A Key for the Microhistological Determination of Plant Fragments Consumed by Carpathian Forest Cervids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Reference Material
2.3. Morphology Diagnostics and Categorization
- Grasses and sedges—monocotyledons of grass-like appearance, such as the Order of Poales, Family Poaceae, Juncaceae, and Cyperaceae.
- Herbaceous plants and leaves of deciduous trees—dicotyledons, including herbs (except for ferns and genera Rubus, Rosa, Vaccinium) and assimilation organs of deciduous trees and shrubs including buds. We grouped herbs and leaves of trees together due to their similar micromorphology, including shape and arrangement of epidermal cells, type and arrangement of stomata, type of venation and presence of variable types of trichomes.
- Genera Rubus, Rosa, Vaccinium. We defined this category because of specific morphological structure regarding amount and type of trichomes, and presence of crystalic inclusions, making them well distinguishable from other herbaceous, and because this category has a high nutritional value as a forage source [52], and thus, a specific importance in evaluating forage of wild cervids [1,25,42].
- Needles—needle-like assimilations organs of coniferous trees.
- Wood and bark–wood and bark tissue of trees and shrubs.
- Ferns and mosses—plants from classes Bryopsida and Polypodiopsida.
- Seeds and fruits—fruits of trees and shrubs, and seeds of agricultural crops.
| Category | Diagnostic Features |
|---|---|
| Grasses and sedges | Elongated rectangular shape of epidermal cells arranged parallel to veins [53]. |
| Epidermal cells aligned in longitudinal strips [46]. | |
| Parallel venation [49]. | |
| Present “short” cells (alone or in pairs) and “long” epidermal cells (except family Juncaceae) [48,49]. | |
| Parallel arrangement of stomata [49] | |
| Presence of dumbbell-shaped guard cells of stomata (Gramineous type) exception of the family Juncaceae, which have kidney-shaped of stomata (Amaryllis type) [47]. | |
| Presence of macrohairs, microhairs, prickle hairs, and papillae, except family Juncaceae which have the sporadic presence of papillae and trichomes [32]. | |
| Herbaceous plants and leaves of deciduous trees | Epidermal cells of variable, mostly irregular-shape (hexagonal, round, elongated) [53], cells of elongated organs have elongated shape. |
| Epidermal cells of leaves arranged diffusely, rarely parallel to the veins [53]. | |
| Usually, reticulated venation. | |
| Kidney-shaped guard cells of stomata (Amaryllis type) arranged irregularly or/and randomly [48]. | |
| Great morphological variability of trichomes, which occur in different taxa [45]. | |
| Presence of different types of crystalline inclusions found in almost all taxa [45]. | |
| Genera Rubus, Rosa, Vaccinium | Venation, shape, and arrangement of epidermal cells identical to category Herbaceous plants and leaves of deciduous trees, except Vaccinium spp. that have also polygonal to rectangular epidermal cells irregularly beaded with straight, thickened, pitted walls [54]. |
| Typical presence of kidney-shaped guard cells of stomata (Amaryllis type) arranged irregularly or/and randomly [45]. | |
| Rubus and Rosa: presence of hollow trichomes (simple-unicellular or stellate) sharply pointed, conical with smooth surface. Simple trichomes are twisted or convoluted (length to width ratio min. 10:1), or they are almost straight, narrowed gradually towards to the apex, bases of trichomes have rectangular shape to hexagonal shape with a rosette of epidermal cells; stellate trichomes (with a smooth surface, with pointed apexes, filiform and hollow arms almost equally long) are smaller compared to simple trichomes; sporadic presence of glandular trichomes with multicellular head or presence of multicellular tongue-shaped trichomes Numerous presences of randomly arranged crystals as druses or styloids, especially typical for Rosa [40,45,54,55,56]. | |
| Vaccinium: presence of multicellular, glandular tongue-shaped trichomes bent over parallel to the midrib when situated to the apex, also presence of glandular trichomes consist of biseriate or /and multiseriate stalks bearing heads of variable size and small conical with wart-like surface [45]. | |
| Needles | Elongated rectangular shape of epidermal cells. |
| Epidermal cells arranged in rows [57] parallel to vein. | |
| Leaves generally have simple venation and have only one or two long veins running down their centre [58]. | |
| Stomata arranged in longitudinal rows forming dense stomatal bands [58]. | |
| Stomatal guard cells sunken below the surface of the epidermis [49]. | |
| Presence of resin canals in the mesophyll [59]. | |
| Wood and bark | Presence of parts of tracheary elements and/or vessels elements, fiber tracheids and cells with accompanied libriform fibers or parenchymal cells, crystal-containing cells and sclereids or their combination [49] |
| Presence of sclereids of various forms such as unbranched (brachysklereids) or sclereids of irregular shapes with a different number of protrusions, as well as large heterogeneous assemblage of sclereids (asterosclerides) [49]. | |
| Typical presence of soft cork cells (thin-walled cells of variable shapes) and/or hard cork cells (thick-walled cells in the shape of a square) [60]. | |
| Presence of phytoliths; calcium oxalate crystals are common in the secondary phloem of conifers [49]. | |
| Ferns and mosses | Fine structure fragments [61]. |
| Cells of ferns have puzzle-like, and mosses have labyrinth-like pattern. | |
| Mosses single-layered fronds (leaves of mosses) have no developed epidermis [62] and is not differentiated from inner tissue. | |
| A thick layer of photosynthetic cells on a leaf surface of mosses forms lamellae; cells are like furrows or ridges that run parallel to each other. | |
| Ferns have open venation and all cells of the lower and upper epidermis contain chloroplasts [61]. | |
| Right (true) vascular tissue contains phloem and xylem absent in mosses [61]. | |
| Exclusively presence of Mnium type of stomata [63]. | |
| Presence of sporangia on the abaxial side of the fern leaves form clusters, sometimes covered by sharps [62]. | |
| Seeds and fruits | Various type of thick wall cells: sclereids, variable oriented layers or clusters of sclereids, irregularly arranged parenchymal cells with a strongly thickened cell wall, cells with pitted walls, parenchymatic cells and/or irregular polyhedrons with internal filling [48], cells usually arranged diffusely. |
| Presence of cross cells with thick lignified walls or/and tube cells (lignified cells elongated parallel with the long axis of the grains). | |
| Sporadic presence of stomata randomly arrangement [49]. | |
| Presence of starch, starch grains, trichomes. |
3. Results
KEY
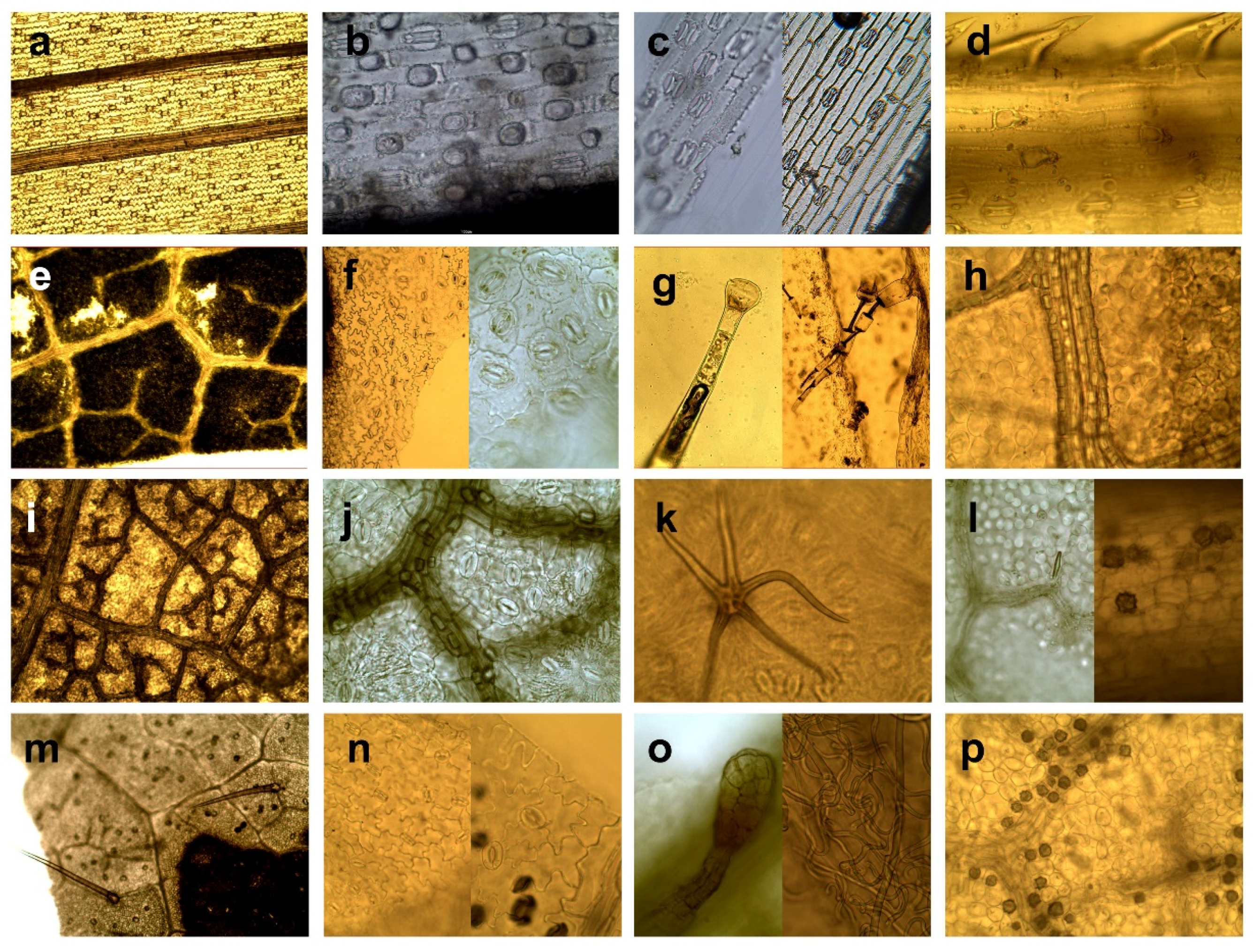
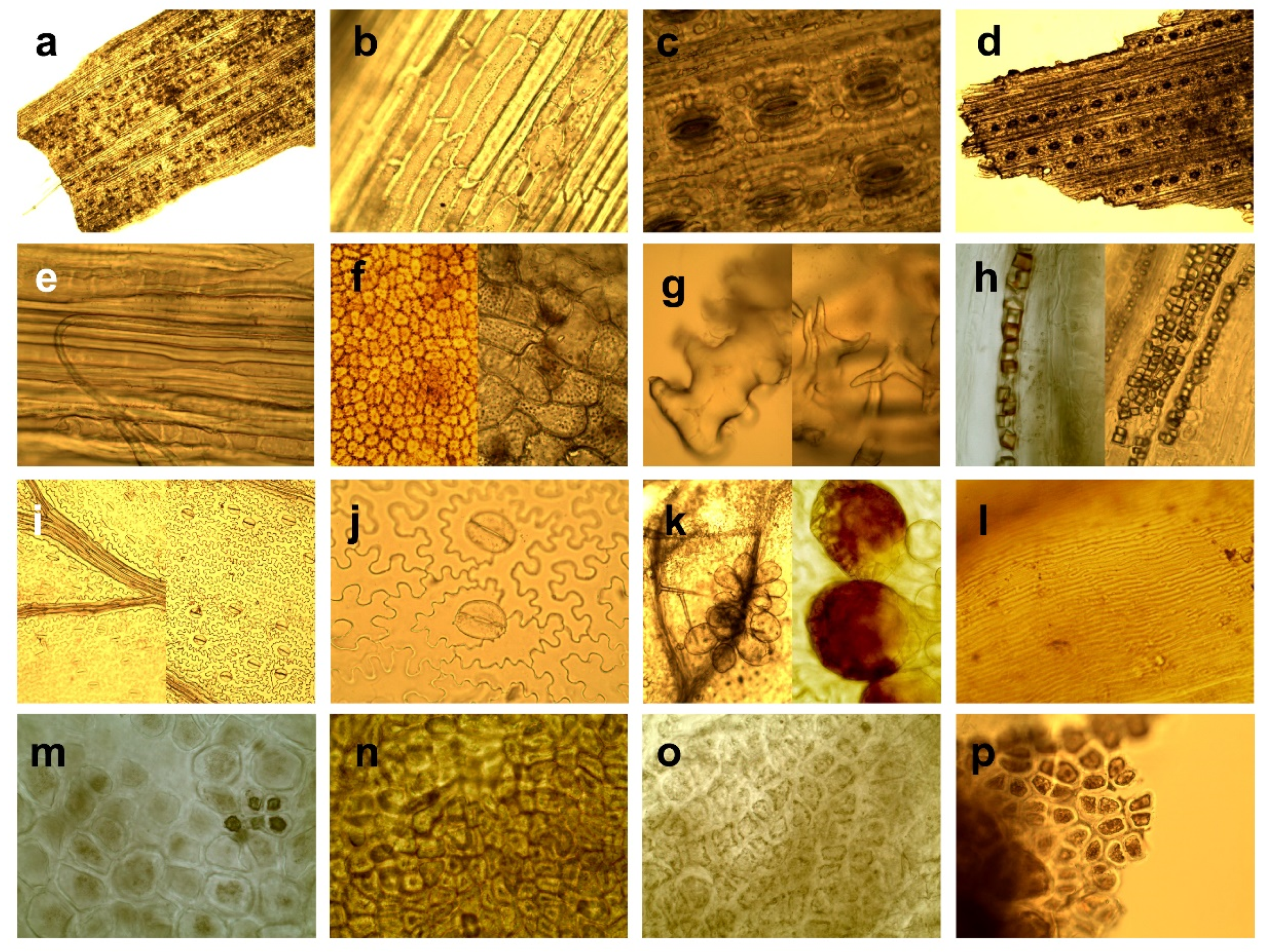
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Spitzer, R.; Felton, A.; Landman, M.; Singh, N.J.; Widemo, F.; Cromsigt, J.P. Fifty years of European ungulate dietary studies: A synthesis. Oikos 2020, 129, 1668–1680. [Google Scholar] [CrossRef]
- D’Aprile, D.; Vacchiano, G.; Meloni, F.; Garbarino, M.; Motta, R.; Ducoli, V.; Partel, P. Effects of Twenty Years of Ungulate Browsing on Forest Regeneration at Paneveggio Reserve, Italy. Forests 2020, 11, 612. [Google Scholar] [CrossRef]
- Hobbs, N.T. Modification of Ecosystems by Ungulates. J. Wildl. Manag. 1996, 60, 695–713. [Google Scholar] [CrossRef]
- Ward, A.I.; White, P.C.; Smith, A.; Critchley, C.H. Modelling the cost of roe deer browsing damage to forestry. For. Ecol. Manag. 2004, 191, 301–310. [Google Scholar] [CrossRef]
- Kropil, R.; Smolko, P.; Garaj, P. Home range and migration patterns of male red deer Cervus elaphus in Western Carpathians. Eur. J. Wildl. Res. 2015, 61, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.N.; Keller, B.J.; Chitwood, M.C.; Hansen, L.P.; Millspaugh, J.J. Diet Composition and Selection of Recently Reintroduced Elk in Missouri. Am. Midl. Nat. 2018, 180, 143–159. [Google Scholar] [CrossRef]
- Smolko, P.; Veselovská, A.; Kropil, R. Seasonal dynamics of forage for red deer in temperate forests: Importance of the habitat properties, stand development stage and overstorey dynamics. Wildl. Biol. 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Smolko, P.; Kropil, R.; Pataky, T.; Veselovská, A.; Merrill, E. Why do migrants move downhill? The effects of increasing predation and density on red deer altitudinal migration in temperate Carpathian forests. Mammal Res. 2018, 63, 297–305. [Google Scholar] [CrossRef]
- Baumgartner, L.L.; Martin, A.C. Plant histology as an aid in squirrel food habits studies. J. Wildl. Manag. 1939, 3, 266–268. [Google Scholar] [CrossRef]
- Dusi, J.L. Methods for determination of food habits by plant microtechniques and histology and their application to cottontail rabbit food habits studies. J. Wildl. Manag. 1949, 13, 295–298. [Google Scholar] [CrossRef]
- Garnick, S.; Barboza, P.S.; Walker, J.W. Assessment of Animal-Based Methods Used for Estimating and Monitoring Rangeland Herbivore Diet Composition. Rangel. Ecol. Manag. 2018, 71, 449–457. [Google Scholar] [CrossRef]
- Christianson, D.; Creel, S. A Review of Environmental Factors Affecting Elk Winter Diets. J. Wildl. Manag. 2010, 71, 164–176. [Google Scholar] [CrossRef]
- Baskin, L.; Danell, K. Ecology of Ungulates; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Habiba, U.; Anwar, M.; Khatoon, R.; Hussain, M.; Khan, K.A.; Khalil, S.; Hussain, A. Feeding habits and habitat use of barking deer (Muntiacus vaginalis) in Himalayan foothills, Pakistan. PLoS ONE 2021, 16, e0245279. [Google Scholar] [CrossRef]
- Green, K.; Davis, N.E.; Robinson, W.A.; McAuliffe, J.; Good, R.B. Diet selection by European hares (Lepus europaeus) in the alpine zone of the Snowy Mountains, Australia. Eur. J. Wildl. Res. 2013, 59, 693–703. [Google Scholar] [CrossRef]
- Katona, K.; Altbäcker, V. Diet estimation by faeces analysis: Sampling optimisation for the European hare. Folia. Zool. 2002, 51, 11–15. [Google Scholar]
- Chapuis, J.L. Comparison of the diets of two sympatric lagomorphs, Lepus europaeus (Pallas) and Oryctolagus cuniculus (L.) in an agroecosystem of the Ile-de-France. Z. Säugetierkunde 1990, 55, 176–185. [Google Scholar]
- Mátrai, K.; Altbäcker, V.; Hahn, I. Seasonal diet of rabbits and their browsing effect on juniper in Bugac Juniper Forest (Hungary). Acta Theriol. 1998, 43, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Alves, J.A.B.; Vingada, J.; Rodrigues, P. The wild rabbit (Oryc-tolagus cuniculus L.) diet on a sand dune area in central Portugal: A contribution towards management. Wildl. Biol. Pract. 2006, 2, 63–71. [Google Scholar] [CrossRef]
- Gębczynska, Z. Food of the roe deer and red deer in the Białowieża Primeval Forest. Acta Theriol. 1980, 25, 487–500. [Google Scholar] [CrossRef] [Green Version]
- Abbas, F.; Picot, D.; Merlet, J.; Cargnelutti, B.; Lourtet, B.; Angibault, J.M.; Verheyden, H. A typical browser, the roe deer, may consume substantial quantities of grasses in open landscapes. Eur. J. Wildl. Res. 2013, 59, 69–75. [Google Scholar] [CrossRef]
- Freschi, P.; Fascetti, S.; Riga, F.; Cosentino, C.; Rizzardini, G.; Musto, M. Diet composition of the Italian roe deer (Capreolus capreolus italicus) (Mammalia: Cervidae) from two protected areas. Eur. Zool. J. 2016, 84, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Mátrai, K.; Kabai, P. Winter plant selection by red and roe deer in a forest habitat in Hungary. Acta Theriol. 1989, 34, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Suter, W.; Suter, U.; Kriisi, B.; Schütz, M. Spatial variation of summer diet of red deer Cervus elaphus in the eastern Swiss Alps. Wildl. Biol. 2004, 10, 43–50. [Google Scholar] [CrossRef]
- Krojerová-Prokešová, J.; Barančeková, M.; Šustr, P.; Heurich, M. Feeding patterns of red deer Cervus elaphus along altitudinal gradient in the Bohemian Forest: Effect of habitat and season. Wildl. Biol. 2010, 16, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Homolka, M.; Heroldová, M. Native red deer and introduced chamois: Foraging habits and competition in a subalpine meadow-spruce forest area. Folia Zool. 2001, 50, 89–98. [Google Scholar]
- Redjadj, C.; Darmon, G.; Maillard, D.; Chevrier, T.; Bastianelli, D.; Verheyden, H.; Saïd, S. Intra-and Interspecific Differences in Diet Quality and Composition in a Large Herbivore Community. PLoS ONE 2014, 9, e84756. [Google Scholar] [CrossRef] [Green Version]
- Hartvig, I.; Howe, A.G.; Schmidt, E.N.; Pertoldi, C.; Nielsen, J.L.; Buttenschøn, R.M. Diet of the European bison (Bison bonasus) in a forest habitat estimated by DNA barcoding. Mammal Res. 2020, 66, 123–136. [Google Scholar] [CrossRef]
- Holechek, J.L.; Gross, B. Training needed for quantifying simulated diets from fragmented range plants. J. Range Manag. 1982, 35, 644–647. [Google Scholar] [CrossRef] [Green Version]
- Carriére, S. Photographic key for the microhistological identification of some arctic vascular plants. Arctic 2002, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Howard, G.S.; Samuel, M.J. Atlas of Epidermal Plant Fragments Ingested by Grazing Animals (No. 1582); Department of Agriculture, Science and Education Administration: Seattle, WT, USA, 1979. [Google Scholar]
- Johnson, M.K. Microhistological Techniques for Food Habits Analysis; U.S. Department of Agriculture: New Orleans, LA, USA, 1983.
- Henderson, J.; Granger, J.; Phillips, G.J. Microhistological Atlas of Greater Yellowstone Moose Browse; Castaway Press: Burbank, CA, USA, 2013. [Google Scholar]
- Middleton, B.; Sanchez, E. Microhistological analysis of food habits in the tropics. Rev. Estud. Vida Local 1993, 3, 41–47. [Google Scholar]
- Lindström, L.I.; Mújica, M.B.; Bóo, R.M. A key to identify perennial grasses in central Argentina based on microhistological characteristics. Can. J. Bot. 1998, 76, 1467–1475. [Google Scholar] [CrossRef]
- Ahmed, T. Photographic Key for the Microhistological Identification of Some Plants of Indian Trans-Himalaya. Not. Sci. Biol. 2015, 7, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Cransac, N.; Cibien, C.; Angibault, J.M.; Morellet, N.; Vincent, J.P.; Hewison, A.J.M. Seasonal variation in the diet of roe deer (Capreolus capreolus) according to sex in a very dense forest (Dourdan, France). Mammalia 2001, 65, 1–12. [Google Scholar] [CrossRef]
- Azorit, C.; Tellado, S.; Oya, A.; Moro, J. Seasonal and specific diet variations in sympatric red and fallow deer of southern Spain: A preliminary approach to feeding behaviour. Anim. Prod. Sci. 2012, 52, 720–727. [Google Scholar] [CrossRef]
- Obidziński, A.; Kiełtyk, P.; Borkowski, J.; Bolibok, L.; Remuszko, K. Autumn-winter diet overlap of fallow, red, and roe deer in forest ecosystems, Southern Poland. Cent. Eur. J. Biol. 2012, 8, 8–17. [Google Scholar] [CrossRef]
- Mátrai, K.; Koltay, A.; Tóth, S.; Vízi, G. Key based on leaf epidermal anatomy for food habit studies of herbivores. Acta Bot. Hung. 1986, 23, 255–271. [Google Scholar]
- Dzieciolowski, R. Food of the red deer in an annual cycle. Acta Theriol. 1967, 12, 503–520. [Google Scholar] [CrossRef] [Green Version]
- Gebert, C.; Verheyden Tixier, H. Variations of diet composition of Red Deer (Cervus elaphus L.) in Europe. Mammal Rev. 2001, 31, 189–201. [Google Scholar] [CrossRef]
- Korschgen, L.J. Histological characteristics of plant leaf epidermis and related structures as an aid in food habits studies. Fed. Aid Proj. 1977, 13, R31. [Google Scholar]
- Reynolds, H.W.; Hansen, R.M.; Peden, D.G. Diets of the slave river lowland bison herd, northwest territories, Canada. J. Wildl. Manag. 1978, 42, 581–590. [Google Scholar] [CrossRef]
- Metcalfe, C.R.; ChaIk, L. Anatomy of the Dicotyledons: Leaves, Stem and Wood in Relation to Taxonomy, with Notes on Economic Uses Volume II; Clarendon Press: Oxford, UK, 1957. [Google Scholar]
- Metcalfe, C.R. Anatomy of the Monocotyledons. 1 Gramineae; Clarendon Press: Oxford, UK, 1960. [Google Scholar]
- Cutler, D.F. Anatomy of the Monocotyledons, IV. Juncales; Clarendon Press: Oxford, UK, 1969. [Google Scholar]
- Esau, K. Anatomy of Seed Plants; John Wiley and Sons: Hoboken, NJ, USA, 1977. [Google Scholar]
- Evert, F.R. Esaus Plant Anatomy; Wiley Interscience: Hoboken, NJ, USA, 2006. [Google Scholar]
- Latham, J.; Staines, B.W.; Gorman, M.L. Comparative feeding ecology of red (Cervus elaphus) and roe deer (Capreolus capreolus) in Scottish plantation forests. J. Zool. 1999, 247, 409–418. [Google Scholar] [CrossRef]
- Lovari, S.; Ferretti, F.; Corazza, M.; Minder, I.; Troiani, N.; Ferrari, C.; Saddi, A. Unexpected consequences of reintroductions: Competition between increasing red deer and threatened Apennine chamois. Anim. Conserv. 2014, 17, 359–370. [Google Scholar] [CrossRef]
- Biel, W.; Jaroszewska, A. The nutritional value of leaves of selected berry species. Sci. Agric. 2017, 74, 405–410. [Google Scholar] [CrossRef]
- Storr, G.M. Microscopic analysis of faeces, a technique for ascertaining the diet of herbivorous animals. Aust. J. Biol. Sci. 1961, 14, 157–164. [Google Scholar] [CrossRef]
- Jackson, B.; Snowdon, D.W. Atlas of Microscopy of Medicinal Plants, Culinary Herbs and Spices; Belhaven Press a Division of Pinter Publishers: London, UK, 1990. [Google Scholar]
- Rahfeld, B. Mikroskopischer Farbatlas Pflanzlicher Drogen; Spektrum Akademischer Verlag: Heidelberg, Germany, 2009. [Google Scholar]
- Tomaszewski, D.; Zieliński, J.; Gawlak, M. Foliar indumentum in central-European Rubus species (Rosaceae) and its contribution to the systematics of the group. Nord. J. Bot. 2014, 32, 1–10. [Google Scholar] [CrossRef]
- Klika, J. Jehličnaté; Nakladatelství Československé Akademie: Praha, Czechoslovakia, 1953. [Google Scholar]
- Foster, A.S.; Gifford, E.M. Comparative Morphology of Vascular Plants; W.H. Freeman and Company: New York, NY, USA, 1959. [Google Scholar]
- Votrubová, O. Anatomie Rostlin; Karolinum: Praha, Czech Republic, 2010. [Google Scholar]
- Požgaj, A. Štruktúra a Vlastnosti Dreva; Príroda a.s.: Bratislava, Slovakia, 1997. [Google Scholar]
- Copeland, E.B. The comparative ecology of San Ramon Polypodiaceae. Philipp. J. Sci. 1907, 2, 1–76. [Google Scholar]
- Novák, J.; Skalický, M. Botanika; Česká Zemědělská Univerzita V Praze: Praha, Czech Republic, 2009. [Google Scholar]
- Scott, T. Concise Encyklopedia Biology; Walter de Gruyter: Berlin, Germany, 1996. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veselovská, A.; Smolko, P.; Kropil, R. A Key for the Microhistological Determination of Plant Fragments Consumed by Carpathian Forest Cervids. Forests 2021, 12, 1229. https://doi.org/10.3390/f12091229
Veselovská A, Smolko P, Kropil R. A Key for the Microhistological Determination of Plant Fragments Consumed by Carpathian Forest Cervids. Forests. 2021; 12(9):1229. https://doi.org/10.3390/f12091229
Chicago/Turabian StyleVeselovská, Alexandra, Peter Smolko, and Rudolf Kropil. 2021. "A Key for the Microhistological Determination of Plant Fragments Consumed by Carpathian Forest Cervids" Forests 12, no. 9: 1229. https://doi.org/10.3390/f12091229
APA StyleVeselovská, A., Smolko, P., & Kropil, R. (2021). A Key for the Microhistological Determination of Plant Fragments Consumed by Carpathian Forest Cervids. Forests, 12(9), 1229. https://doi.org/10.3390/f12091229






