Correlations among Soil Properties, Growth Characteristics, and Ginsenoside Contents in Wild-Simulated Ginseng with Different Ages
Abstract
1. Introduction
2. Materials and Methods
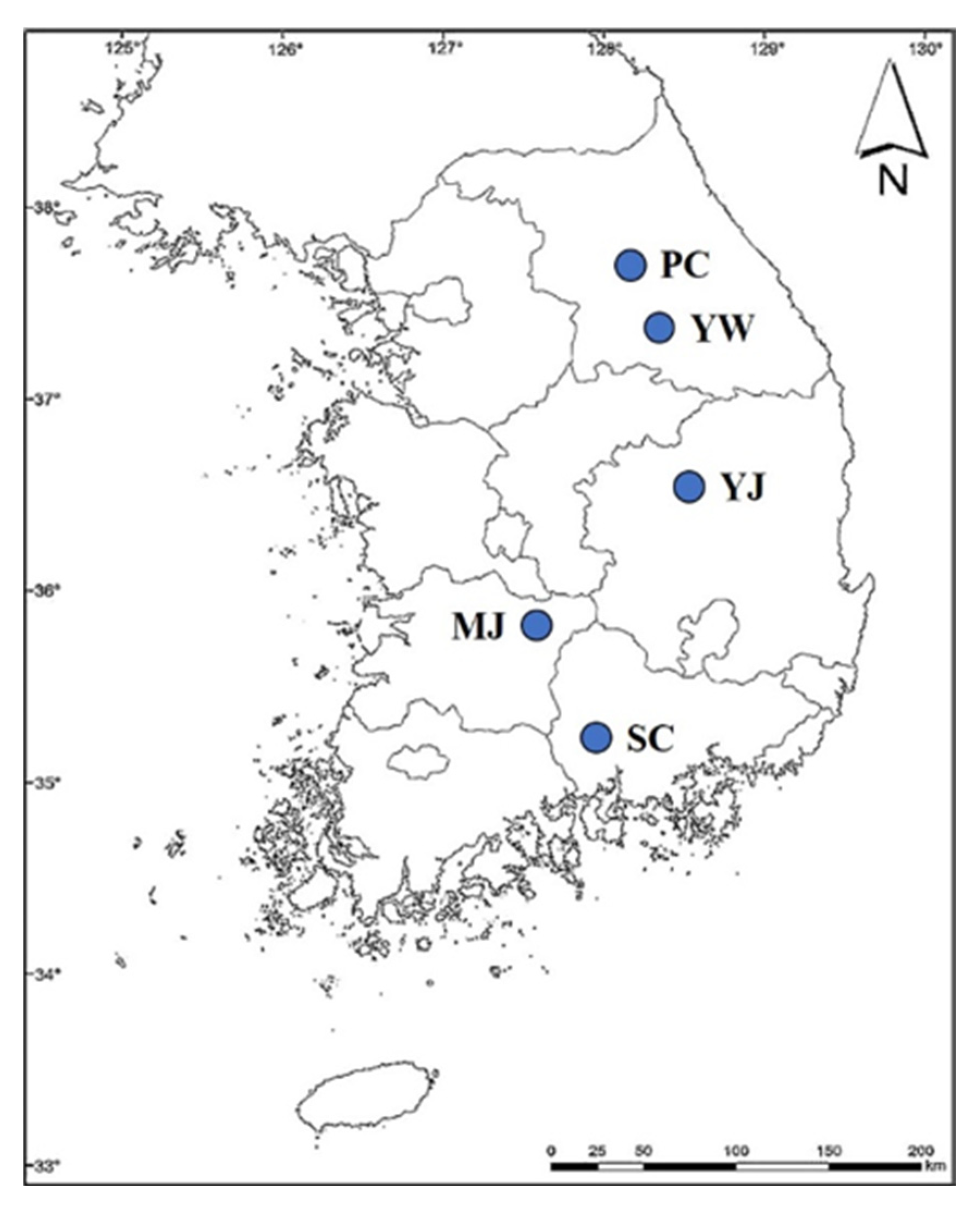
2.1. Determination of Soil Properties in WSG Cultivation Site and Growth Characteristics of WSG
2.2. Sample Extraction and Reagents
2.3. Analysis of Ginsenoside Contents of WSG
2.4. Data Analysis
3. Results and Discussion
3.1. Soil Properties of Cultivation Sites Collecting WSG with Different Ages
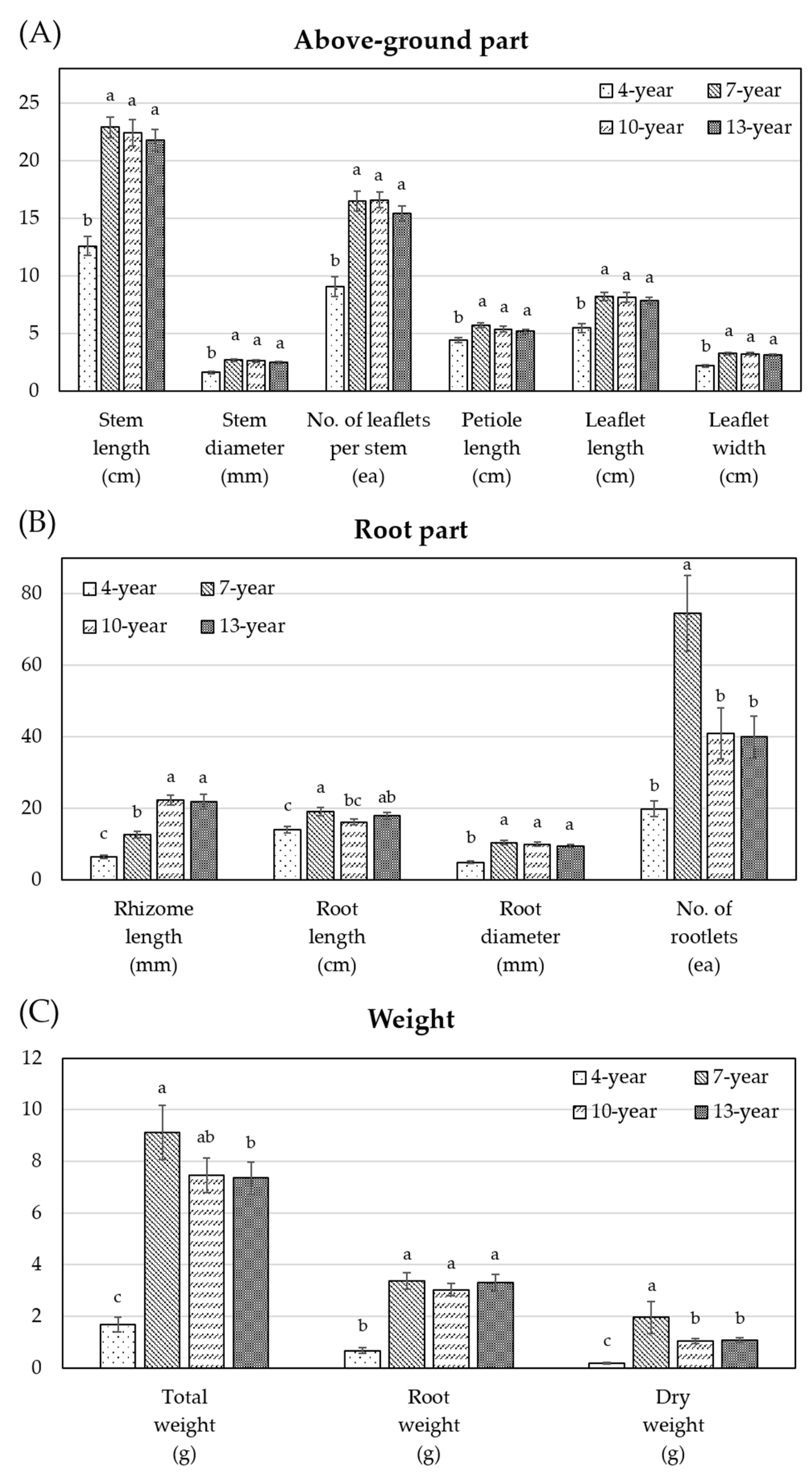
3.2. Growth Characteristics and Ginsenoside Contents of WSG with Different Ages
3.3. Correlations between Growth Characteristics and Ginsenoside Contents of WSG with Different Ages
3.4. Correlation between Soil Properties of Cultivation sites and Ginsenoside Contents of WSG with Different Ages
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nah, S.Y. Ginseng: Recent advances and trends. Korean J. Ginseng Sci. 1997, 21, 1–12. [Google Scholar]
- Kim, J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: A review for use in cardiovascular diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S. Weather characteristic and growth of a forest ginseng cultivation site. J. Korean Soc. For. Sci. 2010, 99, 863–870. [Google Scholar]
- Kim, K.Y.; Um, Y.R.; Jeong, D.H.; Kim, H.J.; Kim, J.J.; Jeon, K.S. The correlation between growth characteristics and location environment of wild-simulated ginseng (Panax ginseng C.A. Meyer). Korean J. Plant Res. 2019, 32, 463–470. [Google Scholar]
- Korea Forest Service (KFS). The Industry Development Countermeasure of Wild-Simulated Ginseng; Korea Forest Service: Daejeon, Republic of Korea, 2019.
- Korea Forest Service (KFS). Production of Forest Products; Korea Forest Service: Daejeon, Republic of Korea, 2021.
- Kim, K.Y.; Um, Y.R.; Eo, H.J.; Park, H.W.; Jeon, K.S.; Kim, H.J. Study on the correlation between the ginsenoside contents and growth characteristics of wild-simulated ginseng with different year-roots (Panax ginseng C.A. Meyer). Korean J. Plant Res. 2020, 33, 255–262. [Google Scholar]
- Lee, M.H.; Jeong, J.H.; Seo, J.W.; Chin, C.G.; Kim, Y.S.; In, J.G.; Yang, D.C.; Yi, J.S.; Choi, Y.E. Enhanced triterpene and phytosterol biosynthesis in Panax ginseng over-expressing squalene synthase gene. Plant Cell. Physiol. 2004, 45, 976–984. [Google Scholar] [CrossRef]
- Richter, R.; Basar, S.; Koch, A.; Konig, W.A. Three sequiterpene hydrocarbons from the roots of Panax ginseng C.A. Meyer (Araliaceae). Phytochemistry 2005, 66, 2708–2713. [Google Scholar] [CrossRef]
- Kim, J.S. Investigation of phenolic, flavonoid, and vitamin contents in different parts of Korean Ginseng (Panax ginseng C.A. Meyer). Prev. Nutr. Food Sci. 2016, 21, 263–270. [Google Scholar] [CrossRef]
- Jin, S.; Jeon, J.H.; Lee, S.; Kang, W.Y.; Seong, S.J.; Yoon, Y.R.; Choi, M.K.; Song, I.S. Detection of 13 ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg3, Rh2, Compound K, 20(S)-protopanaxadiol, and 20(S)-protopanaxatriol) in human plasma and application of the analytical method to human pharmacokinetic studies following two week-repeated administration of red ginseng extract. Molecules 2019, 24, 2618. [Google Scholar]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef]
- Park, C.K.; Jeon, B.S.; Yang, J.W. The chemical components of Korean ginseng. Food Ind. Nutr. 2003, 8, 10–23. [Google Scholar]
- Jeong, B.G.; Jung, G.R.; Kim, M.S.; Moon, H.G.; Park, S.J.; Chun, J. Ginsenoside contents and antioxidant activities of cultivated mountain ginseng (Panax ginseng C.A. Meyer) with different ages. Korean J. Food Preserv. 2019, 26, 90–100. [Google Scholar] [CrossRef]
- Rural Development Administration (RDA). Analysis Manual of Comprehensive Laboratory (Soil, Plant, Water and Liquid Manure); Rural Development Administration: Suwon, Republic of Korea, 2013; pp. 31–53. [Google Scholar]
- Korea Seed and Variety Service (KSVS). Know-How of Characteristics Investigation of the Crops: Ginseng (Panax ginseng Meyer); Korea Seed and Variety Service: Gimcheon, Republic of Korea, 2014. [Google Scholar]
- Nurek, T.; Gendek, A.; Roman, K. Forest residues as a renewable source of energy: Elemental composition and physical properties. Bio. Resour. 2019, 14, 6–20. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, H.J.; Um, Y.R.; Jeon, K.S. Effect of soil properties and soil bacteria community on early growth characteristics of wild-simulated ginseng (Panax ginseng C.A. Meyer) in coniferous and mixed forest. Korean J. Med. Crop Sci. 2020, 28, 183–194. [Google Scholar] [CrossRef]
- Eida, A.A.; Hirt, H.; Saad, M.M. Challenges Faced in Field Application of Phosphate-Solubilizing Bacteria. In Rhizotrophs: Plant Growth Promotion to Bioremediation. Microorganisms for Sustainability; Mehnaz, S., Ed.; Springer: Singapore, 2017; Volume 2, pp. 125–143. [Google Scholar]
- Mardad, I.; Serrano, A.; Soukri, A. Solubilization of inorganic phosphate and production of organic acids by bacteria isolated from a Moroccan mineral phosphate deposit. Afr. J. Microbiol. Res. 2013, 7, 626–635. [Google Scholar]
- Khan, M.; Ahmad, E.; Zaidi, A.; Oves, M. Functional aspect of phosphate-solubilizing bacteria: Importance in crop production. In Bacteria in Agrobiology: Crop Productivity; Maheshwari, D.K., Saraf, M., Aeron, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 237–263. [Google Scholar]
- Huh, J.H.; Yun, Y.B.; Um, Y.R.; Kim, K.Y.; Kim, J.A.; Jeon, K.S. Isolation of nitrogen fixing bacteria from 13 year-old wild-simulated ginseng (Panax ginseng C.A. Meyer) rhizosphere soil. Korean J. Wild Ginseng 2021, 15, 1–11. [Google Scholar]
- Son, H.J.; Kim, Y.G.; Lee, S.J. Isolation, identification and physiological characteristics of biofertilizer resources, insoluble phosphate-solubilizing bacteria. Korean J. Microbiol. 2003, 39, 51–55. [Google Scholar]
- Kim, K.Y.; Jordan, D.; Krishnan, H.B. Rahnella aquatilis, a bacterium isolated from soybean rhizosphere, can solubilize hydroxyapatide. FEMS Microbiol. Lett. 1997, 153, 273–277. [Google Scholar] [CrossRef]
- Eo, J.; Mo, H.S.; Park, K.C. Abiotic factors influencing growth and ginsenoside content of Panax ginseng roots. Hortic. Sci. Technol. 2018, 36, 681–690. [Google Scholar]
- Castagno, L.N.; Sannazzaro, A.I.; Gonzalez, M.E.; Pieckenstain, F.L.; Estrella, M.J. Phosphobacteria as key actors to overcome phosphorus deficiency in plants. Ann. Appl. Biol. 2021, 178, 256–267. [Google Scholar] [CrossRef]
- Choi, Y.E.; Kim, Y.S.; Yi, M.J.; Park, W.G.; Yi, J.S.; Chun, S.R.; Han, S.S.; Lee, S.J. Physiological and chemical characteristics of field-and mountain-cultivated ginseng roots. J. Plant Biol. 2007, 50, 198–205. [Google Scholar] [CrossRef]
- Shin, S.M.; Park, M.S.; Lee, H.S.; Lee, S.E.; Lee, H.E.; Kim, T.H.; Kim, H.J. Global trends in research on wild-simulated ginseng: Quo vadis? Forests 2021, 12, 664. [Google Scholar] [CrossRef]
- National Institute of Forest Science (NIFoS). Standard Guideline for Wild Simulated Ginseng Cultivation; NIFoS: Seoul, Republic of Korea, 2018. [Google Scholar]
- Tian, Y.Z.; Liu, Y.P.; Tian, S.C.; Ge, S.Y.; Wu, Y.J.; Zhang, B.L. Antitumor activity of ginsenoside Rd in gastric cancer via up-regulation of Caspase-3 and Caspase-9. Die Pharm. 2019, 75, 147–150. [Google Scholar]
- Zhang, N.; An, X.; Lang, P.; Wang, F.; Xie, Y. Ginsenoside Rd contributes the attenuation of cardiac hypertrophy in vivo and in vitro. Biomed. Pharmacother. 2019, 109, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Liu, Q.P.; An, P.; Jia, M.; Luan, X.; Tang, J.Y.; Zhang, H. Ginsenoside Rd: A promising natural neutroprotective agent. Phytomedicine 2022, 95, 153883. [Google Scholar] [CrossRef]
- Zhou, A.F.; Zhu, K.; Pu, P.M.; Li, Z.Y.; Zhang, Y.Y.; Shu, B.; Cui, X.J.; Yao, M.; Wang, Y.J. Neuroprotective effect and possible mechanisms of ginsenoside Rd for cerebral ischemia/reperfusion damage in experimental animal: A meta-analysis and systematic review. Oxid. Med. Cell. Longev. 2022, 2022, 7650438. [Google Scholar] [CrossRef]
- Kim, K.Y.; Huh, J.H.; Um, Y.; Jeon, K.S.; Kim, H.J. The comparative of growth characteristics and ginsenoside contents in wild-simulated ginseng (Panax ginseng C.A. Meyer) on different years by soil properties of cultivation regions. Korean J. Plant Res. 2020, 33, 651–658. [Google Scholar]
- Lacava, P.T.; Bogas, A.C.; Cruz, F.P.N. Plant growth promotion and biocontrol by endophytic and rhizospheric microorganisms from the tropics: A review and perspectives. Front. Sustain. Food Syst. 2022, 6, 796113. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]
- Lopes, M.J.S.; Dias-Filho, M.B.; Gurge, E.S.C. Successful plant growth-promoting microbes: Inoculation methods and abiotic factors. Front. Sustain. Food Syst. 2021, 5, 606454. [Google Scholar] [CrossRef]
- Fang, X.; Wang, M.; Zhou, X.; Wang, H.; Wang, H.; Xiao, H. Effects of growth years on ginsenoside biosynthesis of wild ginseng and cultivated ginseng. BMC Genom. 2022, 23, 325. [Google Scholar] [CrossRef] [PubMed]
- Paz-Gonzalez, A.; Vieira, S.R.; Taboada Castro, M.T. The effect of cultivation on the spatial variability of selected properties of an umbric horizon. Geoderma 2000, 37, 273–292. [Google Scholar] [CrossRef]
- You, J.; Liu, X.; Zhang, B.; Xie, Z.; Hou, Z.; Yang, Z. Seasonal changes in soil acidity and related properties in ginseng artificial bed soils under a plastic shade. J. Ginseng Res. 2014, 39, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Konsler, T.R.; Shelton, J.E. Lime and phosphorus effects on American ginseng: I. Growth, soil fertility, and root tissue nutrient status response. JASHS 1990, 115, 570–574. [Google Scholar] [CrossRef]
- Burkhart, E.P. American ginseng (Panax quinquefolius L.) floristic associations in Pennsylvania: Guidance for identifying calcium-rich forest farming sites. Agroforest. Syst. 2013, 87, 1157–1172. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Settineri, G.; Papalia, T.; Mallamaci, C.; Attina, E. Influence of soil properties on bioactive compounds and antioxidant capacity of Brassica rupestris Raf. J. Soil Sci. Plant Nutr. 2019, 19, 808–815. [Google Scholar] [CrossRef]
- Pal, P.K.; Kumar, R.; Guleria, V.; Mahajan, M.; Prasad, R.; Pathania, V.; Gill, B.S.; Singh, D.; Chand, G.; Singh, B.; et al. Crop-ecology and nutritional variability influence growth and secondary metabolites of Stevia rebaudiana Bertoni. BMC Plant Biol. 2015, 15, 67. [Google Scholar] [CrossRef]
- Zhang, T.; Han, M.; Yang, L.; Han, Z.; Cheng, L.; Sun, Z.; Yang, L. The effects of environmental factors on ginsenoside biosynthetic enzyme gene expression and saponin abundance. Molecules 2019, 24, 14. [Google Scholar] [CrossRef]
- Kim, Y.J.; Zhang, D.; Yang, D.C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv. 2015, 33, 717–735. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100255. [Google Scholar] [CrossRef]
- Nam, K.Y. The New Korean Ginseng (Constituent and Its Pharmacological Efficacy); Korea Ginseng and Tobacco Research Institute: Daejeon, Republic of Korea, 1996; pp. 1–10. [Google Scholar]
- Chang, H.K. Changes of saponin contents in Panax ginseng leaves by different harvesting months. Korean J. Food Nutr. 1998, 11, 82–86. [Google Scholar]
- Yang, B.W.; Lee, J.B.; Lee, J.M.; Jo, M.S.; Byun, J.K.; Kim, H.C.; Ko, S.K. The composition of seasonal ginsenoside composition contents in Korean wild simulated ginseng (Panax ginseng) which were cultivated in different areas and various ages. Nat. Prod. Sci. 2019, 25, 1–10. [Google Scholar] [CrossRef]
- Moon, H.K. Quality characteristics and anti-diabetic effect of mountain-cultivated ginseng (Sanyangsam). Ph.D. Thesis, Kyungpook National University, Daegu, Republic of Korea, 2015. [Google Scholar]
- Kang, O.J.; Kim, J.S. Comparison of ginsenoside contents in different parts of Korean ginseng (Panax ginseng C.A. Meyer). Prev. Nutr. Food Sci. 2016, 21, 389–392. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, Y.-B.; Huh, J.-H.; Um, Y. Correlations among Soil Properties, Growth Characteristics, and Ginsenoside Contents in Wild-Simulated Ginseng with Different Ages. Forests 2022, 13, 2065. https://doi.org/10.3390/f13122065
Yun Y-B, Huh J-H, Um Y. Correlations among Soil Properties, Growth Characteristics, and Ginsenoside Contents in Wild-Simulated Ginseng with Different Ages. Forests. 2022; 13(12):2065. https://doi.org/10.3390/f13122065
Chicago/Turabian StyleYun, Yeong-Bae, Jeong-Hoon Huh, and Yurry Um. 2022. "Correlations among Soil Properties, Growth Characteristics, and Ginsenoside Contents in Wild-Simulated Ginseng with Different Ages" Forests 13, no. 12: 2065. https://doi.org/10.3390/f13122065
APA StyleYun, Y.-B., Huh, J.-H., & Um, Y. (2022). Correlations among Soil Properties, Growth Characteristics, and Ginsenoside Contents in Wild-Simulated Ginseng with Different Ages. Forests, 13(12), 2065. https://doi.org/10.3390/f13122065





