Abstract
In boreal forests in Canada, broadleaf stands are characterized by generally well-drained soils and a humus-rich layer. In contrast, spruce-moss stands are often characterized by more poorly drained soils and acidic humus layer. However, presence of these two forest types in various degrees of mixture in stands can be beneficial to spruce seedlings productivity. It was hypothesized that leaf litter and humus from pure spruces-moss stands, pure broadleaf stands, and mixed stand may influence Black spruce (Picea mariana (Mill.) BSP) seedling growth and development differently. A greenhouse experiment was carried out to evaluate the effect of different leaf litter and different humus on spruces seedlings. Our results suggest better development for seedlings grown in humus from mixed stands and pure broadleaf stands compared to humus from pure B. spruce or standard forest nursery substrate. Furthermore, leaf litter from broadleaf trees species, such as species Speckle alder (Alnus rugosa (Du Roi) R.T. Clausen), T. aspen (Populus tremuloides Michx), Willows (Salix spp.) and Paper birch (Betula papyrifera Marsh.), has shown distinct results in the growth and development of B. spruce seedlings in greenhouse. Furthermore, promotion of mixed stand can increase B. spruce productivity by improving the physicochemical composition of the forest floor.
1. Introduction
The fall of broadleaf leaves or coniferous needles on the soil forms litter, which in turn is decomposed in the forest floor, releasing nutrients [1,2]: litter feeds the soil and soil feeds the plants. Remarkably, the heterogeneity of tree species influences understory conditions through the amount of light [3,4,5,6], root density and disposition [1,7,8], as well as transformation of the chemical composition of atmospheric precipitation as it flows through the canopy and reaches the ground, providing metabolites that alter forest humus composition [2,9]. Humus is a stable decomposition product and defined as organic matter at varying degrees of decomposition, above the mineral soil and where root activity is high. Humus and leaf litter present considerable structural and nutritional differences, feeding the plant differently over time [10]. Tree species composition is directly associated with the spatial distribution of organic matter [11,12] and indirectly with the availability of soil nutrients (e.g., C:N, pH, CEC) [13], being able to change microbial communities [14,15,16] and the stability of soil temperature [17]. Differences in litter quantity and quality affect soil nutrient availability and soil microorganisms as a consequence of root-soil nutrient capture interactions [18]. In this way, the contrasted physicochemical and biological properties of humus between pure and mixed species stands may influence differently seedling growth in the understory; therefore, it can stimulate or limit the survival and growth of seedlings of economically desired species.
In the boreal forest, Black spruce (Picea mariana (Mill.) BSP) and Trembling aspen (Populus tremuloides Michx) are among the most abundant commercially important tree species [19,20]. Nonetheless, these two species have contrasting life strategies [7,21,22]. Slow-growing B. spruce is shade tolerant and understory of pure B. spruce stands usually harbor an abundant and diverse flora of nonvascular plants [3,23,24], which also affect directly soil nutrient availability [25]. In opposite, T. aspen is the most abundant of the fast-growing commercial broadleaf tree species [20,26]. Aspen is fast-growing and shade intolerant and understory of broadleaf stands is more favorable to greater abundant and diverse vascular plants [27,28,29,30]. In boreal forests on the Clay Belt in Canada, broadleaf stands are often characterized by a humus-rich layer [12,31], though, these stands have lower throughfall N deposition than coniferous stands [32]. In contrast, spruce stands are characterized by more frequent wet humus conditions, which tend to limit nutrient cycling [33,34]. As a corollary, the presence of these two species in various degrees of mixture in the stand can be beneficial to B. spruce seedling growth [23,24], suggesting that mixed stands may still be more advantageous in terms of soil nutritional availability than pure broadleaf stands and pure conifer stands [35,36], particularly for sites prone to the paludification process.
Paludification consists of the gradual accumulation of organic matter on the soil, associated with the presence of Sphagnum species, leading gradually from productive B. spruce forest stands to a state of low density and low growth due to the reduced availability of nutrients and anoxia due to soil water saturation [37]. The paludification process promotes the formation of peatlands, when the production of some litters and bryophytes is greater than its decomposition. Lower temperatures and higher precipitation contribute to saturating the soil with water, which causes reduced woody productivity [38,39]. The processes of paludification are classified as edaphic or successional. The edaphic paludification occurs when there is depression, therefore, when a deficient drainage favors the maintenance of the water table close to the surface of the ground and, the successional paludification occurs when there is accumulation of the organic layer of the soil results over time, in sites with low or moderately steep slopes. This process is widespread in regions such as the Clay Belt in Quebec and Ontario, Canada [3,26,40]. In this region, the organic horizon of the soil can increase from 20 to 40 cm approximately 100 to 200 years after the fire, which can result in a loss of woody productivity of 50 to 80% [41].
In these regions, silvicultural treatments tend to favor certain trees species and eliminate others, resulting in a simplification of stand composition which can lead to a loss of productivity in the long term [42]. Thus, silvicultural practices can strongly influence the availability of nutrients in forest soils in different ways [43,44,45]. Along these lines, these regions become the focus of different studies to try to increase forest yield, such as B. spruce, endemic to this region.
Different studies reported that litter production was higher in conifer stands mixed with broadleaf than in pure conifer stands [46,47], with a faster decomposition process of organic matter in mixed forests [48,49,50]. This can engender a more favorable plant development due to the nutritional and water availability of forest humus altered by litter [51]. As such, litter has a major impact on soil conditions and, therefore, can have an important influence on seedling recruitment. Many studies analyzed the influence of tree species mixture on wood production [22,52,53,54], and many others have analyzed the effect of litter composition on plant germination [55,56,57]. Considering the quality of the produced organic matter can differ with type and litter of species, these differences can influence the relative amount and quality of organic matter that is incorporated into the forest floor and used by seedlings [58,59]. However, only a few studies have portrayed the development of plants of commercial interest when subjected to different types of litter in humus from different forest stands, especially in young boreal stands sensitive to paludification, leaving a gap to be explored.
It is crucial to know more about how the beneficial effects of leafy litter (i.e., short term effects) on different humus (i.e., long term effects) could justify the maintenance of deciduous trees in a silvicultural scenario in order to maintain or improve the production and growth of desired seedlings in these stands. Thus, a greenhouse experiment was conducted to evaluate the effect of different litter composition and abundance and humus composition on the growth and development of B. spruce seedlings and, eventually, on humus properties. We hypothesized that humus provided by different stand compositions may influence B. spruce seedling growth and development. Moreover, different litters species (e.g., Speckle alder (Alnus rugosa (Du Roi) R.T. Clausen), Paper birch (Betula papyrifera Marsh.), Willow (Salix spp.), T. aspen, B. spruce and control (i.e., nursery substrate) may also have a direct effect on the growth of B. spruce seedlings and show different effects (i.e., interaction effects) when added on different humus. Finally, we tested whether the abundance of these litters will also influence seedling growth, as well as humus pH, moisture, and availability of nutritional elements in the growing substrate before and after treatment.
2. Materials and Methods
2.1. Growing Substrate Source
The growing substrate source for the greenhouse experiment was located in the spruce-moss forest domain, in the Clay belt in western Quebec of Canada [60] (Figure 1A), more specifically in the Plaine du Lac Matagami ecological region within the Abitibi Lowlands [61] (Figure 1B). The region is dominated by stands of B. spruce, but T. aspen is a common occurrence, growing in pure or mixed stands with B. spruce. Specific episodes of the Quaternary, including glaciation, regional glacial re-advances, and marine and lake invasions left in this area thick deposits of silt and clay, on top of which peatlands developed [62] (Figure 1C).
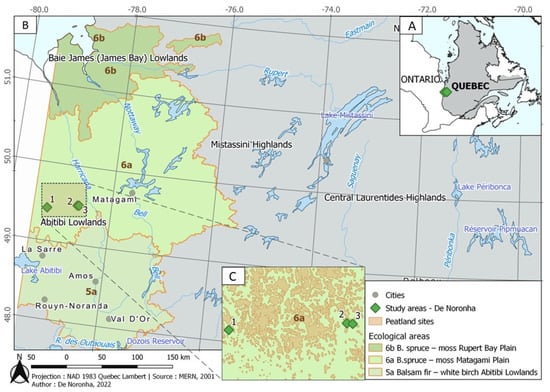
Figure 1.
Study areas: (A). Location of the growing substrates for the greenhouse experiment in Eastern Canada. (B). Location of substrate sources in ecological regions in the boreal Black spruce forest of western Quebec (1: −79.249, 49.419; 2: 78.665, 49.497; 3: −78.655, 49.498). (C). Three distinct areas with young stands treated by pre-commercial thinning and susceptible to the paludification process.
Continental climate of the region is characterized by large variability in temperatures between warm and cold seasons. In summer climate is influenced by moist Atlantic maritime tropical air and by dry maritime arctic air, although in the winter cold continental arctic air masses dominate. Classified as a cool and moderately humid climate, the average annual temperature is 0.1 °C, with the coldest month average being −25.3 °C and the hottest month average being 22.6 °C [63]. Annual rainfall regime consists of 650 mm of rain and 264 cm of snowfall, with 35% of rain received during the growing season [63]. At 3 locations within the area, 3 sets of stands were delimited according to stand composition: pure B. spruce stands, pure T. aspen stands and mixed stands (50% T. aspen/50% B. spruce). All stands were around 35 years old and susceptible to the paludification process. These stands also had comparable abiotic conditions (i.e., trees species, surface deposit, drainage, gentle slope and near peatland zones). The stands were recognized according to their composition: pure B. spruce stands, pure T. aspen stands and mixed stands. They underwent pre-commercial thinning during their younger (10–30-year-old) age.
2.2. Greenhouse Experiment and Sampling Design
To evaluate the long-term influence of leaf litter on growth and development of B. spruce seedlings in different stands, the humic part of the forest floor (i.e., made up of litter incorporated into the soil decomposed over several years, commonly known as humus) was collected in the 3 selected stands (e.g., with trees around each humus collected point composed by B. spruce; pure T. aspen and Mixed B. spruce with T. aspen) in each of the 3 areas. These areas presented many similar abiotic conditions (e.g., drainage, gentle slope, zones close to peat bogs, temperatures and geographic proximity), distinguishing only in the composition of arborescent species, consequently, in the litter of the forest floor. Thus, totaling 9 humus collections from 3 distinct groupings of tree species.
The collected humus was transported to a greenhouse where they were mixed and sieved at 10 mm by each stand category (i.e., pure B. spruce; pure T. aspen and Mixed B. spruce—T. aspen). Sequentially, each of the three categories of humus and the peat substrate (i.e., crushed peat moss that was sieved and artificially fertilized, commonly used by tree nurseries for the production of containerized tree seedlings (i.e., control treatment) was added in containers with a capacity of approximately 462 cm3 each (e.g., every 180 cells in the containers were filled with one of the 4 types of humus, totaling 720 cells). Two-year-old B. spruce seedlings from the Grandes-Piles MFFP nursery (provenance: BS-V2-PLU-1-0) were transported to the greenhouse. The origin of the seeds that formed these seedlings are located in regions of close latitudes, although distant longitudes (−68.341; 48.619), in 2nd generation orchard of Québec MFFP. The substrate from all seedling roots was removed, the rooting system was gently washed and carefully cleaned to remove all residues avoiding affecting the rhizosphere, then the seedlings were transplanted into the nursery containers filled with one of the 4 growing media treatments. Thus, a total of 180 B. spruce seedlings were transplanted in humus from pure B. spruce stands, 180 seedlings in humus from pure T. aspen stands, 180 seedlings in humus from mixed stands, and 180 seedlings in their original peat growing media.
To evaluate the short-term influence of leaf litter composition on the growth and development of B. spruce seedlings growing in different humus, leaf litter from 5 tree species were selected: P. tremuloides (PET), Alnus spp. (Alder sp., AUR), Betula papyrifera (White birch, BOP), Salix spp. (Willow sp., SAL) and P. mariana (BS). Each litter type was collected from 16 m2 tarps previously laid down in pure stands in the Abitibi-Témiscamingue region in the early winter of 2020–2021 (i.e., autumn broadleaf litter and winter B. spruce needles). They were dried for 48 h at 60 °C, crushed at 5 mm, weighed, and separated in proportions equivalent to 100%, 50% and 25% of the total weight (Table 1), and distributed on the surface, without being embedded, over 10 nursery containers for each humus treatment (i.e., each litter amount treatment had 10 seedling replicates and 30 control containers cells per humus). Sequentially, all plants were transferred to the greenhouse at a temperature ranging between 20 and 27 °C where they were watered once a day for about 150 days. Roots and central stems of the 720 plants were measured before being transplanted into the substrate treatments, along with soil pH and percent moisture at 2 cm and 8 cm depth from the soil surface. Every 25 days plants had their central stem height measured along with soil pH and percent moisture, also at 2 cm and 8 cm depth from the soil surface, via the Esimen Digital Plant Test, 12 h after watering.

Table 1.
Proportions (i.e., grams) of litter equivalent to 100%, 50% and 25% total weight of the collected surface of 16 m2, added to each seedling per treatment. PET: T. aspen; BOP: White birch; AUR: Alder sp.; BS: B. spruce; SAL: Willow sp.
Before the start of the greenhouse experiment the 4 types of humus and 5 types of litter were sampled, dried, sieved, and grounded for analysis of their nutrient content. For the humus, the concentrations of total C and N were measured by dry combustion (LECO CR-412, LECO Corporation, St. Joseph, MI, USA), and exchangeable P, K, Ca, Mg, Mn, Cu, Zn, Al, Fe and Mo, Na, S, and cation exchange capacity (CEC) by MehlichIII extraction procedure [64]. Base saturation (BS) was calculated as the sum of bases (i.e., Ca, Mg, K, and Na) over CEC. For litter, the total concentrations of C and N were measured by dry combustion, while total P, K, Ca, Mg, Mn, Cu, Zn, Al, and Fe were measured by inductively coupled plasma emission spectrophotometry (ICP-AES) following complete digestion in concentrated H2SO4, procedure described by [65,66], performed by the MFFP Laboratoire de Chimie Organique et Inorganique.
After 150 days, all B. spruce seedlings were removed from the containers and washed to remove solid residues from the roots and needles. Then, they were cut at the base of the stem, separating the roots from the top, and placed in paper bags separately to dry at 46–48 °C in a drying chamber for 48 h. Stems, needles, and roots were weighed separately. Dry needles of the plants were grounded according to each treatment category (growing media, percent litter and litter type) to analyze their nutrient content following the same procedure as for the litter before the start of the experiment. Humus in the containers were separated by treatment category, then mixed, sieved, and dried. The humus was re-analyzed following the same procedure as before the start of the experiment.
2.3. Statistical Analysis
R software version 3.6.0 was used to perform the analysis. Packages ggplot2 [67] for data visualization, dplyr [68] for data manipulation, nlme [69] for analysis of the generalized linear models, and multicomp [70] for a simultaneous inference. Data were submitted to the Shapiro–Wilk test to verify the normality of the variables, to test whether data followed a Gaussian distribution, and were submitted to the Bartlett test to verify variance homogeneity. Due to the difference only between 2 groups of variables: final dry root and initial pH at 2 cm, they were analyzed with a generalized linear model (GLS) with adjustment of the covariance structure according to treatments when necessary. ANOVA test, then Tukey test, were used to analyze the differences between the treatments of humus, litter and amount of litter added in aerial variables of seedlings (e.g., stem height increment, stem weight gain and needle weight gain) and in seedling root variables (e.g., root growth and root weight gain), and also nutritional variables analyzes, pH and moisture content. Aiming at a better analysis of the effect of the treatments of humus, litter and the amount of litter added on the development and growth of B. spruce seedlings, the increments (i.e., final measurements—initial measurements) were considered in the statistical tests, in addition to the final averages of growth and weight of the plants parts (Table S1). Main and interaction effects between humus, litter, and amount of litter added were considered in the analyses.
3. Results
3.1. Leaf Litter and Humus Nutrients before Treatment
Chemical characteristics of humus and litter before starting the greenhouse treatment are presented in Table 2. Total C concentrations were significantly higher for all litter types compared to humus from different types of stands, including the nursery humus (p = 0.002). Humus from mixed stands and pure T. aspen had a lower C:N ratio compared to humus from pure B. spruce stands and nursery substrate. Nonetheless, PET and BOP litter showed a high C:N ratio compared to other litters (AUR, SAL, and BS). AUR litter showed higher total concentrations for P, Ca, Mg, Al and Fe, while BS needles had lower values for K, Mg and Zn. In general, broadleaf litter had higher values for K, Ca, Mg and Zn when compared to BS litter.

Table 2.
Chemical characteristics of humus and litter before start of the greenhouse experiment. PET: T. aspen; BOP: White birch; SAL: Willow sp; AUR: Alder sp.; BS: B. spruce.
Humus from mixed stands had almost twice the available Mg concentration of humus from pure B. spruce stands. In addition, B. spruce humus had higher concentrations of available Al and Fe compared to others humus types and the nursery substrate had higher concentrations of K, Na, and Cu compared to the others humus types. Base saturation was higher in humus from mixed stands, being higher also in pure stands of T. aspen as compared to B. spruce humus and nursery substrate.
3.2. Seedling Development and Humus Characteristics
The analyses indicate the strongest effect from the humus treatment, followed by a significantly but smaller effect for the type of litter added, and no significant effect of litter amount added on seedling growth (Table S2). Although the triple interaction was significant between the treatments, the effect of amount of litter added had a very weak impact on seedling growth compared to humus amount and litter type (Figure 2). The pH variation measured at 2 cm depth was high, due to the proximity of the deposition of crushed litter on the soil surface, occasionally the amount of crushed litter added exceeded 2 cm of thickness, which caused some variability in the depth of pH measurement for each treatment class.
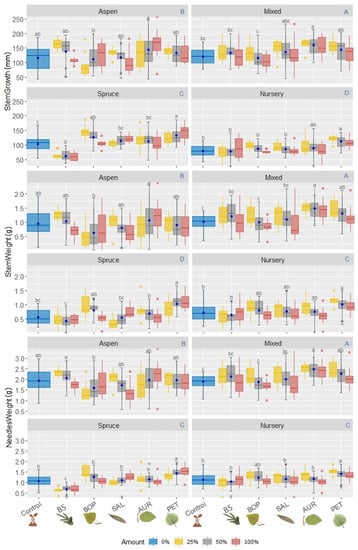
Figure 2.
Boxplot of the aerial parts of B. spruce seedlings: the blue uppercase letters of each block of variables (i.e., StemGrowth, StemWeight and NeedlesWeight) correspond to multiple comparisons of treatments by means of Tukey test for the humus treatment (i.e., humus from pure stands of B. spruce, humus from pure T. aspen stands, humus from mixed B. spruce and T. aspen stands, and MFFP forest nursery soil); the black lowcase letters correspond to the Tukey test on different crushed leaf litter added to the surface of each humus treatment group, with their respective averages per litter group presented by the central circles of each group, and; the colored bars correspond to the percentage of litter addition/non-addition for each litter group. Different lowercase letter colors correspond to the type of litter added. Control: no litter addition; BS: B. spruce; BOP: White birch; SAL: Willow sp; AUR: Alder sp.; PET: T. aspen.
3.2.1. Aerial Parts of B. spruce Seedlings
Humus treatments and some litter type treatments, with some different percent of addition, differed in stem growth response, needle weight and stem weight (Figure 2). Nonetheless, considering the humus treatment in general without addition of litter, there was a correlation between stem height growth gain and stem weight gain (r = 0.76, Pearson), stem height growth gain and needle weight gain (r = 0.82, Pearson), and needle weight and stem weight (r = 0.88, Pearson).
Regarding B. spruce seedlings stem height growth, considering only the 4 different types of humus (i.e., confounding the type of litter added and the amount of litter added), the seedlings that presented the best final heights were those grown in humus from pure stands of T. aspen and Mixed: 235 ± 44 and 239 ± 39 mm (mean ± SD), respectively, with increments of 116 ± 39 and 121 ± 25 mm, respectively, during the experiment. The second highest height growth was for seedlings grown in humus from pure B. spruce stands (final growth average 213 ± 34 mm; average increment 111 ± 26 mm). Seedlings grown in nursery substrate had a lower growth (201 ± 37 mm). The nursery substrate without added litter showed the least response in stem growth, with an average final stem height of 196 ± 25 mm and height increment of 80 ± 18 mm.
Similarly, average dry stem weight gain of seedlings was higher for humus treatments from mixed stands and pure stands of T. aspen (1.19 ± 0.41 and 0.90 ± 0.45 g, respectively) compared to the average stem weight gain of seedlings grown in pure B. spruce and nursery humus (0.68 ± 0.35 and 0.79 ± 0.32 g, respectively). AUR litter added at 25% and 50%, PET litter added at 25%, and BS litter added at 50% showed better effects in humus from Mixed stands with weight gain of 1.52 ± 0.20, 1.50 ± 0.37, 1.46 ± 0.36, and 1.31 ± 0.34 g, respectively. In general, the average weight gain of seedling stems was greater for those grown in humus from Mixed stands with the addition of AUR litter (1.49 ± 0.32 g, considering all % AUR addition). Furthermore, when no litter was added, stem weight gain of seedlings showed the worse performance in pure B. spruce humus (0.56 ± 0.26 g) compared with pure T. aspen humus (0.96 ± 0.42 g) and Mixed (1.03 ± 0.27 g). PET litter also increased stem weight gain in general. It should be noted that for seedlings grown in T. aspen humus, the greatest weight gain was recorded with BS litter compared to the other litters.
In summary, the effect of humus treatment on aerial development of B. spruce seedlings was as follows: Mixed = T. aspen > B. spruce = Nursery (i.e., Control). The litter effect was more noticeable on poor humus than on rich humus (i.e., PET = AUR > BOP = SAL > BS = no addition). Nonetheless, considering only the effect of BS litter, aerial development of B. spruce seedlings was greater, when grown in humus from pure stands of T. aspen and humus from Mixed stands than humus from pure B. spruce stands and the nursery substrate.
3.2.2. Root Development
Humus treatments also influenced seedling root length and weight. There was a difference between the addition and non-addition of litter: seedlings that received crushed litter showed greater root growth (Figure 3). There was a correlation between root weight increment and root elongation (r = 0.82, Pearson). Overall, humus treatments dominated root growth in length and litter treatments dominated root weight gain. Litter abundance had little effect and only in interaction with humus treatment. The results are described in more details below.
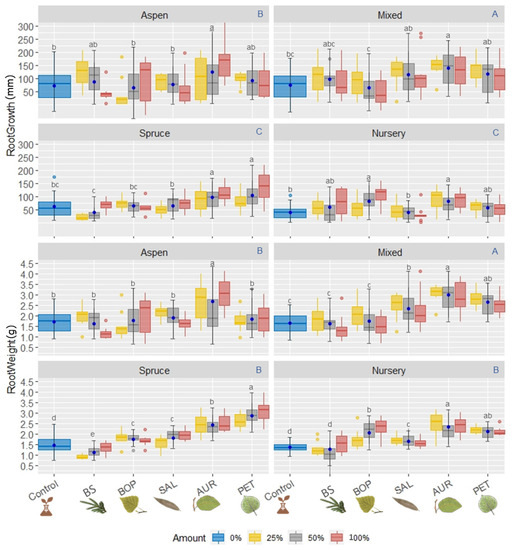
Figure 3.
Boxplot of the underground parts of B. spruce seedlings: the blue uppercase letters of each block of variables (i.e., RootGrowth and RootWeight) correspond to multiple comparisons of treatments by means of Tukey test for the humus treatment (i.e., humus from pure stands of B. spruce, humus from pure T. aspen stands, humus from mixed B. spruce and T. aspen stands, and MFFP forest nursery soil); the black lowcase letters correspond to the Tukey test on different crushed leaf litter added to the surface of each humus treatment group, with their respective averages per litter group presented by the central circles of each group, and; the colored bars correspond to the percentage of litter addition/non-addition for each litter group. Different lowercase letter colors correspond to the type of litter added. Control: no litter addition; BS: B. spruce; BOP: White birch; SAL: Willow sp; AUR: Alder sp.; PET: T. aspen.
Root Growth
There was significant differences in root elongation between humus treatments from Mixed stands and from pure T. aspen stands, considering main effects (i.e., increment of 101 ± 62 and 86 ± 45 mm—mean ± SD—respectively, p = 0.024), although there was no difference in the root length response of seedlings to pure B. spruce humus and from nursery soil (i.e., growth increment of 72 ± 46 and 59 ± 39 mm, respectively, p = 0.27). Therefore, with respect to the humus treatment, root growth follows the order: Mixed > T. aspen > B. spruce = Nursery. Addition of AUR and PET litters showed better performance in root elongation with the B. spruce humus substrate (i.e., growth increment 108 ± 55 and 108 ± 63 mm, respectively). For humus from Mixed stands, greater root length gain was found with AUR, PET and SAL litter addition (i.e., growth increment 139 ± 55, 117 ± 59 and 116 ± 66 mm, respectively) than for T. aspen humus (i.e., 125 ± 54 mm, 92 ± 39 mm and 76 ± 27 mm). In general, AUR litter tended towards greater root growth. Considering only the no litter addition treatment (i.e., control), root elongation were: Mixed = T. aspen > Spruce > Nursery (i.e., 74 ± 39 mm, 72 ± 41 mm, 60 ± 22 mm, and 38 ± 26 mm). For lower amounts of litter added (e.g., 25%), seedling root growth showed the worse performance in nursery and pure B. spruce humus, and for the latter, the root growth performance was even more reduced when BS litter was added. However, in pure T. aspen humus root length increase was observed even when adding BS litter. For moderate amounts of litter added (50%) in T. aspen humus, there was no significant difference in root elongation among litter types.
Root Weight
Regarding root weight gain, considering only the litter treatment: AUR (2.61 ± 0.62 g) > PET (2.37 ± 0.61 g) > SAL (1.94 ± 0.54 g) > BOP (1.84 ± 0.56 g) > no addition (1.52 ± 0.42) > BS (1.49 ± 0.51) (mean ± SD). When no litter was added, root weight gain was not significantly different among T. aspen and Mixed humus (i.e., 1.74 ± 0.40 and 1.66 ± 0.41, respectively), but it was greater than for seedlings grown in humus from B. spruce and nursery (i.e., 1.48 ± 0.37 and 1.37 ± 0.29, respectively). Seedlings had the lowest root weight gain in pure B. spruce humus with the addition of lower or moderate amounts BS litter added (i.e., 25% and 50%, respectively). For the highest amounts of litter added there was no significant difference between root weight gain among the different humus types. For all seedlings grown in pure B. spruce humus, AUR and PET litter improved weight gain values more than the other litters. Furthermore, seedlings grown in humus from Mixed and T. aspen stands with the addition of AUR litter had the highest weight gain (3 ± 0.31 and 2.68 ± 0.39 g, respectively).
3.3. Humus pH
Even though substrate pH showed different values among humus types, there was no significant difference in pH at the 8 cm depth level when compared before and after this experiment among the different humus treatments, litter types, and litter amounts added. However, there was a significant difference in pH at the 2 cm depth level among the different humus treatments, litter types and amounts added (i.e., averaged over all litter types: 0% = −0.39; 25% = 0.17; 50% = 0.05 and 100% addition = −0.06, p-value < 0.001). In general, responses in no or positive variation of humus pH at 2 cm depth were for those concerning litter addition (25% and more; Table 3. No litter addition to humus had negative effect on humus pH at the end of experiment (−0.39 ± 0.13, p-value < 0.001). Humus from pure stands of T. aspen showed the highest average final pH (i.e., 5.56 ± 0.15), followed by humus from mixed stands (i.e., 5.41 ± 0.16). The lowest final pH values came from humus from pure B. spruce stand (i.e., 4.83 ± 0.36) and from nursery soils (i.e., 4.03 ± 0.11) (Table 4). Substrate pH at 2 cm showed the following trend at the end of experiment: B. spruce = T. aspen > Mixed > nursery. For humus from B. spruce, PET litter showed its capability to increase substrate pH at different rates added, also for humus from Mixed stand and nursery at 50% and 100% of addition. In contrast, BS needle litter tended to reduce humus pH from Mixed stands and nursery.

Table 3.
Final pH changes over 150 days (measured at 2 cm depth from the surface) ± SD according to humus category and rate of litter addition. PET: T. aspen; AUR: Alder sp.; SAL: Willow sp; BOP: White birch; BS: B. spruce; Control: no addition.

Table 4.
Final pH ± SD according to the humus treatments with different litter types added.
Pure B. spruce humus with addition of PET, SAL or BOP litter at any addition rate showed an increase in pH at 2 cm depth, but a reduction in pH with the addition of 100% AUR and 100% BS litter (−0.41 ± 0.01 and −0.35 ± 0.01, respectively). PET, AUR and SAL litters were also associated with an increase substrate pH in humus from Mixed and T. aspen stands. Nonetheless, addition of BOP litter caused a decrease in T. aspen humus pH. The absence of litter or the addition of 100% BS litter caused a decrease in substrate pH in all humus treatments. For the substrate from nursery, the addition of PET and SAL litter at all rates of addition generally caused an increase in substrate pH at 2 cm. However, addition of higher amounts (50 and 100%) of BOP and BS litter caused a reduction in pH for this substrate.
3.4. Humus Moisture Content
Moisture content measured at 2 cm depth fluctuated more compared to moisture at 8 cm, due to the added litter thickness that may have exceeded 2 cm in some litter treatments. Highest moisture contents remained higher deeper (i.e., the depth of 8 cm). At the depth of 8 cm, treatments with humus from pure T. aspen and Mixed stands had higher moisture retention (71 ± 14% and 69 ± 16%, respectively) compared to humus from pure B. spruce stands and nursery humus (65 ± 16% and 56 ± 12%, respectively). Substrate moisture content was proportional to the amount of litter added to the substrate (i.e., 100% > 50% > 25% > 0 litter addition) to all humus treatments, except for BS needle addition, which did not increase substrate moisture as the other broadleaf leaf litters (Figure S1). In general, the type of litter added did not display a significant difference in the moisture response at 8 cm, except for BS needle litters, which maintained less moisture in the substrate compared to the other litter treatments.
3.5. Foliage Nutrient Concentrations after Experiment
Higher seedling foliage N, P, and Ca concentrations were measured with humus from pure T. aspen and Mixed stands (p-values ≤ 0.001) Table 5. There was no significant difference in foliar K and Mg values among humus treatments (p-value = 0.72 and 0.58, respectively). Predominantly, humus from Mixed and pure T. aspen stands showed greater correlations between stem growth and root growth and foliar concentrations (Figure S2).

Table 5.
Foliar concentrations of B. spruce seedlings grown in humus from different forest stands and topped with different litter types (mean ± SD from all litter amounts added). PET: T. aspen; AUR: Alder sp.; SAL: Willow sp; BOP: White birch; BS: B. spruce; Control: no addition.
3.6. Humus Substrate Composition at the End of Experiment
Humus from nurseries showed the highest averages C:N ratios were in (i.e., 70.35 ± 6.18, mean ± SD), followed by humus from pure stands of B. spruce (i.e., 40.76 ± 5.96). The lowest mean values of the C:N ratio were for humus from pure stands of T. aspen (i.e., 25.17 ± 1.02) and mixed stands (27.70 ± 3.05). The mixed and pure stands of T. aspen had the highest concentrations of primary macronutrients (i.e., Mixed: N = 13.71 ± 1.24 g·Kg−1, P = 50.88 ± 13.55 mg·Kg−1, K = 181.63 ± 50.88 mg·Kg−1; pure T. aspen: N = 12.14 ± 1.79 g·Kg−1, P = 51.06 ± 11.97 mg·Kg−1, K = 209.21 ± 46.91 mg·Kg−1). Nursery treatments had their average values lower than the other treatments with humus when they did not receive the addition of leaf litter (i.e., N = 6.68 ± 0.63 g·Kg−1; P = 17.81 ± 13.59 mg·Kg−1 and K = 141.94 ± 49.55 mg·Kg−1). The highest final average concentrations of Ca and Mn were for humus from mixed stands (i.e., Ca = 7031.88 ± 366.29 mg·Kg−1 and Mn = 58.75 ± 18.54 mg·Kg−1), but the lowest Mg concentrations were for humus from pure stands of T. aspen (i.e., 894.63 ± 97.40 mg·Kg−1). Furthermore, humus from mixed stands and pure T. aspen showed lower values for Cu (i.e., 5.56 ± 1.06 mg·Kg−1 and 5.25 ± 0.90 mg·Kg−1, respectively). However, the highest Al values were associated with humus from pure B. spruce and pure T. aspen stands (i.e., 1865.00 ± 186.18 mg·Kg−1 and 1872 ± 598.88 mg·Kg−1) (Table S3).
4. Discussion
This study demonstrated that the chemical composition of the litter of different tree species can contribute to the forest floor formation and chemical composition, which is consistent with many other studies (i.e., [71,72,73,74,75,76,77,78,79,80]). However, in this research, litter influences could be observed in the long-term (i.e., by humus) and in the short-term (i.e., fertilization by crushed litter on humus, at different amounts, in 150 days of greenhouse experiment) with litter of distinct species. Furthermore, this humic composition, fertilized or not with crushed litter, influenced the development of B. spruce seedlings aerial and belowground parts.
4.1. Humus Type Effect
Our results showed a better developmental performance for B. spruce seedlings grown in humus from mixed stands and from pure T. aspen stands compared to humus from pure B. spruce stands and from nursery substrate. These results support our hypothesis that the humus provided by different stands compositions can influence the growth of B. spruce seedlings, since each humic composition has different physicochemical characteristics. The humus layer is of paramount importance in the storage and transmission of nutrients to plants [81], being an important factor of forest seedlings development [82,83,84], in addition to water availability [85,86,87,88] and others complex interactions [89,90,91,92,93,94].
The responses of the nutritional properties of humus from different stands, before and after greenhouse experiment, raised some questions, mainly in relation to macronutrients (i.e., N, P, K, Ca, Mg and S). It can be noted that the concentrations of primary macronutrients in the nursery substrate were higher than in the other humus treatments before starting the experiment and, when analyzed after 150 days of experiment, had their values lower than the other humus treatments when they received no addition of crushed litter. It is worth mentioning that nursery substrates usually receive artificial fertilization for greater seedling nutrition, but after this experiment in greenhouse many nutrients from this nursery substrate may have been leached by everyday irrigation. Thus, humus from Mixed and pure T. aspen stands maintained higher concentrations of primary macronutrients throughout the greenhouse experiment for a same irrigation. Nonetheless, some secondary macronutrients (i.e., Mg and S) had a subtle increase in all humus at the end of the experiment, since S is a dominant anion in the leaching and retention process [95,96]. However, humus from Mixed and pure T. aspen stands remained with higher nutrient concentrations compared to humus from pure B. spruce stands and the nursery substrate, and the same was observed for the secondary macronutrient Ca, except for Mg and S. For the macronutrients Mg and S, the pure T. aspen humus presented slightly lower concentrations when compared to the other humus.
The highest concentrations of Al and Fe were found in humus from pure B. spruce stands, followed by humus from pure T. aspen stands. Humus from Mixed stands and the nursery substrate presented the lowest values of Fe and Al, before and after the experiment. Al concentration in humus is known to be controlled by acidity and is it an important indicator of the forest soil acidity status [97]. The negative effects of soil acidification, especially the depletion of major base cations and the liberation of Al3+, are major challenges to plant growth and survival [98]. Base saturation (BS) was higher in humus from Mixed stands as compared to B. spruce humus and the nursery substrate. Commonly, BS increases with increasing soil pH and the availability of Ca2+, Mg2+, and K+. Furthermore, the mobility of metals in soils is pH dependent. Furthermore, the pH values at the end of the experiment were higher for humus from Mixed and pure T. aspen stands than from pure B. spruce stands. This result is consistent with [73] who found higher pH in broadleaf than in conifers stands. Indeed, conifer soils are commonly known for have more acidic soils [99,100,101].
4.2. Litter Type and Litter Addition Rate Effect
Although significant, the F values associated with the amount of litter added to humus treatments and type of litter treatments were among the lowest; in addition, the interactions between the amount of litter and other factors were not significant. Therefore, the amount of litter added effect was very weak compared to the other factors. For all these reasons, litter quantitative effects were not noticed for seedling growth variables. However, the amount of litter had a positive effect on substrate moisture. Thus, the finding in this study that different leaf litter species also have an effect, albeit relatively small, on aerial and underground development of B. spruce seedlings, supports our hypothesis that different leaf litter species have a certain effect on the development of B. spruce seedlings when added to different humus.
All leaf litter types added had a greater positive effect on humus moisture response at greater depth compared to BS needle litter. Furthermore, recent fallen (i.e., less decomposed) undecayed conifer needles have high tensile strength and relatively low moisture content [102]. In addition, BS needles biomass collected in this study, for the same unit of area and for the same period, was relatively smaller compared to others leaf litter of broadleaf species (except SAL). Thus, moisture may also have interfered in the development of B. spruce seedlings in addition to the nutritional and physical properties of pure B. spruce humus. The analysis of the litter nutritional composition indicated that the highest C:N ratios at the beginning of the experiment were for the PET and BOP species and the lowest for AUR, thus indicating a greater decomposition rate of the AUR organic material, indicating certain facilitating effects as measured that litter accumulates and is involved in the humic composition [103]. However, the highest pH values at the end of the experiment were associated with the addition of PET and AUR litter, a factor that also influenced the development of B. spruce seedlings. In addition, with regard to primary macronutrients (N, P, K), humus topped with PET and BOP litter showed the highest P and K values at the end of the experiment, although N concentrations showed considerable oscillations between the different litter treatments, a fact that may also have contributed to the growth performance of B. spruce seedlings. However, AUR and BOP litter presented higher final Fe and Al concentrations in the different humus substrates, which may also indicate a tendency towards soil acidification [97,104,105].
4.3. Management Implications
The seedbed abundance modulates tree commercial species establishment, but it is strongly influenced by the composition of the forest floor [106,107]. Therefore, the effect of management practices on the forest floor must be considered to maintain or improve the nutritional and physicochemical aspects of the forest humus. Along these lines, humus can often be managed for production optimization purposes [85,108,109,110]. Thus, the results of this greenhouse experiment suggested that the promotion of mixed stand, which interferes with humus chemical composition, can increase seedling growth by improving the physicochemical composition of the forest floor (e.g., macronutrients, pH and moisture). Consequently, when managing forest for its desired coniferous vocation by operating a precommercial cutting, a minimum proportion of broadleaved trees should be conserved. However, further research is needed to quantify this minimum proportion of broadleaved trees to maintain the coniferous tree density at acceptable levels.
The coexistence of certain litter types such as Alders and T. aspen can be nutritionally advantageous, being consistent with [107], who evaluated the role of certain substrates and litter in the initial phase of conifers and showed that the survival of seeds and seedlings of coniferous species under T. aspen stands was higher compared to that located under coniferous stands. The predominance of T. aspen litter in forest stands develops a rich soil that favors the growth of herbaceous plants in the subsoil [111], which influenced humus composition as in our experiment. Nonetheless, this study proved that not all types of leaf litter in different amounts have the same effect on the development of B. spruce seedlings and the ideal amount of leaf litter to optimize the growth and development of seedlings for each type of humus remains unclear, suggesting further research to better understand these effects.
5. Conclusions
We demonstrated in this study that the effect of long-term humus enrichment by broadleaf litters is largely dominant and that the short-term effect of different broadleaf litters on forest soil is significant. That indicates the enrichment of leaf litter on humus continues over time, even though the greenhouse experiment took place over a short period. Despite these effects are not necessarily greater if the amount of broadleaf litters is increased, it does promote discussion for spruce-moss forest management to retain some broadleaf species for maximum nutritional benefit. This greenhouse experiment indicated the possibility of natural fertilization of the forest through the maintenance of broadleaf species to improve the development of B. spruce seedlings. Even if mixed stands can generate higher seedling productivity, due to the greater number of interactions between the species and their responses to the humus, the moss-spruce regions are known for the predominance of the B. spruce specie, with a rare presence of broadleaf tree species. However, these rare presences seem to bring a productive advantage in some nutritional aspects. Thus, this is important for a management that aims not to lose the coniferous vocation of the stands and to be able to maintain some broadleaf species in their stands for nutritional complementarity. Finally, research efforts should be continued on the topic of mixed stands versus pure stands associated with forest plantations in the stand and landscape.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/f13111832/s1, Figure S1: Moisture content of the B. spruce seedling substrates in relation with humus substrate treatment, litter type treatment, and amount of litter: the blue uppercase letters correspond to the test to each humus treatment (e.g., B. spruce, Mixed, nursery soils and T. aspen); the black lowercase letters correspond to the test at different litter treatment (e.g., AUR, BOP, Control, BS, PET, SAL); and different colors lowercase letters correspond to the amount litter added on each humus treatment (e.g., no addition, 25%, 50% and 100%); Figure S2: Response curves showing the relationship stem.growth~needle nutrients distribution: Control: no litter addition; EPN: B. spruce; BOP: White birch; SAL: Willow sp; AUR: Alder sp.; PET: T. aspen; Table S1: Final averages ± SD of growth and weight of B. spruce seedling parts: Control: no litter addition; BS: B. spruce; BOP: White birch; SAL: Willow sp; AUR: Alder sp.; PET: T. aspen; Table S2: ANOVA summary A. Black spruce seedling development (e.g., stem growth, stem weight, needle weight and root weight gains, root growth and root weight gain). B. Humus characteristics (e.g., pH change and humus moisture) after 150 days of growth; Table S3: Humus substrate composition at the end of experiment as a function of litter type added and litter addition rate.
Author Contributions
Conceptualization, M.D.N., R.O., M.B., A.L. and Y.B.; methodology and formal analysis, M.D.N.; supervision, R.O. and A.L.; writing—original draft preparation, M.D.N.; writing—review and editing, M.D.N., R.O., M.B., A.L. and Y.B.; funding acquisition, Y.B., A.L., M.B. and R.O. All authors have read and agreed to the published version of the manuscript.
Funding
This project was made possible with funding provided by NSERC (Natural Sciences and Engineering Research Council of Canada): RDCPJ 478742-15, with Greenfirst Produits forestiers, the NSERC-UQAT-UQAM Chair in Sustainable Forest Management, in collaboration with the Direction de la recherche forestière (Ministère des Forêts, de la Faune et des Parcs du Québec (MFFPQ)): 000367-001; Mitacs Accelerate program R70992, and RYAM Forest Management.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank M. Lambert for greenhouse assistance and N. Drouin for laboratory assistance. D. Charron for field assistance and logistics. E. Daigneault for field collaboration in collecting humus and litter. We thank also the Grandes-Piles tree nursery staff for providing the seedlings for the experiment, and the Laboratoire de chimie organique et inorganique of the Ministère des Forêts, de la Faune et des Parcs for the numerous laboratory analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brassard, B.W.; Chen, H.Y.; Bergeron, Y.; Paré, D. Differences in Fine Root Productivity between Mixed-and Single-Species Stands. Funct. Ecol. 2011, 25, 238–246. [Google Scholar] [CrossRef]
- Levia, D.F.; Herwitz, S.R. Interspecific Variation of Bark Water Storage Capacity of Three Deciduous Tree Species in Relation to Stemflow Yield and Solute Flux to Forest Soils. Catena 2005, 64, 117–137. [Google Scholar] [CrossRef]
- Crispo, M.; Jean, M.; Fenton, N.J.; Leduc, A.; Bergeron, Y. Factors Explaining the Composition and Diversity of Vascular Plant Understories along a Transcontinental Gradient in the Canadian Boreal Forest. J. Veg. Sci. 2021, 32, e13058. [Google Scholar] [CrossRef]
- Chávez, V.; Macdonald, S.E. Partitioning Vascular Understory Diversity in Mixedwood Boreal Forests: The Importance of Mixed Canopies for Diversity Conservation. For. Ecol. Manag. 2012, 271, 19–26. [Google Scholar] [CrossRef]
- Messier, C.; Parent, S.; Bergeron, Y. Effects of Overstory and Understory Vegetation on the Understory Light Environment in Mixed Boreal Forests. J. Veg. Sci. 1998, 9, 511–520. [Google Scholar] [CrossRef]
- Mestre, L.; Toro-Manríquez, M.; Soler, R.; Huertas-Herrera, A.; Martínez-Pastur, G.; Lencinas, M.V. The Influence of Canopy-Layer Composition on Understory Plant Diversity in Southern Temperate Forests. For. Ecosyst. 2017, 4, 6. [Google Scholar] [CrossRef]
- Ghotsa Mekontchou, C.; Houle, D.; Bergeron, Y.; Drobyshev, I. Contrasting Root System Structure and Belowground Interactions between Black Spruce (Picea Mariana (Mill.) BSP) and Trembling Aspen (Populus Tremuloides Michx) in Boreal Mixedwoods of Eastern Canada. Forests 2020, 11, 127. [Google Scholar] [CrossRef]
- Adamczyk, B.; Sietiö, O.-M.; Straková, P.; Prommer, J.; Wild, B.; Hagner, M.; Pihlatie, M.; Fritze, H.; Richter, A.; Heinonsalo, J. Plant Roots Increase Both Decomposition and Stable Organic Matter Formation in Boreal Forest Soil. Nat. Commun. 2019, 10, 3982. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Liu, Z. Impacts of Plant Secondary Metabolites from Conifer Litter on the Decomposition of Populus Purdomii Litter. J. For. Res. 2019, 30, 2237–2245. [Google Scholar] [CrossRef]
- Initial Litter Chemical Composition. In Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Berg, B., McClaugherty, C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 67–100. ISBN 978-3-030-59631-6. [Google Scholar]
- Berg, B.; Laskowski, R.; Santo, A.V. Estimated Nitrogen Concentrations in Humus Based on Initial Nitrogen Concentrations in Foliar Litter: A Synthesis. XII. Long-Term Decomposition in a Scots Pine Forest. Can. J. Bot. 2000, 77, 1712–1722. [Google Scholar] [CrossRef]
- Staaf, H. Plant Nutrient Changes in Beech Leaves during Senescence as Influenced by Site Characteristics. Acta Oecol. Oecol. Plant 1982, 3, 161–170. [Google Scholar]
- Priputina, I.V.; Frolova, G.G.; Shanin, V.N.; Myakshina, T.N.; Grabarnik, P.Y. Spatial Distribution of Organic Matter and Nitrogen in the Entic Podzols of the Prioksko-Terrasnyi Reserve and Its Relationship with the Structure of Forest Phytocenoses. Eurasian Soil Sci. 2020, 53, 1021–1032. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, J.C.; Bergeron, Y.; Kembel, S.W.; Fenton, N.J. Dominance of Coniferous and Broadleaved Trees Drives Bacterial Associations with Boreal Feather Mosses. bioRxiv, 2022; bioRxiv preprint. [Google Scholar] [CrossRef]
- Saetre, P. Spatial Patterns of Ground Vegetation, Soil Microbial Biomass and Activity in a Mixed Spruce-Birch Stand. Ecography 1999, 22, 183–192. [Google Scholar] [CrossRef]
- Jean, M.; Holland-Moritz, H.; Melvin, A.M.; Johnstone, J.F.; Mack, M.C. Experimental Assessment of Tree Canopy and Leaf Litter Controls on the Microbiome and Nitrogen Fixation Rates of Two Boreal Mosses. New Phytol. 2020, 227, 1335–1349. [Google Scholar] [CrossRef] [PubMed]
- Rinnan, R.; Michelsen, A.; Jonasson, S. Effects of Litter Addition and Warming on Soil Carbon, Nutrient Pools and Microbial Communities in a Subarctic Heath Ecosystem. Appl. Soil Ecol. 2008, 39, 271–281. [Google Scholar] [CrossRef]
- Vesterdal, L.; Schmidt, I.K.; Callesen, I.; Nilsson, L.O.; Gundersen, P. Carbon and Nitrogen in Forest Floor and Mineral Soil under Six Common European Tree Species. For. Ecol. Manag. 2008, 255, 35–48. [Google Scholar] [CrossRef]
- Forest Regions. The Canadian Encyclopedia. Available online: https://www.thecanadianencyclopedia.ca/en/article/forest-regions (accessed on 17 January 2022).
- Canada, N.R. State-Canadas-Forests-Report. Available online: https://www.nrcan.gc.ca/our-natural-resources/forests/state-canadas-forests-report/16496 (accessed on 30 April 2022).
- Comeau, P.G.; Thomas, K.D. Silviculture of Temperate and Boreal Broadleaf-Conifer Mixtures. In Land Management Handbook; No. 36; Technical Report; Government of British Columbia: Victoria, BC, Canada, 1996. [Google Scholar]
- Légaré, S.; Paré, D.; Bergeron, Y. The Responses of Black Spruce Growth to an Increased Proportion of Aspen in Mixed Stands. Can. J. For. Res. 2004, 34, 405–416. [Google Scholar] [CrossRef]
- Fenton, N.; Bergeron, Y. Dynamic Old-Growth Forests? A Case Study of Boreal Black Spruce Forest Bryophytes. Silva Fenn. 2011, 45, 983–994. [Google Scholar] [CrossRef]
- Qian, H.; Klinka, K.; Økland, R.H.; Krestov, P.; Kayahara, G.J. Understorey Vegetation in Boreal Picea Mariana and Populus Tremuloides Stands in British Columbia. J. Veg. Sci. 2003, 14, 173–184. [Google Scholar] [CrossRef]
- Pacé, M.; Fenton, N.J.; Paré, D.; Bergeron, Y. Ground-Layer Composition Affects Tree Fine Root Biomass and Soil Nutrient Availability in Jack Pine and Black Spruce Forests under Extreme Drainage Conditions. Can. J. For. Res. 2017, 47, 433–444. [Google Scholar] [CrossRef]
- Comeau, P.G. Relationships between Stand Parameters and Understorey Light in Boreal Aspen Stands. J. Ecosyst. Manag. 2002, 1, 2. [Google Scholar]
- Kumar, P.; Chen, H.Y.; Searle, E.B.; Shahi, C. Dynamics of Understorey Biomass, Production and Turnover Associated with Long-Term Overstorey Succession in Boreal Forest of Canada. For. Ecol. Manag. 2018, 427, 152–161. [Google Scholar] [CrossRef]
- Hart, S.A.; Chen, H.Y. Understory Vegetation Dynamics of North American Boreal Forests. Crit. Rev. Plant Sci. 2006, 25, 381–397. [Google Scholar] [CrossRef]
- Canada, L. A Search—Theses Canada. Available online: https://www.bac-lac.gc.ca/eng/services/theses/Pages/item.aspx?idNumber=1033011249 (accessed on 15 March 2022).
- Liu, T.-Y.; Lin, K.-C.; Vadeboncoeur, M.A.; Chen, M.-Z.; Huang, M.-Y.; Lin, T.-C. Understorey Plant Community and Light Availability in Conifer Plantations and Natural Hardwood Forests in Taiwan. Appl. Veg. Sci. 2015, 18, 591–602. [Google Scholar] [CrossRef]
- Kristensen, H.L.; Gundersen, P.; Callesen, I.; Reinds, G.J. Throughfall Nitrogen Deposition Has Different Impacts on Soil Solution Nitrate Concentration in European Coniferous and Deciduous Forests. Ecosystems 2004, 7, 180–192. [Google Scholar] [CrossRef]
- Gundersen, P.; Sevel, L.; Christiansen, J.R.; Vesterdal, L.; Hansen, K.; Bastrup-Birk, A. Do Indicators of Nitrogen Retention and Leaching Differ between Coniferous and Broadleaved Forests in Denmark? For. Ecol. Manag. 2009, 258, 1137–1146. [Google Scholar] [CrossRef]
- Viereck, L.A.; Dyrness, C.T.; Cleve, K.V.; Foote, M.J. Vegetation, Soils, and Forest Productivity in Selected Forest Types in Interior Alaska. Can. J. For. Res. 1983, 13, 703–720. [Google Scholar] [CrossRef]
- Tamm, C.O. Nitrogen-Limited and Nitrogen-Depleted Terrestrial Ecosystems: Ecological Characteristics. In Nitrogen in Terrestrial Ecosystems; Springer: Berlin/Heidelberg, Germany, 1991; pp. 34–49. [Google Scholar]
- Farooq, T.H.; Chen, X.; Shakoor, A.; Li, Y.; Wang, J.; Rashid, M.H.U.; Kumar, U.; Yan, W. Unraveling the Influence of Land-Use Change on Δ13C, Δ15N, and Soil Nutritional Status in Coniferous, Broadleaved, and Mixed Forests in Southern China: A Field Investigation. Plants 2021, 10, 1499. [Google Scholar] [CrossRef]
- Cavard, X.; Bergeron, Y.; Chen, H.Y.; Paré, D.; Laganière, J.; Brassard, B. Competition and Facilitation between Tree Species Change with Stand Development. Oikos 2011, 120, 1683–1695. [Google Scholar] [CrossRef]
- Simard, M.; Lecomte, N.; Bergeron, Y.; Bernier, P.Y.; Paré, D. Forest Productivity Decline Caused by Successional Paludification of Boreal Soils. Ecol. Appl. 2007, 17, 1619–1637. [Google Scholar] [CrossRef]
- Payette, S.; Rochefort, L. Écologie Des Tourbières Du Québec-Labrador; Presses Université Laval: Québec, QC, Canada, 2001. [Google Scholar]
- Paré, D.; Banville, J.L.; Garneau, M.; Bergeron, Y. Soil Carbon Stocks and Soil Carbon Quality in the Upland Portion of a Boreal Landscape, James Bay, Quebec. Ecosystems 2011, 14, 533–546. [Google Scholar] [CrossRef]
- Magnan, G.; Le Stum-Boivin, É.; Garneau, M.; Grondin, P.; Fenton, N.; Bergeron, Y. Holocene Vegetation Dynamics and Hydrological Variability in Forested Peatlands of the Clay Belt, Eastern Canada, Reconstructed Using a Palaeoecological Approach. Boreas 2019, 48, 131–146. [Google Scholar] [CrossRef]
- Simard, M.; Bernier, P.Y.; Bergeron, Y.; Pare, D.; Guérine, L. Paludification Dynamics in the Boreal Forest of the James Bay Lowlands: Effect of Time since Fire and Topography. Can. J. For. Res. 2009, 39, 546–552. [Google Scholar] [CrossRef]
- Lieffers, V.J.; Messier, C.; Stadt, K.J.; Gendron, F.; Comeau, P.G. Predicting and Managing Light in the Understory of Boreal Forests. Can. J. For. Res. 1999, 29, 796–811. [Google Scholar] [CrossRef]
- Ouimet, R.; Duchesne, L.; Tremblay, S. Long-Term Soil Fertility and Site Productivity in Stem-Only and Whole-Tree Harvested Stands in Boreal Forest of Quebec (Canada). Forests 2021, 12, 583. [Google Scholar] [CrossRef]
- Trettin, C.C.; Jurgensen, M.F.; Gale, M.R.; McLaughlin, J.W. Soil Carbon in Northern Forested Wetlands: Impacts of Silvicultural Practices. Carbon Forms Funct. For. Soils 1995, 53711, 437–461. [Google Scholar]
- Prescott, C.E. Effects of Clearcutting and Alternative Silvicultural Systems on Rates of Decomposition and Nitrogen Mineralization in a Coastal Montane Coniferous Forest. For. Ecol. Manag. 1997, 95, 253–260. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Huang, Y. Comparisons of Litterfall, Litter Decomposition and Nutrient Return in a Monoculture Cunninghamia Lanceolata and a Mixed Stand in Southern China. For. Ecol. Manag. 2008, 255, 1210–1218. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, X.; Wen, Y.; Zhu, H.; You, Y.; Qin, Z.; Li, Y.; Huang, X.; Yan, L.; Li, H. Coniferous-Broadleaf Mixture Increases Soil Microbial Biomass and Functions Accompanied by Improved Stand Biomass and Litter Production in Subtropical China. Forests 2019, 10, 879. [Google Scholar] [CrossRef]
- Prescott, C.E.; Zabek, L.M.; Staley, C.L.; Kabzems, R. Decomposition of Broadleaf and Needle Litter in Forests of British Columbia: Influences of Litter Type, Forest Type, and Litter Mixtures. Can. J. For. Res. 2000, 30, 1742–1750. [Google Scholar] [CrossRef]
- Vanguelova, E.I.; Pitman, R.M. Nutrient and Carbon Cycling along Nitrogen Deposition Gradients in Broadleaf and Conifer Forest Stands in the East of England. For. Ecol. Manag. 2019, 447, 180–194. [Google Scholar] [CrossRef]
- Taylor, B.R.; Parsons, W.F.; Parkinson, D. Decomposition of Populus Tremuloides Leaf Litter Accelerated by Addition of Alnus Crispa Litter. Can. J. For. Res. 1989, 19, 674–679. [Google Scholar] [CrossRef]
- Augusto, L.; De Schrijver, A.; Vesterdal, L.; Smolander, A.; Prescott, C.; Ranger, J. Influences of Evergreen Gymnosperm and Deciduous Angiosperm Tree Species on the Functioning of Temperate and Boreal Forests. Biol. Rev. 2015, 90, 444–466. [Google Scholar] [CrossRef] [PubMed]
- Légaré, S.; Paré, D.; Bergeron, Y. Influence of Aspen on Forest Floor Properties in Black Spruce-Dominated Stands. Plant Soil 2005, 275, 207–220. [Google Scholar] [CrossRef]
- Fradette, J. Effet de La Proximité Des Feuillus Sur La Croissance de l’épinette Noire et de l’épinette Blanche Dans Un Contexte de Plantation: Le Dégagement Est-Il Toujours Souhaitable? Ph.D. Thesis, Université du Québec en Abitibi-Témiscamingue, Rouyn Noranda, QC, Canada, 2014. [Google Scholar]
- Felton, A.; Nilsson, U.; Sonesson, J.; Felton, A.M.; Roberge, J.-M.; Ranius, T.; Ahlström, M.; Bergh, J.; Björkman, C.; Boberg, J. Replacing Monocultures with Mixed-Species Stands: Ecosystem Service Implications of Two Production Forest Alternatives in Sweden. Ambio 2016, 45, 124–139. [Google Scholar] [CrossRef]
- Asplund, J.; Hustoft, E.; Nybakken, L.; Ohlson, M.; Lie, M.H. Litter Impair Spruce Seedling Emergence in Beech Forests: A Litter Manipulation Experiment. Scand. J. For. Res. 2018, 33, 332–337. [Google Scholar] [CrossRef]
- Peterson, C.J.; Facelli, J.M. Contrasting Germination and Seedling Growth of Betula Alleghaniensis and Rhus Typhina Subjected to Various Amounts and Types of Plant Litter. Am. J. Bot. 1992, 79, 1209–1216. [Google Scholar] [CrossRef]
- Morsing, J.; Kepfer-Rojas, S.; Baastrup-Spohr, L.; Rodriguez, A.L.; Raulund-Rasmussen, K. Litter Legacy after Spruce Plantation Removal Hampers Initial Vegetation Establishment. Basic Appl. Ecol. 2020, 42, 4–14. [Google Scholar] [CrossRef]
- Uselman, S.M.; Qualls, R.G.; Lilienfein, J. Quality of Soluble Organic C, N, and P Produced by Different Types and Species of Litter: Root Litter versus Leaf Litter. Soil Biol. Biochem. 2012, 54, 57–67. [Google Scholar] [CrossRef]
- Wieder, W.R.; Cleveland, C.C.; Townsend, A.R. Tropical Tree Species Composition Affects the Oxidation of Dissolved Organic Matter from Litter. Biogeochemistry 2008, 88, 127–138. [Google Scholar] [CrossRef]
- Natural Regions. The Canadian Encyclopedia. Available online: https://www.thecanadianencyclopedia.ca/en/article/natural-regions (accessed on 14 January 2022).
- Blouin, J.; Berger, J.-P.; Gosselin, J.; Québec (Province); Direction des Inventaires Forestiers; Québec (Province); Ministère des Ressources Naturelles; de la Faune et des Parcs. Direction des Communications Guide de Reconnaissance des Types Écologiques: Région Écologique 6a, Plaine du Lac Matagami: Région Écologique 6b, Plaine de la Baie de Rupert; Direction des Communications; Ministére des Ressources Naturelles et de la Faune, Forêt Québec, Direction des Inventaires Forestiers: Québec, QC, Canada, 2005; ISBN 978-2-551-22842-3. [Google Scholar]
- Description of Natural Provinces—Province F—Abitibi and James Bay Lowlands. Available online: https://www.environnement.gouv.qc.ca/biodiversite/aires_protegees/provinces/partie4f.htm (accessed on 14 January 2022).
- Sommaire Des Normales Climatiques 1981–2010—Ministère Du Développement Durable, de l’Environnement, de La Faune et Des Parcs. Available online: https://www.environnement.gouv.qc.ca/climat/normales/sommaire.asp?cle=7093376 (accessed on 14 January 2022).
- Tran, T.S.; Simard, R.R. Mehlich III-Extractable Elements. Soil Sampl. Methods Anal. 1993, 43, 49. [Google Scholar]
- Hossner, L.R. Dissolution for Total Elemental Analysis. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 49–64. [Google Scholar]
- Jones, J.B., Jr.; Case, V.W. Sampling, Handling, and Analyzing Plant Tissue Samples. Soil Test. Plant Anal. 1990, 3, 389–427. [Google Scholar]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. RStudio Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. 2021. Available online: https://ggplot2.tidyverse.org/reference/ggplot2-package.html (accessed on 14 January 2022).
- Wickham, H.; François, R.; Henry, L.; Müller, K. RStudio Dplyr: A Grammar of Data Manipulation. 2022. Available online: http://stat599.cwick.co.nz/lectures/02-dplyr.pdf (accessed on 14 January 2022).
- R-core Nlme: Linear and Nonlinear Mixed Effects Models 2022. Available online: https://cran.r-project.org/web/packages/nlme/index.html (accessed on 2 November 2022).
- Hothorn, T.; Bretz, F.; Westfall, P.; Heiberger, R.M.; Schuetzenmeister, A.; Scheibe, S. Multcomp: Simultaneous Inference in General Parametric Models. 2022. Available online: https://cran.r-project.org/web/packages/multcomp/vignettes/generalsiminf.pdf (accessed on 20 January 2022).
- Chomel, M.; Guittonny-Larchevêque, M.; DesRochers, A.; Baldy, V. Effect of Mixing Herbaceous Litter with Tree Litters on Decomposition and N Release in Boreal Plantations. Plant Soil 2016, 398, 229–241. [Google Scholar] [CrossRef]
- Becker, H.; Aosaar, J.; Varik, M.; Morozov, G.; Aun, K.; Mander, Ü.; Soosaar, K.; Uri, V. Annual Net Nitrogen Mineralization and Litter Flux in Well-Drained Downy Birch, Norway Spruce and Scots Pine Forest Ecosystems. Silva Fenn. 2018, 52, 10013. [Google Scholar] [CrossRef]
- Hansson, K.; Olsson, B.A.; Olsson, M.; Johansson, U.; Kleja, D.B. Differences in Soil Properties in Adjacent Stands of Scots Pine, Norway Spruce and Silver Birch in SW Sweden. For. Ecol. Manag. 2011, 262, 522–530. [Google Scholar] [CrossRef]
- Hojjati, S.M.; Hagen-Thorn, A.; Lamersdorf, N.P. Canopy Composition as a Measure to Identify Patterns of Nutrient Input in a Mixed European Beech and Norway Spruce Forest in Central Europe. Eur. J. For. Res. 2009, 128, 13–25. [Google Scholar] [CrossRef]
- Thelin, G.; Rosengren, U.; Callesen, I.; Ingerslev, M. The Nutrient Status of Norway Spruce in Pure and in Mixed-Species Stands. For. Ecol. Manag. 2002, 160, 115–125. [Google Scholar] [CrossRef]
- Daněk, P.; Šamonil, P.; Hort, L. Forest Floor Alteration by Canopy Trees and Soil Wetness Drive Regeneration of a Spruce-Beech Forest. For. Ecol. Manag. 2022, 504, 119802. [Google Scholar] [CrossRef]
- Likulunga, L.E.; Pérez, C.A.R.; Schneider, D.; Daniel, R.; Polle, A. Forest Tree Species Composition and Abiotic Site Conditions Drive Soil Fungal Communities and Functional Groups. bioRxiv, 2021. Available online: https://www.biorxiv.org/content/10.1101/2021.07.21.453256v1.full.pdf (accessed on 20 April 2022).
- Zeller, B.; Legout, A.; Bienaimé, S.; Gratia, B.; Santenoise, P.; Bonnaud, P.; Ranger, J. Douglas Fir Stimulates Nitrification in French Forest Soils. Sci. Rep. 2019, 9, 10687. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Climate Gradients. Substrate Quality versus Climate and Their Interactions. In Plant Litter; Springer: Berlin/Heidelberg, Germany, 2020; pp. 165–187. [Google Scholar]
- Zhou, S.; Butenschoen, O.; Barantal, S.; Handa, I.T.; Makkonen, M.; Vos, V.; Aerts, R.; Berg, M.P.; McKie, B.; Van Ruijven, J. Decomposition of Leaf Litter Mixtures across Biomes: The Role of Litter Identity, Diversity and Soil Fauna. J. Ecol. 2020, 108, 2283–2297. [Google Scholar] [CrossRef]
- Rode, M.W. The Interaction between Organic Layer and Forest Growth and Forest Development on Former Heathland. For. Ecol. Manag. 1999, 114, 117–127. [Google Scholar] [CrossRef]
- Nilsson, M.-C.; Wardle, D.A.; Dahlberg, A. Effects of Plant Litter Species Composition and Diversity on the Boreal Forest Plant-Soil System. Oikos 1999, 86, 16–26. [Google Scholar] [CrossRef]
- Simard, M.-J.; Bergeron, Y.; Sirois, L. Conifer Seedling Recruitment in a Southeastern Canadian Boreal Forest: The Importance of Substrate. J. Veg. Sci. 1998, 9, 575–582. [Google Scholar] [CrossRef]
- Wardle, D.A.; Zackrisson, O.; Nilsson, M.-C. The Charcoal Effect in Boreal Forests: Mechanisms and Ecological Consequences. Oecologia 1998, 115, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Wahl, N.A.; Bens, O.; Schäfer, B.; Hüttl, R.F. Impact of Changes in Land-Use Management on Soil Hydraulic Properties: Hydraulic Conductivity, Water Repellency and Water Retention. Phys. Chem. Earth Parts ABC 2003, 28, 1377–1387. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Hokkanen, T.J.; Järvinen, E.; Pukkala, T. Factors Related to Seedling Growth in a Boreal Scots Pine Stand: A Spatial Analysis of a Vegetation—Soil System. Can. J. For. Res. 1993, 23, 2101–2109. [Google Scholar] [CrossRef]
- Oberhuber, W.; Hammerle, A.; Kofler, W. Tree Water Status and Growth of Saplings and Mature Norway Spruce (Picea Abies) at a Dry Distribution Limit. Front. Plant Sci. 2015, 6, 703. [Google Scholar] [CrossRef]
- Sousa, T.R.; Schietti, J.; Ribeiro, I.O.; Emílio, T.; Fernández, R.H.; ter Steege, H.; Castilho, C.V.; Esquivel-Muelbert, A.; Baker, T.; Pontes-Lopes, A. Water Table Depth Modulates Productivity and Biomass across Amazonian Forests. Glob. Ecol. Biogeogr. 2022, 31, 1571–1588. [Google Scholar] [CrossRef]
- Berg, B. Litter Decomposition and Organic Matter Turnover in Northern Forest Soils. For. Ecol. Manag. 2000, 133, 13–22. [Google Scholar] [CrossRef]
- Henneb, M.; Thiffault, N.; Valeria, O. Regional Climate, Edaphic Conditions and Establishment Substrates Interact to Influence Initial Growth of Black Spruce and Jack Pine Planted in the Boreal Forest. Forests 2020, 11, 139. [Google Scholar] [CrossRef]
- Mason, W.L.; Edwards, C.; Hale, S.E. Survival and Early Seedling Growth of Conifers with Different Shade Tolerance in a Sitka Spruce Spacing Trial and Relationship to Understorey Light Climate. Silva Fenn. 2004, 38, 357–370. [Google Scholar] [CrossRef]
- Boucher, D.; Gauthier, S.; Thiffault, N.; Marchand, W.; Girardin, M.; Urli, M. How Climate Change Might Affect Tree Regeneration Following Fire at Northern Latitudes: A Review. New For. 2020, 51, 543–571. [Google Scholar] [CrossRef]
- Liepe, K.J.; Hamann, A.; Smets, P.; Fitzpatrick, C.R.; Aitken, S.N. Adaptation of Lodgepole Pine and Interior Spruce to Climate: Implications for Reforestation in a Warming World. Evol. Appl. 2016, 9, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Gonçalves, D.S.; Souza, P.A.; de Lucena, F.R.; Silva, R.R. da Brondani, G.E. Luminosity Levels Affect the Initial Seedlings Growth and Nutrient Accumulation in Khaya Senegalensis A. Juss. Cerne 2018, 24, 344–351. [Google Scholar] [CrossRef]
- De Vries, W.; Van der Salm, C.; Reinds, G.J.; Erisman, J.W. Element Fluxes through European Forest Ecosystems and Their Relationships with Stand and Site Characteristics. Environ. Pollut. 2007, 148, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Houle, D.; Marty, C.; Duchesne, L.; Gagnon, C. Humus Layer Is the Main Locus of Secondary SO4 Production in Boreal Forests. Geochim. Cosmochim. Acta 2014, 126, 18–29. [Google Scholar] [CrossRef]
- Lindroos, A.-J.; Derome, J.; Derome, K.; Smolander, A. The Effect of Scots Pine, Norway Spruce and Silver Birch on the Chemical Composition of Stand Throughfall and Upper Soil Percolation Water in Northern Finland. 2011. Available online: https://jukuri.luke.fi/bitstream/handle/10024/516371/Lindroos.pdf?sequence=1&isAllowed=y (accessed on 12 April 2022).
- Driscoll, C.T.; Lawrence, G.B.; Bulger, A.J.; Butler, T.J.; Cronan, C.S.; Eagar, C.; Lambert, K.F.; Likens, G.E.; Stoddard, J.L.; Weathers, K.C. Acidic Deposition in the Northeastern United States: Sources and Inputs, Ecosystem Effects, and Management Strategies: The Effects of Acidic Deposition in the Northeastern United States Include the Acidification of Soil and Water, Which Stresses Terrestrial and Aquatic Biota. BioScience 2001, 51, 180–198. [Google Scholar]
- Jones, D.L.; Kielland, K. Soil Amino Acid Turnover Dominates the Nitrogen Flux in Permafrost-Dominated Taiga Forest Soils. Soil Biol. Biochem. 2002, 34, 209–219. [Google Scholar] [CrossRef]
- Adamczyk, B.; Ahvenainen, A.; Sietiö, O.-M.; Kanerva, S.; Kieloaho, A.-J.; Smolander, A.; Kitunen, V.; Saranpää, P.; Laakso, T.; Straková, P. The Contribution of Ericoid Plants to Soil Nitrogen Chemistry and Organic Matter Decomposition in Boreal Forest Soil. Soil Biol. Biochem. 2016, 103, 394–404. [Google Scholar] [CrossRef]
- Killham, K. Nitrification in Coniferous Forest Soils. Plant Soil 1990, 128, 31–44. [Google Scholar] [CrossRef]
- Kendrick, W.B. The Time Factor in the Decomposition of Coniferous Leaf Litter. Can. J. Bot. 1959, 37, 907–912. [Google Scholar] [CrossRef]
- Urli, M.; Thiffault, N.; Houle, D.; Gauthier, S.; Bergeron, Y. Role of Green Alder in Boreal Conifer Growth: Competitor or Facilitator? Facets 2020, 5, 166–181. [Google Scholar] [CrossRef]
- Nikonov, V.V.; Lukina, N.V.; Polyanskaya, L.M.; Panikova, A.N. Distribution of Microorganisms in the Al–Fe–Humus Podzols of Natural and Anthropogenically Impacted Boreal Spruce Forests. Microbiology 2001, 70, 319–328. [Google Scholar] [CrossRef]
- Giesler, R.; Satoh, F.; Ilstedt, U.; Nordgren, A. Microbially Available Phosphorus in Boreal Forests: Effects of Aluminum and Iron Accumulation in the Humus Layer. Ecosystems 2004, 7, 208–217. [Google Scholar] [CrossRef]
- Caccia, F.D.; Ballaré, C.L. Effects of Tree Cover, Understory Vegetation, and Litter on Regeneration of Douglas-Fir (Pseudotsuga Menziesii) in Southwestern Argentina. Can. J. For. Res. 1998, 28, 683–692. [Google Scholar] [CrossRef]
- Simard, M.-J.; Bergeron, Y.; Sirois, L. Substrate and Litterfall Effects on Conifer Seedling Survivorship in Southern Boreal Stands of Canada. Can. J. For. Res. 2003, 33, 672–681. [Google Scholar] [CrossRef]
- Simola, H. Persistent Carbon Loss from the Humus Layer of Tilled Boreal Forest Soil. Eur. J. Soil Sci. 2018, 69, 303–314. [Google Scholar] [CrossRef]
- Munson, A.D.; Timmer, V.R. Soil Nitrogen Dynamics and Nutrition of Pine Following Silvicultural Treatments in Boreal and Great Lakes-St. Lawrence Plantations. For. Ecol. Manag. 1995, 76, 169–179. [Google Scholar] [CrossRef]
- Prescott, C.E.; Maynard, D.G.; Laiho, R. Humus in Northern Forests: Friend or Foe? For. Ecol. Manag. 2000, 133, 23–36. [Google Scholar] [CrossRef]
- Natalia, S.; Lieffers, V.J.; Landhäusser, S.M. Effects of Leaf Litter on the Growth of Boreal Feather Mosses: Implication for Forest Floor Development. J. Veg. Sci. 2008, 19, 253–260. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).