (FAN085) within the Large Purple Spathoglottis group require further clarification.
3.4. Phylogenetic Analysis Based on Combined Plastid and nrITS Data
Combination of data analysis of the nuclear and plastid gene regions will enhance the phylogenetic signal in the data and increased cladogram resolution. The combined data analyses provided better insight into species delimitation within the four main groupings of Spathoglottis diagnosed in this present work.
The probability value obtained from the ILD test between plastid DNA and nrITS dataset (p-value = 0.14) indicated that the two matrices could be combined for analysis. The combined alignment of all plastid markers and nrITS consisted of 3253 nucleotide characters (with gaps), of which 2489 characters (76.5%) were conserved, 497 characters (15.3%) were parsimony informative, and 267 (8.2%) characters were non-informative. The average sequence divergence for Spathoglottis species from the combined plastid and nuclear data matrix was 3.9%. The average base compositions among all taxa analyzed were A = 29.8%, C = 19.2%, T = 31.3%, and G = 19.7%. The estimated Transition/Transversion bias (R) was 1.44.
Figure 6 shows a combined tree of ML and Bayesian analysis as both trees shared similar tree topology. The ML tree has 1000 replicates with highest log likelihood (−4503.460). There was a total of 1765 positions in the final dataset with highest log likelihood= (−7663.795).
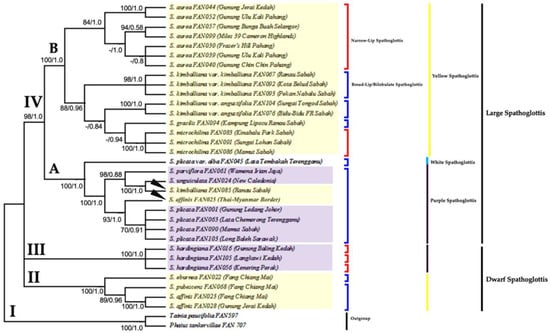
Figure 6.
The combined majority-rule consensus tree results from ML analysis and optimal tree results from Bayesian analysis generated from the combined plastid and nuclear gene dataset. Number below branches are bootstrap percentages ≥ 50 and probability values ≥ 0.5 (BS/PP). Clade II–IV holds all ingroups of genus Spathoglottis; Clade I indicates outgroup. The Dwarf Yellow Spathoglottis (Clade II) formed a basal group; while the Dwarf Purple Spathoglottis (Clade III) is sister to all Large Spathoglottis species. Clade IV that comprises of the Large Spathoglottis groups is monophyletic. Independently, the Large Yellow Spathoglottis group (Subclade IV-B) is monophyletic while the Large Purple Spathoglottis group (Subclade IV-A) is polyphyletic.
Meanwhile, the unweighted parsimony analysis resulted in four most parsimonious trees. The strict-consensus tree is shown in Figure 7.
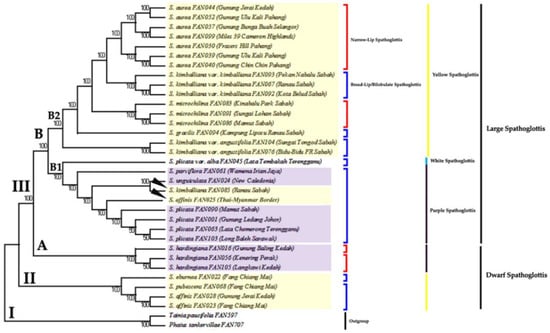
Figure 7.
The consensus tree inferred from two equally parsimonious trees via MP analysis of genus Spathoglottis based on combined plastid and nuclear gene dataset. Number below branches are bootstrap percentages ≥ 50. Branches corresponding to partitions reproduced in less than 50% trees are collapsed. Tree length = 695; consistency index, CI = 0.709; retention index, RI = 0.906; and composite index, RC = 0.711 (0.643) for all sites and parsimony-informative sites (in parentheses). Clade I indicates outgroup. Clade II comprises of the Dwarf Yellow Spathoglottis group, as sister to all Spathoglottis species. Clade III holds the Dwarf Purple Spathoglottis group (Subclade III-A), Large Purple Spathoglottis group (Subclade III-B1) and Large Yellow Spathoglottis group (Subclade III-B2). The Large Spathoglottis group (Clade III-B) is monophyletic.
This combined analysis has produced well-resolved trees and species delimitation among the four groups of Spathoglottis was very clear. All the ML, MP, and Bayesian trees were congruent to each other. In the combined ML and Bayesian tree (Figure 6), there were four major clades. Clade I consists of the outgroup species while Clade II–IV hold all the ingroup Spathoglottis species. Clade II, the Dwarf Yellow Spathoglottis is sister to all Spathoglottis species with maximum branch supports BSML = 100% and PPB = 1.0. The Dwarf Purple Spathoglottis formed Clade III, which was originally placed within Clade IV but was collapsed to a polytomy due to weak branch support (BSML = 48%, PPB < 0.5).
Clade IV is monophyletic with strong (BS) and (PP) supports (BSML = 98%, PPB= 1.0), and was further divided into two subclades according to the colour of the flowers. Subclade IV-A (BSML = 100%, PPB = 1.0) consists of all the Large Purple Spathoglottis species and is polyphyletic; due to the placements of the two yellow-flower Spathoglottis (S. kimballiana_FAN085 and S. affinis_FAN025) within this clade. Eventhough S. kimballiana has distinct morphological attributes and is geographically isolated from S. unguiculata, this two species were connected by having more than four leaves per plant and possessed tough coriaceous sepals; characteristics unique to this two species.
Meanwhile, subclade IV-B that comprises of all the large-yellow Spathoglottis species is monophyletic (BSML = 100% and PPB = 1.0). The S. aurea clade (BSML = 84% and PPB = 1.0) from Peninsular Malaysia is well separated from the Bornean yellow-flower Spathoglottis (BSML = 88% and PPB = 0.96). Spathoglottis aurea were then further grouped based on their flower pollination strategies. The open-flower or insect pollinated S. aurea from Gunung Jerai and Gunung Ulu Kali were well separated from their self-pollinated sisters. While within the Bornean Spathoglottis clade, S. gracilis was observed to be genetically close to S. microchilina and S. kimballiana var. angustifolia. Spathoglottis kimballiana var. angustifolia, a lowland species with large flower is sister to all montane Spathoglottis species possessed medium-sized flower. Spathoglottis microchilina, S. kimballiana var. kimballiana and S. aurea shared the characteristic of having short sidelobes as to compare to the large and auriculate one in S. kimballiana var. angustifolia and S. gracilis.
While for the MP analysis, the tree produced was topologically similar to those of ML and Bayesian’s but was much resolved (Figure 7). The Dwarf Yellow Spathoglottis formed Clade II (BSMP = 100%) as a basal group. Clade III is strongly supported with BSMP = 100%, and it holds together the Dwarf Purple Spathoglottis (Clade III-A; BSMP = 100%) and the Large Spathoglottis group (Clade III-B; BSMP = 100%). From this MP analysis, it was suggested that the Dwarf S. hardingiana is sister to all large Spathoglottis species; whereas its position in the ML and Bayesian trees is rather equivocal. Clade III-A was then divided into two subclades which are Subclade III-B1 (Large Purple Spathoglottis group) and Subclade III-B2 (Large Yellow Spathoglottis group). Both subclades are strongly supported with BSMP = 100%. The arrangements of species within the Large Purple Spathoglottis group were also similar to those of in combined ML and Bayesian tree. While for the Large Yellow Spathoglottis group, the positions of each of the species in this clade were much resolved. The lowland and ecological peculiar Spathoglottis kimballiana var. angustifolia is sister to the rest of yellow-flower montane species. The montane species were consisted of S. gracilis, S. microchilina, S. kimballiana var. kimballiana and S. aurea; and the clade is supported with strong BS value (BSMP = 100%). Spathoglottis gracilis, another large-flower species is sister to the medium-sized species within the montane Spathoglottis group.
From this combined plastid and nuclear analysis, the trees produced were much-resolved with better resolution, strong phylogenetic signals, and maximum clade credibility. Species discrimination was also best described with reduced polytomies. Meanwhile, the two much confused narrow-lip species; S. aurea and S. microchilina were proven to be distantly related and formed different clades separately.
4. Discussion
4.1. The Efficacy of Nuclear and Plastid Gene Regions in Inferring Phylogenetic Relationships
The independent gene analyses together with the combined plastid and nuclear dataset have successfully inferred the phylogenetic relationships of Spathoglottis in Peninsular Malaysia and Borneo. The positions and species boundaries among several debatable taxa were also well clarified and circumscribed. The results obtained from the ML analysis, MP analysis and Bayesian analysis of each of this gene region; coupled with the combined plastid and nuclear dataset have showed that Spathoglottis were constituted of four major groups. These groups are the Dwarf Yellow Spathoglottis, Dwarf Purple Spathoglottis, Large Yellow Spathoglottis, and Large Purple Spathoglottis. Previously, no attempt has been carried out to infer the relationships among species of Spathoglottis, be it based on morphological or molecular aims; thus, any formal sectionals within this genus have never been proposed. Hence, the groupings obtained from this study will be used as the basis for further classifications and subdivisions of this genus.
The nrITS is one of the loci that is commonly used for phylogenetic inference as it provides high copy numbers and relative range of phylogenetic utility [37]. The nrDNA gene is very heterogeneous both in size and nucleotide sequences among wide range of angiosperms [38]. The utility of this gene region in the molecular study of family Orchidaceae is very widespread and is used in various taxonomic levels; especially the low-level taxa such as infrageneric species [39,40,41,42,43,44]. From this present work, it was proven that the ITS region is a powerful tool in providing a clearer understanding of the phylogeny of a major part of genus Spathoglottis. A high level of parsimony informative characters (PIC = 21.3%) was established in this region, thus showing a high level of branch support in the data analysis. The clades were largely resolved, and species delimitation was well-clarified.
Due to its highly conserved region (91.6%), matK can be easily amplified. Partial matK sequences that were used in this present work were able to produce phylogenetic trees that were comparable in resolution and support of the trees. The efficient utility of matK sequences as barcodes for identifying species and in inferring phylogeny has been demonstrated in many molecular studies among the family Orchidaceae [24,45,46,47,48,49]. Results from the matK trees from both independent and combined plastid data analysis showed that separations among the four Spathoglottis groups were good, with strong bootstrap and posterior probabilities supports. However, separation among species, particularly in the Large Yellow Spathoglottis and Large Purple Spathoglottis groups, were not as successful due to the internal branch polytomies (PIC = 4.8%).
The trnL-F region is another plastid marker that is widely used in the systematics of family Orchidaceae [41,46,50,51]. It has been recognized to have phylogenetic utility in a broad range of taxonomic level; from the family down to species, and occasionally to the levels of populations [37]. In this present work, the trnL-F region contains higher PIC values (19.5%) to compare to matK. However, independent analysis of the trnL-F region has shown that the region was unable to resolve the relationships among the four groups of Spathoglottis, eventhough it may confirm the positions of species belonging to each of the group/clade. The trnL-F region is also variable enough to discriminate between species of Spathoglottis; exactly to the population level as observed in the S. aurea clades. Thus, the two plastid gene regions were combined to infer the best evolutionary relationships among species of Spathoglottis from Peninsular Malaysia and Borneo.
4.2. Monophyly of Genus Spathoglottis
Based on the analyses of both independent and combined dataset, the most important finding regarding the phylogenetic relationships of the four groups was: the Dwarf Yellow Spathoglottis, Dwarf Purple Spathoglottis, Large Yellow Spathoglottis and Large Purple Spathoglottis have demonstrated that although they were not all independently monophyletic, they formed well-supported monophyletic groups with strong supports. Each analysis has shown that, independently, the Dwarf Yellow Spathoglottis and Large Yellow Spathoglottis are monophyletic, while the Large Purple Spathoglottis is polyphyletic. In the combined plastid analysis, both the Dwarf Yellow Spathoglottis and Dwarf Purple Spathoglottis were joined together to form the monophyletic Dwarf Spathoglottis group. Meanwhile in the nrITS and combined plastid and nuclear analyses, it was revealed that the Dwarf Yellow Spathoglottis was sister to all Spathoglottis species while S. hardingiana (Dwarf Purple Spathoglottis) is sister to all large-flower Spathoglottis. In all analyses, the Large Spathoglottis group formed a strongly supported monophyletic group by holding both the Large Yellow Spathoglottis and Large Purple Spathoglottis species groups.
The groupings obtained from thes molecular analyses were well-supported and in accordance to the early revisions on the morphological classification of the genus [8,9]. Traditionally, species in Spathoglottis was classified based on their: (1) plant size, (2) flower size, (3) flower colour, (4) lip/midlobe shape, (5) sidelobe shape, and (6) flower-pollination strategies.
The Dwarf Spathoglottis group consists of the miniature Spathoglottis species (≤30 cm tall), which are mainly native to the Indochina region. However, two species escaped the boundary, occuring lower down towards the northernmost part of Peninsular Malaysia (S. hardingiana and S. affinis). From the finding of this present work, this Dwarf Group was then further separated into the Dwarf Purple Spathoglottis and Dwarf Yellow Spathoglottis groups based on the colour of the flowers, lip shapes, and flower resupination. Spathoglottis hardingiana from the Dwarf Purple Spathoglottis group is distinguishable from the rest of Dwarf Yellow Spathoglottis species by having a non-resupinated lip; in which the lip is on the uppermost part of the flower, rather than turning downwards to the normal position as observed in other Spathoglottis species. This trait is autapomorphic for S. hardingiana. In addition, S. hardingiana possessed a narrow lip and almost thread-like without distinct sidelobes; very contrasting to the broad lip and large sidelobes as in S. affinis (bilobulate), S. eburnea (square), and S. pubescens (obcordate).
Meanwhile, the Large Spathoglottis group is a monophyletic group that housed all the large-sized Spathoglottis; particularly referring to the size of the flowers. Almost all the species possessed broad-plicate leaves, except for the two infraspecies; S. kimballiana var. kimballiana and S. kimballiana var. angustifolia with the grass-like leaves. Within this Large Spathoglottis group, the species were then further separated into two groups according to the colour of the flowers, number of leaves, floral bract shape, floral bract texture, sepals texture, lip size, sidelobe lengths, and geological attributes.
The flowers in different shades of purple and possessing a broad/bilobulate lip were placed together in the Large Purple Spathoglottis group. The white form of S. plicata; S. plicata var. alba was also embedded within this clade. Besides the colour of the flowers, the species of Large Purple Spathoglottis group was also characterized by having four or more than four leaves, bilobulate lip, elliptical and soft floral bract, hairy pedicel, thin sepals, short sidelobes, short lip, and generally the plant grows on multisubstrate habitat. However, eventhough the clade was strongly supported by the (BS) and (PP) values; it was showed from all separate gene regions and combined dataset analyses that the Large Purple Spathoglottis is polyphyletic. This is due to the positions of the two yellow-flower Spathoglottis, S. kimballiana_FAN085 and S. affinis_FAN025 within this clade. Ideally, S. kimballiana was supposedly to be placed in the Large Yellow Spathoglottis group while S. affinis nested within the Dwarf Yellow Spathoglottis Complex. These cases of morphological convergence have suggested that the flower colour trait is homoplastic.
The Yellow Spathoglottis group is a monophyletic group placed at the crown position of the phylogenetic trees, evidence of a recent radiation. It was well supported and nests together all the yellow-flower Spathoglottis, which were further separated according to various floral traits and ecological information such as flower size, lip shape, callus architecture, the content of anthocyanin, habitat niches, and geological attributes. Based on the separate plastid markers analyses coupled with the combined dataset, the Large Yellow Spathoglottis were split into the Peninsular Malaysia group (S. aurea) and the Bornean Spathoglottis group (S. gracilis, S. microchilina, S. kimballiana var. kimballiana, S. kimballiana var. angustifolia). However, this Bornean Spathoglottis group was weakly resolved in the independent nrITS, matK, and trnL-F analysis; but largely solved in the combined analyses. Results obtained from this present work have also proven that the two most-conflicting narrow-lip Spathoglottis species; S. aurea and S. microchilina were discriminated as two different species. This was supported by the placements of each of the species in separate clades with strong support values.
4.3. Proposed Taxonomic and Nomenclatural Changes for Spathoglottis plicata var. alba
From the molecular analyses, it was observed that the white-flower S. plicata var. alba had showed a distant genetic divergence from the purple-flower S. plicata. Based on the ML, MP, and Bayesian trees of the nuclear, plastid and combined gene analyses; S. plicata var. alba formed a distinct clade of its own. To see how distant S. plicata var. alba is from S. plicata, the genetic difference between these two entities were calculated. It was shown that the genetic divergence among S. plicata var. alba and S. plicata was 7.8–9.0% for ITS, 0.2% for matK, 0.3% for trnL-F, and 2.8–3.0% for the combined plastid and nuclear genes, respectively. To further support that either S. plicata var. alba can be elevated into a species rank, a separate DNA barcoding gap analysis was conducted. From the DNA barcoding gap analysis, it appears that the maximum boundaries, or referred to as the ‘threshold or gap value’ between different species in genus Spathoglottis were 1.4% for ITS2, 0.2% for matK, 0.5% for trnL-F, and 0.5% for the combined plastid and nuclear genes. Thus, if the values of genetic differences showed between S. plicata var. alba and S. plicata were higher than those threshold values, taxonomic and nomenclatural changes should be considered for S. plicata var. alba.
Supported by both phylogenetic and DNA barcoding gap analyses, it was shown that the white S. plicata var. alba is genetically distant from S. plicata. Hence, it is proposed that the taxonomic status of S. plicata var. alba, be classified from a variety to a species rank.
5. Conclusions
The analyses of independent gene regions coupled with the combined dataset have proven that the genus Spathoglottis is monophyletic. The phylogenetic relationships among the 11 species and three infraspecific taxa of Spathoglottis from Peninsular Malaysia and Borneo were successfully resolved, and species boundaries were successfully circumscribed. Four major groups were determined from these analyses: (1) Dwarf Purple Spathoglottis, (2) Dwarf Yellow Spathoglottis, (3) Large Purple Spathoglottis, and (4) Large Yellow Spathoglottis. From this study, it is proposed that the split in the Dwarf and Large Spathoglottis groups reflects an early differentiation of plant size, flower colors, and flower size. In addition, morphology alone can be misleading for inferring the relationships among groups of interest, as floral morphology is highly flexible.
Author Contributions
Data curation, F.A.N., A.S.O. and K.N.A.M.; formal analysis and investigation, F.A.N., A.S.O., K.S. and R.G.; methodology, F.A.N.; resources, A.S.O., K.S. and R.G.; supervision, A.S.O. and K.S.; validation, A.S.O.; writing—original draft, F.A.N.; writing—review and editing, F.A.N. and A.S.O. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the USM Short Term Grant of assignment No. 304/PBIOLOGI/6315549 awarded to the corresponding author and the fifth author. All funders provided financial supports for the present work but did not contribute any additional role in in the research design, data collections and analysis, and preparation of the manuscript.
Data Availability Statement
Data presented in this article are available on request from the corresponding authors.
Acknowledgments
The authors would like to express our deepest gratitude to the administrative and field personnel of School of Biological Sciences, Universiti Sains Malaysia; Department of Biology, Universiti Putra Malaysia; and Institute for Tropical Biology and Conservation, Universiti Malaysia Sabah for the facilities and assistance provided during the study conducted. Thank you to Forestry Department of Peninsular Malaysia, Forestry Department of Sarawak, Forestry Department of Sabah, Sabah Biodiversity Centre and Kinabalu Park for the research permits granted. Many thanks to Richard Chung (FRIM), Ong Poh Teck (FRIM), Yong Kien Thai (KLU), Nik Norhazrina Nik Mohd Kamil (UKMB) for the assistance provided during visits to the herbaria, to our collaborators, and special tribute to the late Peter O’Byrne for his kind sharing of thoughts and beautiful photos.
Conflicts of Interest
The authors declare no conflict of interest.
References
- POWO, Plants of the World Online (Facilitated by the Royal Botanic Gardens, Kew). Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:325895- (accessed on 15 October 2022).
- Cribb, P.J.; Tang, C.Z. Spathoglottis (Orchidaceae) in Australia and the Pacific Islands. Kew Bull. 1981, 36, 721–729. [Google Scholar] [CrossRef]
- Sosa, V. A molecular and morphological phylogenetic study of subtribe Bletiinae (Epidendreae, Orchidaceae). Syst. Bot. 2007, 32, 34–42. [Google Scholar] [CrossRef]
- Xiang, X.G.; Jin, W.T.; Li, D.Z.; Schuiteman, A.; Huang, W.C.; Li, J.W.; Jin, X.; Li, Z.Y. Phylogenetics of tribe Collabieae (Orchidaceae, Epidendroideae) based on four chloroplast genes with morphological appraisal. PLoS ONE 2014, 9, e98721. [Google Scholar] [CrossRef]
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; Van Den Berg, C.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Dockrill, A. Australian Indigenous Orchids Vol. 1. In: Cribb, P.J.; Tang, C.Z. Spathoglottis (Orchidaceae) in Australia and the Pacific Islands (Eds.). Kew Bull. 1969, 36, 721–729. [Google Scholar]
- Hallê, N. Flore de la Nouvelle-Calêdonie et Dêpendances and Orchidacées. In: Cribb, P.J.; Tang, C.Z. Spathoglottis (Orchidaceae) in Australia and the Pacific Islands (Eds.). Kew Bull. 1977, 36, 721–729. [Google Scholar]
- Holttum, R.E. A Revised Flora of Malaya: Orchids of Malaya, 3rd ed.; Government Printing Office: Singapore, 1964; Volume 1.
- Seidenfaden, G.; Wood, J.J. The Orchids of Peninsular Malaysia and Singapore: A Revision of R.E. Holttum: Orchids of Malaya; Olsen and Olsen in Association with The Kew Royal Botanic Gardens, and Singapore Botanic Gardens: Fredensborg, Denmark, 1992. [Google Scholar]
- Solereder, H.; Meyer, F.J. Systematic anatomy of the monocotyledons. In: Freudenstein, J.V.; Rasmussen, F.N. (Eds.) What does morphology tell us about orchid relationships?—A cladistic analysis. Am. J. Bot. 1930, 86, 225–248. [Google Scholar]
- Williams, N.H. Subsidiary cells in the Orchidaceae: Their general distribution with special reference to development in the Oncidieae. Bot. J. Linn. Soc. 1979, 78, 41–66. [Google Scholar] [CrossRef]
- Pridgeon, A.M.; Stern, W.L.; Benzing, D.H. Tilosomes in roots of Orchidaceae: Morphology and systematic occurrence. Am. J. Bot. 1983, 70, 1365–1377. [Google Scholar] [CrossRef]
- Porembski, S.; Barthlottt, W. Velamen radicum micromorphology and classification of Orchidaceae. Nord. J. Bot. 1988, 8, 117–137. [Google Scholar] [CrossRef]
- Teoh, S.B. Polyploid spore formation in diploid orchid species. Genetica 1984, 63, 53–59. [Google Scholar] [CrossRef]
- Brandham, P. Cytogenetics. In Genera Orchidacearum Vol. 1: General Introduction, Apostasioideae, Cypripedioideae; Pridgeon, A.M., Cribb, P.J., Chase, M.W., Rasmussen, F.N., Eds.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Ginibun, F.C.; Saad, M.R.M.; Hong, T.L.; Othman, R.Y.; Khalid, N.; Bhassu, S. Chloroplast DNA barcoding of Spathoglottis species for genetic conservation. Acta Holticulturae 2010, 878, 453–459. [Google Scholar] [CrossRef]
- Seidenfaden, G.; Smitinand, T. The Orchids of Thailand: A Preliminary List; The Siam Society: Bangkok, Thailand, 1959. [Google Scholar]
- Wood, J.J. Orchids of Borneo Vol. 3: Dendrobium, Dendrochilum and Others; The Sabah Society in Association with The Bentham-Moxon Trust: Kota Kinabalu, Malaysia, 1997. [Google Scholar]
- Chan, C.L.; Lamb, A.; Shim, P.S.; Wood, J.J. Orchids of Borneo Vol. 1 Introduction and a Selection of Species; The Sabah Society in Association with The Bentham-Moxon Trust, England: Kota Kinabalu, Malaysia, 1994. [Google Scholar]
- Comber, J.B. Orchids of Sumatra; Natural History Publications in Association with The Royal Botanic Gardens, Kew and Botanic Gardens, Singapore: Kota Kinabalu, Malaysia, 2001. [Google Scholar]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. Available online: https://sweetgumnybg.org/science/ih (accessed on 27 September 2022).
- Doyle, J.J.; Doyle, J.L. A rapid isolation procedure for small amounts of leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Sun, Y.; Skinner, D.Z.; Liang, G.H.; Hulbert, S.H. Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theor. Appl. Genet. 1994, 89, 26–32. [Google Scholar] [CrossRef]
- Cuénoud, P.; Savolainen, V.; Chatrou, L.W.; Powel, M.; Grayer, R.J.; Chase, M.W. Molecular phylogenetics of Caryophyllales based on nuclear 18S rDNA and plastid rbcL, atpB, and matK DNA sequences. Am. J. Bot. 2002, 89, 132–144. [Google Scholar] [CrossRef]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user friendly biological sequence alignment editor and analysis performer windows 95/98/NT. Nucleic Acid Symp. Ser. Oxf. Acad. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA 6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Fitch, W.M. Towards defining the course of evolution: Minimum change for a specific tree topology. Syst. Zool. 1971, 20, 406–416. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Milne, I.; Wright, F.; Rowe, G.; Marshal, D.F.; Husmeier, D.; McGuire, G. TOPALi: Software for Automatic Identification of Recombinant Sequences within DNA Multiple Alignments. Bioinformatics 2004, 20, 1806–1807. [Google Scholar] [CrossRef]
- Felsenstein, J. Distance methods for inferring phylogenies: A justification. Evolution 1984, 38, 16–24. [Google Scholar]
- Erixon, P.; Svennblad, B.; Bitton, T.; Oxelman, B. Reliability of Bayesian posterior probabilities and bootstrap frequencies in phylogenetics. Syst. Biol. 2003, 52, 665–673. [Google Scholar] [CrossRef]
- Farris, J.S.; Kallersjo, M.; Kluge, A.G.; Bult, C. Constructing Significance Test for Incongruence. Syst. Biol. 1995, 44, 570–572. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*. Phylogenetic Analysis Using Parsimony (* and Other Methods), Version 4b10; Sinauer: Sunderland, MA, USA, 2002.
- Hecht, A.; Glasgow, J.; Jaschke, P.R.; Bawazer, L.A.; Munson, M.S.; Cochran, J.R.; Endy, D.; Salit, M. Measurements of translation initiation from all 64 codons in E. coli. Nucleic Acids Res. 2017, 45, 3615–3626. [Google Scholar] [CrossRef]
- Soltis, D.E.; Soltis, P.S. Phylogenetic relationships among Saxifragaceae sensu lato: A comparison of topologies based on 18S and rbcL sequences. Am. J. Bot. 1997, 84, 504–522. [Google Scholar] [CrossRef]
- Baldwin, B.G.; Sanderson, M.J.; Porter, J.M.; Wojciechowski, M.F.; Campbell, C.S.; Donoghue, M.J. The ITS region of nuclear ribosomal DNA: A valuable source of evidence on angiosperm phylogeny. Ann. Mol. Bot. Gard. 1995, 82, 247–277. [Google Scholar] [CrossRef]
- Aceto, S.; Caputo, P.; Cozzolino, S.; Gaudio, L.; Moretti, A. Phylogeny and evolution of Orchis and allied genera based on ITS DNA variation: Morphological gaps and molecular continuity. Mol. Phylogenetics Evol. 1999, 13, 67–76. [Google Scholar] [CrossRef]
- Van Den Berg, C.; Goldman, D.H.; Freudenstein, J.V.; Pridgeon, A.M.; Cameron, K.M.; Chase, M.W. An overview of the phylogenetic relationships within Eidendroideae inferred from multiple DNA regions and recircumscription of Epidendreae and Arethuseae (Orchidaceae). Am. J. Bot. 2005, 92, 613–624. [Google Scholar] [CrossRef]
- Kocyan, A.; Qiu, Y.L.; Endress, P.K.; Conti, E. A phylogenetic analysis of Apostasioideae (Orchidaceae) based on ITS, trnL–F and matK sequences. Plant Syst. Evol. 2004, 247, 203–213. [Google Scholar] [CrossRef]
- Chochai, A.; Leitch, I.J.; Ingrouille, M.J.; Fay, M.F. Molecular phylogenetics of Paphiopedilum (Cypripedioideae; Orchidaceae) based on nuclear ribosomal ITS and plastid sequences. Bot. J. Linn. Soc. 2012, 170, 176–196. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Yan, H. Remarkable new species of Liparis (Orchidaceae) from China and its phylogenetic implications. PLoS ONE 2013, 8, e78112. [Google Scholar] [CrossRef]
- Xiang, X.-G.; Schuiteman, A.; Li, D.-Z.; Huang, W.-C.; Chung, S.-W.; Li, J.-W.; Zhou, H.-L.; Jin, W.-T.; Lai, Y.-J.; Li, Z.-Y.; et al. Molecular systematics of Dendrobium (Orchidaceae, Dendrobieae) from mainland Asia based on plastid and nuclear sequences. Mol. Phylogenetics Evol. 2013, 69, 950–960. [Google Scholar] [CrossRef]
- Barthet, M.M.; Hilu, K.W. Expression of matK: Functional and evolutionary implications. Am. J. Bot. 2007, 94, 1402–1412. [Google Scholar] [CrossRef]
- Bytebier, B.; Bellstedt, D.U.; Linder, H.P. A molecular phylogeny for the large African orchid genus Disa. Mol. Phylogenetics Evol. 2007, 4, 75–90. [Google Scholar] [CrossRef]
- Khew, G.S.W.; Chia, T.F. Parentage determination of Vanda Miss Joaquim (Orchidaceae) through two chloroplast genes rbcL and matK. AoB Plants 2011, 2011, plr018. [Google Scholar] [CrossRef]
- Yu, J.; Xue, J.H.; Zhou, S.L. New universal matK primers for DNA barcoding angiosperms. J. Syst. Evol. 2011, 49, 176–181. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chou, C.H.; Wang, H.V.; Ko, Y.Z.; Chiang, T.Y.; Chiang, Y.C. Biogeography of the Phalaenopsis amabilis complex inferred from nuclear and plastid DNAs. BioMed. Cent. Plant Biol. 2015, 15, 202. [Google Scholar] [CrossRef]
- Bellstedt, D.U.; Linder, H.P.; Harley, E.H. Phylogenetic relationship in Disa based on non-coding TrnL-TrnF chloroplast sequences: Evidence of numerous repeat regions. Am. J. Bot. 2001, 88, 2088–2100. [Google Scholar] [CrossRef]
- Bellusci, F.; Pellegrino, G.; Palermo, A.M.; Musacchio, A. Phylogenetic relationships in the orchid genus Serapias L. based on non-coding regions of the chloroplast genome. Mol. Phylogenetics Evol. 2008, 47, 986–991. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).