Abstract
Seed maturation not only determines the qualities and yields of seeds, but also affects seed storage and quality preservation. MicroRNAs (miRNAs) are a ubiquitous regulatory factor of gene expression in eukaryotes, which participate in the complex regulatory network of gene expression during seed maturation. However, miRNAs involved in maturation of Tilia tuan are still unknown. To reveal the role of miRNAs in T. tuan, small RNAs were profiled by high-throughput sequencing during seed maturation at five developmental stages. By predicting the target genes of miRNAs, the expression patterns of miRNAs during seed maturation were analyzed to identify those related to seed maturation. A total of 187 known miRNAs belonging to 42 miRNA families were found at five different seed maturation stages. Based on the analysis of unknown sequences, eight novel miRNAs were identified; 11,775 targets of 195 miRNAs were identified. Large numbers of miRNAs with diverse expression patterns, multiple-targeting and co-targeting of many miRNAs, and a complex regulatory network of miRNA-target genes were identified during seed maturation. These miRNAs and their targets may be involved in fatty acid, ABA, and lignin biosynthesis. Our study provides more information about the miRNA regulatory network and deepens our understanding of the function of miRNAs in T. tuan. miRNAs are revealed to be crucial during seed maturation, which provides a basis for further study of the regulatory role of miRNAs during seed maturation.
1. Introduction
The Tilia tuan Szyszyl. contains 80 species and is mainly distributed in the northern temperate zone, and intermittently distributed in Europe, Asia, and North America [1]. Because of its beautiful shape, fragrant blossoms, and high capacity to resist hazardous gases, Tilia tuan may be employed as a landscape tree species while also serving significant ecological roles. This species also has significant economic value, as it can be used as timber, fiber, a source of honey, and garden ornamental. T. tuan seeds are deep dormant, and without germination therapy, they barely germinate in the year after sowing. Dormancy has hampered mating and population growth in this species, and the occurrence of dormancy in T. tuan seeds has been acknowledged by numerous experts [2,3]. Many factors contribute to seed dormancy, including seed coat abnormalities, endogenous inhibitors, and physiological post-ripening of seed embryos [4,5]. In particular, T. tuan seeds have obvious woody pericarp and are difficult to germinate. However, the molecular mechanisms underlying these traits are still unknown.
MicroRNAs (miRNAs), a class of ~20 to 24 nt non-coding small endogenous RNAs, are important and ubiquitous regulators of gene expression in eukaryotes. Through sequence complementarity, miRNAs bind the mRNA of specific targets to form an RNA-induced silencing complex, which negatively regulates gene expression by initiating mRNA degradation or inhibiting mRNA translation [6,7]. In 1993, a small RNA with negative regulation was first identified while studying the embryonic development of Caenorhabditis elegans [8]. In 2002, miRNAs were first discovered in plants [9]. Following that, cloning, sequencing, bioinformatics analysis, and high-throughput sequencing were used to identify a significant number of miRNAs in Arabidopsis thaliana, Oryza sativa, Glycine max, and Gossypium, indicating that they play an important role in plant growth and development as well as stress adaptation. Furthermore, studies have revealed that miRNAs are highly conserved and spatiotemporally regulated in plants and participate in several key processes in plant growth and development, including leaf morphogenesis [10,11], flower differentiation and development [12,13], root formation and development [14,15], and the plant transition from the juvenile to the reproductive stage [16]. In addition, they play a significant regulatory role in plant responses to external stresses such as drought stress [17] and salt stress [18,19].
The seed development and maturation program are, to a major extent, regulated by miRNAs, and transcription factors. The involvement of miRNAs in post-transcriptional regulation of seed and fruit development has been documented in apricot [20], rice [21], soybean [22], and Brassica napus [23]. Transcription factors play crucial roles in regulating lipid biosynthesis and seed size [24,25,26]. For example, miR160 negatively regulates auxin response factors involved in Arabidopsis seed development [27] and floral organs [28]. The Sesamum indicum bHLH transcription factor binds to E- or G-box elements in the FAD2 gene promoter and impacts lipid biosynthesis and accumulation during seed development [29]. MYB89 functions as a negative regulator of seed oil accumulation during maturation in Arabidopsis seeds [30]. Accordingly, miRNA-mediated gene expression influences the GA and ABA signal pathways during seed germination in maize [31]. However, although miRNA-mediated regulatory networks controlling seed development have been revealed in model plants, little is known in T. tuan.
Above all, it is of great significance for the regulation of seed maturation to reveal the complex miRNA-target gene regulatory network, particularly in terms of fatty acid, ABA, and lignin biosynthesis. In this study, we employed RNA-seq to produce a high-confidence full-length transcriptome dataset of T. tuan seed individuals and further used them to identify miRNAs through constructing small RNA libraries in five different seed maturation stages. The related miRNAs present during seed maturation were identified and the target genes were predicted, as well Gene Ontology (GO) and KEGG analyses. Most importantly, specific miRNAs were screened out in fatty acid, ABA, and lignin biosynthesis pathways, with the co-expressed miRNA-target regulatory interactions investigated using transcriptome data. This study provided systematically characterize T. tuan seed related miRNAs and the expanded features of putative targets reveal the miRNA inferred regulatory networks during seed maturation, which provides a theoretical basis for further investigation of molecule function of miRNAs during seed maturation in T. tuan.
2. Materials and Methods
2.1. Plant Materials
The T. tuan seeds were collected from an individual T. tuan with good growth and development, at Beijing Forestry University. Five seed samples (Jun. 1, 1 June 2019; Jul. 1, 1 July 2019; Aug. 2, 2 August 2019; Sept. 2, 2 September 2019; and Oct. 2, 2 October 2019) were obtained in T. tuan at different seed maturation stages (Figure 1A). Three independent biological replicates of thirty seeds at each maturation stage were collected and immediately frozen in liquid nitrogen, and stored at −80 °C for further analysis.
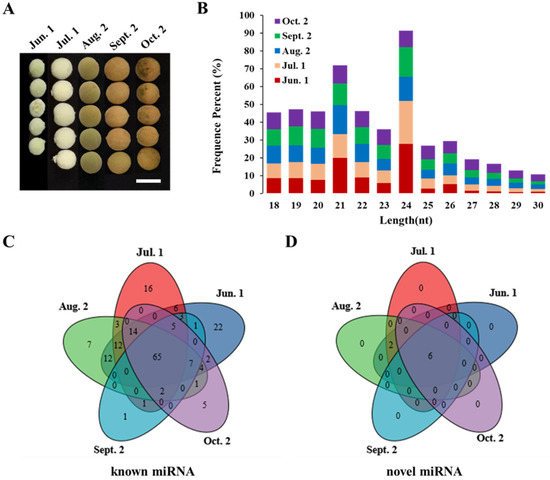
Figure 1.
Overview of small RNAs expressed during seed maturation in T. tuan. (A) Stages of seed maturation used for small RNA sequencing. The images were taken during the sampling period on June 1, July 1, August 2, September 2, and October 2, 2019 (5 time points). Scale bars of 1 cm. (B) Size distribution of small RNA at five different seed maturation time stages in T. tuan. Jun. 1, Jul. 1, Aug. 2, Sept. 2 and Oct. 2 represent T. tuan seed collected at June time point, July time point, August time point, September time point, and October time point 2019, respectively. The abscissa is the length of miRNAs, and the ordinate is the percentage of miRNA at that length. (C,D) Venn diagram showing known miRNAs and novel miRNAs among five samples.
2.2. Construction of RNA Library and Transcriptome Sequencing
Three biological replicates throughout all five seed maturation stages were used for the transcriptome sequencing. A total amount of 1 μg RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using NEBNext® UltraTM RNA Library Prep Kit for Illumina® (New England Biolabs, Ipswich, MA, USA) following manufacturer’s recommendations and index codes were added to attribute sequences to each sample. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using TruSeq PE Cluster Kit v3-cBot-HS (Illumia) according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina Novaseq platform and 150 bp paired-end reads were generated.
Raw reads were filtered by removing reads containing adapter, reads containing ploy-N and low-quality reads. The Trinity software [32] with default parameters and a minimum contig length of 150 bp was used for assembly generation. Transcript levels were determined from the short-read data through RSEM [33], with the resulting full-length transcripts used as a reference sequence. The gene level counts were converted into fragments per kilobase of transcript per million mapped reads (FPKM) values.
2.3. Construction of Small RNA Library and High-Throughput Sequencing
Total RNA was extracted by Novogene Company (Beijing, China) for construction of small RNA library and deep sequencing. Detection of total RNA was done using 1% agarose gel to analyze the degree of RNA degradation and contamination, RNA purity was checked using the NanoPhotometer® spectrophotometer (IMPLEN, Westlake Village, CA, USA), RNA concentration was measured using Qubit® RNA Assay Kit in Qubit® 2.0 Flurometer (Life Technologies, Carlsbad, CA, USA), and RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, USA). After RNA quantification and qualification, a total amount of 3 μg total RNA per sample was used as input material for the small RNA library. Sequencing libraries were generated using NEBNext® Multiplex Small RNA Library Prep Set for Illumina® (New England Biolabs, Ipswich, MA, USA) following manufacturer’s recommendations and index codes were added to attribute sequences to each sample. Briefly, RNA bands corresponding to a size range of 16–30 nt were separated and purified from the acrylamide gel.
The small RNA molecules ligated with 5′ and 3′ adaptors were used for reverse transcription and subsequent PCR. After PCR amplification, the target DNA fragments were separated by polyacrylamide gel electrophoresis, and a cDNA library was obtained. At last, library quality was assessed on the Agilent Bioanalyzer 2100 system using DNA High Sensitivity Chips. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using TruSeq SR Cluster Kit v3-cBot-HS (Illumia) according to the manufacturer’s instructions. After cluster generation, small RNA samples were sequenced using Illumina HiSeqTM 2500 platform (San Diego, CA, USA) and 50 bp single end reads were generated. The sequencing data obtained in FASTQ files were used for further processing.
2.4. Sequence Data Analysis
Raw reads were obtained from the high-throughput sequencing platform. clean reads were obtained by removing reads containing ploy-N, with 5′ adapter contaminants, without 3′ adapter or the insert tag, containing ploy A or T or G or C and low-quality reads from raw data. At the same time, Q20, Q30, and GC-content of the raw reads were calculated. At the end, all the downstream analyses were performed on sequences in the length range of 18–30 nt. Small RNA derived from rRNAs, tRNAs, small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), repeat sRNAs, and ta-siRNAs from the databases Rfam14.7 (http://rfam.xfam.org/; accessed on 11 November 2021), GenBank (https://www.ncbi.nlm.nih.gov/genbank/; accessed on 11 November 2021), and Plant Repeat were further identified by Bowtie [34] without mismatches to analyze their expression and distribution on the reference. The high-quality non-redundant set of reads were used for miRNA identification in each sample.
2.5. Identification of Known and Novel miRNAs
The known miRNAs in T. tuan were identified using miRBase22.1 (http://www.mirbase.org/; accessed on 15 February 2021) was used as reference by software mirdeep2 [35] and srna-tools-cli (http://srna-tools.cmp.uea.ac.uk/; accessed on 15 February 2021) were used to obtain the potential miRNA and draw the secondary structures. Furthermore, the miRNA counts as well as base bias on the first position of identified miRNA with certain length and on each position of all identified miRNA were obtained, respectively. The characteristics of hairpin structure of miRNA precursor can be used to predict novel miRNAs. The available software miREvo [36] and mirdeep2 [35] were integrated to predict novel miRNAs through exploring the secondary structure, the Dicer cleavage site and the minimum free energy of the small RNA unannotated. Only those miRNAs were detected miRNA* to consider as novel miRNAs. At the same time, the identified miRNA counts as well as base bias on the first position with certain length and on each position of all identified miRNA were obtained, respectively.
2.6. Expression Analysis of miRNA
The expression levels of known and novel miRNAs in each sample were statistically analyzed. miRNA expression levels were estimated by TPM (transcript per million) through the following criteria [37] normalization formula: TPM (transcripts per million reads) = (read count × 1,000,000)/total reads. DESeq2 was used to analyze the differential expression of miRNA [38]. p-value was adjusted using qvalue [39]. miRNAs whose expression levels between any two of the five different seed maturation stages varied significantly (|log2 fold change| > 1 and qvalue < 0.01) were assigned as differentially expressed miRNAs by default. Analysis was performed to visualize the expression patterns of miRNAs using the Short Time-series Expression Miner (STEM 1.1) program [40].
2.7. Target Prediction of miRNA and Function Enrichment Analysis
The target genes of known miRNAs and novel miRNAs were predicted by psRobot [41]. Small RNA sequencing results were correlated with the transcriptome data, and target genes corresponding to the differentially expressed key miRNAs were intersected with the differentially expressed genes in the transcriptome. The potential targets of the above key miRNAs were obtained, classified, and functionally annotated. According to the corresponding relationship between miRNAs and their targets, we carried out Gene Ontology (GO) [42] and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis [43] on the targets of each group of differentially expressed miRNAs. Finally, Cytoscape (version 3.9.1, Boston, MA, USA) [44] was used to construct a co-expression network.
3. Results
3.1. Small RNA Sequence Statistics
To explore the biological functions of small RNAs during seed maturation in T. tuan, five samples were selected during seed maturation (Figure 1A). Small RNA sequencing results showed that raw reads were generated (Table 1). After removing contaminant reads, clean reads were obtained and screened against rRNAs, tRNAs, snRNAs, snoRNAs, and mRNAs in the Rfam and NCBI GenBank databases, resulting in clean reads for further analyses (Supplementary Table S1). They accounted for 92.68%, 94.31%, 96.02%, 87.38%, and 88.77% of the total data, respectively, indicating that the quality of the five small RNA libraries constructed was high (Supplementary Table S2). Length distribution analysis of the sequence shows that most of the fragment lengths are 18–30 nt (Figure 1B). The five libraries shared a similar distribution pattern. The most abundant length of small RNA was 24 nt (~28%), followed by 21 nt (20%), which is the typical length of canonical miRNAs. After analyzing of the small RNA length screening, a total of 4,551,867 (Jun. 1), 11,994,904 (Jul. 1), 7,338,458 (Aug. 2), 8,004,524 (Sept. 2), and 6,323,645 (Oct. 2) total reads of each sample small RNA is obtained (Supplementary Table S3). A total of 1,576,482 (34.63%), 7,945,688 (66.24%), 4,785,383 (65.21%), 6,154,905 (76.89%), 5,191,238 (82.09%) reads that can mapped to the reference sequence in the sample (Table 1). Finally, 2,975,385 (Jun. 1), 4,049,216 (Jul. 1), 2,553,075 (Aug. 2), 1,849,619 (Sept. 2), and 1,132,407 (Oct. 2) unannotated unique small RNA sequences were further analyzed to predict novel miRNAs. To further insight into small RNA function, small RNA after length screening were located on the reference sequence by bowtie, and a comparison of small RNA classification tables is provided in Table 2. In terms of quantity, the proportion of miRNAs was larger than that of other types of non-coding RNAs (snRNAs, snoRNAs, and tRNAs). Among the five libraries, the highest 14,225 reads were annotated as known miRNAs and 28,171 reads were annotated as novel miRNAs, accounting for 0.9% and 0.35% of the total sequences, respectively. The ratios of rRNAs were 34.99%, 37.08%, 29.63%, 35.23%, and 21.50%, all much lower than 60%, indicating that our data is high quality of the sample and reliable.

Table 1.
Summary of small RNA sequencing from T. tuan seed.

Table 2.
Non-coding RNAs among the small RNAs.
3.2. Identification of Known miRNAs during Seed Maturation in T. tuan
Comparing to the known miRNAs of 45 selected reference species, 189 unique sequences that showed perfect matches to known miRNAs belonging to 42 miRNA families (with a minimum of two reads) were identified from the five libraries (Figure 1C, Table 3 and Supplementary Table S4). Among them, 23 highly conserved miRNA families were identified in T. tuan, indicating that these conserved miRNAs may have fundamental regulatory roles during seed maturation in T. tuan. Most members of the miRNA family in T. tuan are miR171, which has 21 members, followed by 21, 14, 12, 12, and 11 members from the miR159, miR156, miR395, miR166, and miR167 families, respectively (Supplementary Figure S1). Differential expression of miRNAs was also observed in the five libraries. Among these, 65 miRNAs were expressed in all five libraries, and all of them were conserved. Among those, ttu-miR403 was the most abundant at all five stages, with 130,047, 54,182, 103,165, 46,282, and 12,903 TPM, respectively, followed by ttu-miR394a, ttu-miR156a, and ttu-miR319a; all reads were over 1000 TPM. In addition, the different members in known miRNA families had drastically different expression levels. miR403 (ttu-miR403b) was the most abundant and miR156 (ttu-miR156a) was the second most abundant miRNA in T. tuan seeds (Supplementary Table S5). There are a large number of miRNAs in T. tuan seeds, indicating that many miRNAs are involved in seed maturation. At the same time, we make a statistical analysis of base bias on the first position of identified miRNA with certain length (Supplementary Figure S2A). In T. tuan, the first base in known miRNAs with different lengths varies. For instance, the first nucleic acid is biased to U in 18–21 nt miRNAs, biased toward C in 22–28 nt miRNAs, and biased to U in 29–30 nt miRNAs. Based on statistical analysis of nucleotides at each position of all identified miRNAs, the first and second nucleotides of all known miRNAs in the five libraries were biased towards U, except for those at positions 11 and 21, which were biased towards G; nucleic acids at other sites were biased towards C (Supplementary Figure S3A).

Table 3.
Summary of mapped mature and hairpin in known miRNAs.
3.3. Identification of Novel miRNAs during Seed Maturation in T. tuan
In addition to known miRNAs, novel miRNAs were also identified during seed maturation in T. tuan. The characteristic hairpin structure of miRNA precursors can be used to predict novel miRNAs. Eight novel miRNAs were identified from 2,458 unique small RNAs (Figure 1D, Table 4 and Supplementary Table S6). These all contained fold-back structure, which has the sequence of the typical hairpin structure (Figure 2A). According to the synthesis mechanism of miRNAs, six miRNA star (*) sequences were detected. The length of these novel miRNAs and miRNAs* varied from 18 to 24 nt (Supplementary Table S6). It is worth noting that no miRNA* was detected for ttu-miR09 and ttu-miR10. There were some differences in the expression of novel miRNAs (Figure 2B). Among them, ttu-miR01 was the most richly expressed specific miRNA in T. tuan seeds, which was sequenced in 706,451, 507,841, 721,606, 731,926, and 577,150 TPM of the five libraries, and its expression level was higher than that of some conserved miRNAs. The eight novel miRNAs were divided into two expression patterns. There was synergistic regulation among novel miRNAs, ttu-miR01, ttu-miR04, ttu-miR05, ttu-miR07, ttu-miR08, ttu-miR10 were highly expressed at Jun. 1, Jul. 1, and Aug. 2 stage, and down-regulated at Sept. 2 and Oct. 2 stage. ttu-miR03 was expressed in high abundance only at Sept. 2 and Oct. 2 stage, indicating that novel miRNAs have complementary regulatory functions. Notably, ttu-miR09 were expressed from Jun. 1 to Oct. 2 stage and had two peaks in expression at Jul. 1 and Sept. 2 stage, suggesting that the regulatory mechanism mediated by T. tuan specific miRNAs may play a housekeeping functions role during seed maturation. Expression of miRNA* can be grouped into 4 major patterns (Figure 2C and Supplementary Table S7). The majority of miRNA* were preferentially expressed at Jun. 1, Jul. 1, and Aug. 2 stage while were low expressed at Sept. 2 and Oct. 2 stage. ttu-miR03 and ttu-miR05 were strongly expressed only at Jun. 1 stage then decreased to a low level; ttu-miR08 were strongly expressed only at Jul. 1 stage, and ttu-miR07 was down-regulated after Aug. 2 stage; ttu-miR01 and ttu-miR04 were clustered into one group, they only expressed at Jun. 1 and Aug. 2 stage. We found that the expression pattern of novel miRNA* was significantly different from those of novel miRNAs at different stages, indicating that there was no synergistic regulation of miRNA-miRNA*. Further analysis of the distribution of first base of these novel miRNAs showed that the first nucleotide is biased towards U except in 30 nt novel miRNAs, in which the first nucleic acid is biased towards A (Supplementary Figure S2B). The analysis of the distribution of base bias on each position of these novel miRNAs showed that Nucleotides were also biased towards U at positions 1, 3, 4, 14, and 19 of novel miRNAs; towards A at positions 8, 9, 11, 18, and 20; and towards C at the remaining nucleotide sites (Supplementary Figure S3B).

Table 4.
Summary of mapped mature and hairpin in novel miRNAs.
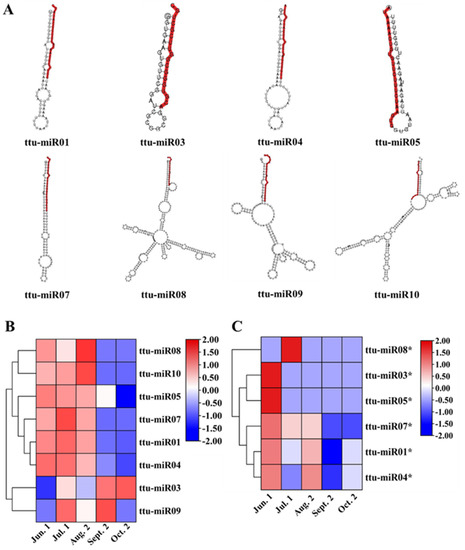
Figure 2.
Characterization of novel miRNAs obtained from T. tuan seed. (A) Examples of stem-loop hairpin secondary structures of predicted novel miRNAs during seed maturation in T. tuan. (Segments corresponding to the mature miRNAs are marked in red). Heat map of expressed novel miRNAs (B) and novel miRNAs* (C) in T. tuan seed at five maturational stages.
3.4. Expression Analysis of miRNAs
To understand the expression pattern of differentially expressed miRNAs during T. tuan seed maturation and provide clues about their potential function. We first compared the expression patterns of miRNAs at different seed maturation stages; several different expression patterns were observed (Figure 3A and Supplementary Figure S4). To obtain a comprehensive expression profile of known and novel miRNAs, we performed a five-stage time series significance analysis of miRNA expression. Three distinct expression modules were discovered. The red pattern was a monotonously falling mode at Jun. 1 and Jul. 1 stage. The purple pattern shows a significant dynamic expression pattern across time, with a significant decline in Jun. 1 and Jul. 1 stage, followed by a continuous increase at Aug. 2 stage and a decline at Sept. 2 stage (Figure 3B). The green pattern was a monotonously falling mode at Jul. 1, Sept. 2, and Oct. 2 stage. To determine miRNAs that were expressed specifically at the seed maturation stage, hierarchical clustering analysis was performed on the normalized read counts of known miRNAs and novel miRNAs, suggesting that strong stage differential expression of most of the miRNAs (Figure 3C). Differential miRNA clustering analysis was used to determine the clustering pattern of differential miRNA expression under different experimental conditions. To obtain the number of conservatively differentially expressed miRNAs, we depicted in a Venn diagram, which directly showed 62 miRNAs differentially expressed in the whole process of seed maturation (Figure 3D), indicating that these miRNAs participate in regulating seed maturation in T. tuan.
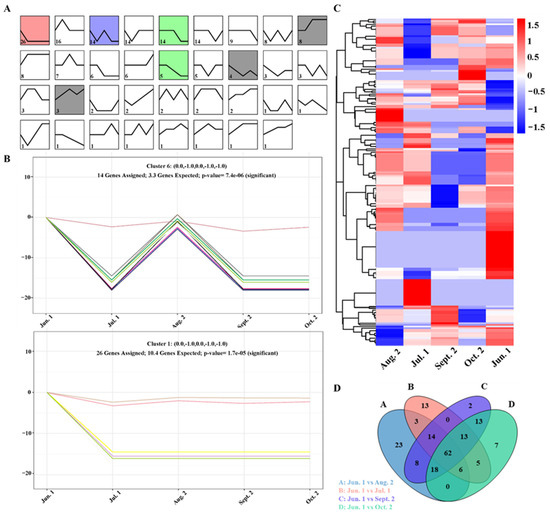
Figure 3.
Expressed analysis of miRNAs during seed maturation in T. tuan. (A) Analysis of miRNAs expression patterns using the Short Time-series Expression Miner (v 1.1, STEM) program. The trend block with color is the trend of significant enrichment, and the different colors are set by the software to distinguish different trends, which have no special significance; the trend block without color is the trend of non-significant enrichment. (B) Expression patterns of known miRNAs and novel miRNAs. (C) Heat map of expressed known miRNAs and novel miRNAs in T. tuan seed at five maturational time stages. (D) Venn diagram showing the number of difference miRNA under selection in the four groups. The big circle represents each comparison combination, the sum of the numbers in each big circle represents the total number of difference miRNA of the comparison combination, and the overlapping part of the circle represents the common number of difference miRNA between combinations.
3.5. Target Prediction of miRNAs and Function Enrichment Analysis
To better understand the regulatory functions of miRNAs during seed maturation in T. tuan. A total of 11,775 miRNA targets were predicted, and 29,288 miRNA-mRNA pairs, including 11,285 targets for 189 known miRNAs and 757 targets for six novel miRNAs (Figure 4A and Supplementary Table S8). Among them, 268 targets are regulated by both known miRNAs and novel miRNAs. According to the annotation results, the regulatory relationship between miRNAs and targets is not always the same. Some miRNAs regulate multiple targets, and a particular target may be regulated by multiple miRNAs. Among the 11,775 miRNA targets, 4.6% were unknown or had no significant similarity to other genes in the database (Supplementary Table S9). In addition, most of the miRNA targets were plant-specific transcription factors or targets that encode signal transduction pathway components and proteins related to plant metabolism, such as auxin response factors, growth-regulating factors, MYB family transcription factors, and various other proteins (squamosa promoter binding protein) or enzymes (alanyl-tRNA synthetase).
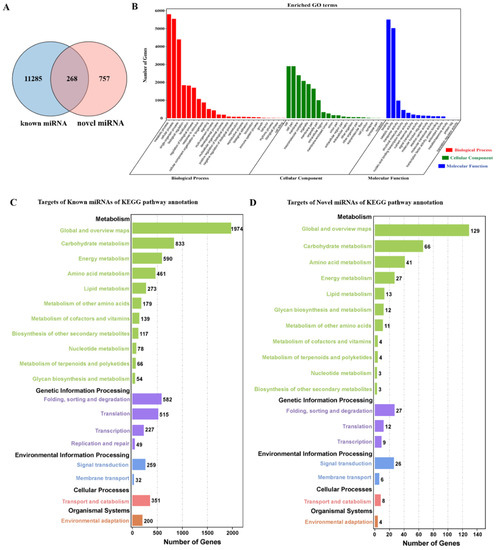
Figure 4.
Target prediction of miRNAs and function enrichment analysis. (A) Venn diagram showing target prediction of known miRNAs and novel miRNAs. (B) Gene ontology (GO) enrichment analysis of the biological functions of targets. Genes were assigned into three main categories: biological processes, cellular components and molecular functions. The y-axis indicates the number of genes in a given category. (C) Histogram of cluster of KEGG pathways of known miRNA targets. The results were summarized in five main categories (black words). (D) Histogram of cluster of KEGG pathways of novel miRNA targets. The results were summarized in five main categories (black words).
To gain a better understanding of the function of targets in T. tuan, we analyzed the function enrichment of gene ontology (GO) and KEGG terms among the genes targeted by miRNAs. GO analysis revealed that the two most abundant types in biological processes were the metabolic process and cellular process; many cellular components were involved, including cell, cell part and membrane; in molecular function, the targets participated in different processes, such as binding, catalytic activity, and transporter activity (Figure 4B). The targets of known miRNAs were annotated by KEGG and matched to 129 different pathways (Figure 4C and Supplementary Figure S5A). Among them, the significantly enriched pathways were MAPK signaling pathway, ABC transporters, Circadian rhythm, and Plant hormone signal transduction. Comparing with targets of known miRNAs, targets of novel miRNAs were annotated by KEGG and matched to 70 different pathways (Figure 4D and Supplementary Figure S5B). The significantly enriched pathways were Plant hormone signal transduction, Glycolysis/Gluconeogenesis, and Glycosylphosphatidylinositol (GPI)-anchor biosynthesis.
3.6. Transcriptional Regulatory miRNA-mRNA Networks
miRNAs and their targets are involved in various biochemical metabolic and signal transduction pathways. To explore the biosynthesis and regulatory mechanism of fatty acid, ABA, and lignin biosynthesis during seed maturation in T. tuan, we analyzed the expression patterns of related miRNAs and targets (Supplementary Table S10). Fatty acid biosynthesis is an important metabolic process during seed development and maturation, and involves lipid transport and metabolism, biosynthesis of secondary metabolites, and carbohydrate transport and metabolism. A total of 123 pairs of miRNAs and targets are involved in fatty acid and lipid metabolism, including 47 miRNAs and 48 target transcripts. These belong to 16 miRNA families (miR156/157, miR159, miR1511, miR162, miR164, miR166, miR167, miR168, miR171, miR172, miR395, miR396, miR2916, miR5141 and miR8155), and the targets include key enzymes of fatty acid synthesis, such as acetyl-CoA carboxylase1 (ACC1), acyl-carrier-protein (MCAMT), long-chain acyl-CoA synthetase (LACS), stearoyl-ACP desaturase, and fatty acyl-ACP thioesterase B (FATB). These enzymes are important for fatty acid and lipid metabolism and play an important role in the initiation and extension of carbon chains and the Kennedy pathway (Figure 5A and Supplementary Table S11). During seed maturation, ttu-miR8155 expression increased from Jun. 1 to Aug. 2 stage, decreased slightly afterward, and reached a maximal level at Oct. 2 stage. Expression of target KR (PB.22270.1) was highest at Jul. 1 stage and then decreased continuously. Expression of ttu-miR171 was downregulated at seed maturation stage, while target LACS7 (PB.5840.1) was upregulated. ttu-miR2916 and target LACS8 (PB.6192.1) were upregulated. In addition, ttu-miR166 exhibited its highest expression at Jun. 1 stage and then decreased slightly but was not expressed at seed maturation stage. Its target FTM1 (PB.23786.1 and PB.24287.1) increased continuously at Jun. 1, stage and reached its highest level at Sept. 2 stage (Figure 5B,C).
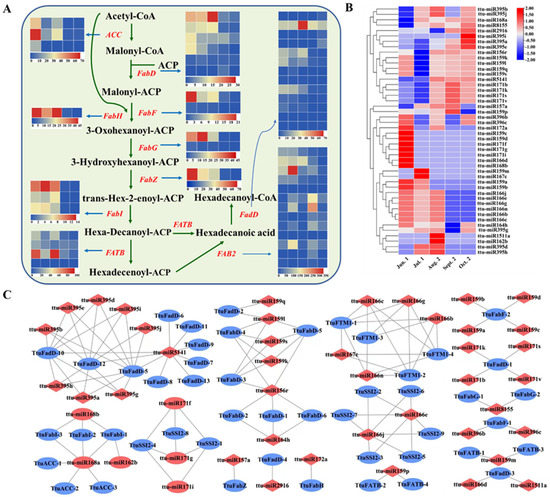
Figure 5.
Enrichment analysis of candidate targets in fatty acid biosynthesis pathway. (A) Represents targets functions of miRNA involved in fatty acid biosynthesis, Heat maps of gene expression levels (FPKM) at five different seed maturation stages. (B) Heat map of expressed miRNAs involved in fatty acid biosynthesis. (C) Represents the view of miRNA-mRNA network involved in fatty acid. The red color represents miRNA and the blue color represents targets.
Similarly, ABA plays a significant role in seed development and maturation. A total of 21 pairs of miRNAs and targets are involved in ABA biosynthesis pathways, including 14 miRNAs and 10 target transcripts. These belong to seven miRNA families (miR156, miR157, miR159, miR160, miR171, miR395, and miR397) and the targets include key enzymes of ABA biosynthesis, such as beta-ring hydroxylase (CYP97A3), zeaxanthin epoxidase (ABA1), 9-cis-epoxycarotenoid dioxygenase (NCED5), and xanthoxin dehydrogenase (ABA2). These enzymes play important roles in ABA biosynthesis and in carotenoid pathway starting from β-carotene (Figure 6A and Supplementary Table S11). miR171 is upregulated while its target NCED5 is downregulated at Aug. 2 and Sept. 2 stage. ABA2 is not expressed from Jun. 1 to Aug. 2 stage; however, it is significantly upregulated at Sept. 2 and Oct. 2 stage. The expression levels of miR160 altered dynamically from Jun. 1 to Oct. 2 stage, with the highest level occurring at Oct. 2 stage. ttu-miR162 was only expressed at Aug. 2 stage, while PYL11 (PB.36139.1) showed an opposite trend at Aug. 2 and Sept. 2 stage, with its highest level observed at Sept. 2 stage (Figure 6B, C). Overall, a large number of genes related to ABA biosynthesis were expressed at Sept. 2 stage, and ABA promoted embryo maturation and seed dormancy.
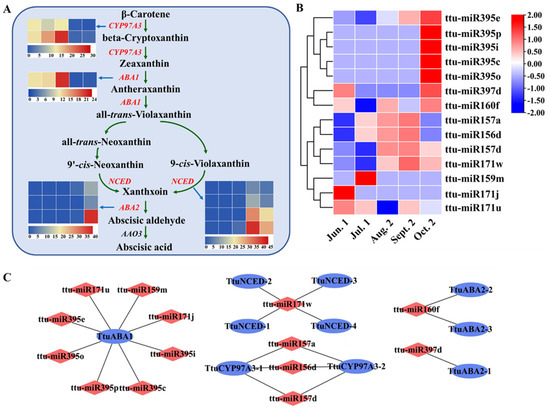
Figure 6.
Enrichment analysis of candidate targets in abscisic acid (ABA) pathway. (A) Represents miRNA targets functions involved in abscisic acid (ABA) biosynthesis. Heat maps of gene expression levels (FPKM) at five different seed maturation stages. (B) Heat map of expressed miRNAs involved in ABA pathway. (C) Represents the view of miRNA-mRNA network involved in abscisic acid (ABA) biosynthesis. The red color represents miRNA and the blue color represents targets.
We also found 62 pairs of miRNAs and targets involved in lignin biosynthesis pathways, including 30 miRNAs and 40 target transcripts. These belong to 12 miRNA families (miR156, miR166, miR171, miR172, miR319, miR393, miR396, miR397, miR4995, miR5139, miR1511, and miR8155), among which miR319 was expressed at the highest level, followed by miR5139, miR1511, and miR8155. These targets include some enzymes important for lignin biosynthesis, such as phenylalanine ammonia-lyase (PAL), trans-cinnamate 4-monooxygenase (C4H), 4-coumarate-CoA ligase (4CL), cinnamoyl-CoA reductase (CCR), cinnamyl alcohol dehydrogenase (CAD), and peroxidase (FOD), which all play important roles in lignin biosynthesis and the phenylalanine pathway (Figure 7A and Supplementary Table S11). ttu-miR4995 targets PAL (PB.5109.1, PB.9417.1, PB.10698.1, PB.12095.1, PB.17886.1, PB.24295.1, and PB.31742.1), which is highly expressed from Jun. 1 to Oct. 2 stage, while targets of ttu-miR4995 were downregulated. ttu-miR166 targets C4H (PB.17044.1), and its expression pattern was antagonistic at Jul. 1 to Aug. 2 stage and synergistic in other periods. ttu-miR5139 targets 4CL (PB.11564.1 and PB.11801.1), which is responsible for p-coumaroyl CoA accumulation. Notably, ttu-miR04 targets FOD (PB.26371.1) which is expressed at at Jul. 1 to Aug. 2 stage then decreased to a low level at Sept. 2 and Oct. 2 stage (Figure 7B, C). These results suggest that these differentially expressed miRNAs may be involved in the biosynthesis of fatty acids, ABA, and lignin in T. tuan seeds through post-transcriptional regulation.
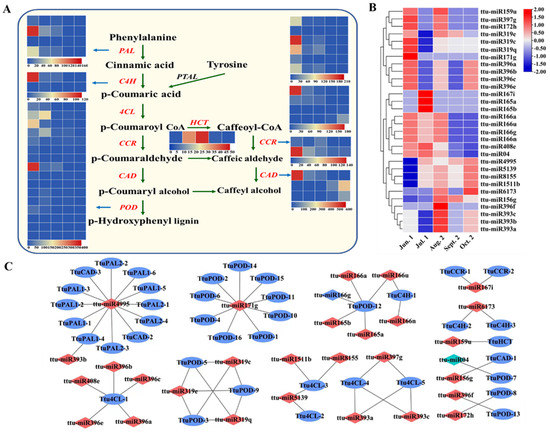
Figure 7.
Enrichment analysis of candidate targets in lignin biosynthesis pathway. (A) Represents miRNA targets functions involved in lignin biosynthesis. Heat maps of gene expression levels (FPKM) at five different seed maturation stages. (B) Heat map of expressed miRNAs involved in lignin biosynthesis pathway. (C) Represents the view of miRNA-mRNA network involved in lignin biosynthesis. The red color represents miRNA and the blue color represents targets.
4. Discussion
To identify the potential role of miRNAs in different seed maturation stages in T. tuan, we used miRBase to identify hairpin precursor sequences of the newly identified miRNAs, which led to the discovery and annotation of 189 known miRNAs and 8 novel miRNAs hairpin sequences. To date, no miRNAs and corresponding hairpin precursor sequences from T. tuan have been deposited in miRBase. These findings have greatly expanded the repertoire of T. tuan miRNA genes and provide supporting evidence for newly discovered miRNAs.
miR403 is a member of a miRNA family unique to dicotyledons [45] that plays vital roles in antiviral defense [46,47], stress resistance [48,49], and growth and development of dicotyledons [50]. It may also play a regulatory role in dicotyledons and participate in the regulation of seed maturation. Our data showed that ttu-miR403b was preferentially expressed at Sept. 2 stage, and its expression level was the highest of all known miRNAs, suggesting that miR403 may also play a major regulatory role in T. tuan seed maturation. In monocotyledonous plants, miR156 has been reported to be the second most abundant miRNA in rice [51], wheat [52], and barley [53]. Previous studies have shown that it regulates seed dormancy by suppressing the gibberellin pathway through depression of the target gene Ideal Plant Architecture 1 (IPA1) in rice [54]. In B. napus seed, the miR156 family is the most abundant family of miRNAs in seeds and mature embryos, most of which are expressed during embryo development. Furthermore, miR156 expression levels increased steadily as the seed maturation [55]. In Phalaenopsis aphrodite, miR156 is also highly expressed in different tissue parts such as leaves, roots, flowers, seeds; members of the miR156 family are highly expressed in the seed library [56]. Functional analysis of miR156 has revealed its crucial role during embryogenesis in Arabidopsis via regulation of SPL [57] and control of grain size, shape, and quality by OsSPL16 in rice [58]. miR156 negative regulatory target SPL during seed development in wheat and maize [59,60]. We identified 14 members of the miR156 family in this study. Compared to Jun. 1 stage, expression of ttu-miR156a, ttu-miR156g, ttu-miR156b, and ttu-miR156f was higher at Aug. 2 and Sept. 2 stage, and significantly increased at Sept. 2 stage, confirming that the miR156 module plays an essential role in T. tuan seed maturation. These findings imply that miR156 plays a conserved role in seed development and maturation in several plant species.
In this study, these novel miRNAs different expression patterns at five stages. It is worth noting ttu-miR01, ttu-miR04, ttu-miR05, ttu-miR07, ttu-miR08, ttu-miR10 was synergistic regulation among novel miRNAs, and ttu-miR03 plays a complementary regulatory role with them. ttu-miR09 were expressed in both seed development stage and seed maturation stage. GO analysis revealed that the significantly enriched pathways were Plant hormone signal transduction, Glycolysis/Gluconeogenesis, and Glycosylphosphatidylinositol (GPI)-anchor biosynthesis (Figure 4B). The regulatory mechanism mediated by T. tuan specific miRNAs may play an important role during seed maturation. In B. napus seeds, novel_mir_1706, novel_mir_1407, novel_mir_173, and novel_mir_104 were significantly down-regulated at 21 DAF and 28 DAF, whereas novel_mir_1081, novel_mir_19 and novel_mir_555 were significantly up-regulated in fatty acid biosynthesis during seed development [61]. These results reveal that different novel miRNAs function at different steps via different regulation routes to co-regulate seed development and maturation. Furthermore, we found that the expression pattern of novel miRNA* was significantly different from those of novel miRNA at different stages, and there was no synergistic regulation between miRNA and miRNA*, suggesting that there is a unique molecular mechanism for miRNA* degradation or its role during seed maturation, which is not known at present. According to previous reports, expression pattern of novel miRNA* was also different from novel miRNA in Arabidopsis, wheat, and B. napus [61,62,63]. This shows that there is no synergistic regulation between miRNA and miRNA* in wide range of species.
Bioinformatics analysis predicted 11,775 targets for 189 known miRNAs and 757 targets for 6 novel miRNAs. A number of the targets were transcription factors, including growth-regulating factors (GRFs), GRAS family transcription factors, MYB family transcription factors, squamosa promoter binding proteins (SPLs), and auxin response factors (ARFs) (Supplementary Table S9). We determined the biological functions of the genes primarily engaged in regulation using GO enrichment after estimating the relevant targets and their activities. Our analyses revealed that energy metabolism, signal transmission, transcription factors, and gene expression were all involved in seed maturation (Figure 4). we found that some miRNAs were involved in the same pathway (e.g., biochemical metabolic and signal transduction pathways) but targeted different genes. Previous reports have indicated that miR156/157 targets Squamosa-promoter binding proteins (SBPs) or SBP-like proteins (SPLs) [16]. Similarly, ttu-miR5139 targets 4-coumarate-CoA ligase, a key lignin synthetase. In addition, ttu-miR171, ttu-miR2916, ttu-miR1511, and ttu-miR5141 jointly target long-chain acyl-CoA synthetase in the fatty acid biosynthesis pathway. We also discovered a phenomenon in which miRNAs target the same genes. ttu-miR171f targets 9-cis-epoxycarotenoid dioxygenase and ttu-miR4995 targets phenylalanine ammonia-lyase. In B. napus, miR173, miR400, and miR396 all target pentatricopeptide (PPR) repeat-containing proteins; miR156, miR394, miR319 “co-target” F-box family proteins and miR160, miR167, miR390 and miR156 co-target various ARFs [55]. Our results are consistent with the study on B. napus mentioned above and indicate that many miRNAs likely play a role in regulating functionally relevant genes or pathways through multiple-targeting and co-targeting of different miRNAs.
miRNAs and targets are involved in many biochemical metabolic and signal transduction pathways. Plant lipids, in which fatty acids (FAs) are esterified to glycerol, are essential components of the cellular membrane, the major structural and functional barrier of cells and intracellular organelles. Lipids are stored in the form of triacylglycerol (TAG), which is a carbon and energy storage material in seeds [64,65]. In plants, acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), long-chain acyl-CoA synthetase, and acyl-[acyl-carrier-protein] desaturase are key enzymes for ab initio synthesis of FAs in plastids [66,67]. In a previous study on tree peony seeds, nine miRNAs were involved in fatty acid biosynthesis [68]. In B. napus seeds, bna-miR156b, bna-miR156g, bna-miR159, bna-miR395b, bna-miR6029, and 19 novel miRNAs were found to be actively involved in fatty acid biosynthesis using high-throughput sequencing [61]. In this study, ttu-miR166 was highly expressed at Jun. 1 stage and expression gradually decreased with seed maturation; by contrast, its target expression pattern showed a decreasing trend overall. Thus, it is speculated that this miRNA negatively regulates lipids synthesis.
Previous studies have shown that miR166 regulates a variety of developmental processes, such as SAM maintenance; root, stem, leaf, flower, and seed development; and rhizome formation [62,69,70,71,72]. However, the exact biological function of miR166 in regulating seed maturation in T. tuan remains unclear. The relative expression of ttu-miR171 was highest at Sept. 2 stage and downregulated at Oct. 2 stage. The expression pattern of target LACS2 was the opposite, gradually increasing as a whole. The miR171 family is a highly conserved family with perfect similarity among different species of angiosperm plants. miR171 regulates members of the SCL transcription factor family. In Arabidopsis, the targets of miR171 are SCL6 (SCL6-II, SCL6-III, and SCL6-IV), which play an essential role in plant root and leaf development, gibberellin response, phytochrome signaling, lateral organ polarity, meristem formation, vascular development, and stress response [73,74,75,76]. We also found that ttu-miR168a regulated ACC synthesis at Jun. 1 stage, ttu-miR8155 regulated FSA synthesis at Jul. 1 stage, and acyl-[acyl-carrier-protein] regulated desaturase synthesis at Aug. 2 and Sept. 2 stage. ttu-miR171 regulates the synthesis of a large number of LACS2 at Oct. 2 stage, indicating these four miRNAs regulate fatty acid biosynthesis in an orderly manner. Further study is needed to elucidate the regulatory mechanisms of miRNAs during seed maturation.
Plant hormones, particularly ABA, are essential regulators of seed dormancy and maturation [77]. Many ABA signal transduction proteins are involved in seed development [78,79]. The inactivation of ABA by 8′-hydroxylase (CYP97A) and the cleavage of carotenoid precursors by 9-cis epoxycarotenoid dioxygenase (NCED) are key steps in regulating metabolism. AtNCED6 and AtNCED9 are necessary for ABA biosynthesis during seed development in Arabidopsis. ABA synthesized in the endosperm and embryo participates in the hormone balance that controls seed dormancy and germination [80]. In this study, miR171 targeted CYP97A, miR162 targeted ABA1 and ABA2, and ttu-miR160 targeted NCED, which participates in ABA biosynthesis during seed maturation (Figure 7). CYP97A3 and ABA1 were highly expressed at Aug. 2 stage, while NCED was highly expressed at Sept. 2 stage and ABA2 was highly expressed at Oct. 2 stage. The accumulation of ABA with seed maturation promotes proper embryo growth and maturation, as well as seed shedding. Members of the miR160 family are conserved and play a crucial role in regulating plant morphology [81], enhancing plant resistance [82], regulating flower and embryo development [31], and affecting hormone levels [83]. Until now, functional studies of miR160 and its targets have mainly focused on vegetative and reproductive growth. miR160 can also negatively regulate AtARF10 and AtARF16 in Arabidopsis, and participate in seed germination and dormancy through the ABA pathway [27,31]. miR162 is involved in a variety of abiotic stress responses in plants. In addition, ABA treatment has been shown to induce miR162 to improve adaptation to drought stress by inhibiting Trehalase precursor 1 (OsTRE1) [84]. In a study on maize, miR162 responded to salt stress, and its accumulation increased 30 min after salt treatment but decreased 5 and 24 h after the treatment [85]. In a study on Panicum virgatum, the accumulation of miR162 changed significantly under drought stress [86]. In cotton, miR162 responds to salinity [87]. miR171 was one of the first members of the miRNA family found in plants. It targets Scarecrow-like protein 6, a transcription factor involved in gibberellin signal transduction and gametophyte development in plants; it is also indispensable in plant sex differentiation [88,89]. These results suggest that miR160, miR162, and other key miRNAs affect ABA biosynthesis by inhibiting relevant targets during seed maturation in T. tuan. Many studies have found that endogenous inhibitors are a significant cause of seed dormancy, and the most prevalent endogenous inhibitor is ABA.
Lignin is a crucial macromolecular organic matter in plants, which occurs in the thickened secondary cell wall [90]. The poor seed coat permeability caused by lignification is the main reason for the dormancy of T. tuan seeds. In the phenylpropanoid biosynthesis pathway, phenylalanine is catalyzed by phenylalanine ammonia-lyase (PAL), trans-cinnamate 4-monooxygenase (C4H), 4-coumarate-CoA ligase (4CL), cinnamyl-alcohol dehydrogenase (CAD), and peroxidase to form p-hydroxyphenyl lignin [91]. Key lignin biosynthesis enzymes are regulated by miRNAs. Previous studies have shown that overexpression of ptr-miR397a in poplar can downregulate the relative expression of 17 laccase genes, resulting in a decrease in lignin content [92]. The overexpression of miR397b in Arabidopsis reduces the relative expression of AtLAC4, resulting in a decrease in lignin content [93]. In this study, the expression of ttu-miR397 was the highest expressed at Jun. 1 stage, and gradually decreased with seed maturation, while expression of the target 4CL exhibited the opposite. In addition, the levels of ttu-miR4995, ttu-miR5139, ttu-miR1511, and ttu-miR8155 increased gradually with seed maturation, and all reached their highest levels at Oct. 2 stage, while the target PAL decreased gradually overall. Thus, the four miRNAs appear to negatively regulate lignin biosynthesis and have a conserved function. We also found that ttu-miR319 was more highly expressed at Jun. 1 stage compared to other miRNAs, and its expression decreased gradually with seed maturation. A number of studies have revealed that miR319 and its targets play a variety of roles in plant developmental processes, such as leaf morphogenesis, flower development, senescence, and jasmonic acid biosynthesis [11,94,95,96]. miR319 targets TCP transcription factors (TCP2, TCP3, TCP4, TCP10, and TCP24) to regulate floral formation, and leaf and gametophyte development [97,98]. In tomato, ectopic expression of miR319 downregulates the expression of several TCPs, resulting in larger leaflets and continuous growth of the leaf margin, while decreased miR319 or increased TCP level resulting in a decrease in leaf size [99]. In Arabidopsis, the target TCP4 of miR319 can directly bind the promoter of VND7, which regulates lignin biosynthesis, to activate its expression, thereby activating formation of the secondary cell wall and programmed cell death [100]. Thus, miR319 plays a significant role in plant developmental processes and may also regulate seed dormancy. These results reveal that some miRNAs may regulate functional genes directly involved during seed maturation, whereas other miRNAs regulate the seed maturation process by acting on a large number of transcription factors. This thoroughly demonstrates that not only has the seed ripening process been altered (Figure 1), but highly sophisticated metabolic changes have also occurred, and that this process is carried out cooperatively by many regulatory networks (Figure 5, Figure 6 and Figure 7).
5. Conclusions
miRNAs with diverse expression patterns, multiple-targeting and co-targeting of many miRNAs, and complex relationships between the expression of miRNAs and targets were identified in this study. We identified and characterized the transcriptome of miRNAs in five different seed maturation stages. A total of 189 known miRNAs belonging to 42 miRNA families and 8 novel miRNAs were identified; 62 miRNAs were differentially expressed in five different seed maturation stages. Further joint analysis of transcriptome data at the same stage of seeds showed that there was an antagonistic correlation between the miRNA expression level and the differential expression of target genes. The relative abundance as well as specific temporal and spatial expression patterns of these miRNAs and their targets suggested that miR403, miR156, miR171, miR172, miR396, miR319, and miR397 are major contributors to the network controlling seed maturation through their pivotal roles in plant development. Our results improve our understanding of miRNA-mRNA networks. This work provides new insights into the regulatory mechanisms of miRNAs and targets, offering critical clues to the molecular mechanisms of fatty acid, ABA, and lignin biosynthesis during seed maturation in T. tuan. Further research elucidating the molecular mechanism underlying the involvement of these miRNAs in growth and development will be important.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13111750/s1, Figure S1: Number of miRNA family members in T. tuan seed; Figure S2: First base composition bias of miRNAs (18–30 nt) in T. tuan; Figure S3: Nucleotide bias at each position of miRNAs in T. tuan; Figure S4: Several distinct expression patterns of known miRNAs and novel miRNAs in T. tuan seed; Figure S5: Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of known miRNAs and novel miRNAs targets; Table S1: List of sequencing and mapping of reads on unigenes; Table S2: Summary of small RNA sequencing statistics; Table S3: Summary of small RNA types and quantities; Table S4: The information of known miRNAs in T. tuan seed; Table S5: List of known miRNA expression levels in five small RNA libraries; Table S6: The information of novel miRNAs in T. tuan seed; Table S7: List of novel miRNA expression levels in five small RNA libraries; Table S8: List of targets prediction results in know miRNAs and novel miRNAs in T. tuan seed; Table S9: List of targets annotation in T. tuan seed; Table S10: List of miRNA targets expression levels in T. tuan seed; Table S11: Key genes expression in crucial pathways in T. tuan seed.
Author Contributions
X.H. participated in performing the experiments, data analysis, drafting and revising the manuscript; L.L. participated in performing the experiments, data analysis, drafting the manuscript; P.L. participated in drafting and revised the manuscript; M.W. participated in performing the experiments; Y.S. participated in conceiving the study and revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by: The Opening Project of State Key Laboratory of Tree Genetics and Breeding (Northeast Forestry University) (No. K2019101). The Major Science and Technology Projects of Inner Mongolia Autonomous Region (No. 2021ZD0008), The Project of Youth talent program of Forestry and grass-land Science and Technology Innovation (No. 2020132606) and the 111 Project (No. B20050).
Data Availability Statement
In this section, the sequencing data of this study have been deposited at the National Center for Biotechnology Information Sequence Read Archive (NCBI, SRA, http://www.ncbi.nlm.gov/sra/; accessed on 23 July 2022) under accession number PRJNA861431, PRJNA861958 and PRJNA878938.
Acknowledgments
We are grateful for the sequence information produced by the U.S. Department of Energy Joint Genome Institute (http://www.jgi.doe.gov; accessed on 5 October 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tang, Y.; Ren, Z. Geographical distribution of tilia linn. J. Syst. Evol. 1996, 34, 254–264. [Google Scholar]
- Yao, W.F.; Shen, Y.B. Effects of gibberellic acid and magnetically treated water on physiological characteristics of Tilia miqueliana seeds. Can. J. Res. 2018, 48, 554–558. [Google Scholar] [CrossRef]
- Shi, F.H.; Shen, Y.B.; Shi, J.S. Protection, development and utilization of Tilia miqueliana seeds. J. For. Eng. 2012, 26, 11–14. [Google Scholar]
- Wu, Y.; Shen, Y.B. The structural and chemical characteristics of the pericarp are important in Tilia miqueliana seed dormancy. New Forests 2021, 52, 878–888. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, Y.B. Dormancy in Tilia miqueliana is attributable to permeability barriers and mechanical constraints in the endosperm and seed coat. Braz. J. Bot. 2021, 44, 725–740. [Google Scholar] [CrossRef]
- Llave, C.; Kasschau, K.D.; Rector, M.A.; Carrington, J.C. Endogenous and silencing-associated small RNAs in plants. Plant Cell 2002, 14, 1605–1619. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jia, T.; Chen, X. The ‘how’ and ‘where’ of plant microRNAs. New Phytol. 2017, 216, 1002–1017. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The, C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Park, W.; Li, J.; Song, R.; Messing, J.; Chen, X. Carpel Factory, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr. Biol. 2002, 12, 1484–1495. [Google Scholar] [CrossRef]
- Gao, R.; Wang, Y.; Gruber, M.Y.; Hannoufa, A. miR156/SPL10 modulates lateral root development, branching and leaf morphology in Arabidopsis by silencingAGAMOUS-LIKE 79. Front. Plant Sci. 2017, 8, 2226. [Google Scholar] [CrossRef]
- Palatnik, J.F.; Allen, E.; Wu, X.; Schommer, C.; Schwab, R.; Carrington, J.C.; Weigel, D. Control of leaf morphogenesis by microRNAs. Nature 2003, 425, 257–263. [Google Scholar] [CrossRef]
- Aukerman, M.J.; Sakai, H. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 2003, 15, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Czech, B.; Weigel, D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 2009, 138, 738–749. [Google Scholar] [CrossRef]
- Li, K.; Liu, Z.; Xing, L.; Wei, Y.; Mao, J.; Meng, Y.; Bao, L.; Han, M.; Zhao, C.; Zhang, D. miRNAs associated with auxin signaling, stress response, and cellular activities mediate adventitious root formation in apple rootstocks. Plant Physiol. Biochem. 2019, 139, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, Y.; Pan, H.; Zhu, J.; Zhu, M.; Xu, T.; Li, Z.; Dong, T. Molecular characterization and target prediction of candidate miRNAs related to abiotic stress responses and/or storage root development in Sweet Potato. Genes 2022, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Park, M.Y.; Conway, S.R.; Wang, J.W.; Weigel, D.; Poethig, R.S. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 2009, 138, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Yu, S.; Kong, D.; Xiong, J.; Ma, X.; Chen, L.; Luo, L. Temporal responses of conserved miRNAs to drought and their associations with drought tolerance and productivity in rice. BMC Genom. 2020, 21, 232. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, H.; Zhang, F.; Jiang, Q.; Yang, S.; Yue, P.; Wang, F.; Zhang, Y.; Li, L.; He, P.; et al. The miR156/SPL module regulates apple salt stress tolerance by activating MdWRKY100 expression. Plant Biotechnol. J. 2021, 19, 311–323. [Google Scholar] [CrossRef]
- Pegler, J.L.; Oultram, J.; Grof, C.; Eamens, A.L. Molecular manipulation of the miR399/PHO2 expression module alters the salt stress response of Arabidopsis thaliana. Plants 2020, 10, 73. [Google Scholar] [CrossRef]
- Niu, J.; Wang, J.; An, J.; Liu, L.; Lin, Z.; Wang, R.; Wang, L.; Ma, C.; Shi, L.; Lin, S. Integrated mRNA and miRNA transcriptome reveal a cross-talk between developing response and hormone signaling for the seed kernels of Siberian apricot. Sci. Rep. 2016, 6, 35675. [Google Scholar] [CrossRef]
- Mutum, R.D.; Kumar, S.; Balyan, S.; Kansal, S.; Mathur, S.; Raghuvanshi, S. Identification of novel miRNAs from drought tolerant rice variety Nagina 22. Sci. Rep. 2016, 6, 30786. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.X.; Liu, Y.F.; Hu, X.Y.; Zhang, W.K.; Ma, B.; Chen, S.Y.; Zhang, J.S. Identification of miRNAs and their target genes in developing soybean seeds by deep sequencing. BMC Plant Biol. 2011, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qiao, Y.; Zhang, J.; Shi, W.; Zhang, J. Genome wide identification of microRNAs involved in fatty acid and lipid metabolism of Brassica napus by small RNA and degradome sequencing. Gene 2017, 619, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Khemka, N.; Singh Rajkumar, M.; Garg, R.; Jain, M. Genome-wide profiling of miRNAs during seed development reveals their functional relevance in seed size/weight determination in chickpea. Plant Direct. 2021, 5, e00299. [Google Scholar] [CrossRef]
- Gupta, M.; Bhaskar, P.B.; Sriram, S.; Wang, P.H. Integration of omics approaches to understand oil/protein content during seed development in oilseed crops. Plant Cell Rep. 2017, 36, 637–652. [Google Scholar] [CrossRef]
- Guo, M.; Simmons, C.R. Cell number counts--the fw2.2 and CNR genes and implications for controlling plant fruit and organ size. Plant Sci. 2011, 181, 1–7. [Google Scholar] [CrossRef]
- Liu, P.P.; Montgomery, T.A.; Fahlgren, N.; Kasschau, K.D.; Nonogaki, H.; Carrington, J.C. Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. Plant J. 2007, 52, 133–146. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.; Wang, Y.; Khanna, K.; Xie, Z.; Owen, H.A.; Zhao, D. The role of floral organs in carpels, an Arabidopsis loss-of-function mutation in MicroRNA160a, in organogenesis and the mechanism regulating its expression. Plant J. 2010, 62, 416–428. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.K.; Shin, J.S.; Suh, M.C. The SebHLH transcription factor mediates trans-activation of the SeFAD2 gene promoter through binding to E- and G-box elements. Plant Mol. Biol. 2007, 64, 453–466. [Google Scholar] [CrossRef]
- Li, D.; Jin, C.; Duan, S.; Zhu, Y.; Qi, S.; Liu, K.; Gao, C.; Ma, H.; Zhang, M.; Liao, Y.; et al. MYB89 transcription factor represses seed oil accumulation. Plant Physiol. 2017, 173, 1211–1225. [Google Scholar] [CrossRef]
- Liu, J.; Guo, X.; Zhai, T.; Shu, A.; Zhao, L.; Liu, Z.; Zhang, S. Genome-wide identification and characterization of microRNAs responding to ABA and GA in maize embryos during seed germination. Plant Biol. 2020, 22, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Shen, Y.; Shi, S.; Tang, T. miREvo: An integrative microRNA evolutionary analysis platform for next-generation sequencing experiments. BMC Bioinform. 2012, 13, 140. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, J.; Li, Z.; Li, X.; Hu, X.; Huang, Y.; Zhao, X.; Liang, C.; Wang, Y.; Sun, L.; et al. Integrated profiling of microRNAs and mRNAs: microRNAs located on Xq27.3 associate with clear cell renal cell carcinoma. PLoS ONE 2010, 5, e15224. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Storey, J.D. The positive false discovery rate: A Bayesian interpretation and the q-value. Ann. Stat. 2003, 31, 2013–2035. [Google Scholar] [CrossRef]
- Ernst, J.; Bar-Joseph, Z. STEM: A tool for the analysis of short time series gene expression data. BMC Bioinform. 2006, 7, 191. [Google Scholar] [CrossRef]
- Wu, H.J.; Ma, Y.K.; Tong, C.; Wang, M.; Wang, X.J. Psrobot: A web-based plant small RNA meta-analysis toolbox. Nucleic Acids Res. 2012, 40, W22–W28. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Jones-Rhoades, M.W.; Bartel, D.P. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol. Cell 2004, 14, 787–799. [Google Scholar] [CrossRef]
- Harvey, J.J.; Lewsey, M.G.; Patel, K.; Westwood, J.; Heimstädt, S.; Carr, J.P.; Baulcombe, D.C. An antiviral defense role of AGO2 in plants. PLoS ONE 2011, 6, e14639. [Google Scholar] [CrossRef]
- Bao, D.; Ganbaatar, O.; Cui, X.; Yu, R.; Bao, W.; Falk, B.W.; Wuriyanghan, H. Down-regulation of genes coding for core RNAi components and disease resistance proteins via corresponding microRNAs might be correlated with successful Soybean mosaic virus infection in soybean. Mol. Plant Pathol. 2018, 19, 948–960. [Google Scholar] [CrossRef]
- Ebrahimi Khaksefidi, R.; Mirlohi, S.; Khalaji, F.; Fakhari, Z.; Shiran, B.; Fallahi, H.; Rafiei, F.; Budak, H.; Ebrahimie, E. Differential expression of seven conserved microRNAs in response to abiotic stress and their regulatory network in Helianthus annuus. Front. Plant Sci. 2015, 6, 741. [Google Scholar] [CrossRef]
- Sun, X.; Xu, L.; Wang, Y.; Yu, R.; Zhu, X.; Luo, X.; Gong, Y.; Wang, R.; Limera, C.; Zhang, K.; et al. Identification of novel and salt-responsive miRNAs to explore miRNA-mediated regulatory network of salt stress response in radish (Raphanus sativus L.). BMC Genom. 2015, 16, 197. [Google Scholar] [CrossRef]
- Zhang, C.; Xian, Z.; Huang, W.; Li, Z. Evidence for the biological function of miR403 in tomato development. Sci. Hortic. 2015, 197, 619–626. [Google Scholar] [CrossRef]
- Sunkar, R.; Zhou, X.; Zheng, Y.; Zhang, W.; Zhu, J.K. Identification of novel and candidate miRNAs in rice by high throughput sequencing. BMC Plant Biol. 2008, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Wang, Y.; Yao, Y.; Xie, C.; Peng, H.; Ni, Z.; Sun, Q. Diverse set of microRNAs are responsive to powdery mildew infection and heat stress in wheat (Triticum aestivum L.). BMC Plant Biol. 2010, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.W.; Shi, B.J.; Huang, C.Y.; Langridge, P.; Baumann, U. Discovery of barley miRNAs through deep sequencing of short reads. BMC Genom. 2011, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Wang, Z.; Zhang, L.; Yao, J.; Hua, K.; Liu, X.; Shi, H.; Zhu, J.K. The grain yield modulator miR156 regulates seed dormancy through the gibberellin pathway in rice. Nat. Commun. 2019, 10, 3822. [Google Scholar] [CrossRef]
- Huang, D.; Koh, C.; Feurtado, J.A.; Tsang, E.W.; Cutler, A.J. MicroRNAs and their putative targets in Brassica napus seed maturation. BMC Genom. 2013, 14, 140. [Google Scholar] [CrossRef]
- Chao, Y.T.; Su, C.L.; Jean, W.H.; Chen, W.C.; Chang, Y.C.; Shih, M.C. Identification and characterization of the microRNA transcriptome of a moth orchid Phalaenopsis aphrodite. Plant Mol. Biol. 2014, 84, 529–548. [Google Scholar] [CrossRef]
- Nodine, M.D.; Bartel, D.P. MicroRNAs prevent precocious gene expression and enable pattern formation during plant embryogenesis. Genes Dev. 2010, 24, 2678–2692. [Google Scholar] [CrossRef]
- Wang, S.; Wu, K.; Yuan, Q.; Liu, X.; Liu, Z.; Lin, X.; Zeng, R.; Zhu, H.; Dong, G.; Qian, Q.; et al. Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 2012, 44, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, Z.; Gao, L.; Wang, L.; Gao, M.; Jiao, Z.; Qiao, H.; Yang, J.; Chen, M.; Yao, L.; et al. Genome-wide identification and characterization of microRNAs in developing grains of Zea mays L. PLoS ONE 2016, 11, e0153168. [Google Scholar] [CrossRef]
- Meng, F.; Liu, H.; Wang, K.; Liu, L.; Wang, S.; Zhao, Y.; Yin, J.; Li, Y. Development-associated microRNAs in grains of wheat (Triticum aestivum L.). BMC Plant Biol. 2013, 13, 140. [Google Scholar] [CrossRef]
- Wang, J.; Jian, H.; Wang, T.; Wei, L.; Li, J.; Li, C.; Liu, L. Identification of microRNAs actively involved in fatty acid biosynthesis in developing Brassica napus Seeds Using High-Throughput Sequencing. Front. Plant Sci. 2016, 7, 1570. [Google Scholar] [CrossRef]
- Tang, X.; Bian, S.; Tang, M.; Lu, Q.; Li, S.; Liu, X.; Tian, G.; Nguyen, V.; Tsang, E.W.; Wang, A.; et al. MicroRNA-mediated repression of the seed maturation program during vegetative development in Arabidopsis. PLoS Genet. 2012, 8, e1003091. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Wei, K.; Wang, M.; Wang, L.; Cui, J.; Zhang, D.; Guo, J.; Zhao, M.; Zheng, Y. Identification and temporal expression analysis of conserved and novel MicroRNAs in the leaves of winter wheat grown in the field. Front Genet. 2019, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.D.; Ohlrogge, J.B. Compartmentation of triacylglycerol accumulation in plants. J. Biol. Chem. 2012, 287, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.D.; Stymne, S.; Ohlrogge, J. Biochemical pathways in seed oil synthesis. Curr. Opin. Plant Biol. 2013, 16, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Ohlrogge, J.B.; Jaworski, J.G. Regulation of fatty acid synthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol 1997, 48, 109–136. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; Debono, A.; Durrett, T.P.; et al. Acyl-lipid metabolism. Arab. Book 2013, 11, e0161. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.D.; Li, S.S.; Shu, Q.Y.; Gu, Z.Y.; Wu, Q.; Feng, C.Y.; Xu, W.Z.; Wang, L.S. Identification of microRNAs and long non-coding RNAs involved in fatty acid biosynthesis in tree peony seeds. Gene 2018, 666, 72–82. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, S.; Han, X.; Ma, J.; Deng, W.; Wang, X.; Guo, H.; Xia, X. Integrated transcriptome and miRNA analysis uncovers molecular regulators of aerial stem-to-rhizome transition in the medical herb Gynostemma pentaphyllum. BMC Genom. 2019, 20, 865. [Google Scholar] [CrossRef]
- Kim, J.; Jung, J.H.; Reyes, J.L.; Kim, Y.S.; Kim, S.Y.; Chung, K.S.; Kim, J.A.; Lee, M.; Lee, Y.; Narry Kim, V.; et al. microRNA-directed cleavage of ATHB15 mRNA regulates vascular development in Arabidopsis inflorescence stems. Plant J. 2005, 42, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Park, C.M. MIR166/165 genes exhibit dynamic expression patterns in regulating shoot apical meristem and floral development in Arabidopsis. Planta 2007, 225, 1327–1338. [Google Scholar] [CrossRef]
- Han, S.Y.; Li, S.G.; Wan, F.; Yang, W.H.; Li, Y.L.; Xu, H.Y. Over-expression of miR166a inhibits cotyledon formation in somatic embryos and promotes lateral root development in seedlings of Larix leptolepis. Plant Cell Tiss. Org. 2016, 127, 461–473. [Google Scholar] [CrossRef]
- Llave, C.; Xie, Z.; Kasschau, K.D.; Carrington, J.C. Cleavage of scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 2002, 297, 2053–2056. [Google Scholar] [CrossRef] [PubMed]
- Bolle, C. The role of GRAS proteins in plant signal transduction and development. Planta 2004, 218, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, B.; Song, S.K.; Heo, J.O.; Yu, N.I.; Lee, S.A.; Kim, M.; Kim, D.G.; Sohn, S.O.; Lim, C.E.; et al. Large-scale analysis of the GRAS gene family in Arabidopsis thaliana. Plant Mol. Biol. 2008, 67, 659–670. [Google Scholar] [CrossRef]
- Wang, L.; Mai, Y.X.; Zhang, Y.C.; Luo, Q.; Yang, H.Q. MicroRNA171c-targeted SCL6-II, SCL6-III, and SCL6-IV genes regulate shoot branching in Arabidopsis. Mol. Plant 2010, 3, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Godin, B.; Bonnet, M.; Sotta, B.; Marion-Poll, A. Maternal synthesis of abscisic acid controls seed development and yield in Nicotiana plumbaginifolia. Planta 2004, 218, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, L.; Liu, X.; Cui, D.; Chen, T.; Zhang, H.; Jiang, C.; Xu, C.; Li, P.; Li, S.; et al. Deep sequencing of maize small RNAs reveals a diverse set of MicroRNA in dry and imbibed seeds. PLoS ONE 2013, 8, e55107. [Google Scholar] [CrossRef]
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef]
- Lefebvre, V.; North, H.; Frey, A.; Sotta, B.; Seo, M.; Okamoto, M.; Nambara, E.; Marion-Poll, A. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 2006, 45, 309–319. [Google Scholar] [CrossRef]
- Turner, M.; Nizampatnam, N.R.; Baron, M.; Coppin, S.; Damodaran, S.; Adhikari, S.; Arunachalam, S.P.; Yu, O.; Subramanian, S. Ectopic expression of miR160 results in auxin hypersensitivity, cytokinin hyposensitivity, and inhibition of symbiotic nodule development in soybean. Plant Physiol. 2013, 162, 2042–2055. [Google Scholar] [CrossRef] [PubMed]
- Pinweha, N.; Asvarak, T.; Viboonjun, U.; Narangajavana, J. Involvement of miR160/miR393 and their targets in cassava responses to anthracnose disease. J. Plant Physiol. 2015, 174, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Mallory, A.C.; Bartel, D.P.; Bartel, B. MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell 2005, 17, 1360–1375. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zuo, Z.; Qiu, J.L. Identification and characterization of ABA-responsive MicroRNAs in rice. J. Genet. Genom. 2015, 42, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Zhang, L.; Wang, H.; Liu, Z.; Zhang, Z.; Zheng, Y. Differential expression of miRNAs in response to salt stress in maize roots. Ann. Bot. 2009, 103, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Stewart, C.N., Jr.; Xiao, P.; Zhang, B. MicroRNA expression analysis in the cellulosic biofuel crop switchgrass (Panicum virgatum) under abiotic stress. PLoS ONE 2012, 7, e32017. [Google Scholar] [CrossRef]
- Salih, H.; Gong, W.; Mkulama, M.; Du, X. Genome-wide characterization, identification, and expression analysis of the WD40 protein family in cotton. Genome 2018, 61, 539–547. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, Y.; Qin, L.; Wang, Y.; Chen, L.; He, Y.; Fei, Z.; Lu, G. Identification of miRNAs and their targets through high-throughput sequencing and degradome analysis in male and female asparagus officinalis. BMC Plant Bio. 2016, 16, 80. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, X.; Cai, W.; Huang, W.; Zhou, X.; Luo, Q.; Yang, H.; Wang, J.; Huang, J. Arabidopsis miR171-targeted scarecrow-like proteins bind to GT cis-elements and mediate gibberellin-regulated chlorophyll biosynthesis under light conditions. PLoS Genet. 2014, 10, e1004519. [Google Scholar] [CrossRef]
- Novaes, E.; Kirst, M.; Chiang, V.; Winter-Sederoff, H.; Sederoff, R. Lignin and biomass: A negative correlation for wood formation and lignin content in trees. Plant Physiol. 2010, 154, 555–561. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, Q.; Wei, H.; Chang, M.J.; Tunlaya-Anukit, S.; Kim, H.; Liu, J.; Song, J.; Sun, Y.H.; Yuan, L.; et al. Ptr-miR397a is a negative regulator of laccase genes affecting lignin content in Populus trichocarpa. Proc. Natl. Acad. Sci. USA 2013, 110, 10848–10853. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhang, S.; Yu, Y.; Luo, Y.C.; Liu, Q.; Ju, C.; Zhang, Y.C.; Qu, L.H.; Lucas, W.J.; Wang, X.; et al. MiR397b regulates both lignin content and seed number in Arabidopsis via modulating a laccase involved in lignin biosynthesis. Plant Biotechnol. J. 2014, 12, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, B.; Shen, W.H.; Huang, H.; Dong, A. TCP transcription factors interact with AS2 in the repression of class-I KNOX genes in Arabidopsis thaliana. Plant J. 2012, 71, 99–107. [Google Scholar] [CrossRef]
- Schommer, C.; Palatnik, J.F.; Aggarwal, P.; Chételat, A.; Cubas, P.; Farmer, E.E.; Nath, U.; Weigel, D. Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol. 2008, 6, e230. [Google Scholar] [CrossRef] [PubMed]
- Nag, A.; King, S.; Jack, T. miR319a targeting of TCP4 is critical for petal growth and development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 22534–22539. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhang, Z.M.; Gao, J.; Zeng, X.; Pan, G.T. The role of miR319 in plant development regulation. Hereditas 2011, 33, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Schommer, C.; Bresso, E.G.; Spinelli, S.V.; Palatnik, J.F. Role of microRNA miR319 in plant development. Signal. Commun. Plants 2012, 15, 29–47. [Google Scholar] [CrossRef]
- Ori, N.; Cohen, A.R.; Etzioni, A.; Brand, A.; Yanai, O.; Shleizer, S.; Menda, N.; Amsellem, Z.; Efroni, I.; Pekker, I.; et al. Regulation of LANCEOLATE by miR319 is required for compound-leaf development in tomato. Nat. Genet. 2007, 39, 787–791. [Google Scholar] [CrossRef]
- Sun, X.; Wang, C.; Xiang, N.; Li, X.; Yang, S.; Du, J.; Yang, Y.; Yang, Y. Activation of secondary cell wall biosynthesis by miR319-targeted TCP4 transcription factor. Plant Biotechnol. J. 2017, 15, 1284–1294. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).