Growing Season Harvests of Shrub Willow (Salix spp.) Have Higher Nutrient Removals and Lower Yields Compared to Dormant-Season Harvests
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
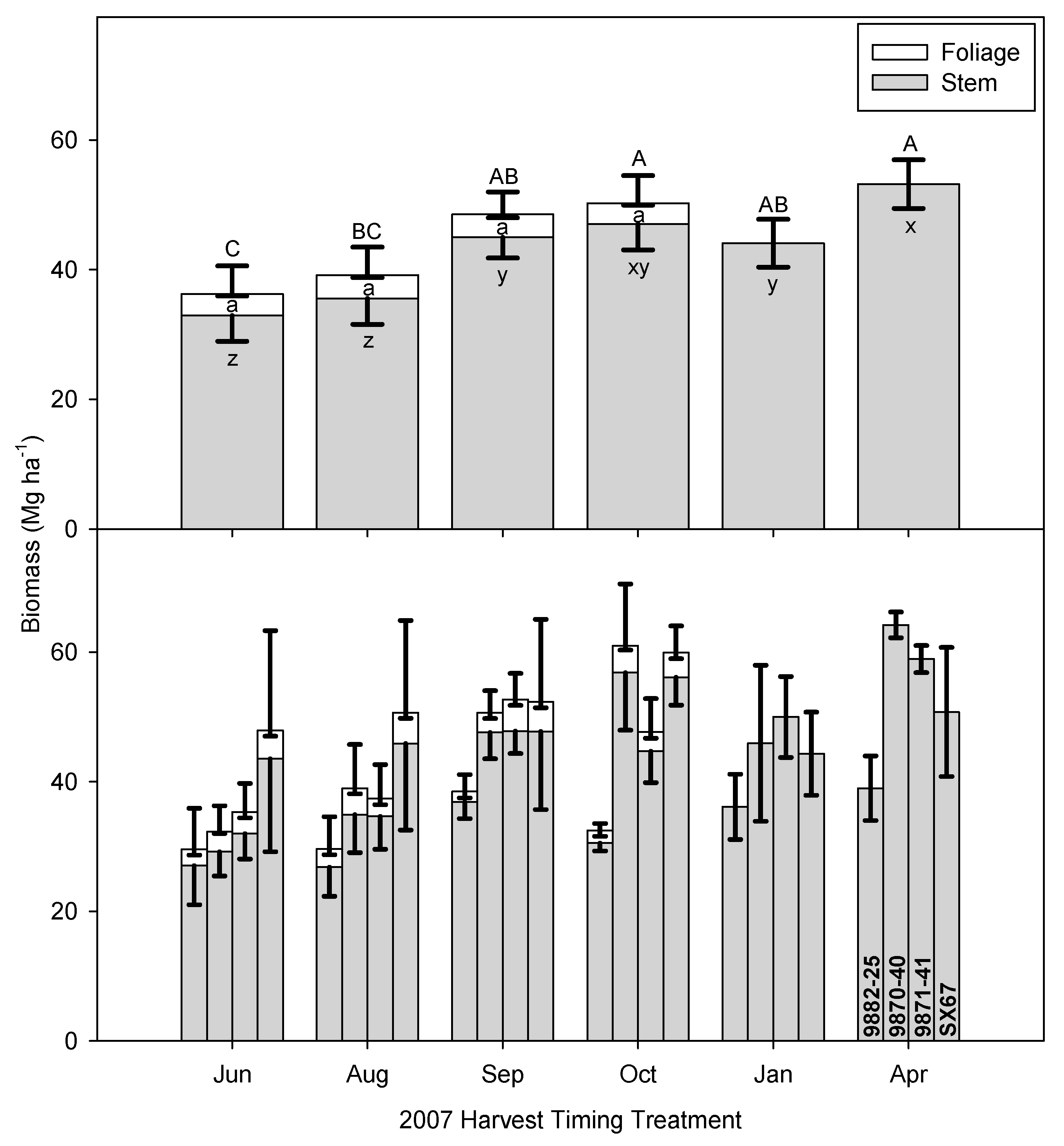
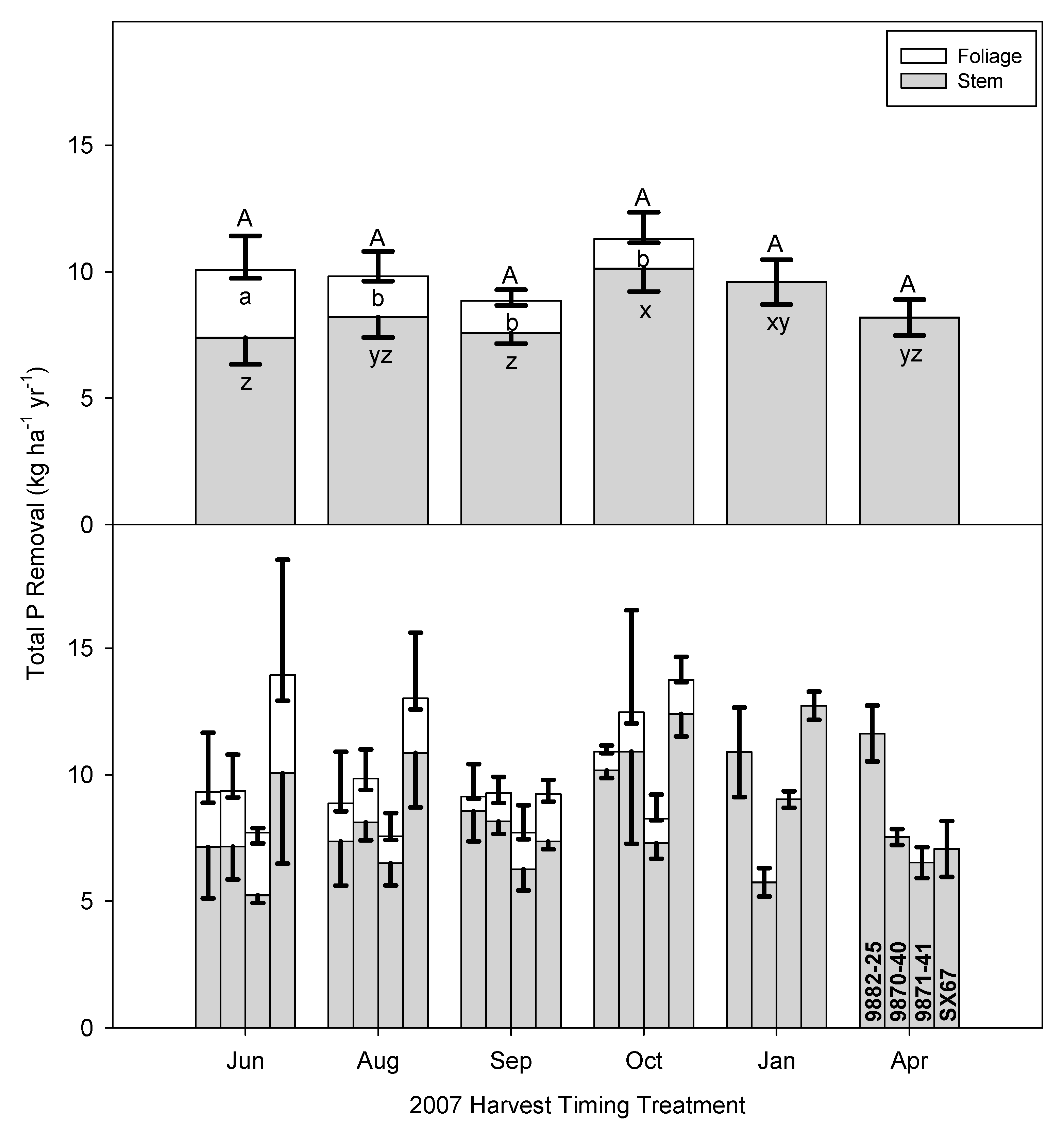
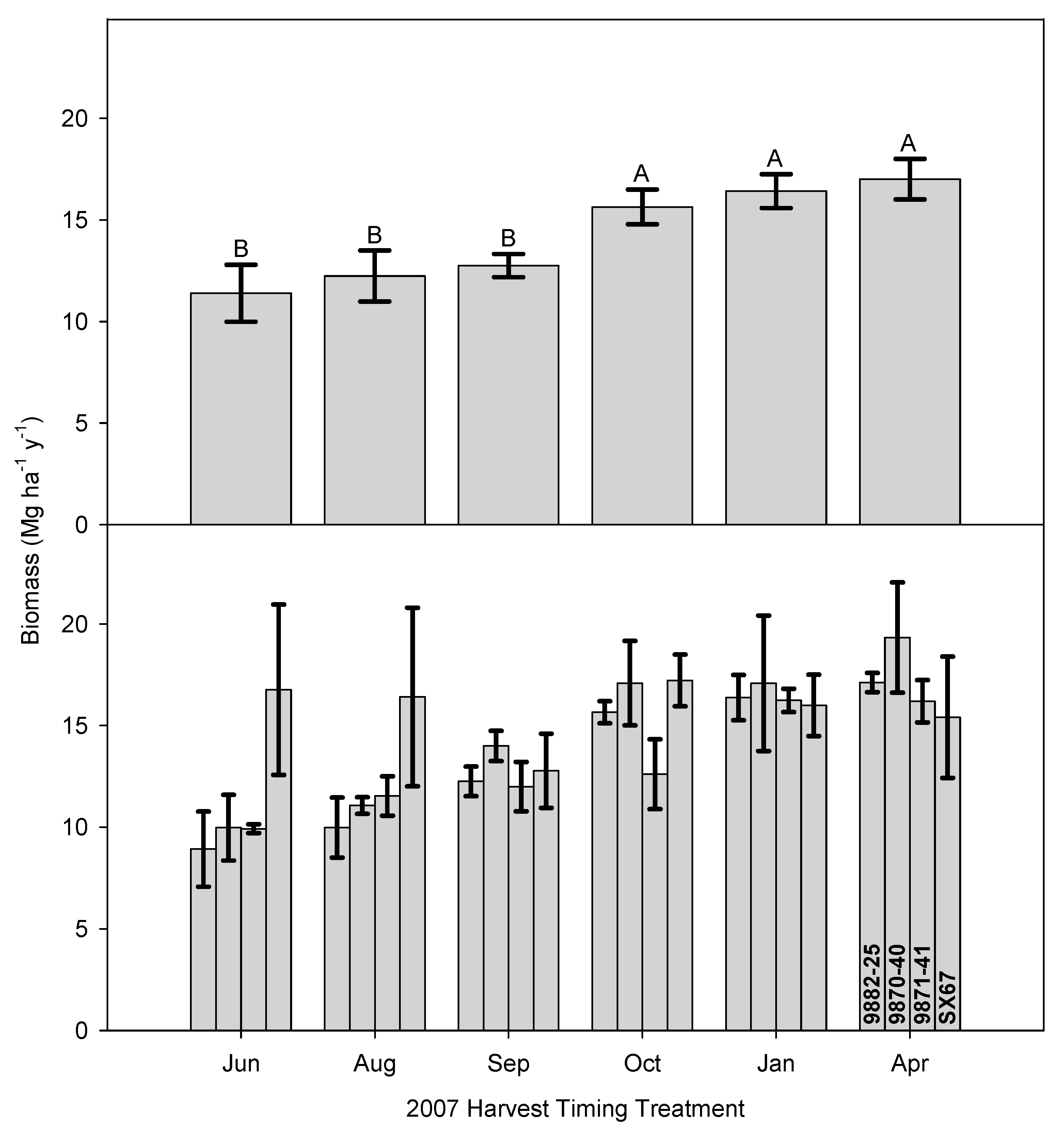
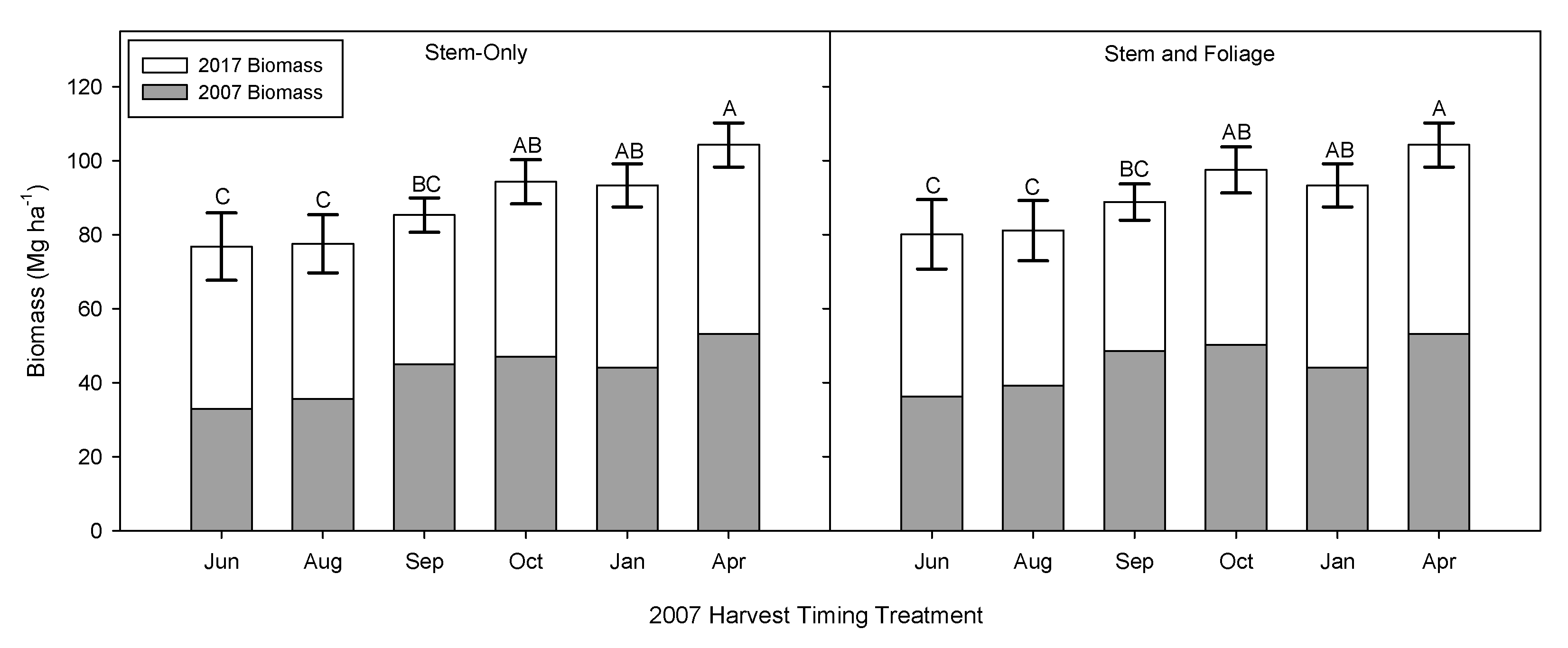
3.1. Time of Harvest Effect on Biomass and Nutrient Removal
3.2. Time of Harvest Effect on Biomass and Second Rotation Biomass
3.3. Implications of Early Harvests on Biomass Production and Regrowth
3.4. Implications of Early Harvests on Nutrient Removal
3.5. Implications of Harvest Dates for Commercial Operations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDOE. U.S. Billion-Ton Update: Biomass Supply for A Bioenergy and Bioproducts Industry; Oak Ridge National Laboratory: Oak Ridge, TN, 2011. [Google Scholar]
- USDOE. Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy. DOE/EE-1440, ORNL/TM-2016/160, 1271651; Langholtz, M., Stokes, B., Eaton, L., Eds.; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2016; p. 448. [Google Scholar]
- Volk, T.A.; Harlow, S.J. BCAP Helps Commercialize Shrub Willow for Bioenergy in Northers New York. Farm Energy. Available online: https://farm-energy.extension.org/bcap-helps-commercialize-shrub-willow-for-bioenergy-in-northern-new-york/ (accessed on 15 November 2022).
- Volk, T.A.; Heavey, J.P.; Eisenbies, M.H. Advances in shrub-willow crops for bioenergy, renewable products, and environmental benefits. Food Energy Secur. 2016, 5, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Buchholz, T.; Volk, T. Profitability of Willow Biomass Crops Affected by Incentive Programs. BioEnergy Res. 2013, 6, 53–64. [Google Scholar] [CrossRef]
- Stoof, C.R.; Richards, B.K.; Woodbury, P.B.; Fabio, E.S.; Brumbach, A.R.; Cherney, J.; Das, S.; Geohring, L.; Hansen, J.; Hornesky, J.; et al. Untapped Potential: Opportunities and Challenges for Sustainable Bioenergy Production from Marginal Lands in the Northeast USA. BioEnergy Res. 2015, 8, 482–501. [Google Scholar] [CrossRef]
- Campbell, S.P.; Frair, J.L.; Gibbs, J.P.; Volk, T.A. Use of short-rotation coppice willow crops by birds and small mammals in central New York. Biomass Bioenergy 2012, 47, 342–353. [Google Scholar] [CrossRef]
- Smart, L.B.; Cameron, K.D. Genetic improvement of willow (Salix spp.) as a dedicated bioenergy crop. In Genetic Improvement of Bioenergy Crops; Vermerris, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 377–396. [Google Scholar]
- Heavey, J.P.; Volk, T.A. Living snow fences show potential for large storage capacity and reduced drift length shortly after planting. Agrofor. Syst. 2014, 88, 803–814. [Google Scholar] [CrossRef]
- Mirck, J.; Volk, T.A. Response of three shrub willow varieties (Salix spp.) to storm water treatments with different concentrations of salts. Bioresour. Technol. 2010, 101, 3484–3492. [Google Scholar] [CrossRef]
- Fox, T.R. Sustained productivity in intensively managed forest plantations. For. Ecol. Manag. 2000, 138, 187–202. [Google Scholar] [CrossRef]
- Garrett, L.G.; Smaill, S.J.; Addison, S.L.; Clinton, P.W. Globally relevant lessons from a long-term trial series testing universal hypothesis of the impacts of increasing biomass removal on site productivity and nutrient pools. For. Ecol. Manag. 2021, 494, 119325. [Google Scholar] [CrossRef]
- Worrell, R.; Hampson, A. The influence of some forest operations on the sustainable management of forest soils—A review. For. Int. J. For. Res. 1997, 70, 61–85. [Google Scholar] [CrossRef]
- Heilman, P.; Norby, R.J. Nutrient cycling and fertility management in temperate short rotation forest systems. Biomass Bioenergy 1998, 14, 361–370. [Google Scholar] [CrossRef]
- Burger, J.; Scott, D. Soil interpretations for sustainable forest management in the southeastern United States. In Soil Science—Past, Present, and Future, Proceedings of the Joint Meeting of the Czech Society of Soil Science and the Soil Science Society of America, Prague, Czech Republic, 16–20 September 2001; Virginia Polytechnic Institute and State University: Prague, Czech Republic, 2001; pp. 65–72. [Google Scholar]
- Burger, J.A. Soil and long-term site productivity values. In Bioenergy from Sustainable Forestry: Guiding Principles and Practice; Richardson, J., Björheden, R., Hakkila, P., Lowe, A.T., Smith, C.T., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; Volume 71, ISBN 1-4020-0676-4. [Google Scholar]
- NRCS. Soil Biology and Land Management; USDA-NRCS: Washington, DC, USA, 2004; p. 20. [Google Scholar]
- Abbas, D.; Current, D.; Phillips, M.; Rossman, R.; Hoganson, H.; Brooks, K.N. Guidelines for harvesting forest biomass for energy: A synthesis of environmental considerations. Biomass Bioenergy 2011, 35, 4538–4546. [Google Scholar] [CrossRef]
- Eisenbies, M.; Volk, T.; Amidon, T.; Bergey, S.; Bold-Erdene, Z.; Clark, R.; DeSouza, D.; Ebadian, M.; Emerson, R.; Gantz, C.; et al. Improved Advanced Biomass Logistics Utilizing Woody and other Feedstocks in the Northeast and Pacific Northwest; Final Report: EE0006638, 1768177; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2020. [Google Scholar]
- De Souza, D.P.L. “Nutrient Removal in Willow Biomass Crops is Impacted Over Multiple Rot” by Daniel Pegoretti Leite de Souza. Dissertation; State University of New York College of Environmental Science and Forestry: Syracuse, NY, USA, 2019. [Google Scholar]
- Eisenbies, M.H.; Volk, T.A.; Posselius, J.; Foster, C.; Shi, S.; Karapetyan, S. Evaluation of a Single-Pass, Cut and Chip Harvest System on Commercial-Scale, Short-Rotation Shrub Willow Biomass Crops. BioEnergy Res. 2014, 7, 1506–1518. [Google Scholar] [CrossRef]
- Berhongaray, G.; El Kasmioui, O.; Ceulemans, R. Comparative analysis of harvesting machines on an operational high-density short rotation woody crop (SRWC) culture: One-process versus two-process harvest operation. Biomass Bioenergy 2013, 58, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Energy from Willow. Scottish Agricultural College. 2000. Available online: https://www.ecologieforum.eu/download/Willow_s.pdf (accessed on 9 January 2020).
- Eisenbies, M.; Volk, T.; DeSouza, D.; Kallen, K. Cut and chip harvester material capacity and fuel performance on commercial-scale willow fields for varying ground and crop conditions. Glob. Change Biol. Bioenergy 2020, 12, 380–395. [Google Scholar] [CrossRef] [Green Version]
- Von Fircks, Y.; Sennerby-Forsse, L. Seasonal fluctuations of starch in root and stem tissues of coppiced Salix viminalis plants grown under two nitrogen regimes. Tree Physiol. 1998, 18, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Von Fircks, Y.; Ericsson, T.; Sennerby-Forsse, L. Seasonal variation of macronutrients in leaves, stems and roots of Salix dasyclados Wimm. grown at two nutrient levels. Biomass Bioenergy 2001, 21, 321–334. [Google Scholar] [CrossRef]
- Brereton, N.J.B.; Pitre, F.E.; Shield, I.; Hanley, S.J.; Ray, M.J.; Murphy, R.J.; Karp, A. Insights into nitrogen allocation and recycling from nitrogen elemental analysis and 15N isotope labelling in 14 genotypes of willow. Tree Physiol. 2014, 34, 1252–1262. [Google Scholar] [CrossRef] [Green Version]
- Frank, J.; Therasme, O.; Volk, T.A.; Brown, T.; Malmsheimer, R.W.; Fortier, M.-O.; Eisenbies, M.H.; Ha, H.; Heavey, J. Integrated Stochastic Life Cycle Assessment and Techno-Economic Analysis for Shrub Willow Production in the Northeastern United States. Sustainability 2022, 14, 9007. [Google Scholar] [CrossRef]
- Hytönen, J. Biomass Production and Nutrition of Short Rotation Plantations. Ph.D. Thesis, The Finish Forest Research Institute, Helsinki, Finland, 1996. [Google Scholar]
- Ceulemans, R.; McDonald, A.J.S.; Pereira, J.S. A comparison among eucalypt, poplar and willow characteristics with particular reference to a coppice, growth-modelling approach. Biomass Bioenergy 1996, 11, 215–231. [Google Scholar] [CrossRef]
- Steinbeck, K. Intensively managed short rotation coppice forests. In 1978: Energy and the Southern Forest; Choong, E.T., Chamber, J.L., Eds.; Baton Rouge, LA, USA, 1978; Available online: https://digitalcommons.lsu.edu/cgi/viewcontent.cgi?article=1025&context=pafs (accessed on 9 January 2020).
- Strong, T.F.; Zavitovski, J. Effect of Harvesting Season on Hybrid Poplar Coppictng. In Intensive Plantation Culture: 12 Years Research; Hansen, E.A., Ed.; 1983; p. 4. Available online: https://www.fs.usda.gov/research/treesearch/18838 (accessed on 9 January 2020).
- De Souza, D.P.L.; Gallagher, T.; Mitchell, D.; McDonald, T.; Smidt, M. Determining the effects of felling method and season of year on the regeneration of short rotation coppice. Int. J. For. Eng. 2016, 27, 53–65. [Google Scholar] [CrossRef]
- Cunniff, J.; Purdy, S.J.; Barraclough, T.J.P.; Castle, M.; Maddison, A.L.; Jones, L.E.; Shield, I.F.; Gregory, A.S.; Karp, A. High yielding biomass genotypes of willow (Salix spp.) show differences in below ground biomass allocation. Biomass Bioenergy 2015, 80, 114–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubátová, P.; Száková, J.; Břendová, K.; Kroulíková-Vondráčková, S.; Mercl, F.; Tlustoš, P. Effects of summer and winter harvesting on element phytoextraction efficiency of Salix and Populus clones planted on contaminated soil. Int. J. Phytoremediation 2018, 20, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Bollmark, L. Accumulation and Mobilisation of Nutrient Reserves in Salix Viminalis; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2000; ISBN 978-91-576-5889-0. [Google Scholar]
- Hytönen, J. Effect of cutting season, stump height and harvest damage on coppicing and biomass production of willow and birch. Biomass Bioenergy 1994, 6, 349–357. [Google Scholar] [CrossRef]
- Da Ros, L.M.; Soolanayakanahally, R.Y.; Guy, R.D.; Mansfield, S.D. Phosphorus storage and resorption in riparian tree species: Environmental applications of poplar and willow. Environ. Exp. Bot. 2018, 149, 1–8. [Google Scholar] [CrossRef]
- USDA Natural Resources Conservation Service Web Soil Survey. Available online: https://websoilsurvey.sc.egov.usda.gov/App/HomePage.htm (accessed on 9 January 2020).
- Hanna, W.E. Soil Survey of Madison County; USDA Soil Conservation Service, Cornell University Agricultural Experiment Station: New York, NY, USA, 1981. [Google Scholar]
- Arevalo, C.; Volk, T.; Bevilacqua, E.; Abrahamson, L. Development and validation of aboveground biomass estimations for four Salix clones in central New York. Biomass Bioenergy 2007, 31, 1–12. [Google Scholar] [CrossRef]
- ASABE. Moisture Measurement—ANSI/ASABE Standards S358.2; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2012; p. 1. [Google Scholar]
- Carmer, S.; Swanson, M. An evaluation of ten pairwise multiple comparison procedures by Monte Carlo methods. J. Am. Stat. Assoc. 1973, 68, 66–74. [Google Scholar] [CrossRef]
- SAS. Institute SAS/STAT 9.2 User’s Guide, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2009. [Google Scholar]
- Sleight, N.J.; Volk, T.A.; Johnson, G.A.; Eisenbies, M.H.; Shi, S.; Fabio, E.S.; Pooler, P.S. Change in Yield Between First and Second Rotations in Willow (Salix spp.) Biomass Crops is Strongly Related to the Level of First Rotation Yield. BioEnergy Res. 2016, 9, 270–287. [Google Scholar] [CrossRef]
- Fabio, E.S.; Smart, L.B. Effects of nitrogen fertilization in shrub willow short rotation coppice production—A quantitative review. GCB Bioenergy 2018, 10, 548–564. [Google Scholar] [CrossRef]
- Tharakan, P.J.; Robison, D.J.; Abrahamson, L.P.; Nowak, C.A. Multivariate approach for integrated evaluation of clonal biomass production potential. Biomass Bioenergy 2001, 21, 237–247. [Google Scholar] [CrossRef]
- Weih, M. Genetic and environmental variation in spring and autumn phenology of biomass willows (Salix spp.): Effects on shoot growth and nitrogen economy. Tree Physiol. 2009, 29, 1479–1490. [Google Scholar] [CrossRef]
- Fixen, P.; Bruulsema, T.; Jensen, T.; Mikkelsen, R.; Murrell, T.; Phillips, S.; Rund, Q.; Stewart, W. The fertility of North American soils, 2010. Better Crops Plant Food 2010, 94, 6–8. [Google Scholar]
- Fromm, J. Wood formation of trees in relation to potassium and calcium nutrition. Tree Physiol. 2010, 30, 1140–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducrey, M.; Turrel, M. Influence of cutting methods and dates on stump sprouting in Holm oak (Quercus ilex L.) coppice. Ann. Sci. For. 1992, 49, 449–464. [Google Scholar] [CrossRef] [Green Version]
- Eisenbies, M.H.; Volk, T.A.; Posselius, J.; Shi, S.; Patel, A. Quality and Variability of Commercial-Scale Short Rotation Willow Biomass Harvested Using a Single-Pass Cut-and-Chip Forage Harvester. BioEnergy Res. 2014, 8, 546–559. [Google Scholar] [CrossRef]
- Kenney, K.L.; Smith, W.A.; Gresham, G.L.; Westover, T.L. Understanding biomass feedstock variability. Biofuels 2013, 4, 111–127. [Google Scholar] [CrossRef]
- Adegbidi, H.G.; Volk, T.A.; White, E.H.; Abrahamson, L.P.; Briggs, R.D.; Bickelhaupt, D.H. Biomass and nutrient removal by willow clones in experimental bioenergy plantations in New York State. Biomass Bioenergy 2001, 20, 399–411. [Google Scholar] [CrossRef]
- Hangs, R.D.; Schoenau, J.J.; Van Rees, K.C.J.; Bélanger, N.; Volk, T.; Jensen, T. First Rotation Biomass Production and Nutrient Cycling within Short-Rotation Coppice Willow Plantations in Saskatchewan, Canada. BioEnergy Res. 2014, 7, 1091–1111. [Google Scholar] [CrossRef] [Green Version]
- Labrecque, M.; Teodorescu, T.I. High biomass yield achieved by Salix clones in SRIC following two 3-year coppice rotations on abandoned farmland in southern Quebec, Canada. Biomass Bioenergy 2003, 25, 135–146. [Google Scholar] [CrossRef]
- Quaye, A.K.; Volk, T.A.; Hafner, S.; Leopold, D.J.; Schirmer, C. Impacts of paper sludge and manure on soil and biomass production of willow. Biomass Bioenergy 2011, 35, 2796–2806. [Google Scholar] [CrossRef]
- Dickmann, D.I.; Isebrands, J.G.; Eckenwalder, J.E.; Richardson, J.E. (Eds.) Poplar Culture in North America; NRC Research Press: Ottawa, ON, Canada, 2001. [Google Scholar]
- Lodhiyal, L.S.; Lodhiyal, N. Nutrient Cycling and Nutrient Use Efficiency in Short Rotation, High Density Central Himalayan Tarai Poplar Plantations. Ann. Bot. 1997, 79, 517–527. [Google Scholar] [CrossRef]
- Ericsson, T. Nutrient Dynamics and Requirements of Forest Crops. N. Z. J. For. Sci. 1994, 24, 133–168. [Google Scholar]
- Fabio, E.S.; Volk, T.A.; Miller, R.O.; Serapiglia, M.J.; Kemanian, A.R.; Montes, F.; Kuzovkina, Y.A.; Kling, G.J.; Smart, L.B. Contributions of environment and genotype to variation in shrub willow biomass composition. Ind. Crops Prod. 2017, 108, 149–161. [Google Scholar] [CrossRef]
- Peterson, G.M.; Galbraith, J.K. The Concept of Marginal Land. J. Farm Econ. 1932, 14, 295–310. [Google Scholar] [CrossRef]
- Khanna, M.; Chen, L.; Basso, B.; Cai, X.; Field, J.L.; Guan, K.; Jiang, C.; Lark, T.J.; Richard, T.L.; Spawn-Lee, S.A.; et al. Redefining marginal land for bioenergy crop production. GCB Bioenergy 2021, 13, 1590–1609. [Google Scholar] [CrossRef]
- Heavey, J.P.; Volk, T.A. EcoWillow 2.0. Available online: https://www.esf.edu/willow/download.htm (accessed on 9 January 2020).
- Hausenbuiller, R.L. Soil Science: Principles and Practices, 2nd ed.; Wm. C. Brown Company Publishers: Dubuque, IA, USA, 1978; ISBN 0-697-05853-0. [Google Scholar]
- Emerson, R.M.; Hernandez, S.; Williams, C.L.; Lacey, J.A.; Hartley, D.S. Improving bioenergy feedstock quality of high moisture short rotation woody crops using air classification. Biomass Bioenergy 2018, 117, 56–62. [Google Scholar] [CrossRef]
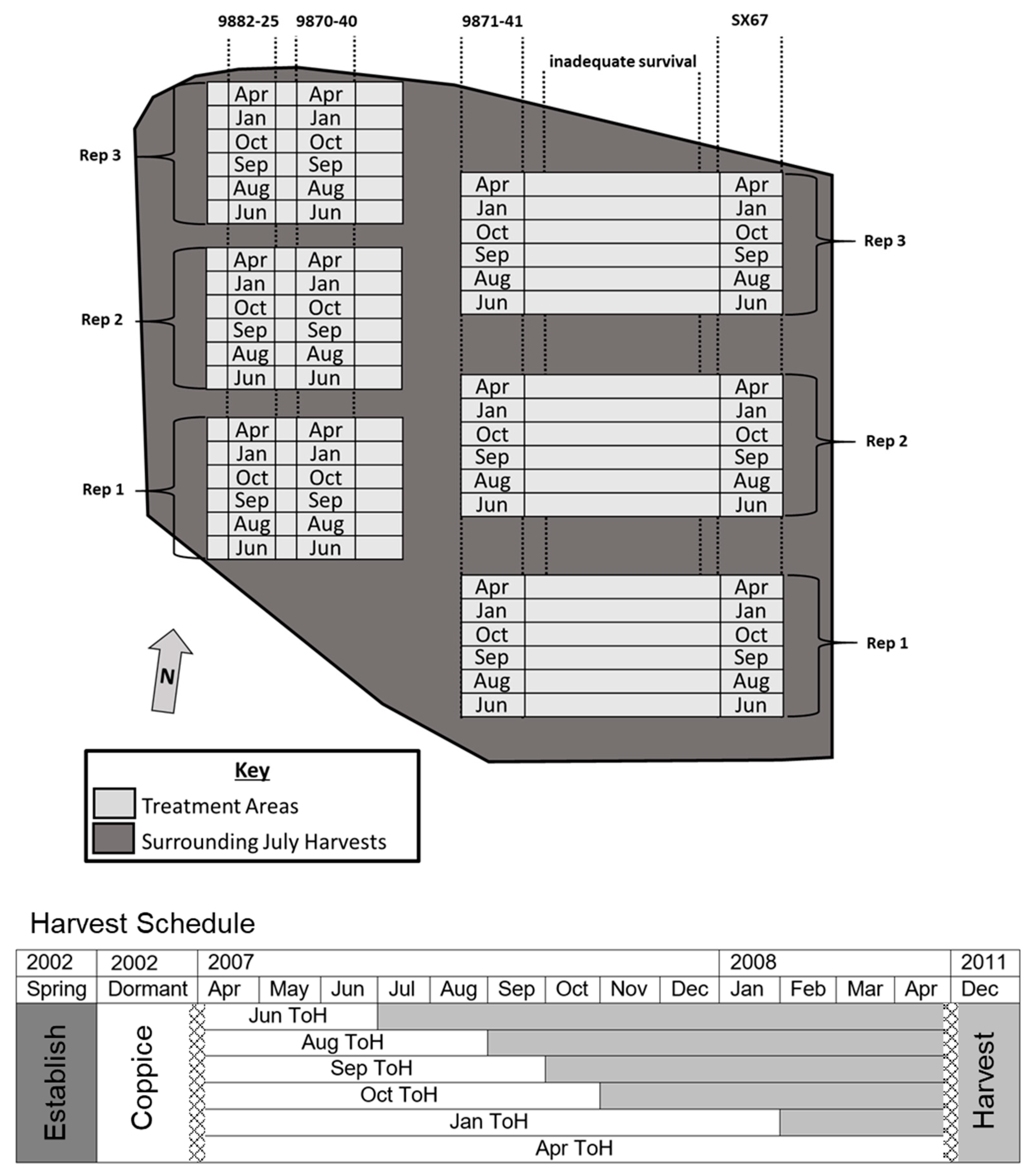


| Cultivar ID * | Diversity Group | Species/Pedigree | Note |
|---|---|---|---|
| 9882-25 | PUR | S. purpurea L. | narrower strips |
| 9870-40 | MIYA | S. miyabeana Seem. | |
| 9871-41 | MIYA | S. miyabeana Seem. | |
| 95311 | ERIO | S. eriocephala Michx. | low survival, removed |
| SX61 | MIYA | S. miyabeana Seem. | low survival, removed |
| SX67 | MIYA | S. miyabeana Seem. |
| Time of Harvest Treatment | Harvest Date | Plant Stage | Stem Age at First Harvest (Months) | GDD * for Entire First Rotation | Stem Age at Second Harvest in January 2011 (Months) | GDD * for Entire Second Rotation |
|---|---|---|---|---|---|---|
| June | 6–8 June 2007 | After full leaf out | 54 | 7187 | 54 | 6745 |
| August | 2–8 August 2007 | After bud set | 56 | 7739 | 52 | 6146 |
| September | 13–18 September 2007 | Starting senescence | 57 | 8164 | 51 | 5747 |
| October | 15–19 October 2007 | Mid-fall (leaves dropping) | 58 | 8366 | 50 | 5552 |
| January | 21–24 January 2008 | Dormant | 61 | 8417 | 47 | 5508 |
| April | 10–14 April 2008 | Before leaf out began | 64 | 8420 | 44 | 5460 |
| Biomass | Time of Harvest (ToH) | Cultivar (C) | ToHxC |
|---|---|---|---|
| p-Values | |||
| 2007 Stem | <0.0001 | 0.2013 | 0.3546 |
| 2007 Foliage | 0.8868 | 0.0469 | 0.0794 |
| 2007 Total | 0.0068 | 0.2375 | 0.4023 |
| 2011 Annual yield | <0.0001 | 0.3233 | 0.1488 |
| Cumulative 2007–2011 | 0.0027 | 0.3038 | 0.2707 |
| DF | 5 | 3 | 15 |
| Denominator DF | 40 | 6 | 40 |
| Element | Time of Harvest (ToH) | Cultivar (C) | ToHxC |
|---|---|---|---|
| p-Values | |||
| Combined stem and foliage | |||
| N (kg ha−1 year−1) | 0.0293 | 0.0792 | 0.0249 |
| P (kg ha−1 year−1) | 0.0514 | 0.0879 | 0.0891 |
| K (kg ha−1 year−1) | 0.0002 | 0.0592 | 0.1043 |
| Stem | |||
| N (kg ha−1 year−1) | <0.0001 | 0.0655 | 0.0062 |
| P (kg ha−1 year−1) | 0.0108 | 0.0717 | 0.0419 |
| K (kg ha−1 year−1) | 0.0724 | 0.0501 | 0.1331 |
| Foliage | |||
| N (kg ha−1 year−1) | 0.0345 | 0.1233 | 0.1429 |
| P (kg ha−1 year−1) | 0.0076 | 0.1534 | 0.1646 |
| K (kg ha−1 year−1) | 0.0363 | 0.0589 | 0.1069 |
| DF | 5 | 3 | 15 |
| Denominator DF | 40 | 6 | 40 |
| Harvest Date | N | P | K | |||
|---|---|---|---|---|---|---|
| Wood | Foliage | Wood | Foliage | Wood | Foliage | |
| ------------------------------------------------------------ mg/kg ------------------------------------------------------------- | ||||||
| June | 3.37 (0.18) C | 26.92 (0.70) A | 0.57 (0.04) | 3.43 (0.11) A | 3.18 (0.18) A | 14.44 (0.40) A |
| August | 3.53 (0.17) BC | 22.71 (0.64) B | 0.66 (0.04) | 2.04 (0.08) B | 3.42 (0.20) A | 12.12 (0.74) B |
| September | 3.55 (0.09) BC | 18.97 (0.68) C | 0.59 (0.03) | 1.61 (0.06) C | 2.63 (0.13) B | 9.38 (0.27) C |
| October | 4.12 (0.17) AB | 17.25 (0.51) C | 0.63 (0.03) | 1.70 (0.09) C | 2.21 (0.10) BC | 8.31 (0.78) C |
| Novembwer | 4.53 (0.50) A | 0.60 (0.06) | 2.36 (0.23) B | |||
| April | 3.41 (0.26) C | 0.49 (0.04) | 1.88 (0.14) C | |||
| Harvest Date | First Rotation | Second Rotation | Gross Revenue over Two Rotations | ||
|---|---|---|---|---|---|
| Biomass Wet | Gross Revenue * | Biomass Wet | Gross Revenue * | ||
| Mg ha−1 | USD ha−1 | Mg ha−1 | USD ha−1 | USD ha−1 | |
| June | 65.8 | 2006.9 | 82.6 | 2519.3 | 4526.2 |
| August | 71.2 | 2171.6 | 79.1 | 2412.6 | 4584.2 |
| September | 76.3 | 2327.2 | 76.0 | 2318.0 | 4645.2 |
| October | 82.5 | 2516.3 | 89.2 | 2720.6 | 5236.9 |
| January | 80.2 | 2446.1 | 93.0 | 2836.5 | 5282.6 |
| April | 91.7 | 2796.9 | 96.2 | 2934.1 | 5731.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Souza, D.P.; Eisenbies, M.H.; Volk, T.A. Growing Season Harvests of Shrub Willow (Salix spp.) Have Higher Nutrient Removals and Lower Yields Compared to Dormant-Season Harvests. Forests 2022, 13, 1936. https://doi.org/10.3390/f13111936
De Souza DP, Eisenbies MH, Volk TA. Growing Season Harvests of Shrub Willow (Salix spp.) Have Higher Nutrient Removals and Lower Yields Compared to Dormant-Season Harvests. Forests. 2022; 13(11):1936. https://doi.org/10.3390/f13111936
Chicago/Turabian StyleDe Souza, Daniel P., Mark H. Eisenbies, and Timothy A. Volk. 2022. "Growing Season Harvests of Shrub Willow (Salix spp.) Have Higher Nutrient Removals and Lower Yields Compared to Dormant-Season Harvests" Forests 13, no. 11: 1936. https://doi.org/10.3390/f13111936
APA StyleDe Souza, D. P., Eisenbies, M. H., & Volk, T. A. (2022). Growing Season Harvests of Shrub Willow (Salix spp.) Have Higher Nutrient Removals and Lower Yields Compared to Dormant-Season Harvests. Forests, 13(11), 1936. https://doi.org/10.3390/f13111936







