Plant Growth and Microbiota Structural Effects of Rhizobacteria Inoculation on Mahogany (Swietenia macrophylla King [Meliaceae]) under Nursery Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains Identification
2.2. Rhizobacteria Functional Validation Bioassay
2.3. Functional Evaluation of Rhizobacteria on S. macrophylla under Nursery Conditions
2.4. Bacterial Structure
2.5. Statistical Analyses
3. Results
3.1. Taxonomic Assignation and Validation of In Vitro Growth-Promoting Effect of Rhizobacteria
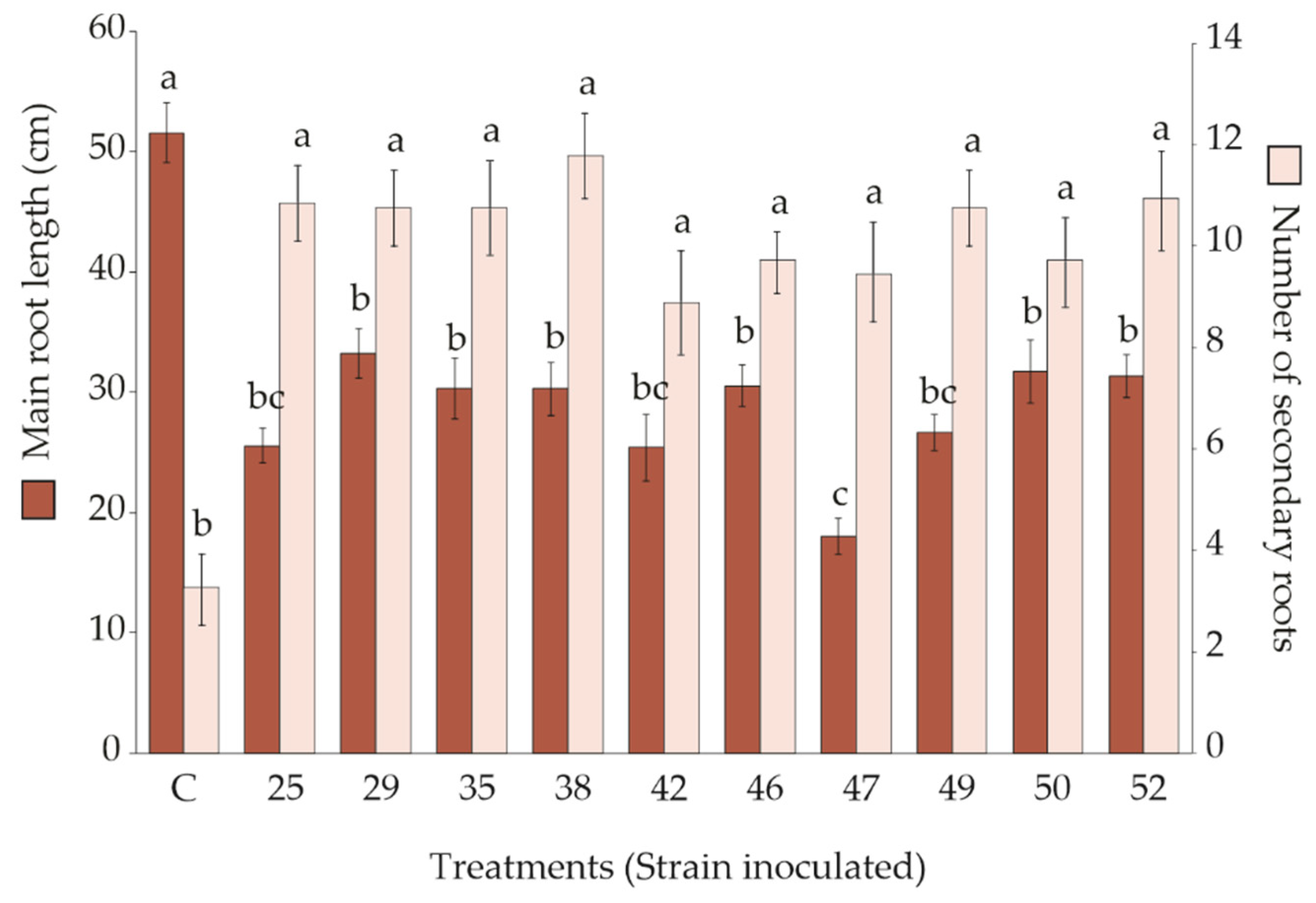
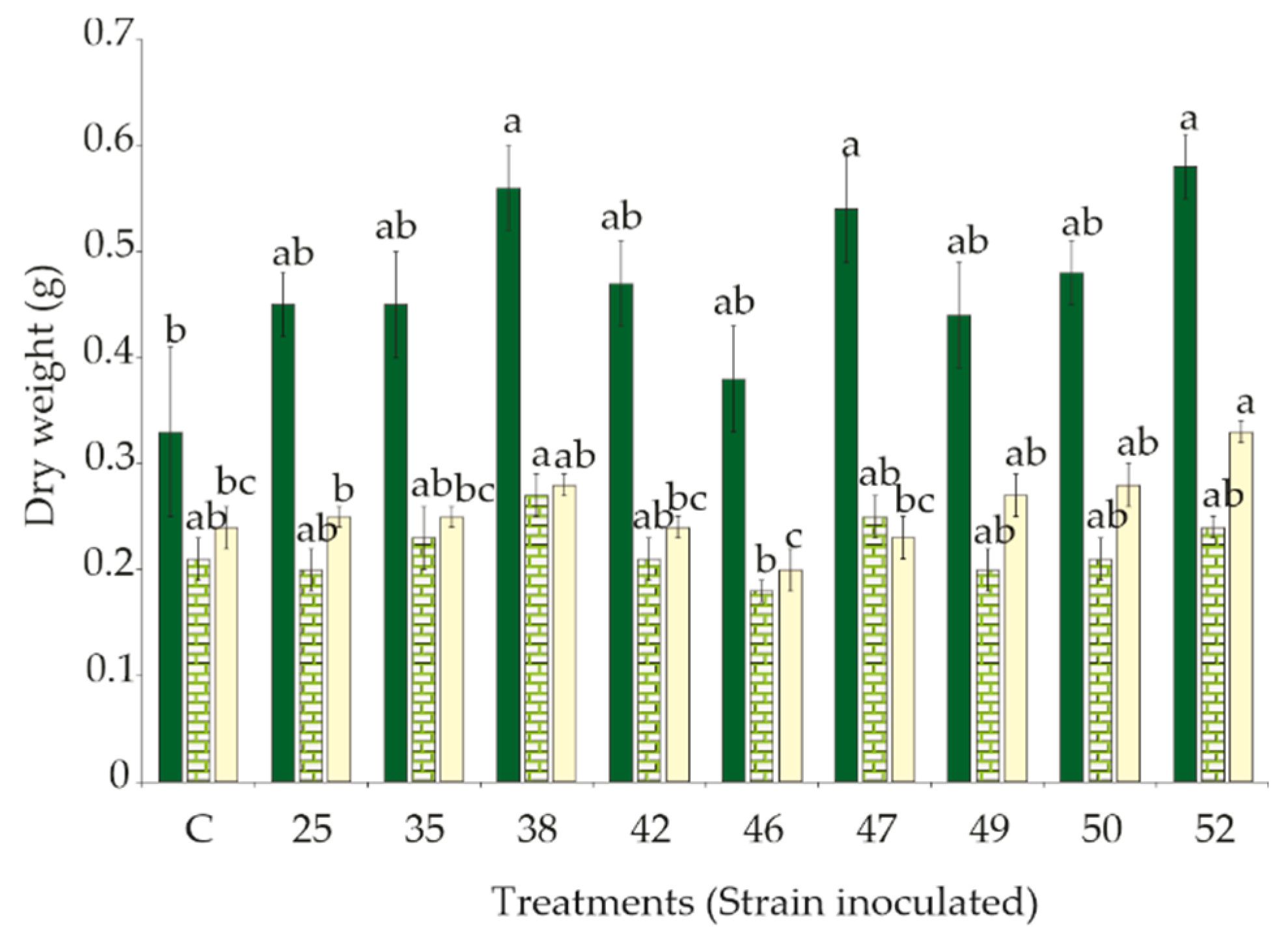
3.2. Effect of Rhizobacteria Strains Inoculation on S. macrophylla
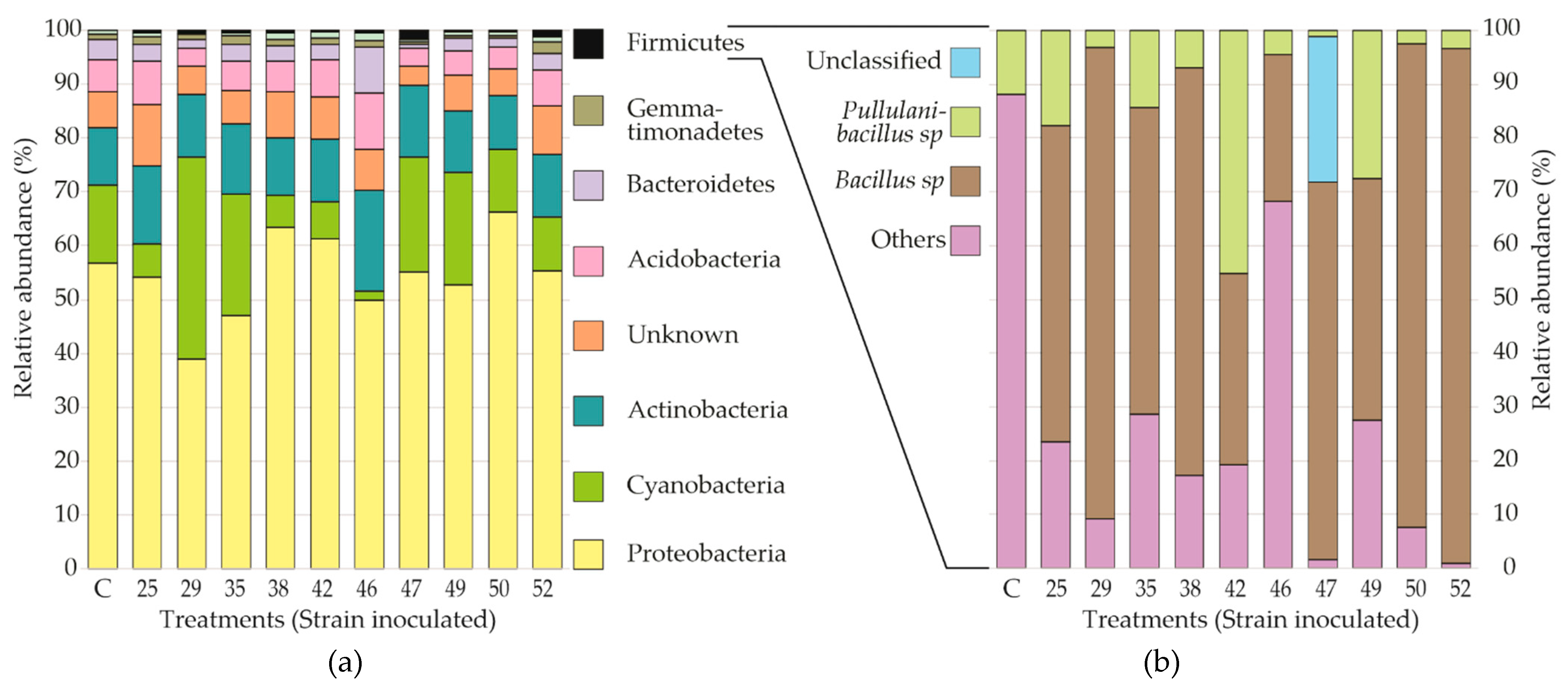
3.3. Bacterial Communities in the Rhizosphere of Seedling S. macrophylla in the Nursery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva Guzmán, J.A.; Ramírez Arango, A.M.; Fuentes Talavera, F.J.; Rodriíguez Anda, R.; Turrado Saucedo, J.; Georg Richter, H. Diagnóstico de La Industria de Transformación Primaria de Las Maderas Tropicales de México. Rev. Mex. Ciencias For. 2018, 6, 202–221. [Google Scholar] [CrossRef][Green Version]
- SEMARNAT. Anuario Estadístico de La Producción Forestal 2017, 1st ed.; Secretaría de Medio Ambiente y Recursos Naturales: Guadalajara, México, 2018; 284p. [Google Scholar]
- Gilman, E.F.; Harchick, C. Root System Morphology Influences Lateral Stability of Swietenia Mahagoni. Arboric. Urban For. 2014, 40, 27–35. [Google Scholar] [CrossRef]
- Mexal, J.G.; Cuevas Rangel, R.A.; Negreros-Castillo, P.; Paraguirre Lezama, C. Nursery Production Practices Affect Survival and Growth of Tropical Hardwoods in Quintana Roo, Mexico. For. Ecol. Manag. 2002, 168, 125–133. [Google Scholar] [CrossRef]
- García de Salamone, I.E.; Hynes, R.K.; Nelson, L.M. Cytokinin Production by Plant Growth Promoting Rhizobacteria and Selected Mutants. Can. J. Microbiol. 2001, 47, 404–411. [Google Scholar] [CrossRef]
- López-Bucio, J.; Campos-Cuevas, J.C.; Hernández-Calderón, E.; Velásquez-Becerra, C.; Farías-Rodríguez, R.; Macías-Rodríguez, L.I.; Valencia-Cantero, E. Bacillus Megaterium Rhizobacteria Promote Growth and Alter Root-System Architecture Through an Auxin- and Ethylene-Independent Signaling Mechanism in Arabidopsis Thaliana. Mol. Plant-Microbe Interact. 2007, 20, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Khan, A.L.; You, Y.-H.; Kim, J.-G.; Kamran, M.; Lee, I.-J. Gibberellin Production by Newly Isolated Strain Leifsonia Soli SE134 and Its Potential to Promote Plant Growth. J. Microbiol. Biotechnol. 2014, 24, 106–112. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for Isolating and Characterizing ACC Deaminase-Containing Plant Growth-Promoting Rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef]
- Zhang, L.; Hurek, T.; Reinhold-Hurek, B. A NifH-Based Oligonucleotide Microarray for Functional Diagnostics of Nitrogen-Fixing Microorganisms. Microb. Ecol. 2007, 53, 456–470. [Google Scholar] [CrossRef]
- Khan, A.A.; Jilani, G.; Akhtar, M.S.; Saqlan, S.M.; Rasheed, M. Phosphorus Solubilizing Bacteria: Occurrence, Mechanisms and Their Role in Crop Production. J. Agric. Biol. Sci. 2009, 1, 48–58. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Plant Growth-Promoting Rhizobacteria Affect the Growth and Nutrient Uptake of Fraxinus Americana Container Seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 4617–4625. [Google Scholar] [CrossRef]
- Chanway, C.P.; Holl, F.B. First Year Field Performance of Spruce Seedlings Inoculated with Plant Growth Promoting Rhizobacteria. Can. J. Microbiol. 1993, 39, 1084–1088. [Google Scholar] [CrossRef]
- Cely, M.V.T.; Siviero, M.A.; Emiliano, J.; Spago, F.R.; Freitas, V.F.; Barazetti, A.R.; Goya, E.T.; Lamberti, G.D.S.; dos Santos, I.M.O.; De Oliveira, A.G.; et al. Inoculation of Schizolobium Parahyba with Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Increases Wood Yield under Field Conditions. Front. Plant Sci. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sitepu, I.R.; Aryanto; Ogita, N.; Osaki, M.; Santoso, E.; Tahara, S.; Hashidoko, Y. Screening of Rhizobacteria from Dipterocarp Seedlings and Saplings for the Promotion of Early Growth of Shorea Selanica Seedlings. Tropics 2007, 16, 245–252. [Google Scholar] [CrossRef]
- Mafia, R.G.; Alfenas, A.C.; Maffia, L.A.; Ferreira, E.M.; Binoti, D.H.B.; Mafia, G.M.V. Plant Growth Promoting Rhizobacteria as Agents in the Biocontrol of Eucalyptus Mini-Cutting Rot. Trop. Plant Pathol. 2009, 34, 10–17. [Google Scholar] [CrossRef][Green Version]
- Trápani, A.I.; Ulla, E.L.; Amigo, J.A.; Jimenez, P. Response of Cedrela Fissilis Vell. to Inoculation with Plant Growth-Promoting Rhizobacteria in Nurseries. Revista Agronómica del Noroeste Argentina 2021, 41, 93–98. [Google Scholar]
- Alexandre, F.S.; Della Flora, L.V.; Henrique, I.G.; da Silva, D.C.; Mercedes, A.P.; Cardoso Silva, A.; Silva de Oliveira, A.; Bondespacho da Silva, M.P.; Formelh Ronning, B.P.; Dreher, D.R.; et al. Arbuscular Mycorrhizal Fungi (Rhizophagus Clarus) and Rhizobacteria (Bacillus Subtilis) Can Improve the Clonal Propagation and Development of Teak for Commercial Plantings. Front. Plant Sci. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Asif, M.; Mughal, A.H.; Bisma, R.; Mehdi, Z.; Saima, S.; Ajaz, M.; Malik, M.A.; Masood, A.; Sidique, S. Application of Different Strains of Biofertilizers for Raising Quality Forest Nursery. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3680–3686. [Google Scholar] [CrossRef]
- Muthukumar, T.; Udaiyan, K. Coinoculation of Bioinoculants Improve Acacia Auriculiformis Seedling Growth and Quality in a Tropical Alfisol Soil. J. For. Res. 2018, 29, 663–673. [Google Scholar] [CrossRef]
- Tailor, A.J.; Joshi, B.H. Harnessing Plant Growth Promoting Rhizobacteria Beyond Nature: A Review. J. Plant Nutr. 2014, 37, 1534–1571. [Google Scholar] [CrossRef]
- Gao, N.; Zhang, H.; Xiong, R.; Fang, L.; Shen, W.; Senoo, K. Different Strategies for Colonization and Prevalence after Inoculation with Plant Growth-Promoting Rhizobacteria Revealed by a Monitoring Method. Soil Sci. Plant Nutr. 2022, 68, 442–453. [Google Scholar] [CrossRef]
- Vasconcellos, R.L.F.; Romagnoli, E.M.; Taketani, R.G.; Santos, S.N.; Zucchi, T.D.; Melo, I.S. Impact of Inoculation with Pseudomonas Aestus CMAA 1215T on the Non-Target Resident Bacterial Community in a Saline Rhizosphere Soil. Curr. Microbiol. 2021, 78, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Elisea, F.I. Capacidad de Solubilización de Fosfato de Aluminio Por Bacterias Edáficas Promotoras de Crecimiento Vegetal. Bachelor’s Thesis, Universidad Michoacana de San Nicolás de Hidalgo, Uruapan, Mexico, 2015; 96p. [Google Scholar]
- Becerra-Lucio, A.A. Capacidad Solubilizadora de Fósforo En Bacterias Edáficas Promotoras Del Crecimiento Vegetal Aisladas En Uruapan, Michoacán. Bachelor’s Thesis, Universidad Michoanana de San Nicolás de Hidalgo, Uruapan, Mexico, 2015; 86p. [Google Scholar]
- Cutting, S.; Vander Horn, P. Genetic Analysis. In Molecular Biological Methods for Bacillus (Modern Microbiological Methods); Hardwood, C.R., Cutting, S.M., Eds.; Wiley: Chichester, UK, 1990; p. 581. ISBN 0471923931. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Shahzad, Z.; Kellermeier, F.; Armstrong, E.M.; Rogers, S.; Lobet, G.; Amtmann, A.; Hills, A. EZ-Root-VIS: A Software Pipeline for the Rapid Analysis and Visual Reconstruction of Root System Architecture. Plant Physiol. 2018, 217. [Google Scholar] [CrossRef]
- Martínez, C.R.C.; Gutiérrez, E.J.C.; Arrazate, C.H.A. (Eds.) Protocolo de Multiplicación y Conservación in Vitro de Cuatro Especies Forestales Tropicales de Semillas Recalcitrantes CASO: Caoba (Swietenia Macrophylla), 1st ed. 2019. Available online: https://www.gob.mx/cms/uploads/attachment/file/126297/Protocolo_de_multiplicacion_y_conservacion_in_vitro_de_cuatro_especies_forestales_tropicales_de_semillas_recalcitrantes.pdf (accessed on 1 January 2019).
- Angulo, V.C.; Sanfuentes, E.A.; Rodríguez, F.; Sossa, K.E. Caracterización de Rizobacterias Promotoras de Crecimiento En Plántulas de Eucalyptus Nitens. Rev. Argent. Microbiol. 2014, 46, 338–347. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- de Souza, N.L.; Rocha, S.S.; Narezzi, N.T.; Tiepo, A.N.; de Oliveira, A.L.M.; Oliveira, H.C.; Bianchini, E.; Pimenta, J.A.; Stolf-Moreira, R. Differential Impacts of Plant Growth-Promoting Bacteria (PGPB) on Seeds of Neotropical Tree Species with Contrasting Tolerance to Shade. Trees 2020, 34, 121–132. [Google Scholar] [CrossRef]
- González, P.; Sossa, K.; Rodríguez, F.; Sanfuentes, E. Rhizobacteria Strains as Promoters of Rooting in Hybrids of Eucalyptus Nitens × Eucalyptus Globulus. Chil. J. Agric. Res. 2018, 78, 3–12. [Google Scholar] [CrossRef]
- Jha, K.S.; Saraf, M. Effect of Plant Growth Promoting Rhizobacteria on Seed Germination Behaviour and Seedling Vigor of Jatropha Curcas. Int. J. Biotechnol. Biosci. 2011, 1, 161–169. [Google Scholar]
- Verbon, E.H.; Liberman, L.M. Beneficial Microbes Affect Endogenous Mechanisms Controlling Root Development. Trends Plant Sci. 2016, 21, 218–229. [Google Scholar] [CrossRef]
- Benková, E.; Bielach, A. Lateral Root Organogenesis—From Cell to Organ. Curr. Opin. Plant Biol. 2010, 13, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Dobbelaere, S.; Croonenborghs, A.; Thys, A.; Vande Broek, A.; Vanderleyden, J. Phytostimulatory Effect of Azospirillum Brasilense Wild Type and Mutant Strains Altered in IAA Production on Wheat. Plant Soil 1999, 6, 155–164. [Google Scholar] [CrossRef]
- Ortíz-Castro, R.; Martínez-Trujillo, M.; López-Bucio, J. N -Acyl-L-Homoserine Lactones: A Class of Bacterial Quorum-Sensing Signals Alter Post-Embryonic Root Development in Arabidopsis Thaliana. Plant. Cell Environ. 2008, 31, 1497–1509. [Google Scholar] [CrossRef]
- Ortiz-Castro, R.; López-Bucio, J.S.; López-Bucio, J. Physiological and Molecular Mechanisms of Bacterial Phytostimulation. In Advances in PGPR Research; Sing, H.B., Sarma, B.K., Keswani, C., Eds.; CABI: Wallingford, UK, 2017; pp. 16–28. [Google Scholar]
- Pérez-Flores, P.; Valencia-Cantero, E.; Altamirano-Hernández, J.; Pelagio-Flores, R.; López-Bucio, J.; García-Juárez, P.; Macías-Rodríguez, L. Bacillus Methylotrophicus M4-96 Isolated from Maize (Zea Mays) Rhizoplane Increases Growth and Auxin Content in Arabidopsis Thaliana via Emission of Volatiles. Protoplasma 2017, 254, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- García, J.A.L.; Domenech, J.; Santamaría, C.; Camacho, M.; Daza, A.; Mañero, F.J.G. Growth of Forest Plants (Pine and Holm-Oak) Inoculated with Rhizobacteria: Relationship with Microbial Community Structure and Biological Activity of Its Rhizosphere. Environ. Exp. Bot. 2004, 52, 239–251. [Google Scholar] [CrossRef]
- dos Santos, R.F.; da Cruz, S.P.; Botelho, G.R.; Flores, A.V. Inoculation of Pinus Taeda Seedlings with Plant Growth-Promoting Rhizobacteria. Floresta e Ambient. 2018, 25, 1–7. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Patel, J.S.; Yadav, S.K.; Bajpai, R.; Teli, B.; Rashid, M. PGPR Secondary Metabolites: An Active Syrup for Improvement of Plant Health. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Sharma, V., Salwan, R., Al-Ani, L.K.T., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 195–208. ISBN 978-0-12-818469-1. [Google Scholar]
- Vivas, M.; Kemler, M.; Slippers, B. Maternal Effects on Tree Phenotypes: Considering the Microbiome. Trends Plant Sci. 2015, 20, 541–544. [Google Scholar] [CrossRef]
- Becerra-Lucio, A.A.; Labrín-Sotomayor, N.Y.; Becerra-Lucio, P.A.; Trujillo-Elisea, F.I.; Chávez-Bárcenas, A.T.; Machkour-M’Rabet, S.; Peña-Ramírez, Y.J. Diversity and Interactomics of Bacterial Communities Associated with Dominant Trees during Tropical Forest Recovery. Curr. Microbiol. 2021. [Google Scholar] [CrossRef]
- Estrada-Medina, H.; Canto-Canché, B.B.; De los Santos-Briones, C.; O’Connor-Sánchez, A. Yucatán in Black and Red: Linking Edaphic Analysis and Pyrosequencing-Based Assessment of Bacterial and Fungal Community Structures in the Two Main Kinds of Soil of Yucatán State. Microbiol. Res. 2016, 188–189, 23–33. [Google Scholar] [CrossRef]
- Pajares, S.; Campo, J.; Bohannan, B.J.M.; Etchevers, J.D. Environmental Controls on Soil Microbial Communities in a Seasonally Dry Tropical Forest. Appl. Environ. Microbiol. 2018, 84, e00342-18. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trujillo-Elisea, F.I.; Labrín-Sotomayor, N.Y.; Becerra-Lucio, P.A.; Becerra-Lucio, A.A.; Martínez-Heredia, J.E.; Chávez-Bárcenas, A.T.; Peña-Ramírez, Y.J. Plant Growth and Microbiota Structural Effects of Rhizobacteria Inoculation on Mahogany (Swietenia macrophylla King [Meliaceae]) under Nursery Conditions. Forests 2022, 13, 1742. https://doi.org/10.3390/f13101742
Trujillo-Elisea FI, Labrín-Sotomayor NY, Becerra-Lucio PA, Becerra-Lucio AA, Martínez-Heredia JE, Chávez-Bárcenas AT, Peña-Ramírez YJ. Plant Growth and Microbiota Structural Effects of Rhizobacteria Inoculation on Mahogany (Swietenia macrophylla King [Meliaceae]) under Nursery Conditions. Forests. 2022; 13(10):1742. https://doi.org/10.3390/f13101742
Chicago/Turabian StyleTrujillo-Elisea, Flor I., Natalia Y. Labrín-Sotomayor, Patricia A. Becerra-Lucio, Angel A. Becerra-Lucio, Jorge E. Martínez-Heredia, Ana T. Chávez-Bárcenas, and Yuri J. Peña-Ramírez. 2022. "Plant Growth and Microbiota Structural Effects of Rhizobacteria Inoculation on Mahogany (Swietenia macrophylla King [Meliaceae]) under Nursery Conditions" Forests 13, no. 10: 1742. https://doi.org/10.3390/f13101742
APA StyleTrujillo-Elisea, F. I., Labrín-Sotomayor, N. Y., Becerra-Lucio, P. A., Becerra-Lucio, A. A., Martínez-Heredia, J. E., Chávez-Bárcenas, A. T., & Peña-Ramírez, Y. J. (2022). Plant Growth and Microbiota Structural Effects of Rhizobacteria Inoculation on Mahogany (Swietenia macrophylla King [Meliaceae]) under Nursery Conditions. Forests, 13(10), 1742. https://doi.org/10.3390/f13101742





