Comparison of Stomatal Structure and Distribution between Ovules and Leaves in Ginkgo biloba
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Collection and Treatment
2.2. Scanning Electron Microscopic Observations
2.3. Confocal Laser Scanning Microscopic
2.4. Semi-Thin Sectioning
3. Results
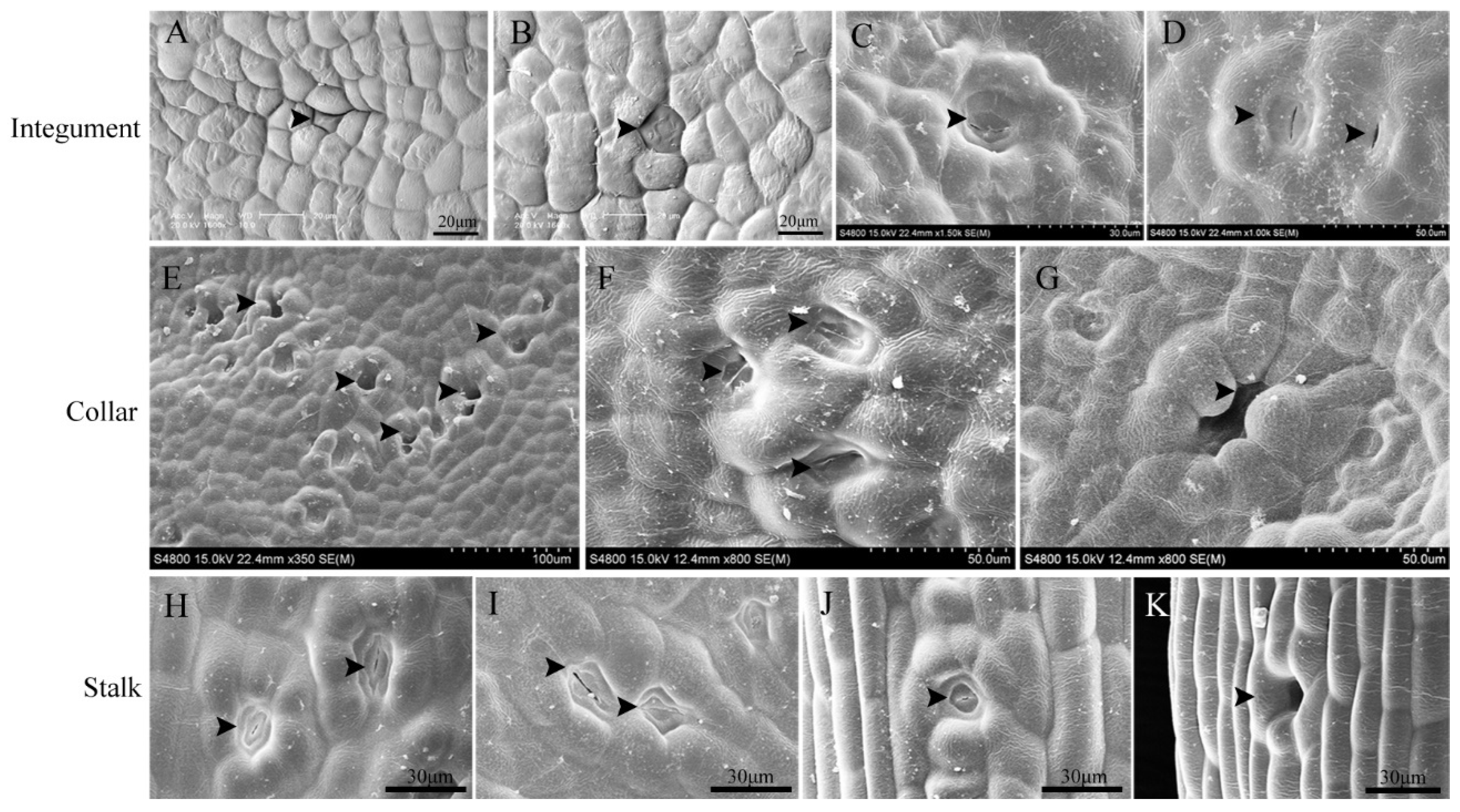
3.1. Stomatal Distribution on Ovules
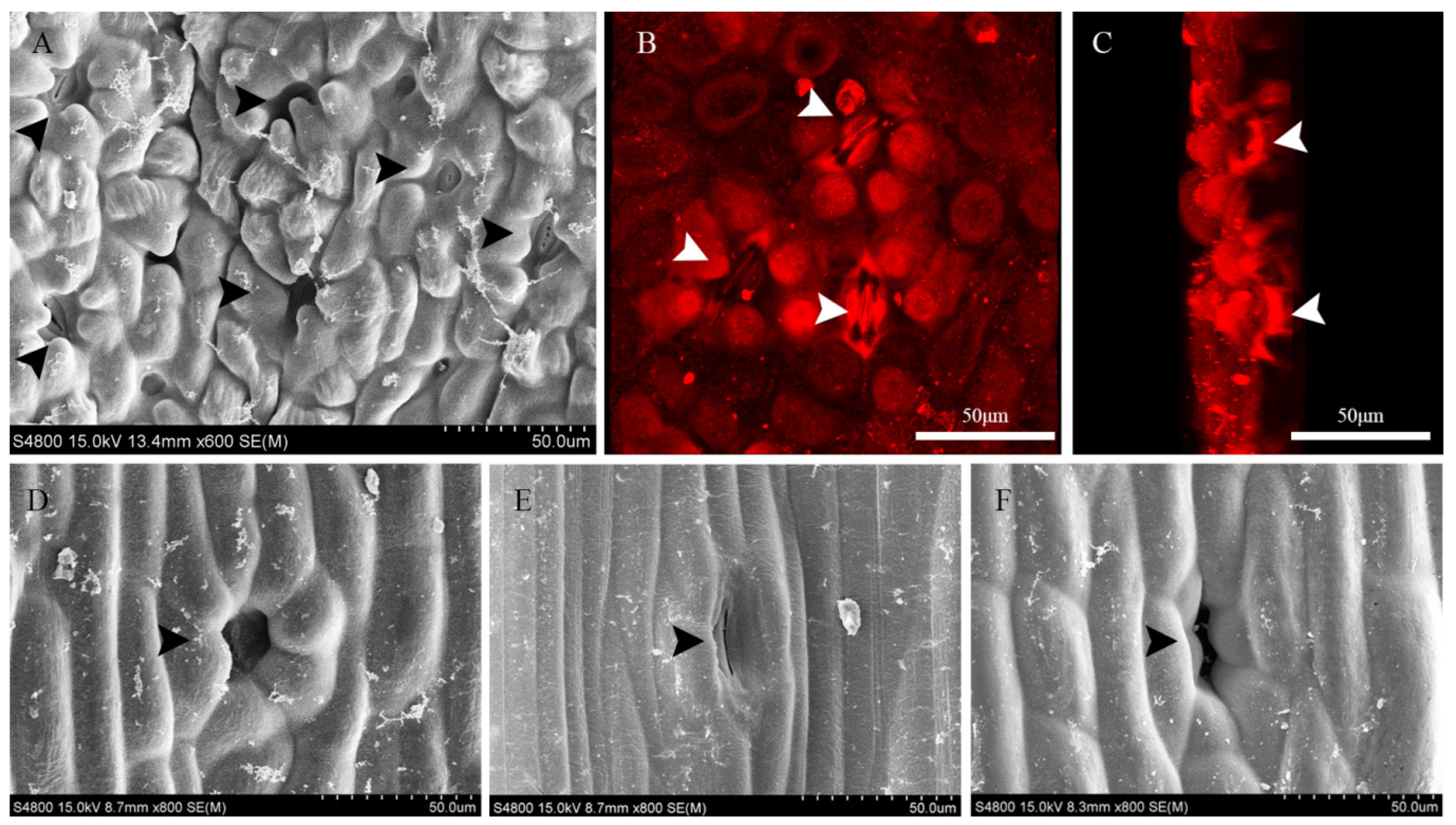
3.2. Stomatal Development and Ovular Morphology
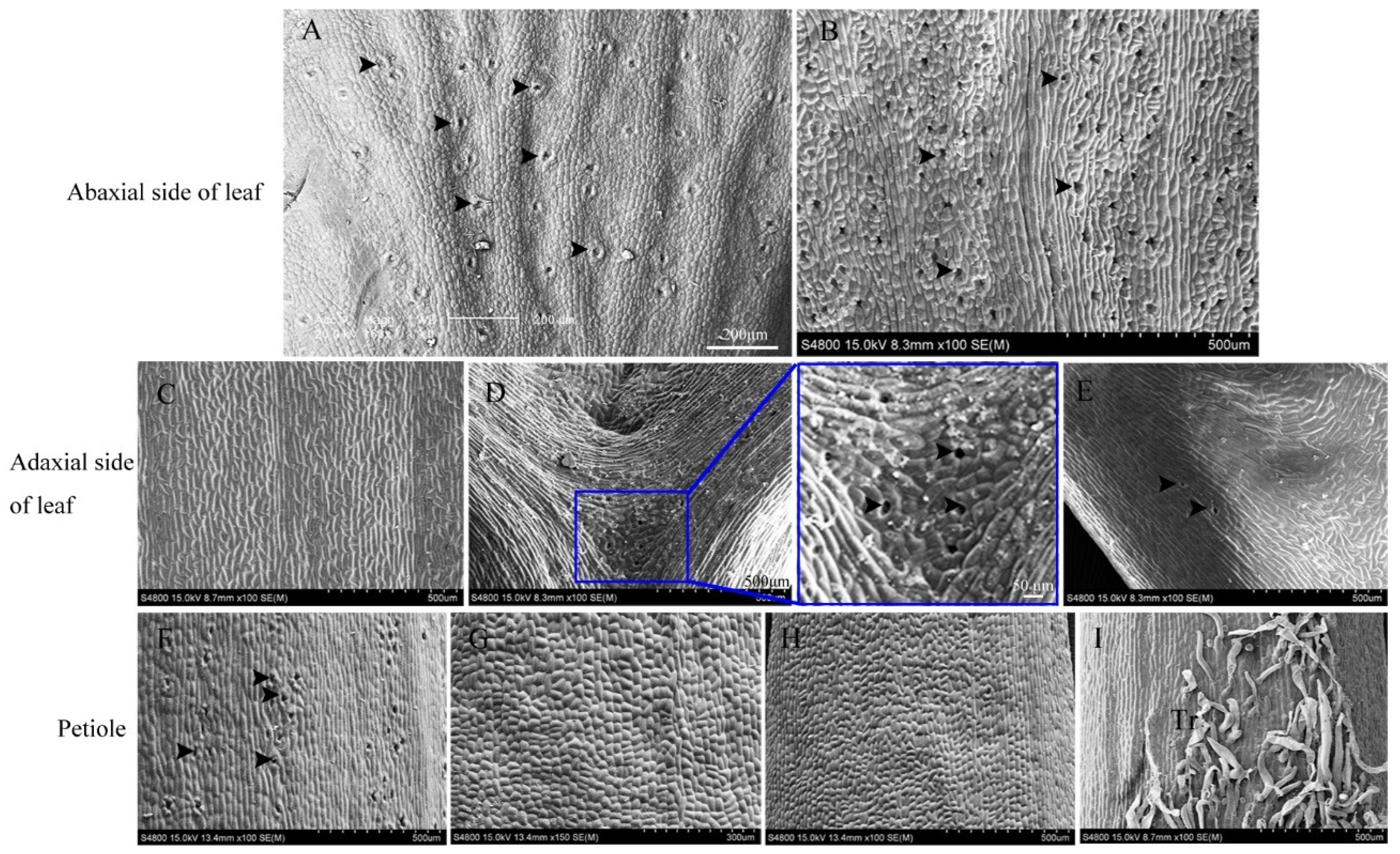
3.3. Stomatal Distribution on Leaves
3.4. Stomatal Morphology on Leaves
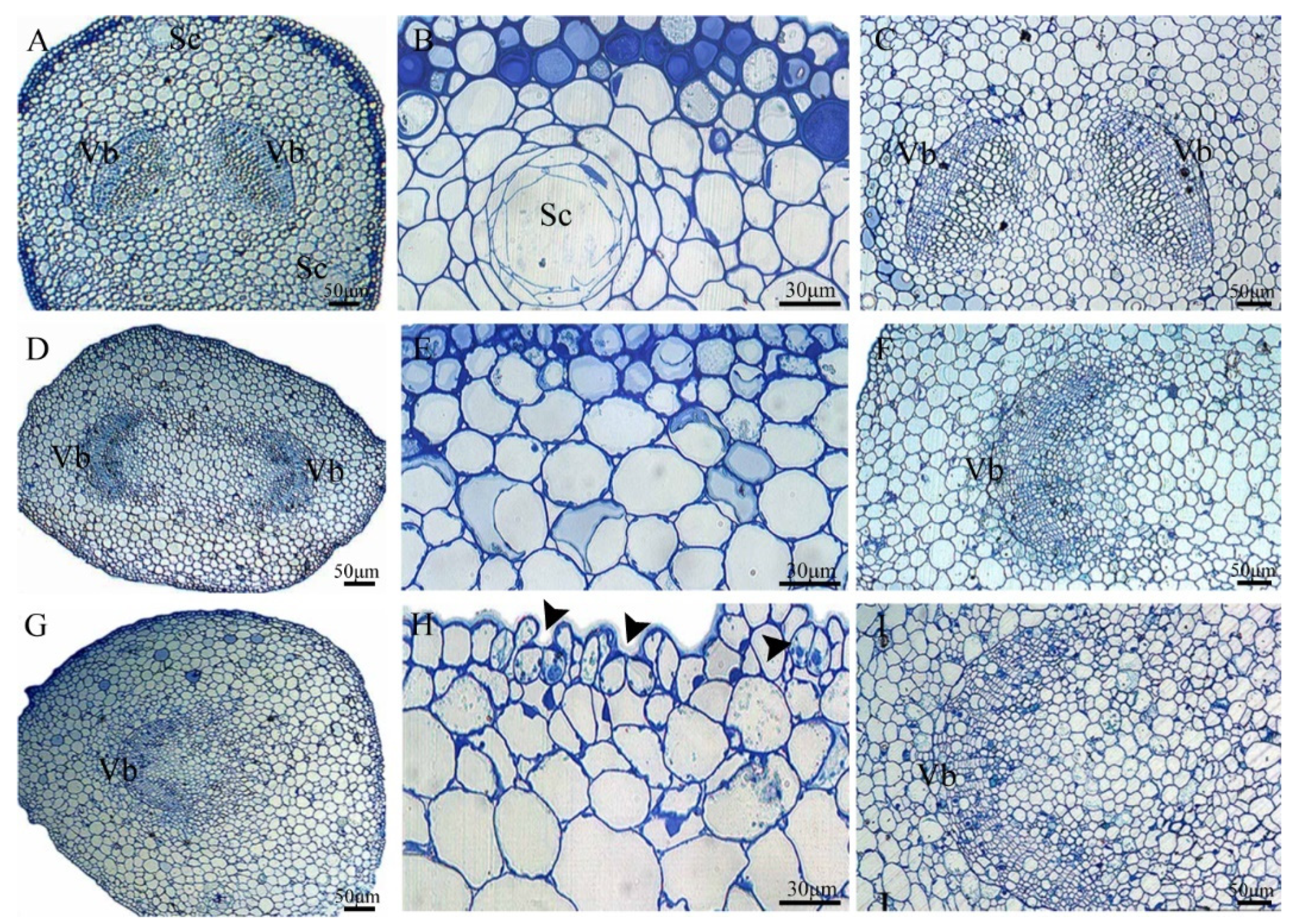
3.5. Anatomical Characteristics of the Ovule Stalk, General Stalk, and Petiole
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ackerly, D.D.; Donoghue, M.J. Leaf Size, Sapling Allometry, and Corner’s Rules: Phylogeny and Correlated Evolution in Maples. Am. Nat. 1998, 152, 767–791. [Google Scholar] [CrossRef]
- Guan, Z.J.; Zhang, S.B.; Guan, K.Y.; Li, S.Y.; Hu, H. Leaf Anatomical Structures of Paphiopedilum and Cypripedium and Their Adaptive Significance. J. Plant Res. 2011, 124, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Beerling, D.J. CO2-forced Evolution of Plant Gas Exchange Capacity and Water-use Efficiency over the Phanerozoic. Geobiology 2009, 7, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Pittermann, J. The Evolution of Water Transport in Plants: An Integrated Approach. Geobiology 2010, 8, 112–139. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, K.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light Regulation of Stomatal Movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef]
- Franks, P.J.; Drake, P.L.; Beerling, D.J. Plasticity in Maximum Stomatal Conductance Constrained by Negative Correlation between Stomatal Size and Density: An Analysis Using Eucalyptus globulus. Plant Cell Environ. 2009, 32, 1737–1748. [Google Scholar] [CrossRef]
- Roelfsema, M.R.G.; Hedrich, R. In the Light of Stomatal Opening: New Insights into ‘the Watergate’. New Phytol. 2005, 167, 665–691. [Google Scholar] [CrossRef]
- Bergmann, D.C.; Sack, F.D. Stomatal Development. Annu. Rev. Plant Biol. 2007, 58, 163–181. [Google Scholar] [CrossRef]
- Kim, T.H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard Cell Signal Transduction Network: Advances in Understanding Abscisic Acid, CO2, and Ca2+ Signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef]
- Christodoulakis, N.S.; Menti, J.; Galatis, B. Structure and Development of Stomata on the Primary Root of Ceratonia siliqua L. Ann. Bot. 2002, 89, 23–29. [Google Scholar] [CrossRef]
- Horner, H.T.; Healy, R.A.; Cervantes-Martinez, T.; Palmer, R.C. Floral Nectary Fine Structure and Development in Glycine max L. Int. J. Plant Sci. 2003, 164, 675–690. [Google Scholar] [CrossRef]
- Wang, L.; Hasenstein, K.H. Seed Coat Stomata of Several Iris Species. Flora 2016, 224, 24–29. [Google Scholar] [CrossRef]
- Wang, L.; Xia, X.; Jiang, H.; Lu, Z.G.; Cui, J.W.; Cao, F.L.; Jin, B. Genome-wide Identification and Characterization of Novel lncRNAs in Ginkgo biloba. Trees 2018, 32, 1429–1442. [Google Scholar] [CrossRef]
- Douglas, A.W.; Stevenson, D.W.; Little, D.P. Ovule Development in Ginkgo biloba L., with Emphasis on the Collar and Nucellus. Int. J. Plant Sci. 2007, 168, 1207–1236. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Lin, M.M.; Lu, Y.; Jiang, X.X.; Jin, B. An Embryological Study and Systematic Significance of the Primitive Gymnosperm Ginkgo biloba. J. Syst. Evol. 2011, 49, 353–361. [Google Scholar] [CrossRef]
- Mao, D.Y.; Tang, H.; Xiao, N.; Wang, L. Uncovering the Secrets of Secretory Fluids during the Reproductive Process in Ginkgo biloba. Crit. Rev. Plant Sci. 2022, 41, 161–175. [Google Scholar] [CrossRef]
- Jin, B.; Wang, D.; Lu, Y.; Jiang, X.X.; Zhang, M.; Zhang, L.; Wang, L. Female Short Shoot and Ovule Development in Ginkgo biloba L. with Emphasis on Structures Associated with Wind Pollination. Int. Sch. Res. Not. 2012, 2012, 230685. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Quan, C.; Liu, Y.S. Tertiary Ginkgo Ovulate Organs with Associated Leaves from North Dakota, USA, and Their Evolutionary Significance. Int. J. Plant Sci. 2012, 173, 67–80. [Google Scholar] [CrossRef]
- Friedman, W.E.; Goliber, T.E. Photosynthesis in the Female Gametophyte of Ginkgo biloba. Am. J. Bot. 1986, 73, 1261–1266. [Google Scholar] [CrossRef]
- Wang, Y.D.; Guignard, G.; Thévenard, F.; Dilcher, D.; Barale, G.; Mosbrugger, V.; Yang, X.J.; Mei, S.W. Cuticular Anatomy of Sphenobaiera huangii from the Lower Jurassic of Hubei, China. Am. J. Bot. 2005, 92, 709–721. [Google Scholar] [CrossRef]
- Quan, C.; Sun, G.; Zhou, Z. A New Tertiary Ginkgo from the Wuyun Formation of Jiayin, Heilongjiang, Northeastern China and Its Paleoenvironmental Implications. Am. J. Bot. 2010, 97, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Rudall, P.J.; Rowland, A.; Bateman, R.M. Ultrastructure of Stomatal Development in Ginkgo biloba. Int. J. Plant Sci. 2012, 173, 849–860. [Google Scholar] [CrossRef]
- Ren, S.; Ma, K.; Lu, Z.; Chen, G.; Cui, J.; Tong, P.; Wang, L.; Teng, N.; Jin, B. Transcriptomic and Metabolomic Analysis of the Heat-Stress Response of Populus tomentosa Carr. Forests 2019, 10, 383. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Z.G.; Li, W.X.; Xu, J.; Luo, K.G.; Lu, W.C.; Zhang, L.; Jin, B. Global Comparative Analysis of Expressed Genes in Ovules and Leaves of Ginkgo biloba L. Tree Genet. Genomes 2016, 12, 29. [Google Scholar] [CrossRef]
- Arbuckle, J.L. IBM SPSS Amos 20 User’s Guide; Amos Development Corporation, SPSS Inc.: Chicago, IL, USA, 2011; pp. 226–229. [Google Scholar]
- Jia, Z.; Zhao, B.; Liu, S.; Lu, Z.; Chang, B.; Jiang, H.; Cui, H.; He, Q.; Li, W.; Jin, B.; et al. Embryo Transcriptome and miRNA Analyses Reveal the Regulatory Network of Seed Dormancy in Ginkgo biloba. Tree Physiol. 2021, 41, 571–588. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The Role of Stomata in Sensing and Driving Environmental Change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Gray, J.E.; Hetherington, A.M. Plant Development: YODA the Stomatal Switch. Curr. Biol. 2004, 14, 488–490. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.S.; Xie, X.Z. Regulation of Stomatal Development in Plants. Hereditas 2011, 33, 131–137. [Google Scholar] [CrossRef]
- Lv, H.F. Comparative Study on the Stomatic Distribution on Plants Setereasea purpurea Boom, Zebrina pendula Schnizi and Commelina communis Linn. Chin. Bull. Bot. 2000, 17, 375–380. [Google Scholar]
- Xu, Y.; Yang, Y.; Wang, Y.F. Dynamic Change of the Stomata Size in the Hypocotyls of Mock pad-chol. J. Anhui Agric. Sci. 2010, 38, 11102–11103, 11164. [Google Scholar]
- Azad, A.K.; Sawa, Y.; Ishikawa, T.; Shibata, H. Temperature-dependent Stomatal Movement in Tulip Petals Controls Water Transpiration during Flower Opening and Closing. Ann. Appl. Biol. 2007, 150, 81–87. [Google Scholar] [CrossRef]
- Tobe, H. Floral Structure of Cardiopteris with Special Emphasis on the Gynoecium: Systematic and Evolutionary Implications. J. Plant Res. 2012, 125, 361–369. [Google Scholar] [CrossRef]
- Peschel, S.; Beyer, M.; Knoche, M. Surface Characteristics of Sweet Cherry Fruit: Stomata-number, Distribution, Functionality and Surface Wetting. Sci. Hortic. 2003, 97, 265–278. [Google Scholar] [CrossRef]
- Paiva, É.A.S.; Lemos-Filho, J.P.; Oliveira, D.M.T. Imbibition of Swietenia macrophylla Seeds: The Role of Stomata. Ann. Bot. 2006, 98, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.J.; Thomas, S.; Kang, Y.X.; Wang, L.B.; Yu, H.Y.; Zhang, X. Discovery and Evolutionary Significance of Homalanthus Populifolius Seed Coat Stomata. Bull. Bot. Res. 2015, 35, 660–664. [Google Scholar]
- Wang, Q.; Jiang, Y.; Mao, X.; Yu, W.; Lu, J.; Wang, L. Integration of Morphological, Physiological, Cytological, Metabolome and Transcriptome Analyses Reveal Age Inhibited Accumulation of Flavonoid Biosynthesis in Ginkgo biloba Leaves. Ind. Crops Prod. 2022, 187, 115405. [Google Scholar] [CrossRef]
- Petrík, P.; Petek, A.; Konôpková, A.; Bosela, M.; Fleischer, P.; Frýdl, J.; Kurjak, D. Stomatal and Leaf Morphology Response of European Beech (Fagus sylvatica L.) Provenances Transferred to Contrasting Climatic Conditions. Forests 2020, 11, 1359. [Google Scholar] [CrossRef]
- Petrik, P.; Petek-Petrik, A.; Kurjak, D.; Mukarram, M.; Klein, T.; Gömöry, D.; Střelcová, K.; Frýdl, J.; Konôpková, A. Interannual Adjustments in Stomatal and Leaf Morphological Traits of European beech (Fagus sylvatica L.) Demonstrate Its Climate Change Acclimation Potential. Plant Biol. 2022; Early View. [Google Scholar] [CrossRef]
- Pan, Z.H.; Wu, Z.J.; Pu, F.T. Anatomical Studies of Petiole in Ligusticum from China. Acta Bot. Yunnanica 1992, 14, 143–149. [Google Scholar]
- Chang, S.H.; Lu, L.D.; Gao, W.J.; Deng, C.L.; Liu, S.X.; Hou, C.L. Comparative Anatomical Studies on Petioles of Osmanthus. J. Trop. Subtrop. Bot. 2008, 16, 10–18. [Google Scholar]
- Fujita, K.; Takagi, S.; Terashima, I. Leaf Angle in Chenopodium album is Determined by Two Processes: Induction and Cessation of Petiole Curvature. Plant Cell Environ. 2008, 31, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Sarlikioti, V.; de Visser, P.H.B.; Buck-Sorlin, G.H.; Marcelis, L.F.M. How Plant Architecture Affects Light Absorption and Photosynthesis in Tomato: Towards An Ideotype for Plant Architecture Using A Functional–structural Plant Model. Ann. Bot. 2011, 108, 1065–1073. [Google Scholar] [CrossRef]
- Liu, D.D.; Chao, W.M.; Turgeon, R. Transport of Sucrose, not Hexose, in the Phloem. J. Exp. Bot. 2012, 63, 4315–4320. [Google Scholar] [CrossRef]
- Bloemen, J.; McGuire, M.A.; Aubrey, D.P.; Teskey, R.O.; Steppe, K. Transport of Root-respired CO2 via the Transpiration Stream Affects Aboveground Carbon Assimilation and CO2 Efflux in Trees. New Phytol. 2013, 197, 555–565. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, S. The Missing Link in Ginkgo Evolution. Nature 2003, 423, 821–822. [Google Scholar] [CrossRef]

| Integument | Collar | Ovule Stalk (Abaxial Side) | Leaf Lamina (Abaxial Side) | Petiole (Abaxial Side) | |
|---|---|---|---|---|---|
| Density (mm2) | 32.00 ± 3.27 c | 218.00 ± 51.41 a | 149.67 ± 13.47 b | 112.00 ± 11.78 b | 28.00 ± 3.27 c |
| Length (μm) | 18.88 ± 1.50 b | 23.37 ± 1.15 b | 18.19 ± 4.29 b | 22.07 ± 1.22 b | 31.97 ± 3.54 a |
| Width (μm) | 9.30 ± 0.85 bc | 11.32 ± 0.65 a | 8.16 ± 0.71 c | 10.07 ± 0.67 ab | 10.69 ± 0.67 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wang, D.; Sheng, X.; Zhang, C.; Li, W.; Xiao, N.; Wang, L.; Lu, Z. Comparison of Stomatal Structure and Distribution between Ovules and Leaves in Ginkgo biloba. Forests 2022, 13, 1801. https://doi.org/10.3390/f13111801
Chen S, Wang D, Sheng X, Zhang C, Li W, Xiao N, Wang L, Lu Z. Comparison of Stomatal Structure and Distribution between Ovules and Leaves in Ginkgo biloba. Forests. 2022; 13(11):1801. https://doi.org/10.3390/f13111801
Chicago/Turabian StyleChen, Siming, Di Wang, Xi Sheng, Chengyu Zhang, Wei Li, Nan Xiao, Li Wang, and Zhaogeng Lu. 2022. "Comparison of Stomatal Structure and Distribution between Ovules and Leaves in Ginkgo biloba" Forests 13, no. 11: 1801. https://doi.org/10.3390/f13111801
APA StyleChen, S., Wang, D., Sheng, X., Zhang, C., Li, W., Xiao, N., Wang, L., & Lu, Z. (2022). Comparison of Stomatal Structure and Distribution between Ovules and Leaves in Ginkgo biloba. Forests, 13(11), 1801. https://doi.org/10.3390/f13111801






