Conservation of Tree Species Richness in a Traditional Agroforestry Landscape in the Vhembe Biosphere Reserve, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection—Fieldwork
2.3. Data Collection—Focus Group Discussion
2.4. Vegetation Analysis
2.4.1. Species Richness in Different Land-Uses
2.4.2. The Variance of Species Richness among Different Land-Uses
2.4.3. Drivers of Species Richness in Different Land-Uses
2.4.4. Species Richness Distribution in Different Land-Uses
2.5. Social-Ecological Data Analysis—Focus Group Discussion
3. Results
3.1. Species Richness in Different Land-Uses
3.2. The Variance of Species Richness among Different Land-Uses
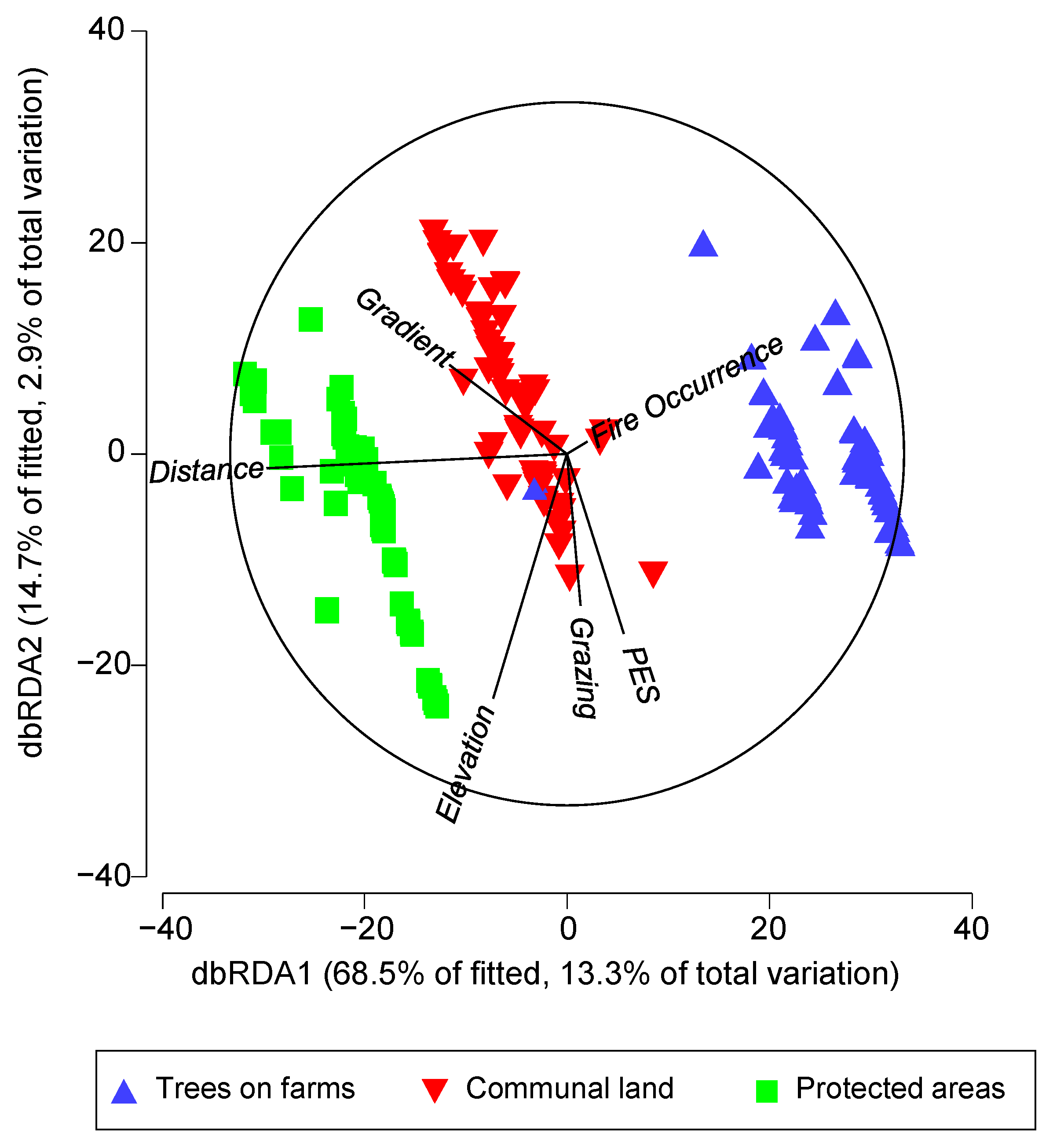
3.3. Drivers of Species Richness in Different Land-Uses
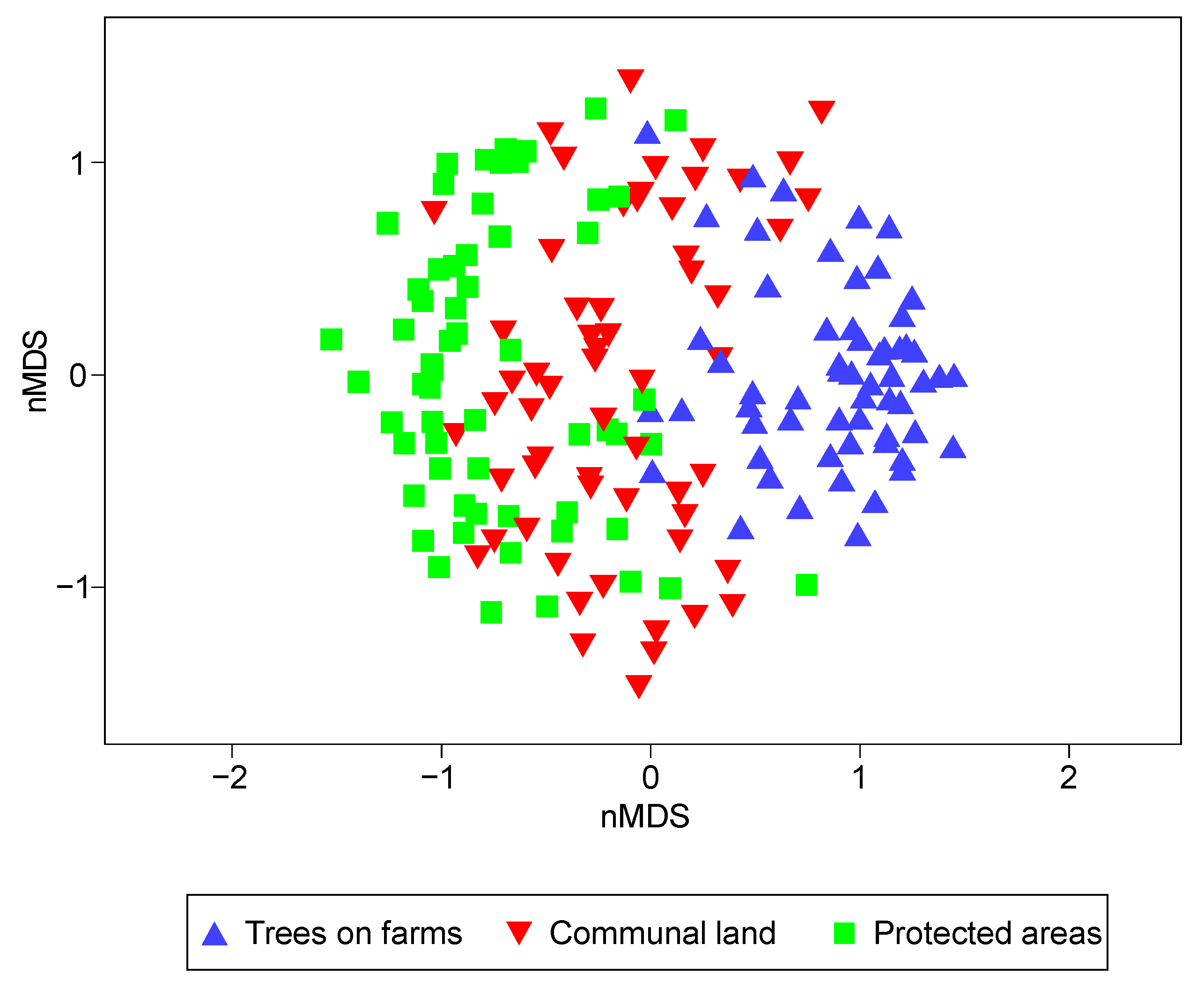
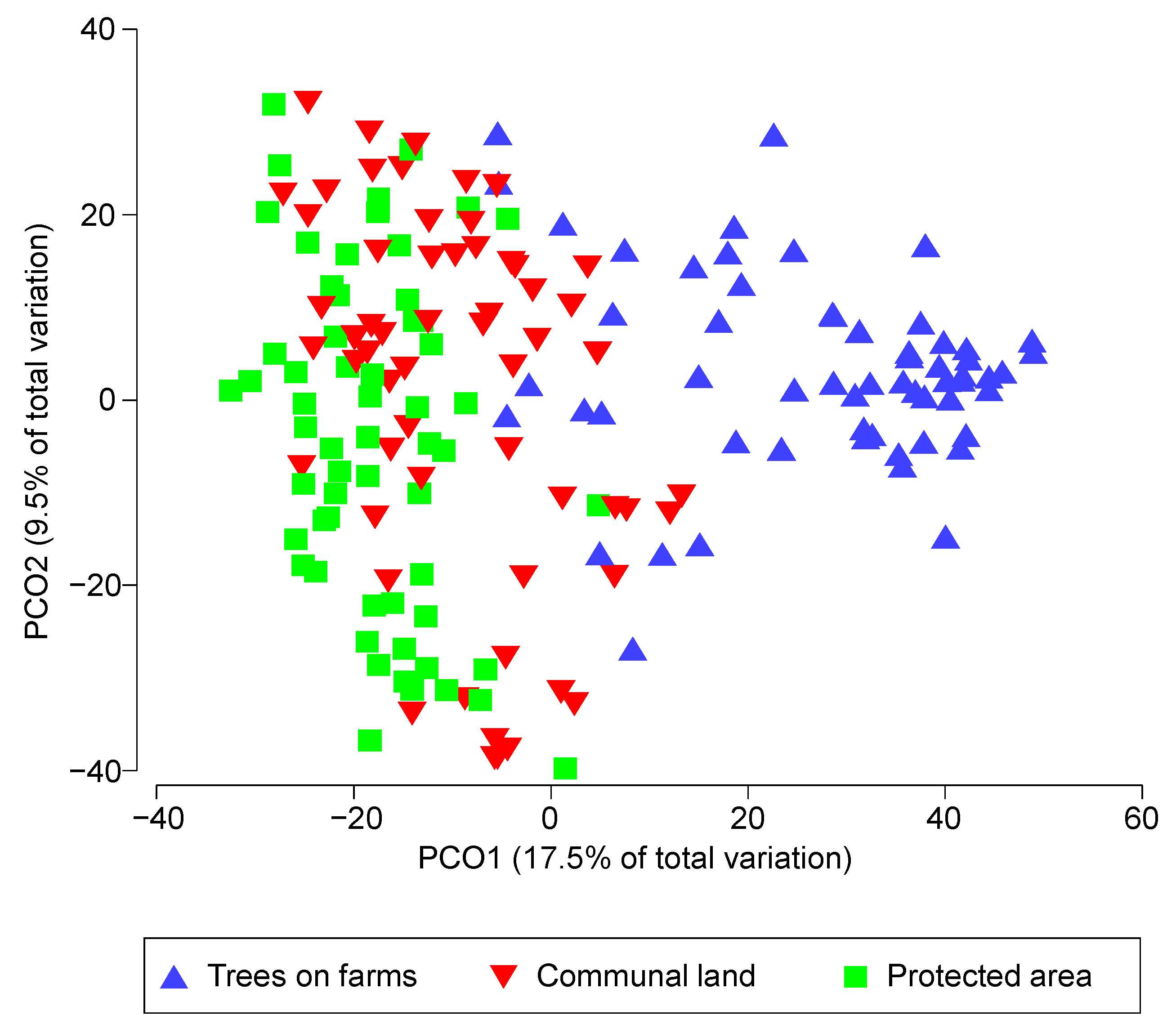
3.4. Species Abundance Distribution in Different Land-Uses
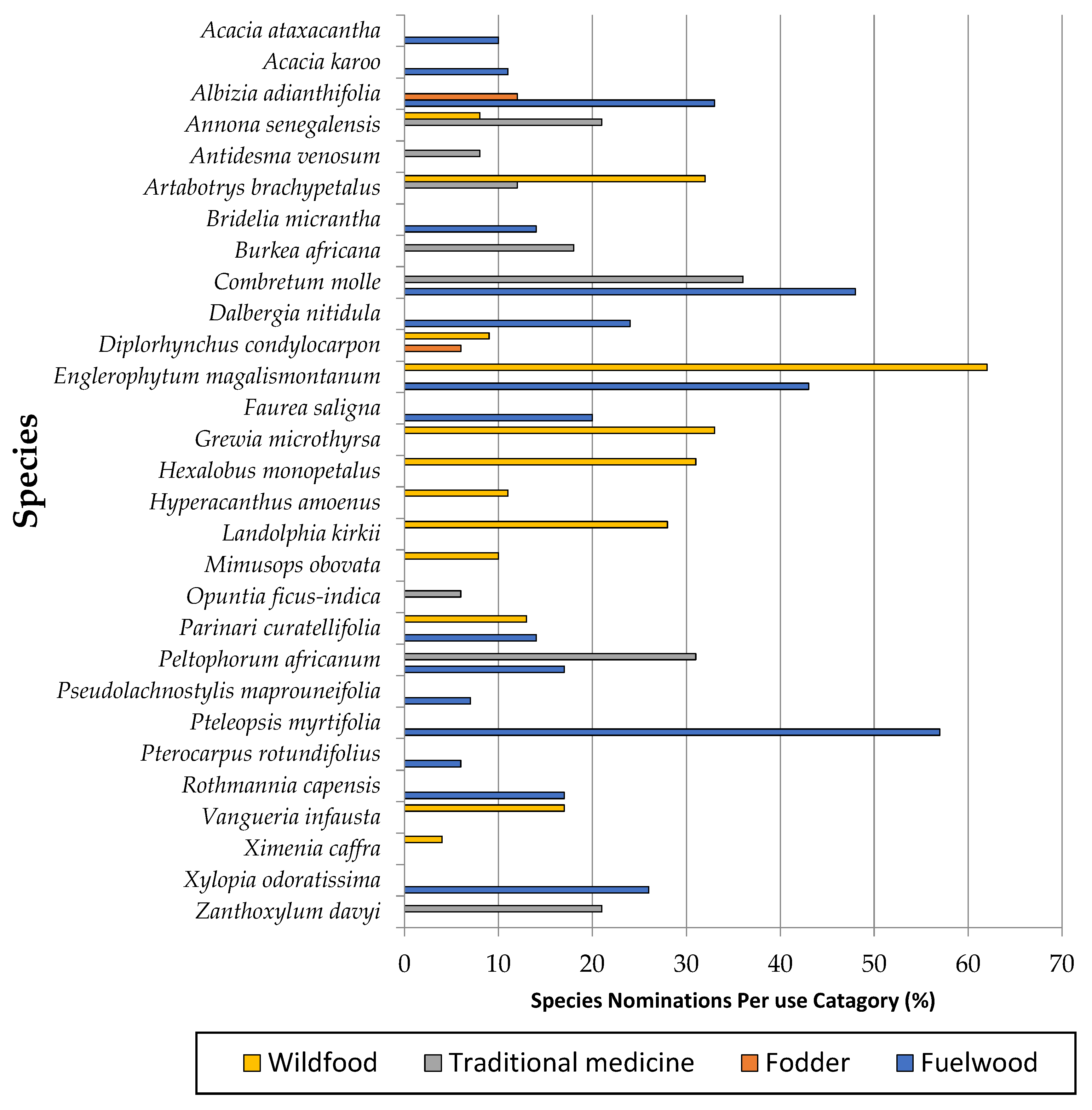
3.5. Tree Species Use and Conservation Strategies
3.5.1. Theme 1: National Conservation Policy
3.5.2. Theme 2: Community Conservation Policy
3.5.3. Theme 3: Conservation Practices
4. Discussion
4.1. Species Richness in Different Land-Uses
4.2. Drivers of Species Richness Variance in Different Land-Uses
4.3. Tree Species Richness, Uses and Conservation Strategies
4.4. Implications for Conservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasethe, M.T.; Semenya, S.S.; Potgieter, M.J.; Maroyi, A. The utilization and management of plant resources in rural areas of the Limpopo Province, South Africa. J. Ethnobiol. Ethnomed. 2013, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Scheiter, S.; Gaillard, C.; Martens, C.; Erasmus, B.; Pfeiffer, M. How vulnerable are ecosystems in the Limpopo province to climate change? S. Afr. J. Bot. 2018, 116, 86–95. [Google Scholar] [CrossRef]
- Turpie, J.K.; Forsythe, K.J.; Knowles, A.; Blignaut, J.; Letley, G. Mapping and valuation of South Africa’s ecosystem services: A local perspective. Ecosyst. Serv. 2017, 27, 179–192. [Google Scholar] [CrossRef]
- Makhado, R.A.; Potgieter, M.J.; Wessels, D.C.; Saidi, A.T.; Masehela, K.L. Use of Mopane Woodland Resources and Associated Woodland Management Challenges in Rural Areas of South Africa. Ethnobot. Res. Appl. 2012, 10, 369–379. [Google Scholar]
- Santos, P.Z.F.; Crouzeilles, R.; Sansevero, J.B.B. Can agroforestry systems enhance biodiversity and ecosystem service provision in agricultural landscapes? A meta-analysis for the Brazilian Atlantic Forest. For. Ecol. Manag. 2019, 433, 140–145. [Google Scholar] [CrossRef]
- Araia, M.G.; Chirwa, P.W.; Syampungani, S. Do strictly protected areas protect vulnerable local tree species better than human land use? Disentangling conservation value from biodiversity value. J. Nat. Conserv. 2020, 58, 125919. [Google Scholar] [CrossRef]
- Bucheli, P.; Vandreé, J.; Bokelmann, W. Agroforestry systems for biodiversity and ecosystem services: The case of the Sibundoy Valley in the Colombian province of Putumayo. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2017, 13, 380–397. [Google Scholar] [CrossRef]
- Plieninger, T.; Muñoz-Rojas, J.; Buck, L.E.; Scherr, S.J. Agroforestry for sustainable landscape management. Sustain. Sci. 2020, 15, 1255–1266. [Google Scholar] [CrossRef]
- Viswanath, S.; Lubina, P.A.; Subbanna, S.; Sandhya, M.C. Traditional Agroforestry systems and practices: A review. Adv. Agric. Res. Technol. J. 2018, 2, 18–29. [Google Scholar]
- Sheppard, J.P.; Bohn Reckziegel, R.; Borrass, L.; Chirwa, P.W.; Cuaranhua, C.J.; Hassler, S.K.; Hoffmeister, S.; Kestel, F.; Maier, R.; Mälicke, M.; et al. Agroforestry: An Appropriate and Sustainable Response to a Changing Climate in Southern Africa? Sustainability 2020, 12, 6796. [Google Scholar] [CrossRef]
- Pinard, F.; Joetzjer, E.; Kindt, R.; Kehlenbeck, K. Are coffee agroforestry systems suitable for circa situm conservation of indigenous trees? A case study from Central Kenya. Biodivers. Conserv. 2014, 23, 467–495. [Google Scholar] [CrossRef]
- Chavan, S.B.; Newaj, R.; Rizvi, R.H.; Ajit; Prasad, R.; Alam, B.; Handa, A.K.; Dhyani, S.K.; Jain, A.; Tripathi, D. Reduction of global warming potential vis-à-vis greenhouse gases through traditional agroforestry systems in Rajasthan, India. Environ. Dev. Sustain. 2021, 23, 4573–4593. [Google Scholar] [CrossRef]
- Rendón-Sandoval, F.J.; Casas, A.; Moreno-Calles, A.I.; Torres-García, I.; García-Frapolli, E. Traditional Agroforestry Systems and Conservation of Native Plant Diversity of Seasonally Dry Tropical Forests. Sustainability 2020, 12, 4600. [Google Scholar] [CrossRef]
- Ayisi, K.K.; Belete, A.; Addo-Bediako, A.; Ramudzuli, M.R.; Mkhari, J.J.; Mollel, N. Traditional agroforestry practice in Limpopo Province of South Africa. J. Hum. Ecol. 2018, 62, 24–34. [Google Scholar]
- Department of Agriculture, Forestry and Fisheries. Agroforestry Strategy Framework for South Africa. 2017. Available online: https://www.forestry.co.za/uploads/File/industry_info/policy%20legislation/Agroforestry%20Strategy%20Framework%20Approved%20fourth%20Draft%20-%2009%20Feb%202017.pdf (accessed on 15 August 2022).
- Sheppard, J.P.; Chamberlain, J.; Agúndez, D.; Bhattacharya, P.; Chirwa, P.W.; Gontcharov, A.; Sagona, W.C.J.; Shen, H.; Tadesse, W.; Mutke, S. Sustainable Forest Management Beyond the Timber-Oriented Status Quo: Transitioning to Co-production of Timber and Non-wood Forest Products—A Global Perspective. Curr. For. Rep. 2020, 6, 26–40. [Google Scholar] [CrossRef]
- Musakwa, W.; Wang, S.; Wei, F.; Malapane, O.L.; Thomas, M.M.; Mavengahama, S.; Zeng, H.; Wu, B.; Zhao, W.; Nyathi, N.A.; et al. Survey of Community Livelihoods and Landscape Change along the Nzhelele and Levuvhu River Catchments in Limpopo Province, South Africa. Land 2020, 9, 91. [Google Scholar] [CrossRef]
- FAO. Southern Africa’s Forests and People: Investing in a Sustainable Future: Successes, Challenges and Ways Forward; FAO: Rome, Italy, 2015; ISBN 9780620667098. [Google Scholar]
- Kori, E.; Onyango Odhiambo, B.D.; Chikoore, H. A geomorphodiversity map of the Soutpansberg Range, South Africa. Landf. Anal. 2019, 38, 13–24. [Google Scholar] [CrossRef]
- Constant, N.L.; Tshisikhawe, M.P. Hierarchies of knowledge: Ethnobotanical knowledge, practices and beliefs of the Vhavenda in South Africa for biodiversity conservation. J. Ethnobiol. Ethnomed. 2018, 14, 56. [Google Scholar] [CrossRef]
- Ramarumo, L.J.; Maroyi, A. An inventory of useful threatened plant species in Vhembe Biosphere Reserve, Limpopo Province, South Africa. Biodiversitas 2020, 21, 2146–2158. [Google Scholar] [CrossRef]
- Makhubele, L.; Chirwa, P.W.; Araia, M.G. The influence of forest proximity to harvesting and use of provisioning ecosystem services from tree species in traditional agroforestry landscapes. Int. J. Sustain. Dev. World Ecol. 2022, 29, 812–826. [Google Scholar] [CrossRef]
- Araia, M.G.; Chirwa, P.W. Revealing the Predominance of Culture over the Ecological Abundance of Resources in Shaping Local People’s Forest and Tree Species Use Behavior: The Case of the Vhavenda People, South Africa. Sustainability 2019, 11, 3143. [Google Scholar] [CrossRef]
- Alavi, H.; Hąbek, P. Addressing Research Design Problem in Mixed Methods Research. Manag. Syst. Prod. Eng. 2016, 21, 62–66. [Google Scholar] [CrossRef]
- Migiro, S.; Magangi, B.A. Mixed methods: A review of literature and the future of the new research paradigm. Afr. J. Bus. Manag. 2011, 5, 3757–3764. [Google Scholar]
- Leavy, P. Research Design: Quantitative, Qualitative, Mixed Methods, Arts-Based, and Community-Based Participatory Research Approaches; Guilford Press: New York, NY, USA; London, UK, 2017; ISBN 9781462530038. [Google Scholar]
- Berg, B.L.; Lune, H. Qualitative Research Methods for the Social Sciences; Pearson: Harlow, UK, 2017; ISBN 129-2-16439-5. [Google Scholar]
- Dhanya, B.; Sathish, B.N.; Viswanath, S.; Purushothaman, S. Ecosystem services of native trees: Experiences from two traditional agroforestry systems in Karnataka, Southern India. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2014, 10, 101–111. [Google Scholar] [CrossRef]
- Maponya, P.; Venter, S.L.; Du Plooy, C.P.; Backeberg, G.R.; Mpandeli, S. Research, Extension Services and Training as Key Drivers to Agroforestry Adoption in Limpopo Province, South Africa. In Proceedings of the 3rd World Irrigation Forum, International Commission on Irrigation and Drainage (ICID), Bali, Indonesia, 1–9 September 2019. [Google Scholar]
- Anderson, M.; Gorley, R.N.; Clarke, R.K. Permanova+ for Primer: Guide to Software and Statistical Methods; PRIMER-Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Devkota, K.; dos Santos, C.F.; Blochtein, B. Mustard plants distant from forest fragments receive a lower diversity of flower-visiting insects. Basic Appl. Ecol. 2020, 47, 35–43. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Primer-E Ltd.: Plymouth, UK, 1994. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- da Silva, T.C.; Cruz, M.P.; Sousa Araújo, T.A.d.; Schwarz, M.L.; Albuquerque, U.P. Methods in Research of Environmental Perception. In Methods and Techniques in Ethnobiology and Ethnoecology; Albuquerque, U.P.D., Cruz da Cunha, L.V.F., Lucena, R.F.P.D., Alves, R.R.N., Eds.; Humana Press: New York, NY, USA, 2014; pp. 99–109. ISBN 978-1-4614-8635-0. [Google Scholar]
- Roller, M.R. A Quality Approach to Qualitative Content Analysis: Similarities and Differences Compared to Other Qualitative Methods. Forum Qualitative Sozialforschung/Forum: Qualitative Social Research, Vol 20, No 3 (2019): Qualitative Content Analysis I. Forum Qual. Soc. Res. 2019, 20. [Google Scholar] [CrossRef]
- Bengtsson, M. How to plan and perform a qualitative study using content analysis. Nurs. Open 2016, 2, 8–14. [Google Scholar] [CrossRef]
- Tazebew, E.; Asfaw, Z. Tree species diversity, preference and management in smallholder coffee farms of Western Wellega, Ethiopia. J. Biol. Agric. Healthc. 2018, 8, 43–53. [Google Scholar]
- Eyasu, G.; Tolera, M.; Negash, M. Woody species composition, structure, and diversity of homegarden agroforestry systems in southern Tigray, Northern Ethiopia. Heliyon 2020, 6, e05500. [Google Scholar] [CrossRef]
- Bendix, J.; Wiley, J.J.; Commons, M.G. Intermediate disturbance and patterns of species richness. Phys. Geogr. 2017, 38, 393–403. [Google Scholar] [CrossRef]
- Silva Pedro, M.; Rammer, W.; Seidl, R. A disturbance-induced increase in tree species diversity facilitates forest productivity. Landsc. Ecol. 2016, 31, 989–1004. [Google Scholar] [CrossRef]
- Bhandari, S.K.; Maraseni, T.; Timilsina, Y.P.; Parajuli, R. Species composition, diversity, and carbon stock in trees outside forests in middle hills of Nepal. For. Policy Econ. 2021, 125, 102402. [Google Scholar] [CrossRef]
- Milián-García, I.; Sánchez-Cárdenas, S.; Wencomo-Cárdenas, H.B.; Ramírez-Suárez, W.M.; Navarro-Boulandier, M. Study of biodiversity components in the agroecological farm La Paulina, Perico municipality, Cuba. Pastos Forrajes 2018, 41, 47–51. [Google Scholar]
- Dominati, E.J.; Maseyk, F.J.; Mackay, A.D.; Rendel, J.M. Farming in a changing environment: Increasing biodiversity on farm for the supply of multiple ecosystem services. Sci. Total Environ. 2019, 662, 703–713. [Google Scholar] [CrossRef]
- Iverson, A.L.; Gonthier, D.J.; Pak, D.; Ennis, K.K.; Burnham, R.J.; Perfecto, I.; Ramos Rodriguez, M.; Vandermeer, J.H. A multifunctional approach for achieving simultaneous biodiversity conservation and farmer livelihood in coffee agroecosystems. Biol. Conserv. 2019, 238, 108179. [Google Scholar] [CrossRef]
- Sagastuy, M.; Krause, T. Agroforestry as a Biodiversity Conservation Tool in the Atlantic Forest? Motivations and Limitations for Small-Scale Farmers to Implement Agroforestry Systems in North-Eastern Brazil. Sustainability 2019, 11, 6932. [Google Scholar] [CrossRef]
- Borsari, B. Agrobiodiversity as Necessary Standard for the Design and Management of Sustainable Farming Systems. J. Agro Sci. 2022, 10, 92–101. [Google Scholar] [CrossRef]
- Duriaux-Chavarría, J.-Y.; Baudron, F.; Gergel, S.E.; Yang, K.F.; Eddy, I.M.S.; Sunderland, T. More people, more trees: A reversal of deforestation trends in Southern Ethiopia. Land Degrad. Dev. 2021, 32, 1440–1451. [Google Scholar] [CrossRef]
- Mylliemngap, W. Agrobiodiversity and Natural Resource Management in Traditional Agricultural Systems of Northeast India; SSRN: Rochester, NY, USA, 2021. [Google Scholar]
- Yashmita-Ulman; Singh, M.; Kumar, A.; Sharma, M. Conservation of Plant Diversity in Agroforestry Systems in a Biodiversity Hotspot Region of Northeast India. Agric. Res. 2021, 10, 569–581. [Google Scholar] [CrossRef]

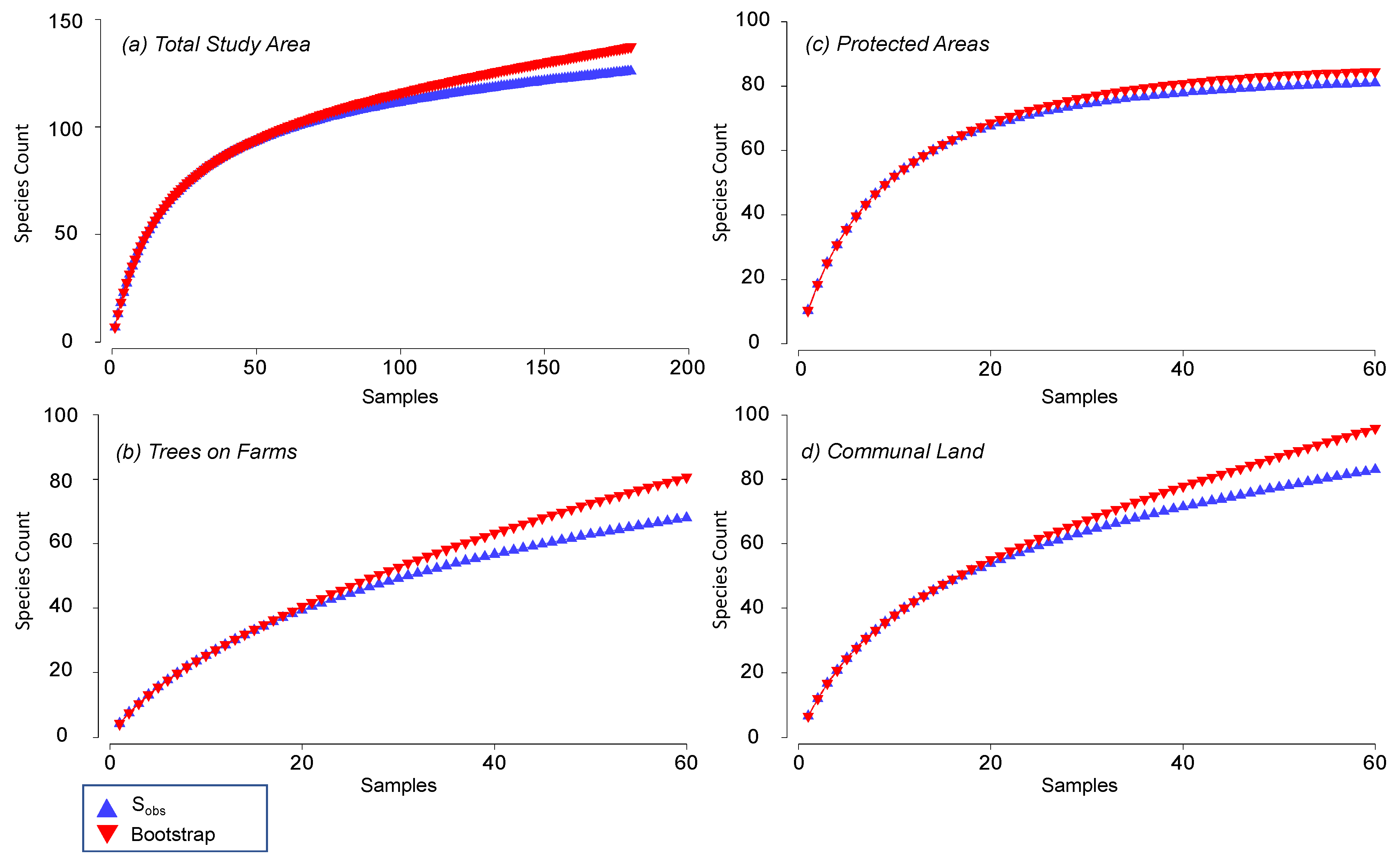

| Land-Uses | Number of Species | Sampling Effectiveness | |
|---|---|---|---|
| SOb | SBoot | (%) | |
| Trees on farms | 68 | 80.6 | 84.4 |
| Communal land | 83 | 95.9 | 86.5 |
| Protected areas | 81 | 84.5 | 95.8 |
| Overall | 126 | 137.4 | 91.7 |
| Source | df | SS | MS | Pseudo-F | P (Perm) | Unique Terms |
|---|---|---|---|---|---|---|
| Vi | 3 | 33,220 | 11,073 | 1.9945 | 0.016 | 997 |
| Lu | 2 | 81,632 | 40,816 | 18.015 | 0.001 | 998 |
| VixLu | 6 | 33,311 | 5551 | 2.4504 | 0.001 | 997 |
| Res | 168 | 3.81 × 105 | 2265 | |||
| Total | 179 | 5.29 × 105 |
| Pairwise Land-Uses | t | P (Perm) | Av.SBray |
|---|---|---|---|
| (Trees on farms & Communal land) | 4.48 | 0.001 | 20.99 |
| (Trees on farms & Protected area) | 5.41 | 0.001 | 17.29 |
| (Communal land & Protected area) | 2.34 | 0.001 | 24.87 |
| Pairwise Land-Uses | t | P (Perm) | Within (Land-Uses Av. D (%)) |
|---|---|---|---|
| (Trees on farms & Communal land) | 4.4022 | 0.001 | Trees on farms (45) |
| (Trees on farms & Protected areas) | 4.2802 | 0.001 | Communal land (52) |
| (Communal land & Protected areas) | 0.4508 | 0.673 | Protected areas (51) |
| Marginal Tests | ||
|---|---|---|
| Variable | p | p (%) |
| Elevation | 0.001 | 2.6 |
| Gradient | 0.001 | 5.5 |
| Fire occurrence | 0.384 | 0.6 |
| Grazing | 0.001 | 1.6 |
| PES | 0.001 | 1.5 |
| Distance | 0.001 | 12.2 |
| Trees on Farms (A.Sim = 14.9) | Communal Land (A.Sim = 10.88) | Protected Areas (A.Sim = 14.9) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Av.A | Av.S | C.% | Av.A | Av.S | C.% | Av.A | Av.S | C.% |
| Afzelia quanzensis | 0.45 | 0.39 | 69.95 | ||||||
| Albizia adianthifolia | 0.45 | 0.41 | 66.35 | 0.72 | 1.23 | 41.75 | |||
| Brachylaena huillensis | 0.57 | 0.57 | 67.5 | ||||||
| Bridelia micrantha | 0.90 | 1.23 | 52.14 | 0.88 | 0.88 | 47.81 | |||
| Combretum molle | 0.48 | 0.50 | 62.62 | 0.85 | 0.83 | 59.45 | |||
| Englerophytum magalismontanum | 0.78 | 0.50 | 70.95 | ||||||
| Mangifera indica | 3.25 | 9.80 | 65.79 | ||||||
| Parinari curatellifolia | 1.05 | 1.73 | 33.27 | ||||||
| Persea americana | 0.95 | 2.16 | 80.28 | ||||||
| Pseudolachnostylis maprouneifolia | 0.45 | 0.64 | 58.01 | 0.77 | 0.60 | 63.56 | |||
| Pteleopsis myrtifolia | 1.33 | 2.30 | 21.13 | 1.97 | 3.11 | 21.37 | |||
| Senegalia ataxacantha | 0.45 | 0.36 | 73.30 | ||||||
| Tabernaemontana elegans | 1.33 | 2.15 | 40.87 | 0.70 | 0.87 | 53.78 | |||
| Average Dissimilarity | (TOF & CL) = 97% | (TOF & PA) = 97% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TOF | CL | TOF | PA | ||||||
| Species | Av.A | Av.A | Av.D | C.% | Av.A | Av.A | Av.D | C.% | |
| Afzelia quanzensis | 0.02 | 0.45 | 1.84 | 51.53 | |||||
| Albizia adianthifolia | 0.13 | 0.45 | 2.10 | 45.74 | 0.13 | 0.72 | 2.75 | 36.55 | |
| Annona senegalensis | 0.08 | 0.25 | 1.21 | 66.24 | |||||
| Artabotrys brachypetalus | 0.08 | 0.30 | 1.36 | 61.05 | 0.08 | 0.33 | 1.30 | 60.72 | |
| Brachylaena discolor | 0.12 | 0.33 | 1.75 | 53.34 | |||||
| Brachylaena huillensis | --- | 0.57 | 1.93 | 48.8 | |||||
| Bridelia micrantha | 0.08 | 0.90 | 3.84 | 33.18 | 0.08 | 0.88 | 3.19 | 27.72 | |
| Citrus x sinensis | 0.30 | --- | 1.09 | 70.84 | |||||
| Combretum molle | 0.03 | 0.48 | 1.87 | 49.64 | 0.03 | 0.85 | 2.70 | 39.34 | |
| Croton sylvaticus | 0.02 | 0.37 | 1.03 | 68.88 | |||||
| Cussonia spicata | --- | 0.37 | 1.16 | 65.64 | |||||
| Dalbergia nitidula | 0.02 | 0.5 | 1.60 | 50.44 | |||||
| Dichrostachys cinerea | 0.03 | 0.33 | 1.59 | 54.99 | |||||
| Englerophytum magalismontanum | 0.12 | 0.22 | 1.13 | 68.57 | 0.12 | 0.78 | 2.63 | 42.06 | |
| Faurea saligna | --- | 0.37 | 1.07 | 66.74 | |||||
| Ficus sur | 0.07 | 0.27 | 1.18 | 63.24 | |||||
| Hexalobus monopetalus | --- | 0.28 | 1.13 | 67.41 | |||||
| Hyperacanthus amoenus | |||||||||
| Maesa lanceolata | --- | 0.33 | 0.98 | 70.91 | |||||
| Mangifera indica | 3.25 | --- | 12.17 | 12.58 | 3.25 | --- | 10.10 | 10.41 | |
| Musa acuminita | 1.00 | --- | 3.26 | 36.55 | 1.00 | --- | 2.77 | 33.71 | |
| Parinari curatellifolia | 0.05 | 0.28 | 1.57 | 56.61 | 0.05 | 1.05 | 3.95 | 21.09 | |
| Peltophorum africanum | 0.03 | 0.22 | 1.29 | 62.38 | |||||
| Persea americana | 0.95 | --- | 3.95 | 29.22 | 0.95 | --- | 3.23 | 24.43 | |
| Pseudolachnostylis maprouneifolia | 0.10 | 0.45 | 2.35 | 38.99 | 0.10 | 0.77 | 2.63 | 44.77 | |
| Psidium guajava | 0.30 | 0.07 | 1.49 | 58.14 | 0.30 | 0.17 | 1.48 | 55.19 | |
| Pteleopsis myrtifolia | 0.35 | 1.33 | 5.92 | 25.14 | 0.35 | 1.97 | 6.4 | 17.02 | |
| Pterocarpus angolensis | 0.03 | 0.43 | 1.31 | 59.38 | |||||
| Rothmannia capensis | --- | 0.35 | 0.99 | 69.9 | |||||
| Sclerocarya birrea | 0.30 | 0.25 | 2.24 | 41.3 | 0.30 | 0.12 | 1.52 | 53.66 | |
| Scolopia Mundii | --- | 0.38 | 1.17 | 64.45 | |||||
| Senegalia ataxacantha | 0.05 | 0.45 | 2.19 | 43.57 | 0.05 | 0.43 | 1.37 | 58.03 | |
| Strychnos madagascariensis | 0.02 | 0.47 | 1.26 | 62.02 | |||||
| Syzygium cordatum | 0.07 | 0.57 | 1.97 | 46.8 | |||||
| Tabernaemontana elegans | 0.25 | 1.33 | 6.24 | 19.03 | 0.25 | 0.70 | 3.04 | 30.86 | |
| Trichilia emetica | 0.28 | 0.05 | 1.29 | 63.72 | 0.28 | 0.05 | 1.04 | 67.82 | |
| Vachellia sieberiana | 0.02 | 0.30 | 1.45 | 59.64 | 0.02 | 0.42 | 1.39 | 56.62 | |
| Vitex ferruginea | --- | 0.32 | 1.23 | 64.99 | |||||
| Ximenia caffra | --- | 0.28 | 1.10 | 69.71 | |||||
| Xylopia parviflora | 0.18 | 0.37 | 1.91 | 47.71 | 0.18 | 0.32 | 1.60 | 52.09 | |
| Average Dissimilarity | (CL & PA) = 89% | ||||||||
| CL | PA | ||||||||
| Species | Av.A | Av.A | Av.D | C.% | |||||
| Afzelia quanzensis | 0.45 | 0.18 | 1.68 | 49.57 | |||||
| Albizia adianthifolia | 0.45 | 0.72 | 2.79 | 31.42 | |||||
| Annona senegalensis | |||||||||
| Artabotrys brachypetalus | 0.30 | 0.33 | 1.56 | 51.32 | |||||
| Brachylaena discolor | 0.33 | 0.13 | 1.37 | 57.82 | |||||
| Brachylaena huillensis | 0.23 | 0.57 | 2.10 | 39.15 | |||||
| Bridelia micrantha | 0.90 | 0.88 | 4.20 | 17.46 | |||||
| Citrus x sinensis | |||||||||
| Combretum molle | 0.48 | 0.85 | 3.07 | 24.96 | |||||
| Croton sylvaticus | 0.07 | 0.37 | 1.01 | 68.05 | |||||
| Cussonia spicata | 0.18 | 0.37 | 1.43 | 56.28 | |||||
| Dalbergia nitidula | 0.20 | 0.5 | 1.81 | 45.72 | |||||
| Dichrostachys cinerea | 0.33 | 0.15 | 1.44 | 54.68 | |||||
| Englerophytum magalismontanum | 0.22 | 0.78 | 2.51 | 34.24 | |||||
| Faurea saligna | --- | 0.37 | 0.96 | 69.13 | |||||
| Ficus sur | 0.17 | 0.27 | 1.17 | 61.89 | |||||
| Hexalobus monopetalus | 0.28 | 0.20 | 1.21 | 60.57 | |||||
| Hyperacanthus amoenus | 0.12 | 0.23 | 0.92 | 70.16 | |||||
| Maesa lanceolata | 0.08 | 0.33 | 1.11 | 65.69 | |||||
| Mangifera indica | |||||||||
| Musa acuminita | |||||||||
| Parinari curatellifolia | 0.28 | 1.05 | 3.61 | 21.52 | |||||
| Peltophorum africanum | 0.22 | 0.18 | 1.16 | 63.19 | |||||
| Persea americana | |||||||||
| Pseudolachnostylis maprouneifolia | 0.45 | 0.77 | 2.97 | 28.29 | |||||
| Psidium guajava | |||||||||
| Pteleopsis myrtifolia | 1.33 | 1.97 | 6.40 | 7.19 | |||||
| Pterocarpus angolensis | 0.05 | 0.43 | 1.23 | 59.21 | |||||
| Rothmannia capensis | |||||||||
| Sclerocarya birrea | 0.25 | 0.12 | 1.11 | 64.44 | |||||
| Scolopia Mundii | 0.02 | 0.38 | 1.09 | 66.91 | |||||
| Senegalia ataxacantha | 0.45 | 0.43 | 2.27 | 36.79 | |||||
| Strychnos madagascariensis | 0.2 | 0.47 | 1.55 | 53.06 | |||||
| Syzygium cordatum | 0.18 | 0.57 | 1.98 | 43.69 | |||||
| Tabernaemontana elegans | 1.33 | 0.70 | 4.94 | 12.74 | |||||
| Trichilia emetica | |||||||||
| Vachellia sieberiana | 0.30 | 0.42 | 2.06 | 41.46 | |||||
| Vitex ferruginea | |||||||||
| Ximenia caffra | |||||||||
| Xylopia parviflora | 0.37 | 0.32 | 1.75 | 47.68 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhubele, L.; Chirwa, P.W.; Sheppard, J.P.; Tshidzumba, R.P.; Araia, M.G.; Kahle, H.-P. Conservation of Tree Species Richness in a Traditional Agroforestry Landscape in the Vhembe Biosphere Reserve, South Africa. Forests 2022, 13, 1766. https://doi.org/10.3390/f13111766
Makhubele L, Chirwa PW, Sheppard JP, Tshidzumba RP, Araia MG, Kahle H-P. Conservation of Tree Species Richness in a Traditional Agroforestry Landscape in the Vhembe Biosphere Reserve, South Africa. Forests. 2022; 13(11):1766. https://doi.org/10.3390/f13111766
Chicago/Turabian StyleMakhubele, Lucky, Paxie W. Chirwa, Jonathan P. Sheppard, Ratsodo P. Tshidzumba, Mulugheta G. Araia, and Hans-Peter Kahle. 2022. "Conservation of Tree Species Richness in a Traditional Agroforestry Landscape in the Vhembe Biosphere Reserve, South Africa" Forests 13, no. 11: 1766. https://doi.org/10.3390/f13111766
APA StyleMakhubele, L., Chirwa, P. W., Sheppard, J. P., Tshidzumba, R. P., Araia, M. G., & Kahle, H.-P. (2022). Conservation of Tree Species Richness in a Traditional Agroforestry Landscape in the Vhembe Biosphere Reserve, South Africa. Forests, 13(11), 1766. https://doi.org/10.3390/f13111766






