Fire Impacts on Recruitment Dynamics in a Seasonal Tropical Forest in Continental Southeast Asia
Abstract
:1. Introduction
- Is regeneration response among species affected by fire intensity?
- Are functional trait distributions in recruits different during periods with and without fire?
2. Materials and Methods
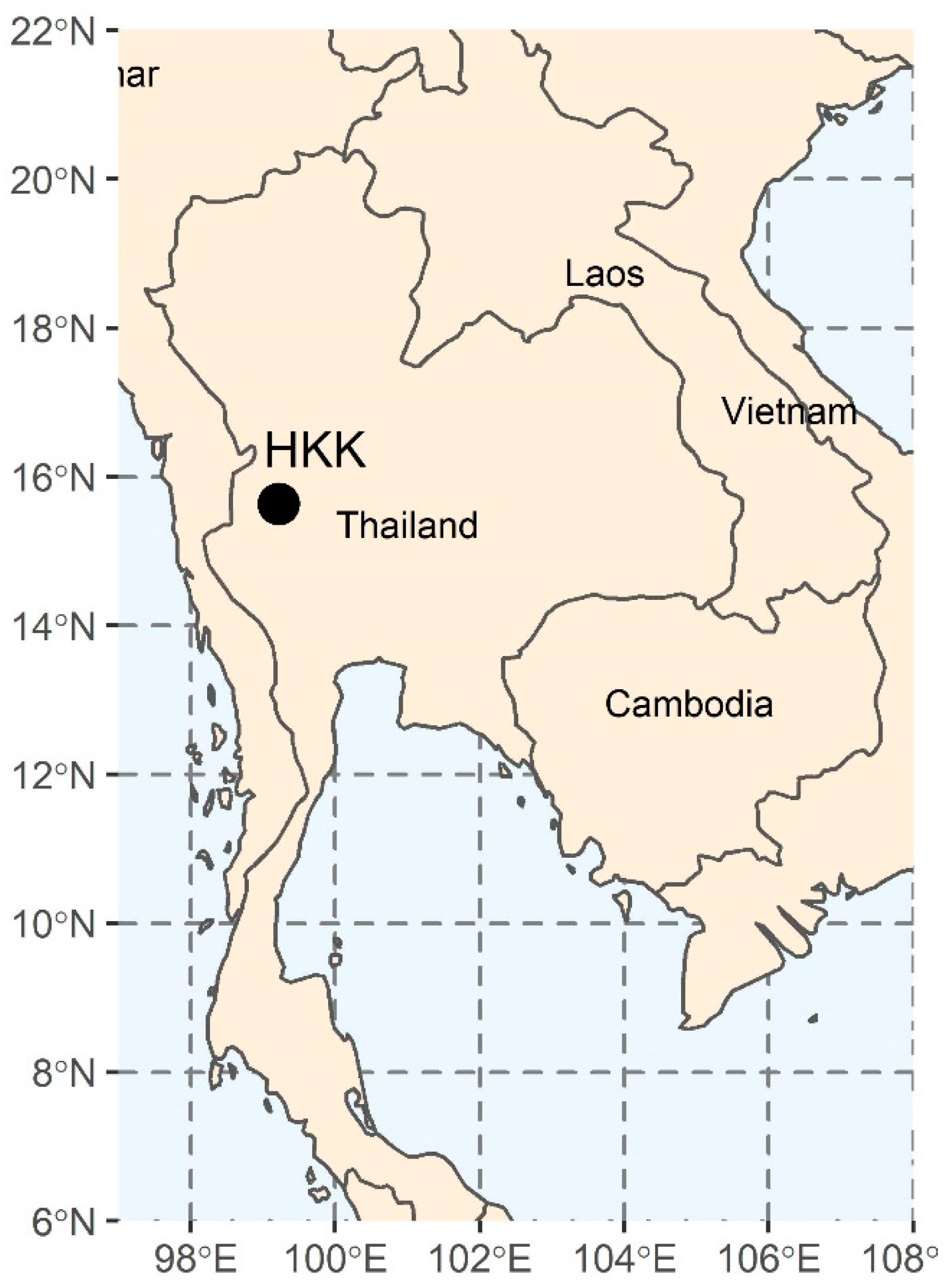
2.1. Study Area
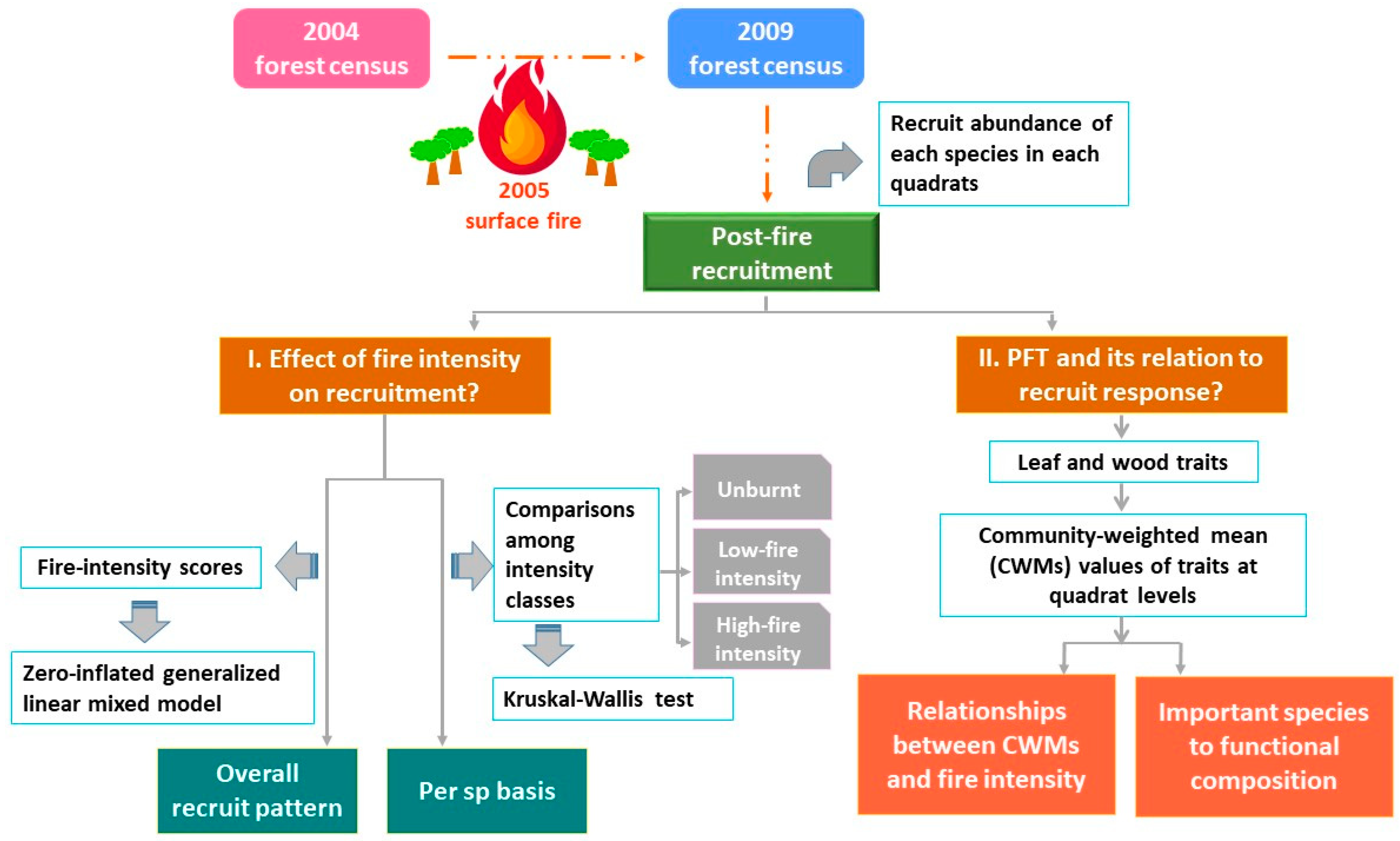
2.2. Workflow and Data
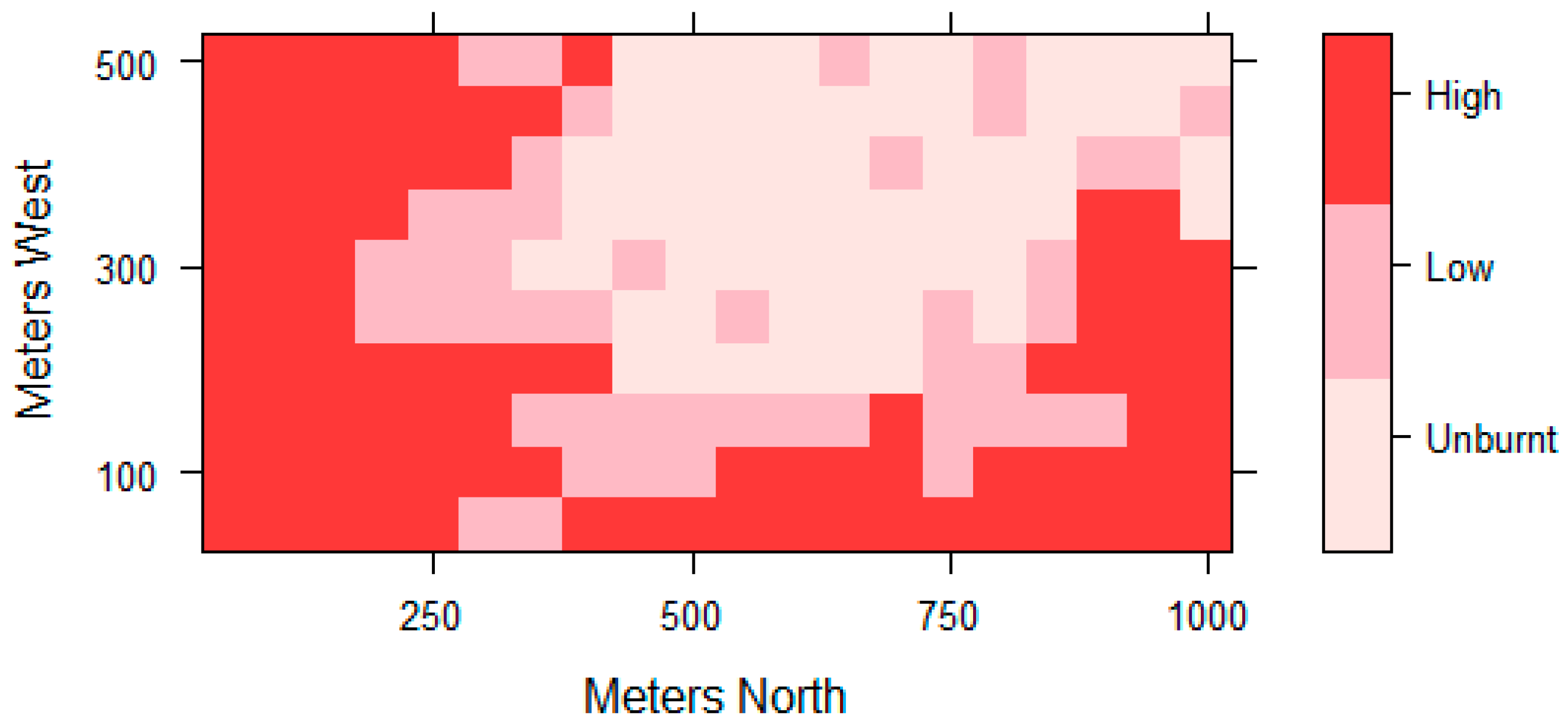
2.2.1. Fire Intensity Classification
2.2.2. Functional Trait Dataset and Their Measurement
2.3. Data Analysis
2.3.1. The Effect of Fire Intensity on Recruit Abundance for All Species and on a per Species Basis
zi = ~ fire intensity, family = Poisson
2.3.2. Specific Plant Functional Trait and Its Relation to Recruit Response
3. Results
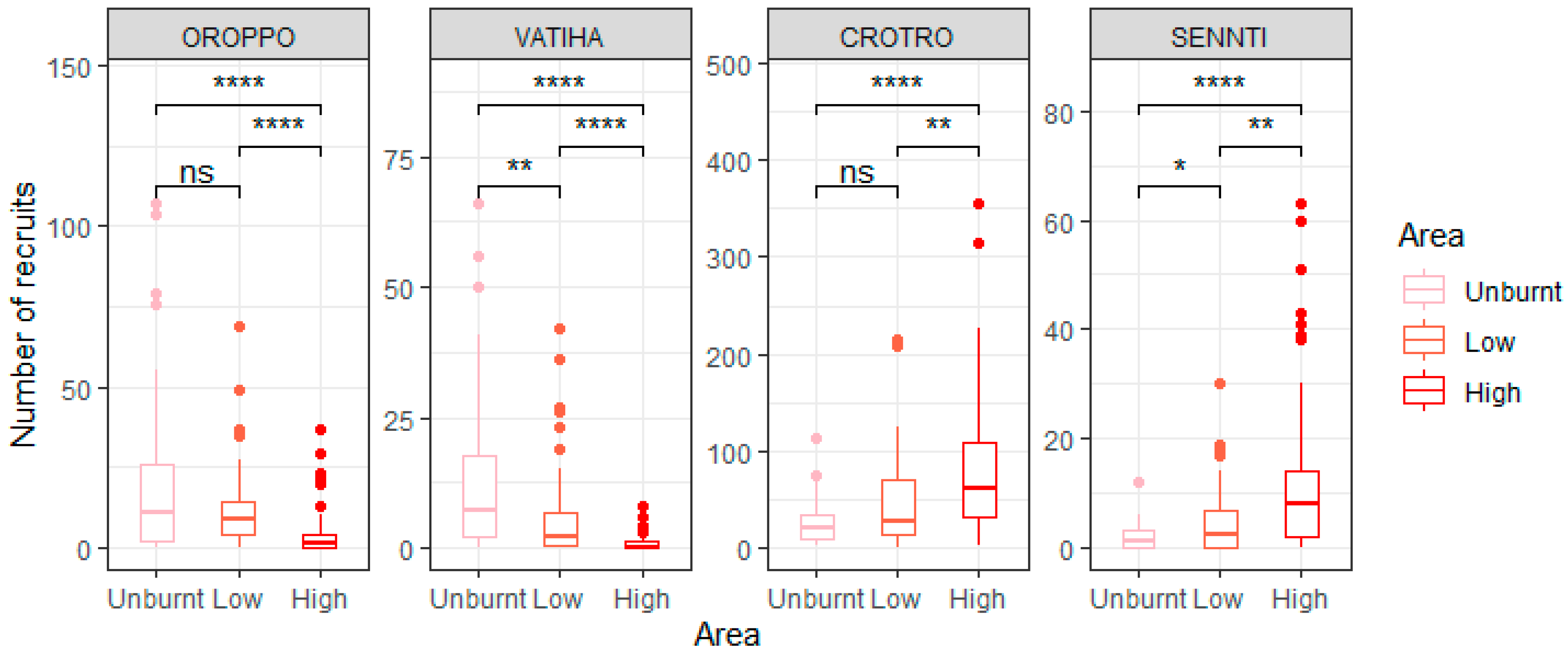
3.1. The Effect of Fire Intensity on Recruit Abundance for Overall Species and on a per Species Basis
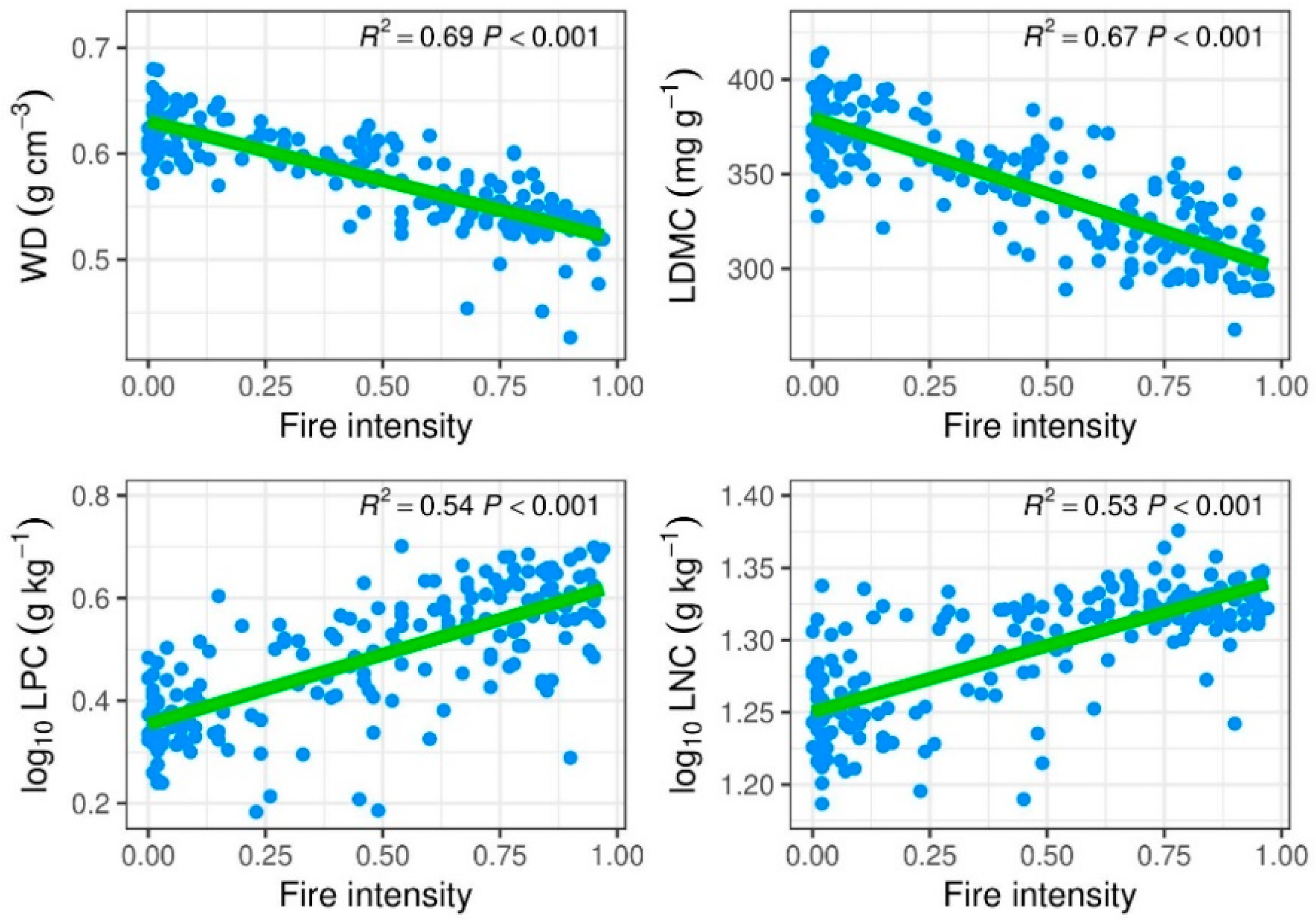
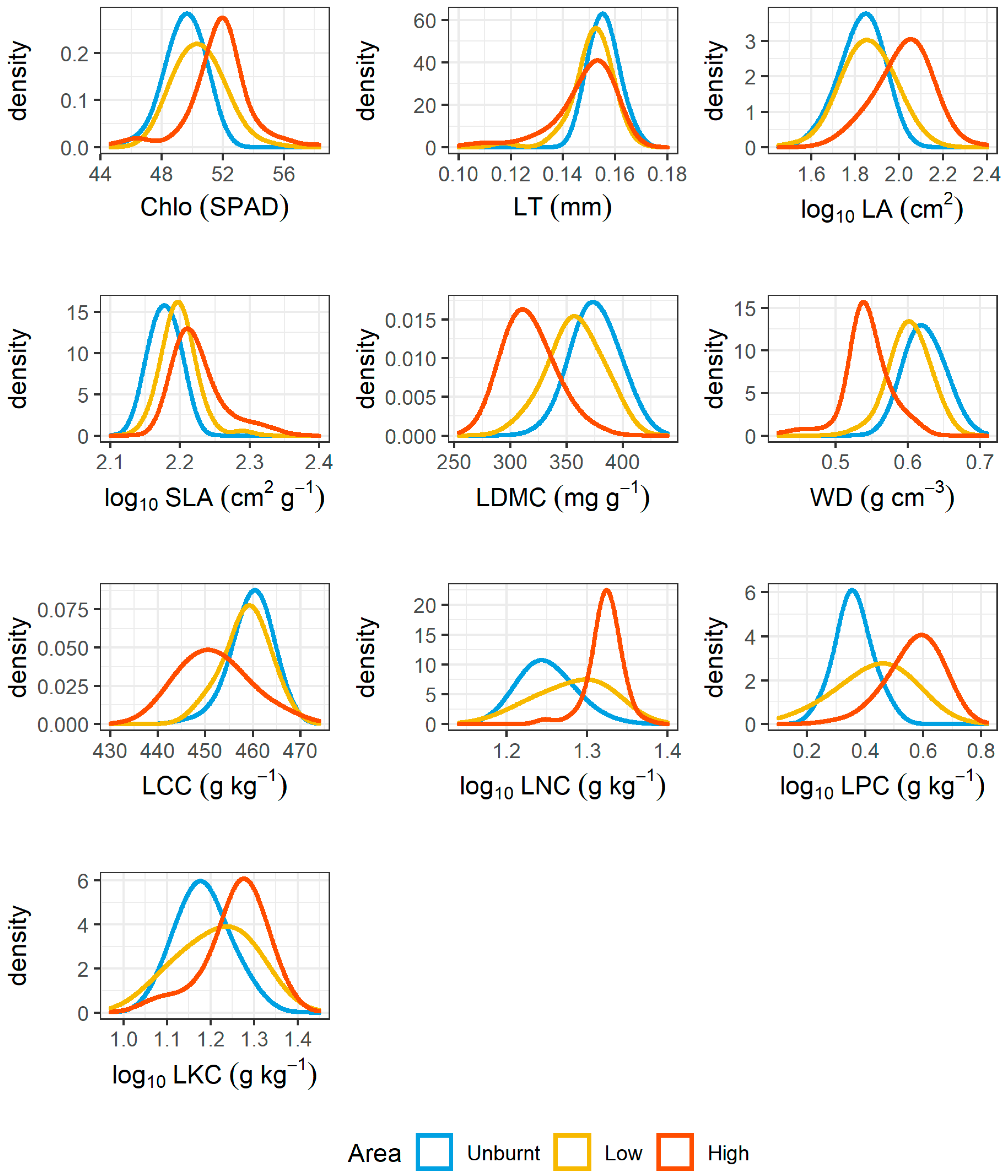
3.2. Post-Fire Community Trait Responses and Relationships with Recruitments
4. Discussion
4.1. Effect of Fire Intensity on Recruitment Patterns
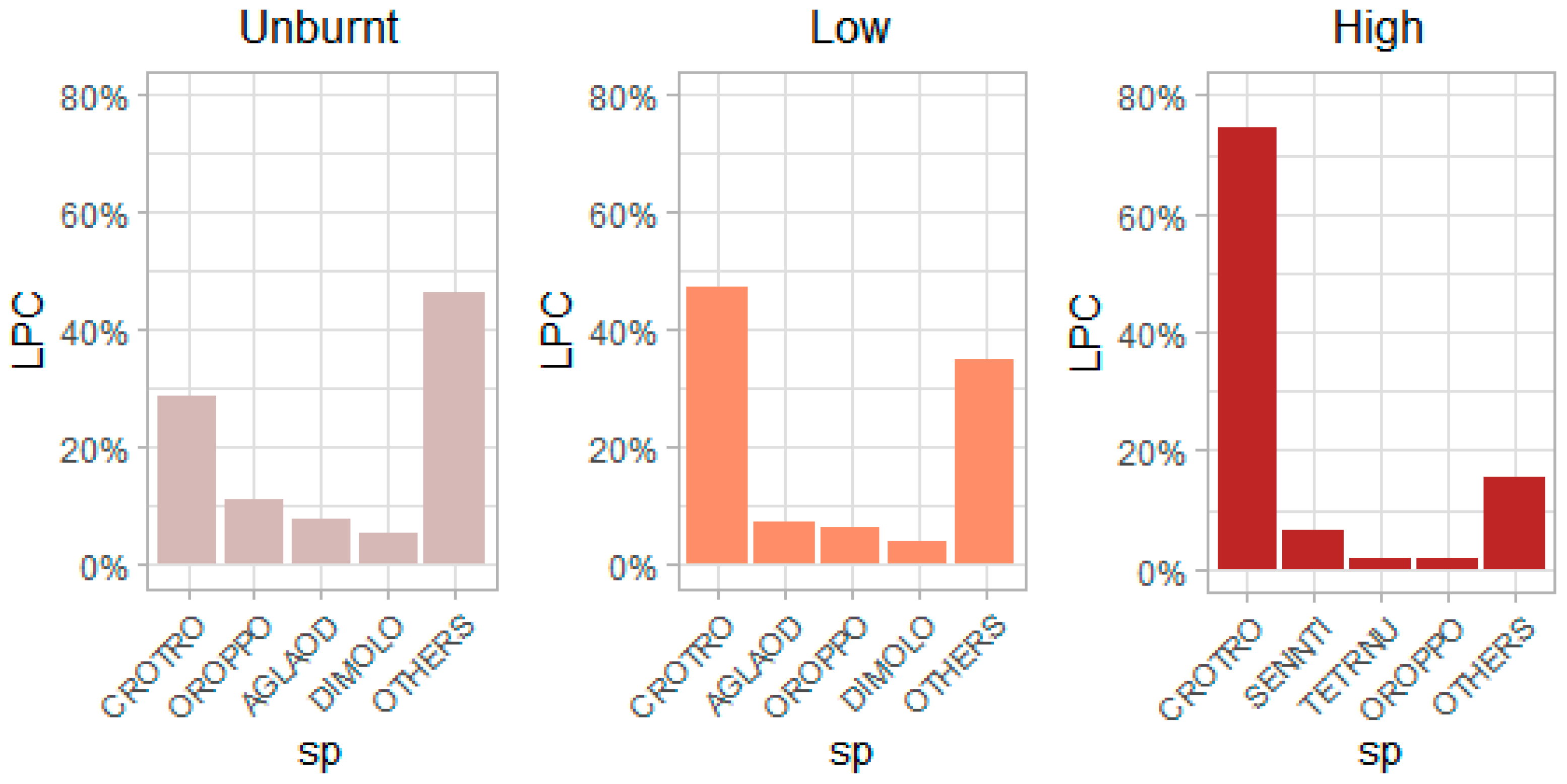
4.2. Effect of Fire in Relation to Functional Traits and the Key Species in Determining Functional Compositions
4.3. Current Fire Disturbance and Forecasting Its Impact on Species and Functional Compositions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Model of Zero-Inflated Generalized Linear Mixed Model
References
- Woods, P. Effects of logging, drought, and fire on structure and composition of tropical forests in Sabah, Malaysia. Biotropica 1989, 21, 290–298. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Platt, W.J. Effects of long-term fire exclusion on tree species composition and stand structure in an old-growth Pinus palustris (Longleaf pine) forest. Plant Ecol. 1999, 140, 15–26. [Google Scholar] [CrossRef]
- Oliveras, I.; Malhi, Y.; Salinas, N.; Huaman, V.; Urquiaga-Flores, E.; Kala-Mamani, J.; Quintano-Loaiza, J.A.; Cuba-Torres, I.; Lizarraga-Morales, N.; Román-Cuesta, R.-M. Changes in forest structure and composition after fire in tropical montane cloud forests near the Andean treeline. Plant Ecol. Divers. 2014, 7, 329–340. [Google Scholar] [CrossRef]
- McLauchlan, K.K.; Higuera, P.E.; Miesel, J.; Rogers, B.M.; Schweitzer, J.; Shuman, J.K.; Tepley, A.J.; Varner, J.M.; Veblen, T.T.; Adalsteinsson, S.A.; et al. Fire as a fundamental ecological process: Research advances and frontiers. J. Ecol. 2020, 108, 2047–2069. [Google Scholar] [CrossRef]
- Verma, S.; Singh, D.; Mani, S.; Jayakumar, S. Effect of forest fire on tree diversity and regeneration potential in a tropical dry deciduous forest of Mudumalai Tiger Reserve, Western Ghats, India. Ecol. Process. 2017, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Kodandapani, N.; Cochrane, M.; Sukumar, R. A comparative analysis of spatial, temporal, and ecological characteristics of forest fires in seasonally dry tropical ecosystems in the Western Ghats, India. For. Ecol. Manag. 2008, 256, 607–617. [Google Scholar] [CrossRef]
- Cochrane, M.A.; Schulze, M.D. Fire as a recurrent event in tropical forests of the eastern Amazon: Effects on forest structure, biomass, and species composition. Biotropica 1999, 31, 2–16. [Google Scholar] [CrossRef]
- González, M.E.; Veblen, T.T.; Sibold, J.S. Influence of fire severity on stand development of Araucaria araucana–Nothofagus pumilio stands in the Andean cordillera of south-central Chile. Austral Ecol. 2010, 35, 597–615. [Google Scholar] [CrossRef]
- Mundo, I.A.; Holz, A.; González, M.E.; Paritsis, J. Fire History and Fire Regimes Shifts in Patagonian Temperate Forests. In Dendroecology: Tree-Ring Analyses Applied to Ecological Studies; Amoroso, M.M., Daniels, L.D., Baker, P.J., Camarero, J.J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 211–229. [Google Scholar]
- Johnstone, J.F.; Chapin, F.S. Effects of soil burn severity on post-fire tree recruitment in boreal Forest. Ecosystems 2006, 9, 14–31. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Murphy, B.P.; Baker, P.J. The existence of a fire-mediated tree-recruitment bottleneck in an Asian savanna. J. Biogeogr. 2019, 46, 745–756. [Google Scholar] [CrossRef]
- Otterstrom, S.M.; Schwartz, M.W.; Velázquez-Rocha, I. Responses to fire in selected tropical dry forest trees. Biotropica 2006, 38, 592–598. [Google Scholar] [CrossRef]
- Baker, P.J.; Bunyavejchewin, S.; Oliver, C.D.; Ashton, P.S. Disturbance history and historical stand dynamics of a seasonal tropical forest in western Thailand. Ecol. Monogr. 2005, 75, 317–343. [Google Scholar] [CrossRef]
- Amoroso, M.M.; Daniels, L.D.; Baker, P.J.; Camarero, J.J. Dendroecology: Tree-Ring Analyses Applied to Ecological Studies; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Syaufina, L.; Ainuddin, A.N. Impacts of Fire on SouthEast Asia tropical forests biodiversity: A review. Asian J. Plant Sci. 2011, 10, 238–244. [Google Scholar] [CrossRef]
- Baker, P.J.; Bunyavejchewin, S.; Robinson, A.P. The impacts of large-scale, low-intensity fires on the forests of continental South-east Asia. Int. J. Wildland Fire 2008, 17, 782–792. [Google Scholar] [CrossRef]
- Stott, P. The spatial pattern of dry season fires in the savanna forests of Thailand. J. Biogeogr. 1986, 13, 345–358. [Google Scholar] [CrossRef]
- Murphy, P.G.; Lugo, A.E. Ecology of tropical dry forest. Annu. Rev. Ecol. Syst. 1986, 17, 67–88. [Google Scholar] [CrossRef]
- Baker, P.J.; Bunyavejchewin, S. Fire behavior and fire effects across the forest landscape of continental Southeast Asia. In Tropical Fire Ecology: Climate Change, Land Use, and Ecosystem Dynamics; Springer: Berlin/Heidelberg, Germany, 2009; pp. 311–334. [Google Scholar]
- Williams, L.J.; Bunyavejchewin, S.; Baker, P.J. Deciduousness in a seasonal tropical forest in western Thailand: Interannual and intraspecific variation in timing, duration and environmental cues. Oecologia 2008, 155, 571–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trouvé, R.; Bunyavejchewin, S.; Baker, P.J. Disentangling fire intensity and species’ susceptibility to fire in a species-rich seasonal tropical forest. J. Ecol. 2020, 108, 1664–1676. [Google Scholar] [CrossRef]
- Geber, M.A.; Griffen, L.R. Inheritance and natural selection on functional traits. Int. J. Plant Sci. 2003, 164, 21–42. [Google Scholar] [CrossRef] [Green Version]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Diaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Steege, H.T.; Morgan, H.D.; Heijden, M.G.A.V.d.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef] [Green Version]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. Available online: https://www.nature.com/articles/nature02403#supplementary-information (accessed on 1 February 2019). [CrossRef] [PubMed]
- Chai, Y.; Yue, M.; Wang, M.; Xu, J.; Liu, X.; Zhang, R.; Wan, P. Plant functional traits suggest a change in novel ecological strategies for dominant species in the stages of forest succession. Oecologia 2016, 180, 771–783. [Google Scholar] [CrossRef]
- Bunyavejchewin, S.; Lafrankie, J.V.; Pattapong, P.; Kanzaki, M.; Itoh, A.; Yamakura, T.; Ashton, P. Topographic analysis of a large-scale research plot in seasonal dry evergreen forest at Huai Kha Khaeng Wildlife Sanctuary, Thailand. Tropics 1998, 8, 45–60. [Google Scholar] [CrossRef] [Green Version]
- Baillie, I.C.; Bunyavejchewin, S.; Kaewfoo, M.; Baker, P.J.; Hallett, S.H. Stoichiometry of cationic nutrients in Phaeozems derived from skarn and Acrisols from other plant materials in lowland forests of Thailand. Geoderma Reg. 2018, 12, 1–9. [Google Scholar] [CrossRef]
- Bunyavejchewin, S.; LaFrankie, J.V.; Baker, P.J.; Davies, S.J.; Ashton, P.J. Forest Trees of Huai Kha Khaeng Wildlife Sanctuary, Thailand: Data from the 50-Hectare Forest Dynamics Plot; National Parks, Wildlife & Plant Conservation Department: Bangkok, Thailand, 2009. [Google Scholar]
- Condit, R. Tropical Forest Census Plot; Springer: Berlin, Germany, 1998. [Google Scholar]
- Anderson-Teixeira, K.J.; Davies, S.J.; Bennett, A.C.; Gonzalez-Akre, E.B.; Muller-Landau, H.C.; Joseph Wright, S.; Abu Salim, K.; Almeyda Zambrano, A.M.; Alonso, A.; Baltzer, J.L.; et al. CTFS-ForestGEO: A worldwide network monitoring forests in an era of global change. Glob. Chang. Biol. 2015, 21, 528–549. [Google Scholar] [CrossRef] [Green Version]
- Davies, S.J.; Abiem, I.; Abu Salim, K.; Aguilar, S.; Allen, D.; Alonso, A.; Anderson-Teixeira, K.; Andrade, A.; Arellano, G.; Ashton, P.S.; et al. ForestGEO: Understanding forest diversity and dynamics through a global observatory network. Biol. Conserv. 2021, 253, 108907. [Google Scholar] [CrossRef]
- Center for Tropical Forest Science. The CTFS R Package. Available online: http://ctfs.arnarb.harvard.edu/Public/CTFSRPackage (accessed on 30 June 2019).
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; Benthem, K.J.v.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 15 April 2019).
- Sulisetijono, S.; Arumingtyas, E.L.; Mastuti, R.; Indriyani, S. Physical and chemical treatments to break seed dormancy on lerak (Sapindus rarak DC.). Int. J. Agric. Environ. Res. 2016, 2, 936–947. [Google Scholar]
- Moreira, B.; Pausas, J.G. Tanned or burned: The role of fire in shaping physical seed dormancy. PLoS ONE 2012, 7, e51523. [Google Scholar] [CrossRef] [Green Version]
- Ne’eman, G. Regeneration of natural pine forest—Review of work done after the 1989 fire in Mount Carmel, Israel. Int. J. Wildland Fire 1997, 7, 295–306. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Mooney, H.A. Principles of Terrestrial Ecosystem Ecology; Springer: New York, NY, USA, 2002. [Google Scholar]
- Raison, R.J. A review of the role of fire in nutrient cycling in Australian native forests, and of methodology for studying the fire-nutrient interaction. Aust. J. Ecol. 1980, 5, 15–21. [Google Scholar] [CrossRef]
- Sabiiti, E.N.; Wein, R.W. Fire and Acacia seeds: A hypothesis of colonization success. J. Ecol. 1987, 75, 937–946. [Google Scholar] [CrossRef]
- Peterson, D.W.; Reich, P.B. Fire frequency and tree canopy structure influence plant species diversity in a forest-grassland ecotone. Plant Ecol. 2008, 194, 5–16. [Google Scholar] [CrossRef]
- Reilly, M.J.; Wimberly, M.C.; Newell, C.L. Wildfire effects on plant species richness at multiple spatial scales in forest communities of the southern Appalachians. J. Ecol. 2006, 94, 118–130. [Google Scholar] [CrossRef]
- Muscarella, R.; Uriarte, M.; Aide, T.M.; Erickson, D.L.; Forero-Montaña, J.; Kress, W.J.; Swenson, N.G.; Zimmerman, J.K. Functional convergence and phylogenetic divergence during secondary succession of subtropical wet forests in Puerto Rico. J. Veg. Sci. 2016, 27, 283–294. [Google Scholar] [CrossRef]
- Boukili, V.K.; Chazdon, R.L. Environmental filtering, local site factors and landscape context drive changes in functional trait composition during tropical forest succession. Perspect. Plant Ecol. Evol. Syst. 2017, 24, 37–47. [Google Scholar] [CrossRef]

| Variable | Abbreviation | Measurement Unit |
|---|---|---|
| Leaf chlorophyll content | Chlo | SPAD unit |
| Leaf thickness | LT | mm |
| Leaf area | LA | cm2 |
| Leaf dry matter content | LDMC | mg g−1 |
| Specific leaf area | SLA | cm2 g−1 |
| Leaf total carbon concentration | LCC | g kg−1 |
| Leaf total potassium concentration | LKC | g kg−1 |
| Leaf total nitrogenconcentration | LNC | g kg−1 |
| Leaf total phosphorus concentration | LPC | g kg−1 |
| Functional Trait | CWM Pre-Fire | CWM Post-Fire | p Value | Significance Level |
|---|---|---|---|---|
| Chlo (SPAD) | 50.8 | 50.8 | 0.7545 | ns |
| LT (mm) | 0.153 | 0.151 | 0.0541 | ns |
| log10 LA (cm2) | 1.97 | 1.92 | 3.10 × 10−8 | **** |
| log10 SLA (cm2 g−1) | 2.21 | 2.2 | 0.8239 | ns |
| LDMC (mg g−1) | 340 | 345 | 0.0319 | * |
| WD (g cm−3) | 0.569 | 0.582 | 0.0001 | **** |
| LCC (g kg−1) | 455 | 456 | 0.1275 | ns |
| LNC (g kg−1) | 1.3 | 1.29 | 0.0694 | ns |
| LPC (g kg−1) | 0.499 | 0.473 | 8.64 × 10−5 | **** |
| LKC (g kg−1) | 1.23 | 1.22 | 0.5581 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewsong, K.; Johnson, D.J.; Bunyavejchewin, S.; Baker, P.J. Fire Impacts on Recruitment Dynamics in a Seasonal Tropical Forest in Continental Southeast Asia. Forests 2022, 13, 116. https://doi.org/10.3390/f13010116
Kaewsong K, Johnson DJ, Bunyavejchewin S, Baker PJ. Fire Impacts on Recruitment Dynamics in a Seasonal Tropical Forest in Continental Southeast Asia. Forests. 2022; 13(1):116. https://doi.org/10.3390/f13010116
Chicago/Turabian StyleKaewsong, Kanokporn, Daniel J. Johnson, Sarayudh Bunyavejchewin, and Patrick J. Baker. 2022. "Fire Impacts on Recruitment Dynamics in a Seasonal Tropical Forest in Continental Southeast Asia" Forests 13, no. 1: 116. https://doi.org/10.3390/f13010116
APA StyleKaewsong, K., Johnson, D. J., Bunyavejchewin, S., & Baker, P. J. (2022). Fire Impacts on Recruitment Dynamics in a Seasonal Tropical Forest in Continental Southeast Asia. Forests, 13(1), 116. https://doi.org/10.3390/f13010116






