The Current and Future Potential Geographical Distribution and Evolution Process of Catalpa bungei in China
Abstract
:1. Introduction
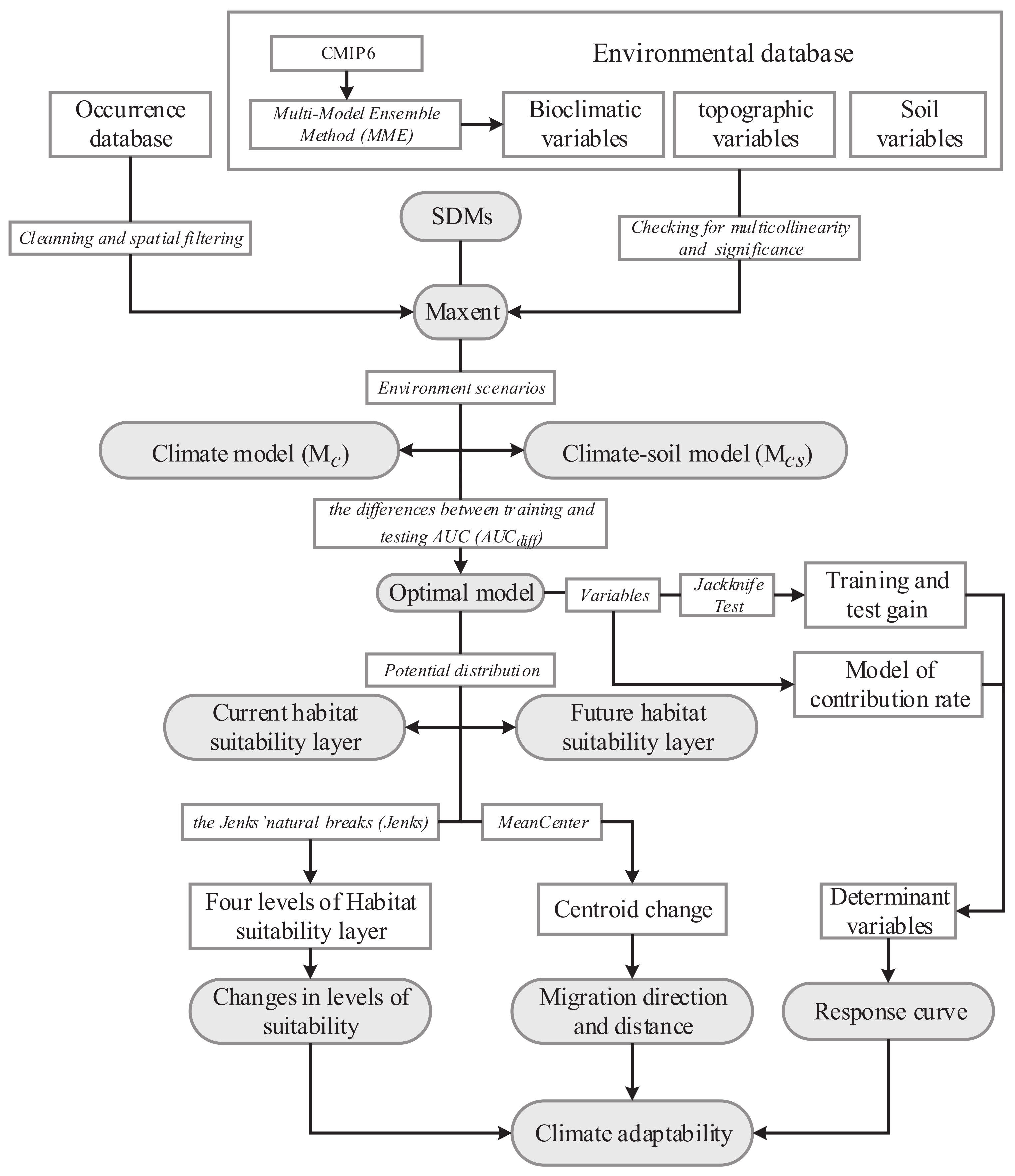
2. Materials and Methods
2.1. Collecting Species Occurrence Data
2.2. Environmental Data
2.3. Construction and Validation of Maxent
3. Results
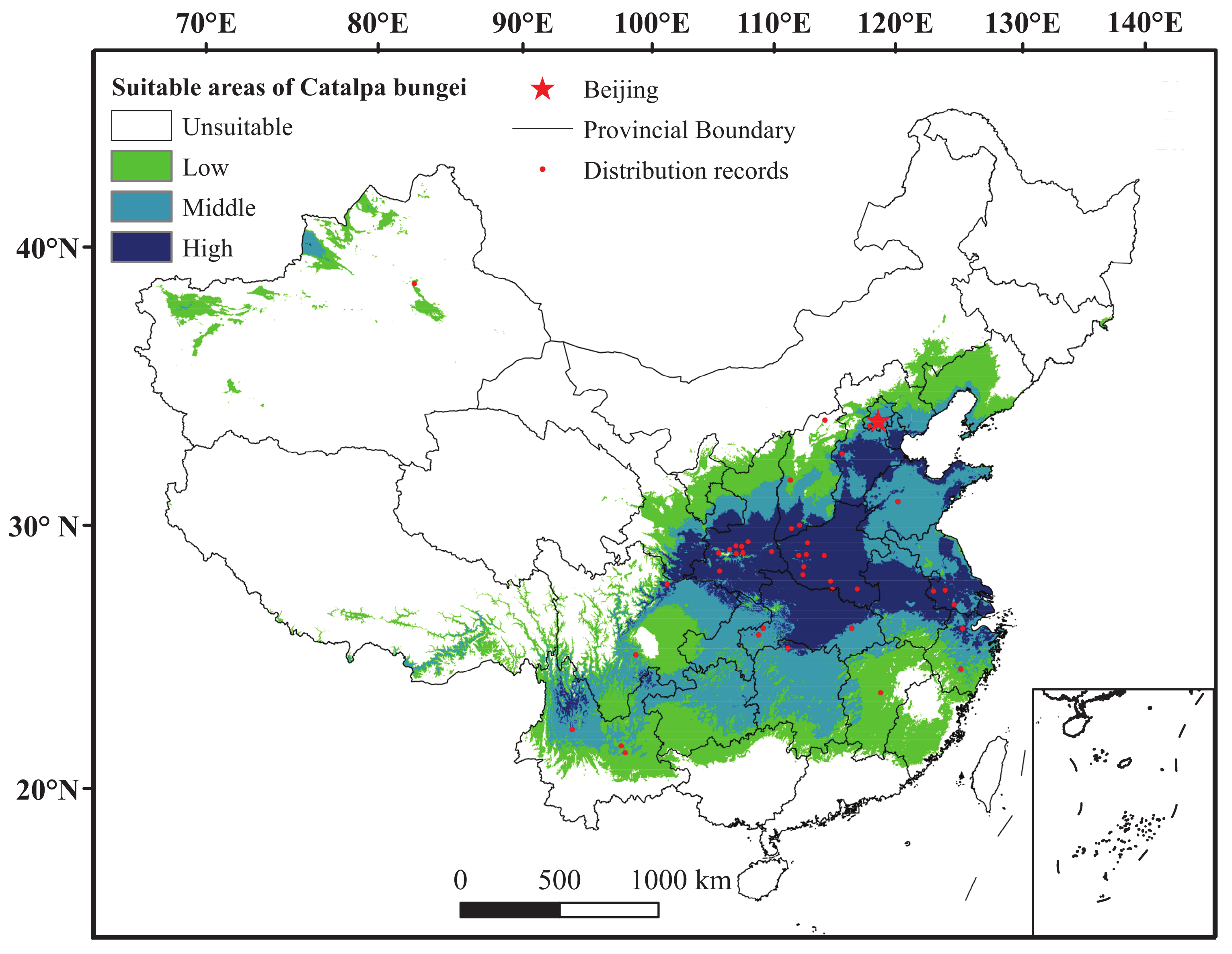
3.1. Comparison and Evaluation of Maxent under Current Climate
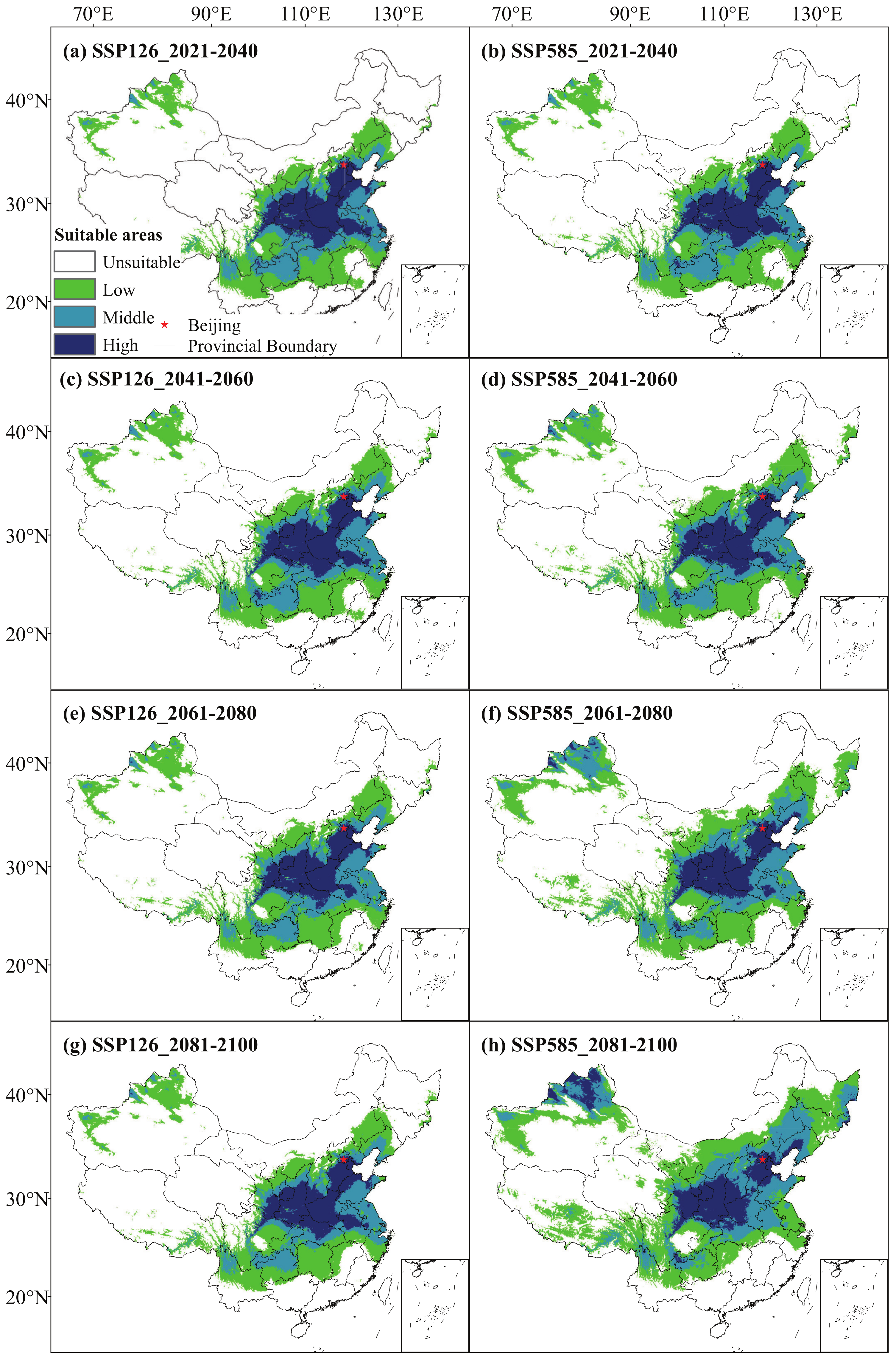
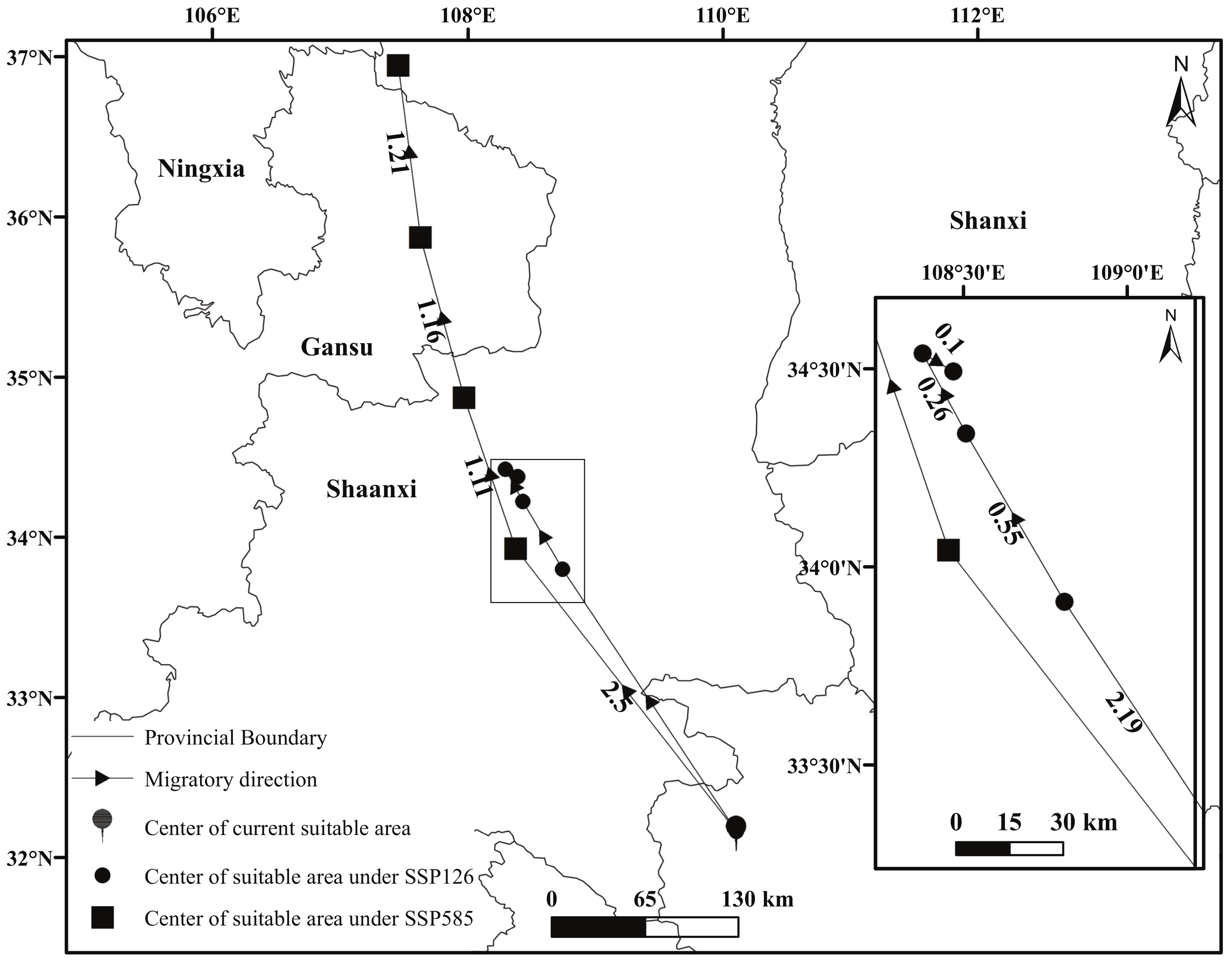
3.2. Potential Distribution of C. bungei under Future Climate
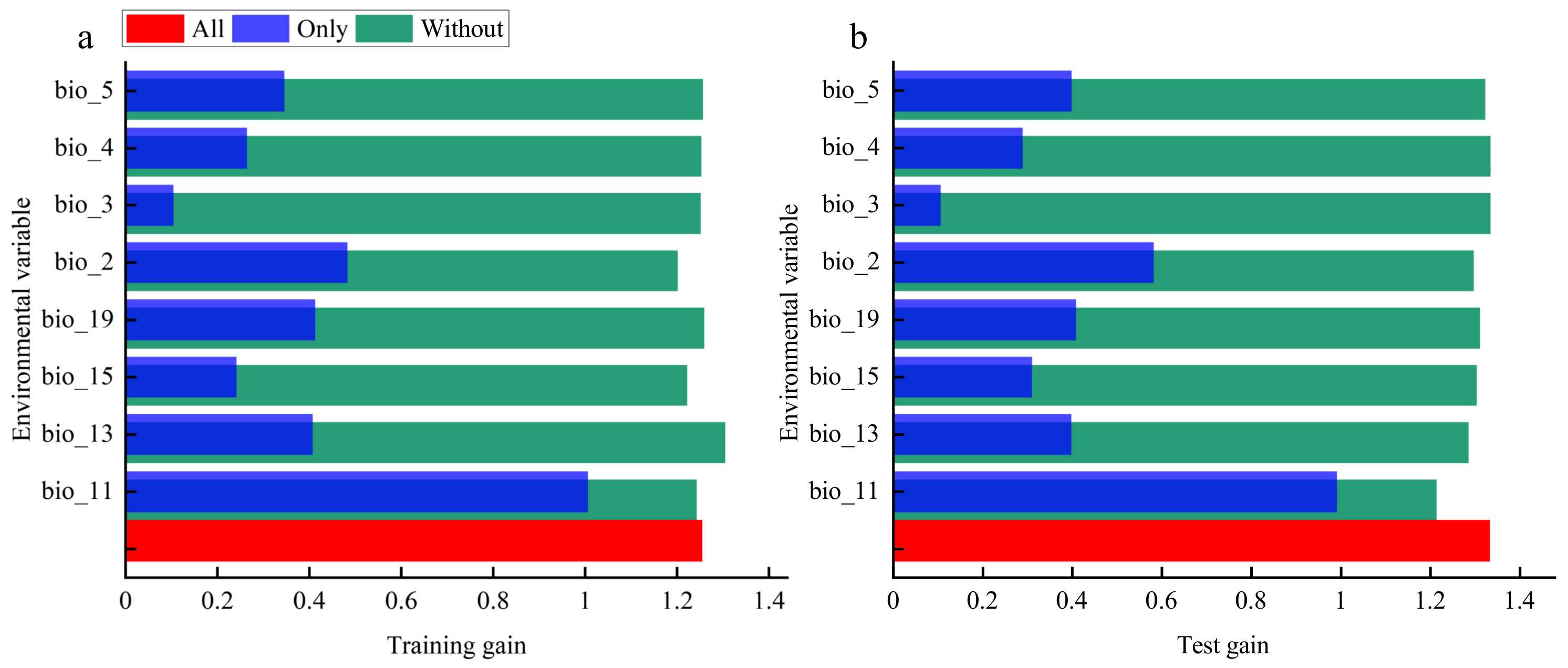
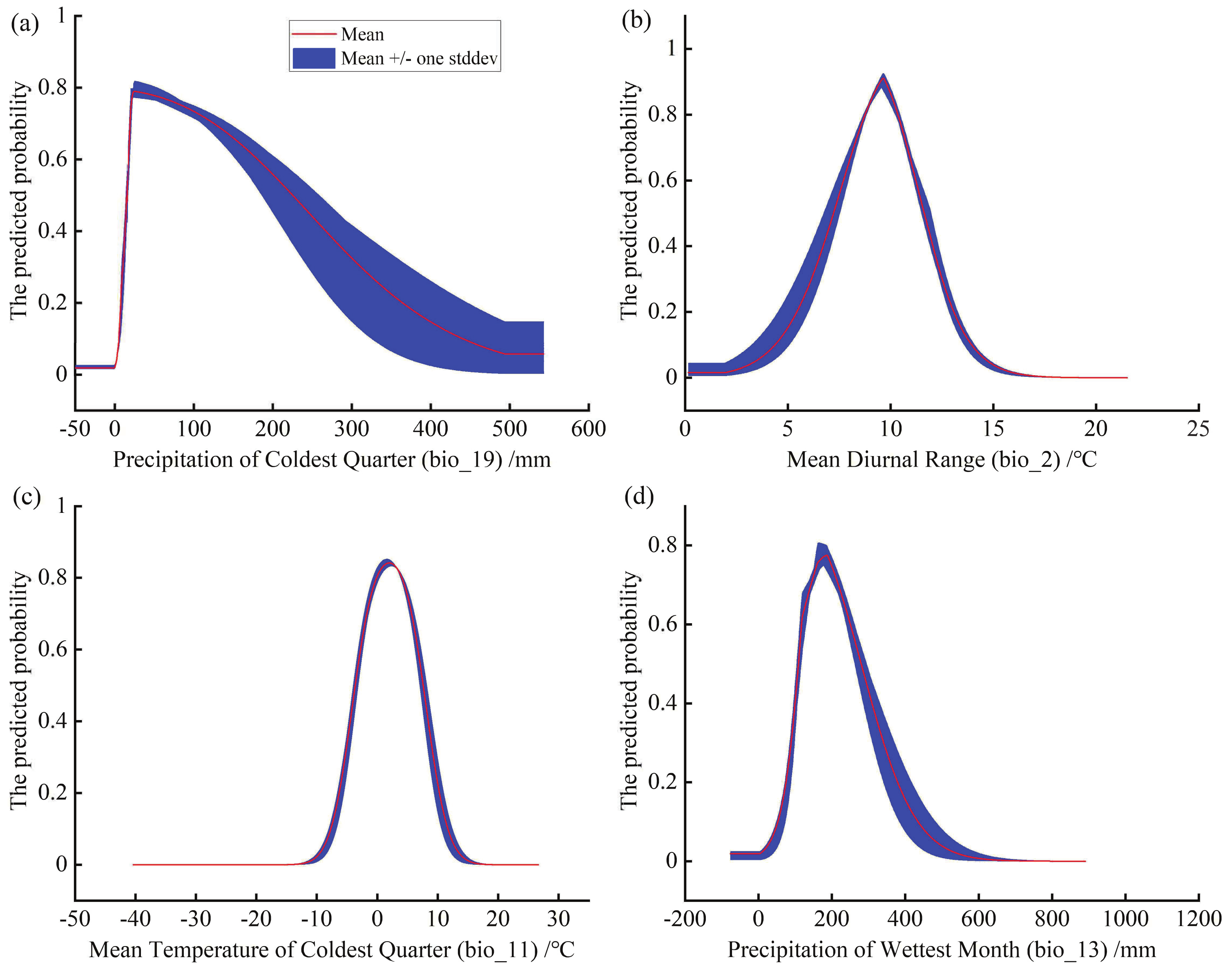
3.3. The Dominant Environmental Factors Influencing Potential Distribution of C. bungei
4. Discussion
4.1. Model Evaluation
4.2. Key Environmental Variables and Current Spatial Distribution
4.3. Potential Distribution of C. bungei under Future Climate
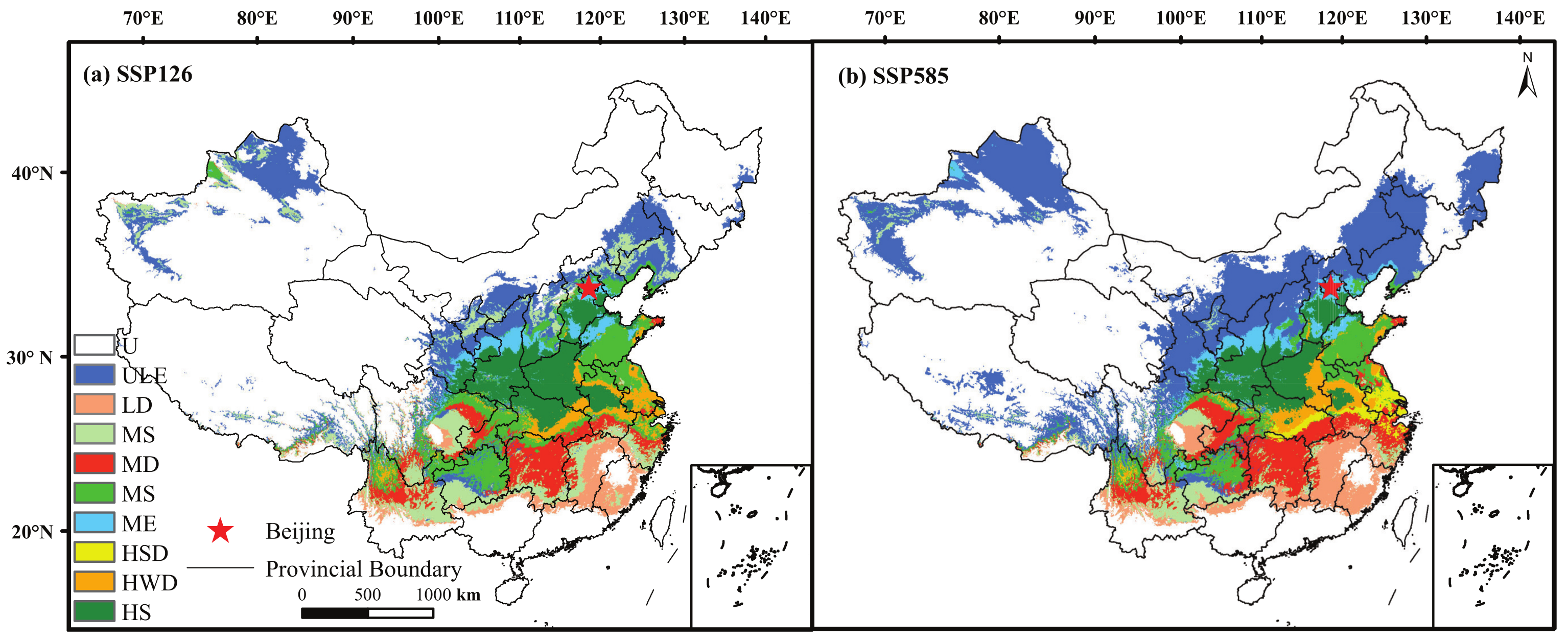
4.4. The Distribution of Plantation and Its Growth Subregions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Çoban, H.O.; Örücü, Ö.K.; Arslan, E.S. MaxEnt Modeling for Predicting the Current and Future Potential Geographical Distribution of Quercus libani Olivier. Sustainability 2020, 12, 2671. [Google Scholar] [CrossRef] [Green Version]
- Arslan, E.S.; Akyol, A.; Örücü, Ö.K.; Sarıkaya, A.G. Distribution of rose hip (Rosa canina L.) under current and future climate conditions. Reg. Environ. Chang. 2020, 20, 107. [Google Scholar] [CrossRef]
- Moritz, C.; Agudo, R. The future of species under climate change: Resilience or decline? Science 2013, 341, 504–508. [Google Scholar] [CrossRef]
- Varol, T.; Canturk, U.; Cetin, M.; Ozel, H.B.; Sevik, H. Impacts of climate change scenarios on European ash tree (Fraxinus excelsior L.) in Turkey. Forest Ecol. Manag. 2021, 491, 119199. [Google Scholar] [CrossRef]
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A. Pervasive shifts in forest dynamics in a changing world. Science 2020, 368, 964. [Google Scholar] [CrossRef] [PubMed]
- Working Groups—IPCC. 2021. Available online: https://www.ipcc.ch/working-groups (accessed on 1 November 2021).
- Svenning, J.C.; Sandel, B. Disequilibrium vegetation dynamics under future climate change. Am. J. Bot. 2013, 100, 1266–1286. [Google Scholar] [CrossRef] [PubMed]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef]
- Koo, K.; Park, S.; Seo, C. Effects of Climate Change on the Climatic Niches of Warm-Adapted Evergreen Plants: Expansion or Contraction? Forests 2017, 8, 500. [Google Scholar] [CrossRef] [Green Version]
- Warren, R.; VanDerWal, J.; Price, J.; Welbergen, J.A.; Atkinson, I.; Ramirez-Villegas, J.; Osborn, T.J.; Jarvis, A.; Shoo, L.P.; Williams, S.E.; et al. Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nat. Clim. Chang. 2013, 3, 678–682. [Google Scholar] [CrossRef] [Green Version]
- Pecchi, M.; Marchi, M.; Burton, V.; Giannetti, F.; Moriondo, M.; Bernetti, I.; Bindi, M.; Chirici, G. Species distribution modelling to support forest management. A literature review. Ecol. Model. 2019, 411, 108817. [Google Scholar] [CrossRef]
- Booth, T.H. Species distribution modelling tools and databases to assist managing forests under climate change. For. Ecol. Manag. 2018, 430, 196–203. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Briscoe Runquist, R.D.; Lake, T.; Tiffin, P.; Moeller, D.A. Species distribution models throughout the invasion history of Palmer amaranth predict regions at risk of future invasion and reveal challenges with modeling rapidly shifting geographic ranges. Sci. Rep. 2019, 9, 2426. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Yu, Y.; Zhang, J.; Li, Z.; Zhang, Y.; Volis, S. Conservation strategy for aquatic plants: Endangered Ottelia acuminata (Hydrocharitaceae) as a case study. Biodivers. Conserv. 2019, 28, 1533–1548. [Google Scholar] [CrossRef]
- Still, S.M.; Frances, A.L.; Treher, A.C.; Oliver, L. Using Two Climate Change Vulnerability Assessment Methods to Prioritize and Manage Rare Plants: A Case Study. Nat. Area J. 2015, 35, 106–121. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Lissovsky, A.A.; Dudov, S.; Obolenskaya, E. Advantages and limitations of application of the species distribution modeling methods. 1.A general approach. Zhurnal Obs. Biol. 2020, 81, 123–134. [Google Scholar] [CrossRef]
- Gebrewahid, Y.; Abrehe, S.; Meresa, E.; Eyasu, G.; Abay, K.; Gebreab, G.; Kidanemariam, K.; Adissu, G.; Abreha, G.; Darcha, G. Current and future predicting potential areas of Oxytenanthera abyssinica (A. Richard) using MaxEnt model under climate change in Northern Ethiopia. Ecol. Process. 2020, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Wen, Z.; Liu, Y.; Guo, Q.; Jiang, Y.; Ren, H.; Fan, Y.; Yang, Y. Integrating Habitat Suitability and the Near-Nature Restoration Priorities into Revegetation Plans Based on Potential Vegetation Distribution. Forests 2021, 12, 218. [Google Scholar] [CrossRef]
- Lim, C.; Yoo, S.; Choi, Y.; Jeon, S.; Son, Y.; Lee, W. Assessing Climate Change Impact on Forest Habitat Suitability and Diversity in the Korean Peninsula. Forests 2018, 9, 259. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Ma, L.; Li, Y.; Wang, S.A.; Li, L.; Yang, R.; Ma, Y.; Wang, Q. Transcriptome profiling of indole-3-butyric acid-induced adventitious root formation in softwood cuttings of the Catalpa bungei variety ‘YU-1’at different developmental stages. Genes Genom. 2016, 38, 145–162. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, C.; Chen, W.; Yang, G.; Wang, J.; Nan, X.; Wang, C. Evaluation on the Resistance of Different Catalpa bungei Clones against Omphis aplagialis. J. Northwest For. Univ. 2020, 35, 133–140. [Google Scholar]
- Zhao, K.; Wu, J.; Cheng, Y.; Wang, X.; Liao, D.; Liu, W. Study on cuttage breeding of Catalpa bungei clone shoots. J. Cent. South Univ. For. Technol. 2010, 30, 66–69. [Google Scholar]
- Xiao, Y.; Ma, W.; Lu, N.; Wang, Z.; Wang, N.; Zhai, W.; Kong, L.; Qu, G.; Wang, Q.; Wang, J. Genetic Variation of Growth Traits and Genotype-by-Environment Interactions in Clones of Catalpa bungei and Catalpa fargesii f. duclouxii. Forests 2019, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Zhai, W.; Ma, W.; Wang, Q.; Wang, P.; Wang, J. Combined Selection of Excellent Families and Plus Trees of Catalpa bungei at Nursery Stage. J. Northwest For. Univ. 2012, 27, 68–71. [Google Scholar]
- Wu, L.; Wang, J.; Lin, J. Study on Plant Resources of Catalpa bungei. Agric. Sci. China 2010, 28, 91–96. (In Chinese) [Google Scholar]
- Li, Y.; Li, Z.; Miao, X.; Wang, Z.; Wang, J.; Ma, W. Bioassay for Salt Tolerance of Six Catalpa ovata Provenances. For. Res. 2019, 32, 106–114. [Google Scholar] [CrossRef]
- Jia, X.; Shao, M.; Zhu, Y.; Luo, Y. Soil moisture decline due to afforestation across the Loess Plateau, China. J. Hydrol. 2017, 546, 113–122. [Google Scholar] [CrossRef]
- Huang, J.; Li, G.; Li, J.; Zhang, X.; Yan, M.; Du, S. Projecting the Range Shifts in Climatically Suitable Habitat for Chinese Sea Buckthorn under Climate Change Scenarios. Forests 2017, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Fourcade, Y.; Engler, J.O.; Rödder, D.; Secondi, J. Mapping species distributions with MAXENT using a geographically biased sample of presence data: A performance assessment of methods for correcting sampling bias. PLoS ONE 2014, 9, e97122. [Google Scholar] [CrossRef] [Green Version]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, B.; Xu, Y.; Han, Z. CMIP6 Evaluation and Projection of Temperature and Precipitation over China. Adv. Atmos. Sci. 2021, 38, 817–830. [Google Scholar] [CrossRef]
- Yazdandoost, F.; Moradian, S.; Izadi, A.; Aghakouchak, A. Evaluation of CMIP6 precipitation simulations across different climatic zones: Uncertainty and model intercomparison. Atmos. Res. 2020, 250, 05369. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhang, J.; Liang, J. Projected Temperature and Precipitation Changes over Major Land Regions of the Belt and Road Initiative under the 1.5 °C and 2 °C Climate Targets by the CMIP6 Multi-Model Ensemble. Clim. Environ. Res. 2021, 26, 374–390. [Google Scholar]
- Fordham, D.A.; Wigley, T.M.L.; Brook, B.W. Multi-model climate projections for biodiversity risk assessments. Ecol. Appl. 2011, 21, 3317–3331. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Liang, Y.; Pandey, R.; Papeş, M. Collinearity in ecological niche modeling: Confusions and challenges. Ecol. Evol. 2019, 9, 10365–10376. [Google Scholar] [CrossRef] [Green Version]
- Rodda, G.H.; Jarnevich, C.S.; Reed, R.N. Challenges in identifying sites climatically matched to the native ranges of animal invaders. PLoS ONE 2011, 6, e14670. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Graham, J.; West, A.M.; Evangelista, P.H. Using district-level occurrences in MaxEnt for predicting the invasion potential of an exotic insect pest in India. Comput. Electron. Agric. 2014, 103, 55–62. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Hijmans, R.J. Cross-validation of species distribution models: Removing spatial sorting bias and calibration with a null model. Ecology 2012, 93, 679–688. [Google Scholar] [CrossRef]
- Fois, M.; Cuena-Lombraña, A.; Fenu, G.; Bacchetta, G. Using species distribution models at local scale to guide the search of poorly known species: Review, methodological issues and future directions. Ecol. Model. 2018, 385, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Foden, W.B.; Young, B.E.; Akçakaya, H.R.; Garcia, R.A.; Hoffmann, A.A.; Stein, B.A.; Thomas, C.D.; Wheatley, C.J.; Bickford, D.; Carr, J.A.; et al. Climate change vulnerability assessment of species. Wires. Clim. Chang. 2019, 10, e551. [Google Scholar] [CrossRef] [Green Version]
- Bradie, J.; Leung, B. A quantitative synthesis of the importance of variables used in MaxEnt species distribution models. J. Biogeogr. 2017, 44, 1344–1361. [Google Scholar] [CrossRef]
- Thuiller, W.; Guéguen, M.; Renaud, J.; Karger, D.N.; Zimmermann, N.E. Uncertainty in ensembles of global biodiversity scenarios. Nat. Commun. 2019, 10, 1446. [Google Scholar] [CrossRef]
- Beale, C.M.; Lennon, J.J.; Gimona, A. Opening the climate envelope reveals no macroscale associations with climate in European birds. Proc. Natl. Acad. Sci. USA 2008, 105, 14908–14912. [Google Scholar] [CrossRef] [Green Version]
- Shukla, P.K.; Baradevanal, G.; Rajan, S.; Fatima, T. MaxEnt prediction for potential risk of mango wilt caused by Ceratocystis fimbriata Ellis and Halst under different climate change scenarios in India. J. Plant Pathol. 2020, 102, 765–773. [Google Scholar] [CrossRef]
- Kumar, S.; Neven, L.G.; Zhu, H.; Zhang, R. Assessing the global risk of establishment of Cydia pomonella (Lepidoptera: Tortricidae) using CLIMEX and MaxEnt niche models. J. Econ. Entomol. 2015, 108, 1708–1719. [Google Scholar] [CrossRef]
- Title, P.O.; Bemmels, J.B. ENVIREM: An expanded set of bioclimatic and topographic variables increases flexibility and improves performance of ecological niche modeling. Ecography 2018, 41, 291–307. [Google Scholar] [CrossRef] [Green Version]
- Feng, X. The Position and Development of Catalpa bungei in Forestry Ecological System. Farm. Consult. 2017, 22, 78. (In Chinese) [Google Scholar]
- Zhao, F.; Lin, G.H.; Zhao, Z.Z. The response of temporal and spatial change of vegetation index to hydrothermal condition in the Three-Rivers Headwaters Region. Pratacult. Sci. 2011, 28, 1095–1100. [Google Scholar]
- Li, W.; Li, C.; Liu, X.; He, D.; Bao, A.; Yi, Q.; Wang, B.; Liu, T. Analysis of spatial-temporal variation in NPP based on hydrothermal conditions in the Lancang-Mekong River Basin from 2000 to 2014. Environ. Monit. Assess. 2018, 190, 321. [Google Scholar] [CrossRef]
- Han, E.; Luo, W.; Zhao, H.; Tang, D.; Zhang, W.; Liu, M.; Gao, H. A Study on Seedling Culture and Afforestation techniques of Catalpa bungei. J. Northwest For. Univ. 2002, 17, 19–23. [Google Scholar]
- Chen, H.; Fan, X.; Qiao, S.; Zhang, X. Key Points of High Yield Cultivation Techniques of Catalpa bungei in Jianghan Plain. Hubei For. Sci. Technol. 2013, 42, 77–78. (In Chinese) [Google Scholar]
- Peng, F.; Zhang, L.; Zhang, L.; Hao, M.; Liang, Y.; Liu, Q. Status and Protection Strategy of Yuntai Mountain Catalpa Tree Resources in Lianyungang. J. Jiangsu For. Sci. Technol. 2013, 40, 27–30. (In Chinese) [Google Scholar]
- Pei, F.; Zhou, Y.; Xia, Y. Assessing the Impacts of Extreme Precipitation Change on Vegetation Activity. Agriculture 2021, 11, 487. [Google Scholar] [CrossRef]
- Li, S.; Wei, F.; Wang, Z.; Shen, J.; Liang, Z.; Wang, H.; Li, S. Spatial Heterogeneity and Complexity of the Impact of Extreme Climate on Vegetation in China. Sustainability 2021, 13, 5748. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Y.; He, G.; Xue, D.; Li, C. Coupling effects of water and fertilizer on seedling growth and nutrient status of Catalpa bungei. J. Beijing For. Univ. 2018, 40, 58–67. [Google Scholar]
- Wu, D.; Zhao, X.; Liang, S.; Zhou, T.; Huang, K.; Tang, B.; Zhao, W. Time-lag effects of global vegetation responses to climate change. Glob. Change Biol. 2015, 21, 3520–3531. [Google Scholar] [CrossRef]
- Li, G.; Wen, Z.; Guo, K.; Du, S. Simulating the Effect of Climate Change on Vegetation Zone Distribution on the Loess Plateau, Northwest China. Forests 2015, 6, 2092–2108. [Google Scholar] [CrossRef] [Green Version]
- Jiangyue, L.; Hong, C.; Tong, L.; Chi, Z. The potential geographical distribution of Haloxylon across Central Asia under climate change in the 21st century. Agr. For. Meteorol. 2019, 275, 243–254. [Google Scholar] [CrossRef]
- Xie, C.; Huang, B.; Jim, C.Y.; Han, W.; Liu, D. Predicting differential habitat suitability of Rhodomyrtus tomentosa under current and future climate scenarios in China. For. Ecol. Manag. 2021, 501, 119696. [Google Scholar] [CrossRef]
- Guo, K.; Jiang, X.; Xu, G. Potential Suitable Area of Cyclobalanopsis glauca and Impact of Climate Change on Its Distribution. Chin. J. Ecol. 2021, 40, 2563–2574. (In Chinese) [Google Scholar]
- Fan, Z.; Fan, B. Shifts of the mean centers of potential vegetation ecosystems under future climate change in Eurasia. Forests 2019, 10, 873. [Google Scholar] [CrossRef] [Green Version]
- Corlett, R.T.; Westcott, D.A. Will plant movements keep up with climate change? Trends Ecol. Evol. 2013, 28, 482–488. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhang, X.; Qin, H.; Wang, G.; Wang, W. Impacts of climate change on the suitable habitats and spatial migration of Xanthoceras sorbifolia. China Environ. Sci. 2020, 40, 4597–4606. [Google Scholar]
- Wang, S.; Guo, H.; Wang, X.; Fan, W. Dispersal Limitation Versus Environment Filtering in the Assembly of Plant Communities in the Ziwu Mountains. Sci. Agric. Sin. 2013, 46, 4733–4744. [Google Scholar]
- Sun, R.; Chen, S.; Su, H. Spatiotemporal variation of NDVI in different ecotypes on the Loess Plateau and its response to climate change. Geogr. Res 2020, 39, 1200–1214. [Google Scholar]
- Chen, I.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.C.; Zarnetske, P.L.; Skelly, D.K. Moving forward: Dispersal and species interactions determine biotic responses to climate change. Ann. N. Y. Acad. Sci. 2013, 1297, 44–60. [Google Scholar] [CrossRef]
- Peng, F.; Hao, M.; Liang, Y.; Wang, L. Current Situation and Development Strategy of Catalpa bungei Germplasm Resources in China. J. For. Eng. 2011, 25, 1–5. (In Chinese) [Google Scholar]
- Guan, Z.; Zhao, J.; Qiu, Q.; Ma, W.; Wang, S.; Feng, X.; Su, Y.; Wang, J.; Li, J.; He, Q. Growth Law and Optimal Growth Model of Catalpa bungei Plantation -A Case Study of Catalpa bungei in Luoning County, Henan Province. For. Eng. 2021, 37, 1–10. (In Chinese) [Google Scholar]
- The Henan Forestry Administration. The Upgrading Project Planning of Henan Forestry Ecological Construction ( 2013–2017 ); The Henan Forestry Administration: Zhengzhou, China, 2013; p. 25. [Google Scholar]

| GCMs | Country | Applicability of Temperature/Precipitation | Bioclimatic Variables (see Table 2) | MME |
|---|---|---|---|---|
| BCC-CSM2-MR | China | Poor/Good | Yes | Adopted |
| CNRM-CM6-1 | France | Good/Good | Yes | Adopted |
| CNRM-ESM2-1 | France | Poor/Poor | Yes | Rejected |
| CanESM5 | Canada | Poor/Poor | No | Rejected |
| GFDL-ESM4 | America | Good/Poor | Yes | Rejected |
| IPSL-CM6A-LR | France | Poor/Poor | Yes | Rejected |
| MIROC-ES2L | Japan | Good/Good | Yes | Adopted |
| MIROC6 | Japan | Poor/Poor | Yes | Rejected |
| MRI-ESM2-0 | Japan | Good/Good | Yes | Adopted |
| Variable Abbreviation | Variable description | Unit |
|---|---|---|
| bio_1 | Annual mean temperature | C |
| bio_2 | Mean diurnal range (mean of monthly (maxtemp–mintemp) | C |
| bio_3 | Isothermality (bio_2/bio_7 × 100) | - |
| bio_4 | Temperature seasonality (standard deviation × 100) | - |
| bio_5 | Max temperature of warmest month | C |
| bio_6 | Min temperature of coldest month | C |
| bio_7 | Temperature annual range (bio_5-bio_6) | C |
| bio_8 | Mean temperature of wettest quarter | C |
| bio_9 | Mean temperature of driest quarter | C |
| Bio_10 | Mean temperature of warmest quarter | C |
| bio_11 | Mean temperature of coldest quarter | C |
| bio_12 | Annual precipitation | mm |
| bio_13 | Precipitation of wettest month | mm |
| bio_14 | Precipitation of driest month | mm |
| bio_15 | Precipitation seasonality (coefficient of variation) | mm |
| bio_16 | Precipitation of wettest quarter | mm |
| bio_17 | Precipitation of driest quarter | mm |
| bio_18 | Precipitation of warmest quarter | mm |
| bio_19 | Precipitation of coldest uarter | mm |
| S-Type | Soil type | - |
| Slp | Slope | - |
| Asp | Aspect | - |
| Alt | Altitude | m |
| Order | Raw Dataset | Post-Processing Dataset | Evaluation Principles |
|---|---|---|---|
| 1 | VS | VS | Correlation coefficient r ≤ 0.8 |
| 2 | VS | VS | Jackknife test |
| 3 | VS | VS | MIN|AUC-AUC| |
| Order | Variables | Variable Description | Unit |
|---|---|---|---|
| 1 | bio_2 | Mean of monthly (maxtemp–mintemp) | C |
| 2 | bio_3 | Isothermality (bio_2 / bio_7 × 100) | - |
| 3 | bio_4 | Temperature seasonality (standard deviation × 100) | - |
| 4 | bio_5 | Max temperature of warmest month | C |
| 5 | bio_11 | Mean temperature of coldest quarter | C |
| 6 | bio_13 | Precipitation of wettest month | mm |
| 7 | bio_15 | Precipitation seasonality (coefficient of variation) | mm |
| 8 | bio_19 | Precipitation of coldest quarter | mm |
| Period | Climate Scenario | Area of Suitable Areas at Different Levels/× 10 km (Variation Relative to Previous Period/%) | |||
|---|---|---|---|---|---|
| Unsuitable | Low | Middle | High | ||
| 1970–2000 | 6.65 | 1.17 | 1.09 | 0.7 | |
| 2021–2040 | SSP126 | 6.26 (−5.85) | 1.6 (36.33) | 0.99 (−8.97) | 0.76 (8.6) |
| SSP585 | 6.2 (−6.68) | 1.68 (42.6) | 0.99 (−9.12) | 0.74 (6.15) | |
| 2041–2060 | SSP126 | 6.14 (−1.94) | 1.72 (7.36) | 1.02 (2.45) | 0.74 (−2.77) |
| SSP585 | 5.94 (−4.18) | 1.89 (13.01) | 1.08 (9.33) | 0.69 (−6.88) | |
| 2061–2080 | SSP126 | 6.22 (1.29) | 1.74 (1.04) | 0.99 (−2.38) | 0.67 (−9.9) |
| SSP585 | 5.44 (−8.39) | 2.1 (10.95) | 1.33 (22.92) | 0.73 (6.28) | |
| 2081–2100 | SSP126 | 6.23 (0.28) | 1.72 (−1.14) | 0.96 (−3.03) | 0.7 (4.85) |
| SSP585 | 4.86 (−10.71) | 2.4 (14.03) | 1.54 (15.84) | 0.81 (10.59) | |
| Order | Variables | Model Contribution Rate (%) | The Permutation Importance (%) |
|---|---|---|---|
| 1 | bio 11 | 50.9 | 56.7 |
| 2 | bio 13 | 20.5 | 7.7 |
| 3 | bio 19 | 12.9 | 9.7 |
| 4 | bio 4 | 6.4 | 0 |
| 5 | bio 15 | 4.2 | 9.2 |
| 6 | bio 2 | 3 | 8.4 |
| 7 | bio 5 | 2 | 8.2 |
| 8 | bio 3 | 0 | 0 |
| The Growth Subregions | Climate Scenarios | bio_11/C | bio_13/mm | bio_19/mm | bio_2 /C |
|---|---|---|---|---|---|
| U | SSP126 | −8.94 (−10.98–8.24) | 101.9 (96.42–104.97) | 23.5 (22.98–24.23) | 13.07 (13.02–13.1) |
| SSP585 | −7.39 (−11–3.97) | 106.33 (97.96–114.03) | 25.16 (24.79–25.45) | 12.98 (12.8–13.09) | |
| LUE | SSP126 | −6.08 (−8.18–5.41) | 103.53 (98.62–107.25) | 17.16 (16.65–17.6) | 12.11 (12.11–12.13) |
| SSP585 | −5.72 (−9.2–2.28) | 102.79 (95.29–108.61) | 15.06 (14.37–16.06) | 12.31 (12.22–12.38) | |
| LD | SSP126 | 9.24 (7.81–9.86) | 264.78 (253.84–276.63) | 152.28 (146.74–159.31) | 8.89 (8.68–9.03) |
| SSP585 | 10.97 (8.43–13.5) | 269.14 (255.3–281.68) | 151.65 (148.38–153.92) | 8.39 (8.23–8.64) | |
| LS | SSP126 | 2.48 (0.98-3.06) | 181.88 (174.54-188.6) | 64.38 (62.08-67.41) | 10.34 (9.97–10.47) |
| SSP585 | 6.73 (4.14–9.44) | 203.02 (194.69–211.01) | 68.76 (67.36–69.89) | 9.79 (9.18–10.1) | |
| MD | SSP126 | 7.88 (6.23-8.54) | 218.91 (209.4-227.79) | 123.59 (117.94-130.27) | 8.6 (8.4–8.73) |
| SSP585 | 8.85 (6.16–11.48) | 223.2 (209.79–235.7) | 119.11 (114.38–122.05) | 8.34 (8.17–8.61) | |
| MS | SSP126 | 3.81 (2.12–4.46) | 199.58 (189.08–207.63) | 60.16 (57.02–63.48) | 9.36 (9.28–9.42) |
| SSP585 | 4.63 (1.83–7.38) | 203.56 (190.8–212.53) | 48.86 (46.2–51.38) | 9.62 (9.48–9.77) | |
| ME | SSP126 | −0.41 (−2.49–0.29) | 159.32 (148.83–164.84) | 16.16 (15.43–16.82) | 10.99 (10.82–11.55) |
| SSP585 | 0.1 (−3.11–3.2) | 156.57 (142.8–163.98) | 17.99 (16.73–19.57) | 11.05 (10.89–11.49) | |
| HSD | SSP126 | 6.37 (4.72–7.03) | 190.83 (177.03–199.46) | 131.58 (127.08–138.75) | 8.22 (8.09–8.56) |
| SSP585 | 7.48 (4.72–10.09) | 202.28 (189.35–220.09) | 126.59 (117.94–132.69) | 8.23 (8.06–8.49) | |
| HWD | SSP126 | 5.74 (4.01–6.41) | 197.94 (188.33–206.62) | 100.37 (94.77–106.09) | 8.73 (8.6–9) |
| SSP585 | 6.07 (3.32–8.77) | 196.41 (185.88–203.8) | 72.63 (66.46–77.44) | 9.32 (9.12–9.73) | |
| HS | SSP126 | 3.35 (1.45–4.02) | 166.89 (157.35–174.17) | 39.29 (36.53–41.67) | 10.06 (9.9–10.48) |
| SSP585 | 4.02 (1.01–6.87) | 164.02 (151.27–173.1) | 32.62 (29.64–35.23) | 10.17 (9.97–10.64) |
| Order | Bases of Plantation | Center | Growth Subregions |
|---|---|---|---|
| 1 | Taihang Mountain in Hebei Province | 3434′ N–4043′ N | ULE, ME, HS |
| 11014′ E–11433′ E | |||
| 2 | Yantai and Qixia city, Shandong province | 37′54 N, 12138′ E | MS, HWD |
| 3 | Lianyungang, Yuntai Mountain, Jiangsu province | 3461′ N, 11917′ E | MS, HWD |
| 4 | Jingmen, Hubei province | 3103′ N, 1122′ E | HS |
| 5 | Luanchuan and Luoning city, Henan province | 3411′ N, 1116′ E | HS |
| 6 | Lijiang city, Yunnan province | 2688′ N, 10023′ E | MD, HWD, HSD |
| 7 | Xingren, Anshun and Guiding city, Guizhou province | 2544′ N–2659′ N | ME, MS |
| 10521′ E–10724′ E | |||
| 8 | Funiu Mountain and Dabai Tongbai Mountain in Henan province | 3102′ N–3414′ N | HS |
| 11109′ E–11674′ E |
| Bases of Plantation | Climate Scenarios | bio_11/C | bio_13/mm | bio_19/mm | bio_2 /C |
|---|---|---|---|---|---|
| 1 | ssp126 | −2.73 (−4.27–2.15) | 156.9 (144.37–165.65) | 10.59 (10.26–10.91) | 12.58 (12.31-12.67) |
| ssp585 | −1.47 (−4.27–1.53) | 163.45 (144.37–175.33) | 11.14 (10.26–12.43) | 12.45 (12.31–12.59) | |
| 2 | ssp126 | 1.19 (−0.72–1.85) | 194.42 (180.48–202.56) | 36.98 (35.09–37.98) | 8.04 (7.69–9.32) |
| ssp585 | 2.28 (−0.72–5.01) | 200.79 (180.48–217.7) | 37.98 (35.09–41.35) | 8.02 (7.67–9.32) | |
| 3 | ssp126 | 3.25 (1.27–3.97) | 224.93 (211.34–237.25) | 55.68 (52.11–58.35) | 9.44 (9.21–10.06) |
| ssp585 | 4.27 (1.27–7) | 229.68 (211.34–244.47) | 57.85 (52.11–62.64) | 9.42 (9.18–10.06) | |
| 4 | ssp126 | 6.67 (4.99–7.31) | 184.55 (175.16–193.66) | 81.09 (74.72–86.2) | 8.52 (8.4–8.74) |
| ssp585 | 7.62 (4.99–10.26) | 182.53 (175.16–188.01) | 81.51 (74.72–85.88) | 8.52 (8.34–8.74) | |
| 5 | ssp126 | 3.36 (1.32–4.07) | 153.16 (144.84–161.94) | 30.47 (28.05–32.48) | 10.66 (10.58–10.71) |
| ssp585 | 4.37 (1.32–7.19) | 158.66 (144.84–168.15) | 31.37 (28.05–34.13) | 10.64 (10.55–10.78) | |
| 6 | ssp126 | 6.12 (4.28–6.78) | 147.88 (143.61–154.36) | 55.72 (54.27–57.27) | 11.04 (10.05–11.37) |
| ssp585 | 7.24 (4.28–9.89) | 145.79 (140.2–153.74) | 54.37 (52.8–55.71) | 11.14 (10.05–11.61) | |
| 7 | ssp126 | 7.28 (5.96–7.85) | 226.51 (220.81–237.89) | 57.27 (55.5–60.33) | 8.16 (7.48–8.42) |
| ssp585 | 8.24 (5.96–10.62) | 225.76 (214.33–238.65) | 54.88 (54.48–55.5) | 8.23 (7.48–8.74) | |
| 8 | ssp126 | 4.52 (2.63–5.21) | 174.47 (164.45–181.85) | 42.19 (38.86–45.12) | 10.6 (10.42–11.06) |
| ssp585 | 5.49 (2.63–8.26) | 176.38 (164.45–188.39) | 43.61 (38.86–47.44) | 10.57 (10.37–11.06) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, S.; Zhu, T.; Wang, J.; Yan, D. The Current and Future Potential Geographical Distribution and Evolution Process of Catalpa bungei in China. Forests 2022, 13, 96. https://doi.org/10.3390/f13010096
Jian S, Zhu T, Wang J, Yan D. The Current and Future Potential Geographical Distribution and Evolution Process of Catalpa bungei in China. Forests. 2022; 13(1):96. https://doi.org/10.3390/f13010096
Chicago/Turabian StyleJian, Shengqi, Tiansheng Zhu, Jiayi Wang, and Denghua Yan. 2022. "The Current and Future Potential Geographical Distribution and Evolution Process of Catalpa bungei in China" Forests 13, no. 1: 96. https://doi.org/10.3390/f13010096
APA StyleJian, S., Zhu, T., Wang, J., & Yan, D. (2022). The Current and Future Potential Geographical Distribution and Evolution Process of Catalpa bungei in China. Forests, 13(1), 96. https://doi.org/10.3390/f13010096







