The Distribution and Migration of 137Cs in Oak (Quercus serrata) and Cedar (Cryptomeria japonica) Forest Organic Fractions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Preparation
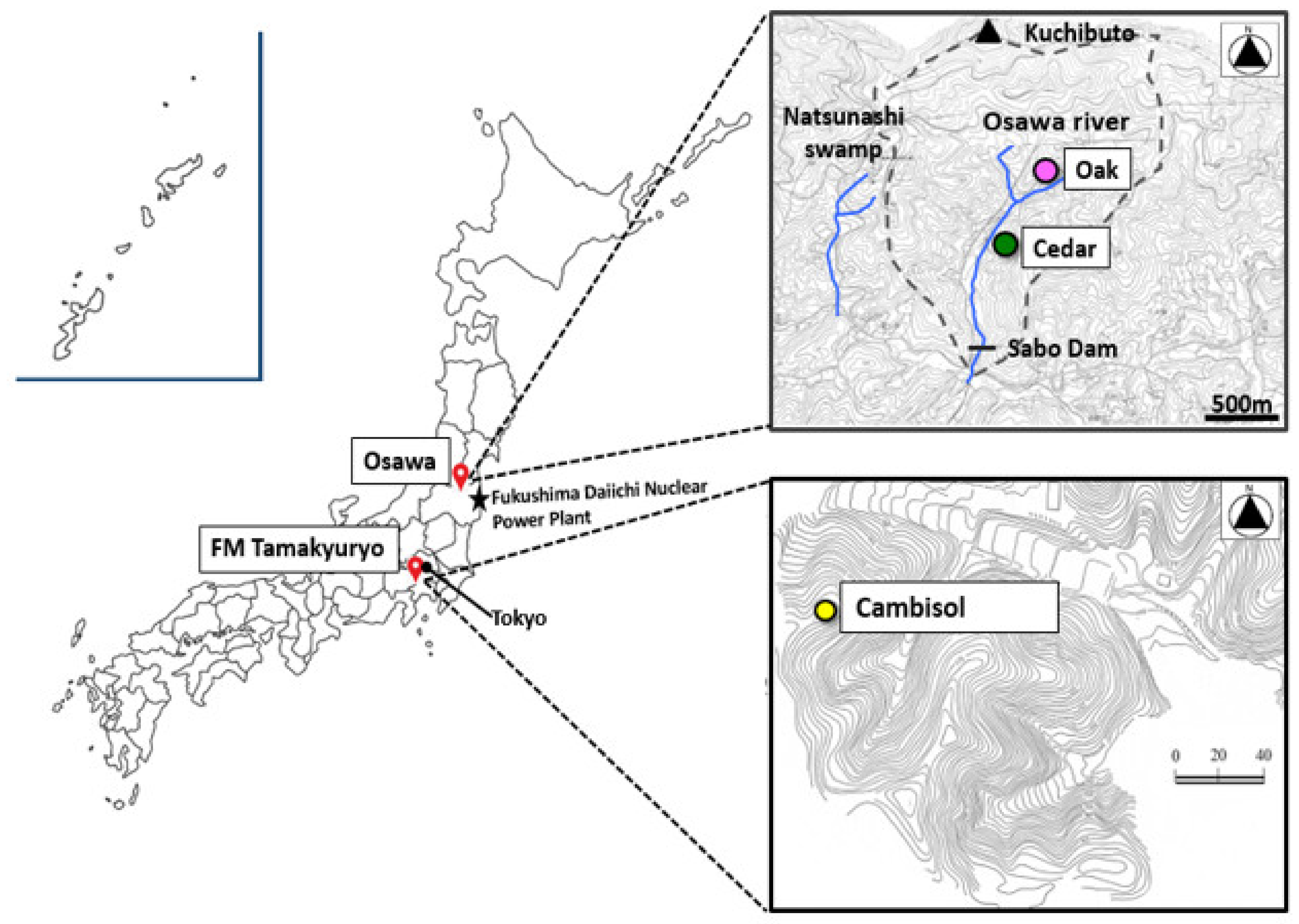
2.1.1. Study Sites
2.1.2. Sampling Methods
2.2. Experimental Setup
2.3. Laboratory Analyses
2.3.1. Soil Physicochemical Analyses
| Soil Group (WRB, 2006) | Depth (cm) | Bulk Density (kg m−3) | pH (H2O) |
|---|---|---|---|
| Cambisol | 0–1 | 450 | 4.9 |
| 1–2 | 450 | 5.1 | |
| 2–5 | 450 | 5.5 | |
| 5–10 | 550 | 5.6 |
| Soil Column | Depth | TC a | TN b | C/N c | 137Cs | 137Cs Retention f | |
|---|---|---|---|---|---|---|---|
| Treatment | (cm) | (kg m−2) | (Bq kg−1) d | (Bq m−2) e | (%) | ||
| Oak-L | 0–1 | 2.20 | 0.16 | 13.8 | 355 a | 1596 a | 56 |
| 1–2 | 1.78 | 0.14 | 12.7 | 168 b | 756 b | 26 | |
| 2–5 | 1.47 | 0.12 | 12.3 | 67 b | 302 b | 11 | |
| 5–10 | 1.23 | 0.11 | 11.2 | 38 b | 207 b | 7 | |
| Oak-F | 0–1 | 2.61 | 0.21 | 12.4 | 3050 a | 13713 a | 91 |
| 1–2 | 1.89 | 0.16 | 11.8 | 211 b | 947 b | 6 | |
| 2–5 | 1.40 | 0.12 | 11.7 | 50 b | 225 b | 2 | |
| 5–10 | 1.75 | 0.14 | 12.5 | 24 b | 131 b | 1 | |
| Cedar-L | 0–1 | 2.01 | 0.16 | 12.6 | 409 a | 1842 a | 55 |
| 1–2 | 1.99 | 0.16 | 12.4 | 212 b | 953 b | 29 | |
| 2–5 | 1.28 | 0.10 | 12.8 | 66 c | 295 c | 9 | |
| 5–10 | 1.25 | 0.10 | 12.5 | 40 c | 221 c | 7 | |
| Cedar-F | 0–1 | 2.24 | 0.17 | 13.2 | 1523 a | 6855 a | 71 |
| 1–2 | 1.87 | 0.14 | 13.4 | 300 b | 1349 b | 14 | |
| 2–5 | 1.83 | 0.14 | 13.1 | 202 b | 908 b | 10 | |
| 5–10 | 1.64 | 0.13 | 12.6 | 81 b | 446 b | 5 | |
2.3.2. Measurement of 137Cs Activity
2.4. Estimation of Soil Depth Distribution Parameters
2.5. Statistical Analysis
3. Results and Discussion
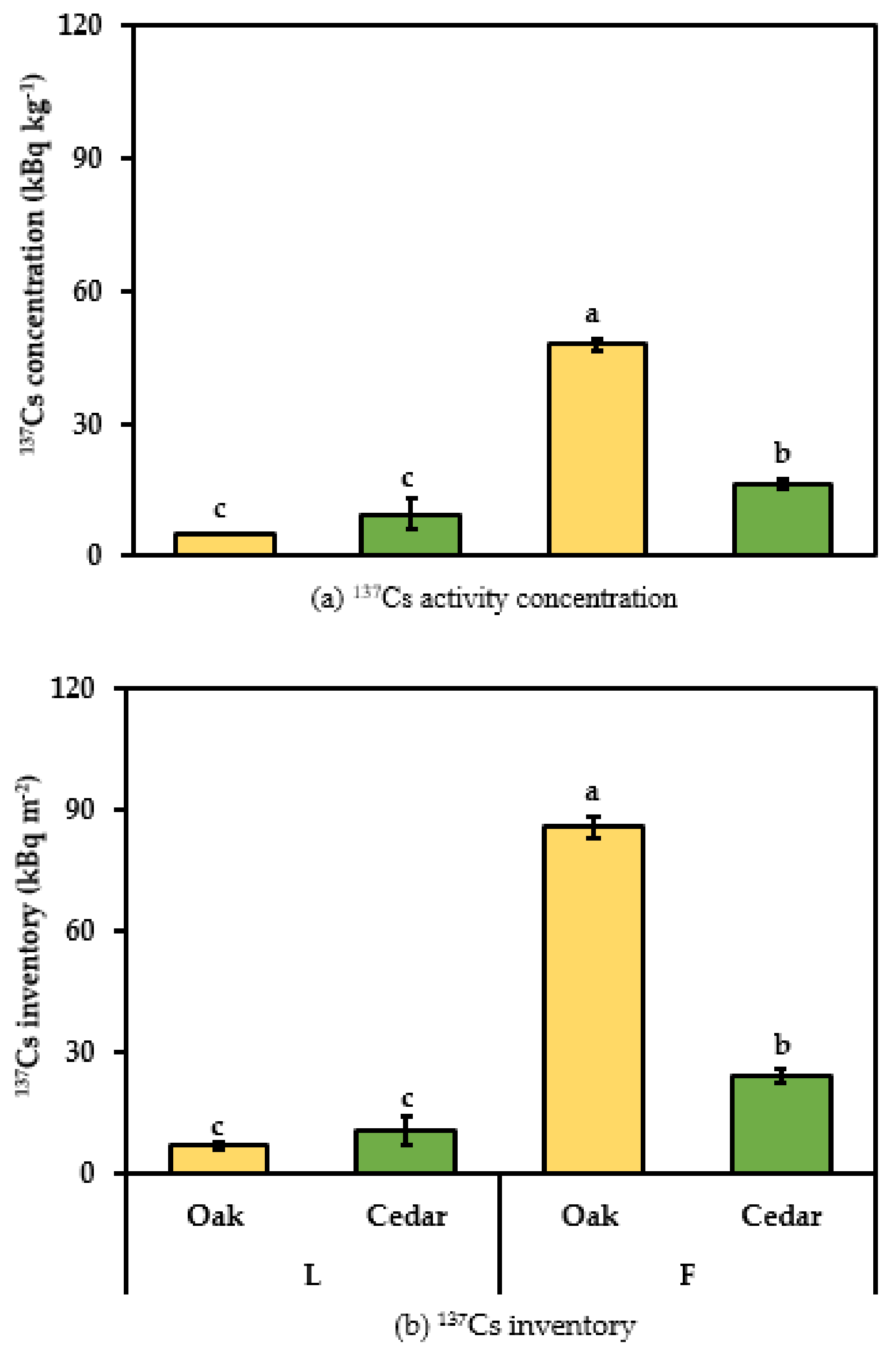
3.1. Cs Retention in Forest Litter Organic Layer Fractions
3.2. Radioactive 137Cs in Soil Profile Layers
3.3. Depth Distribution of 137Cs in Soil Profile Layers
3.4. The Effect of Soil Properties on 137Cs Retention in Soils
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koarashi, J.; Atarashi-Andoh, M.; Matsunaga, T.; Sato, T.; Nagao, S.; Nagai, H. Factors affecting vertical distribution of Fukushima accident-derived radiocesium in soil under different land-use conditions. Sci. Total Environ. 2012, 431, 392–401. [Google Scholar] [CrossRef]
- JAEA. Airborne Monitoring in the Distribution Survey of Radioactive Substances (FY 2011–FY 2016 The Ministry of Education, Culture, Sports, Science and Technology, the U.S. Department of Energy, and the Secretariat of the Nuclear Regulation Authority). Available online: https://emdb.jaea.go.jp/emdb/en/portals/b1010301/ (accessed on 19 June 2020).
- Onda, Y.; Gomi, T.; Mizugaki, S.; Nonoda, T.; Sidle, R.C.; Onda, Y.; Gomi, T.; Mizugaki, S.; Nonoda, T.; Sidle, R.C. An overview of the field and modelling studies on the effects of forest devastation on flooding and environmental issues. Hydrol. Process 2010, 24, 527–534. [Google Scholar] [CrossRef]
- Ono, K.; Hiradate, S.; Morita, S.; Hirai, K. Fate of organic carbon during decomposition of different litter types in Japan. Biogeochemistry 2013, 112, 7–21. [Google Scholar] [CrossRef]
- Imamura, N.; Komatsu, M.; Ohashi, S.; Hashimoto, S.; Kajimoto, T.; Kaneko, S.; Takano, T. Temporal changes in the radiocesium distribution in forests over the five years after the Fukushima Daiichi Nuclear Power Plant accident. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Omari, A.; Toda, H.; Choi, D. Efficacy of soil moisture conditions on the formation of ectomycorrhizal colonization and 137Cs absorption. Landsc. Ecol. Eng. 2020, 16, 87–95. [Google Scholar] [CrossRef]
- Smith, W.B.; Miles, P.D.; Perry, C.H.; Pugh, S.A. Forest Resource of the United States, 2007; Gen. Tech. Rep. WO-78; U.S. Department of Agriculture, Forest Service, Washington Office: Washington, DC, USA, 2009. [CrossRef] [Green Version]
- Forsberg, S.; Strandmark, M. Migration and chemical availability of 137Cs and 90 Sr in Swedish long-term experimental pastures. Water Air Soil Pollut. 2001, 127, 157–171. [Google Scholar] [CrossRef]
- Konopleva, I.; Klemt, E.; Konoplev, A.; Zibold, G. Migration and bioavailability of 137Cs in forest soil of southern Germany. J. Environ. Radioact. 2009, 100, 315–321. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Walling, D.E. The distribution of fallout 137Cs and 210Pb in undisturbed and cultivated soils. Appl. Radiat. Isot. 1997, 48, 677–690. [Google Scholar] [CrossRef]
- Teramage, M.T.; Onda, Y.; Patin, J.; Kato, H.; Gomi, T.; Nam, S. Vertical distribution of radiocesium in coniferous forest soil after the Fukushima nuclear power plant accident. J. Environ. Radioact. 2014, 137, 37–45. [Google Scholar] [CrossRef]
- Forestry Agency of Japan. Preliminary Results of Surveys of Distributions of Radioactive Elements in Forest Ecosystems. Available online: http://www.rinya.maff.go.jp/j/press/hozen/111227_2.html (accessed on 5 June 2021). (In Japanese)
- Hashimoto, S.; Ugawa, S.; Nanko, K.; Shichi, K. The total amounts of radioactively contaminated materials in forests in Fukushima, Japan. Sci. Rep. 2012, 2, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensah, A.D.; Terasaki, A.; Aung, H.P.; Toda, H.; Suzuki, S.; Tanaka, H.; Onwona-Agyeman, S.; Omari, R.A.; Bellingrath-Kimura, S.D. Influence of soil characteristics and land use type on existing fractions of radioactive 137Cs in fukushima soils. Environments 2020, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.; Toda, H.; Guy, R.D. Characteristics of 137Cs accumulation by Quercus serrata seedlings infected with ectomycorrhizal fungi. J. For. Res. 2018, 23, 21–27. [Google Scholar] [CrossRef]
- Koarashi, J.; Atarashi-Andoh, M. Low 137 Cs retention capability of organic layers in Japanese forest ecosystems affected by the Fukushima nuclear accident. J. Radioanal. Nucl. Chem. 2019, 320, 179–191. [Google Scholar] [CrossRef]
- Koarashi, J.; Atarashi-Andoh, M.; Takeuchi, E.; Nishimura, S. Topographic heterogeneity effect on the accumulation of Fukushima-derived radiocesium on forest floor driven by biologically mediated processes. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koarashi, J.; Atarashi-Andoh, M.; Ishizuka, S.; Miura, S.; Saito, T.; Hirai, K.; HiraiI, K. Quantitative aspects of heterogeneity in soil organic matter dynamics in a cool-temperate Japanese beech forest: A radiocarbon-based approach. Glob. Chang. Biol. 2009, 15, 631–642. [Google Scholar] [CrossRef]
- Ono, K.; Hirai, K.; Morita, S.; Ohse, K.; Hiradate, S. Organic carbon accumulation processes on a forest floor during an early humification stage in a temperate deciduous forest in Japan: Evaluations of chemical compositional changes by 13C NMR and their decomposition rates from litterbag experiment. Geoderma 2009, 151, 351–356. [Google Scholar] [CrossRef]
- Prescott, C.E.; Zabek, L.M.; Staley, C.L.; Kabzems, R. Decomposition of broadleaf and needle litter in forests of British Columbia: Influences of litter type, forest type, and litter mixtures. Can. J. For. Res. 2000, 30, 1742–1750. [Google Scholar] [CrossRef]
- Zhang, D.; Hui, D.; Luo, Y.; Zhou, G. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Agroforestree Database: A Tree Reference and Selection Guide. Version 4; World Agroforestry Centre: Nairobi, Kenya, 2009. [Google Scholar]
- Japan Meteorological Agency. Nihonmatsu and Hachiojii AMeDAS data. Available online: http://www.data.jma.go.jp/ (accessed on 1 February 2018).
- FAO. World Reference Base for Soil World Reference Base for Soil Resources 2006 A Framework for International Classification, Correlation and Communication; World Soil Reports 103; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Haque, M.; Ghose, S.; Islam, S. A Laboratory Based Study on the Movement of Radiocaesium in Some Soil Columns by Gamma Spectrometer. J. Bangladesh Acad. Sci. 1970, 35, 141–151. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H.; Blake, G.R.; Hartge, K.H. Bulk Density. In Methods of Soil Analysis, Part 1—Physical and Mineralogical Methods; SSSA Book Series SV-5.1; Klute, A., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1986; pp. 363–382. [Google Scholar] [CrossRef]
- Committee of Soil Standard Methods for Analyses and Measurements. Soil Standard Methods for Analyses and Measurements; Hakuyusha: Tokyo, Japan, 1986; p. 354. (In Japanese) [Google Scholar]
- Walling, D.E.; He, Q. Improved Models for Estimating Soil Erosion Rates from Cesium-137 Measurements. J. Environ. Qual. 1999, 28, 611–622. [Google Scholar] [CrossRef]
- Beck, H.L. Environmental Gamma Radiation from Deposited Fission Products, 1960–1964. Health Phys. 1966, 12, 313–322. [Google Scholar] [CrossRef]
- Kato, H.; Onda, Y.; Teramage, M. Depth Distribution of 137Cs, 134Cs, and 131I in Soil Profile after Fukushima Dai-Ichi Nuclear Power Plant Accident. J. Environ. Radioact. 2012, 111, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, Ö.; Yaprak, G. Vertical Distributions and Gamma Dose Rates of 40K, 232Th, 238U and 137Cs in the Selected Forest Soils in Izmir, Turkey. Radiat. Prot. Dosimetry 2008, 131, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Porto, P.; Walling, D.E.; Ferro, V. Validating the Use of Caesium-137 Measurements to Estimate Soil Erosion Rates in a Small Drainage Basin in Calabria, Southern Italy. J. Hydrol. 2001, 248, 93–108. [Google Scholar] [CrossRef]
- Nakanishi, T.; Matsunaga, T.; Koarashi, J.; Atarashi-Andoh, M. 137Cs vertical migration in a deciduous forest soil following the Fukushima Dai-ichi Nuclear Power Plant accident. J. Environ. Radioact. 2014, 128, 9–14. [Google Scholar] [CrossRef]
- Cannell, M.G.R. World Forest Biomass and Primary Production Data; Academic Press: London, UK; New York, NY, USA, 1982; ISBN 9780121587802. [Google Scholar]
- Rafferty, B.; Brennan, M.; Dawson, D.; Dowding, D. Mechanisms of 137Cs migration in coniferous forest soils. J. Environ. Radioact. 2000, 48, 131–143. [Google Scholar] [CrossRef]
- Koarashi, J.; Atarashi-Andoh, M.; Matsunaga, T.; Sanada, Y. Forest Type Effects on the Retention of Radiocesium in Organic Layers of Forest Ecosystems Affected by the Fukushima Nuclear Accident. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Schimmack, W.; Bunzl, K.; Dietl, F.; Klotz, D. Infiltration of radionuclides with low mobility (137Cs and 60Co) into a forest soil. Effect of the irrigation intensity. J. Environ. Radioact. 1994, 24, 53–63. [Google Scholar] [CrossRef]
- Sigurgeirsson, M.A.; Arnalds, O.; Palsson, S.E.; Howard, B.J.; Gudnason, K. Radiocaesium fallout behaviour in volcanic soils in Iceland. J. Environ. Radioact. 2005, 79, 39–53. [Google Scholar] [CrossRef]
- Bunzl, K.; Schimmack, W.; Krouglov, S.V.; Alexakhin, R.M. Changes with time in the migration of radiocesium in the soil, as observed near Chernobyl and in Germany, 1986–1994. Sci. Total Environ. 1995, 175, 49–56. [Google Scholar] [CrossRef]
- Ivanov, Y.A.; Lewyckyj, N.; Levchuk, S.E.; Prister, B.S.; Firsakova, S.K.; Arkhipov, N.P.; Arkhipov, A.N.; Kruglov, S.V.; Alexakhin, R.M.; Sandalls, J.; et al. Migration of 137Cs and 90Sr from Chernobyl fallout in Ukrainian, Belarussian and Russian soils. J. Environ. Radioact. 1997, 35, 1–21. [Google Scholar] [CrossRef]
- Takahashi, J.; Tamura, K.; Suda, T.; Matsumura, R.; Onda, Y. Vertical distribution and temporal changes of 137Cs in soil profiles under various land uses after the Fukushima Dai-ichi Nuclear Power Plant accident. J. Environ. Radioact. 2015, 139, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Chibowski, S.; Zygmunt, J. The influence of the sorptive properties of organic soils on the migration rate of 137Cs. J. Environ. Radioact. 2002, 61, 213–223. [Google Scholar] [CrossRef]
- Cremers, A.; Elsen, A.; De Preter, P.; Maes, A. Quantitative analysis of radiocaesium retention in soils. Nature 1988, 335, 247–249. [Google Scholar] [CrossRef]
- Rigol, A.; Vidal, M.; Rauret, G. An overview of the effect of organic matter on soil-radiocaesium interaction: Implications in root uptake. J. Environ. Radioact. 2002, 58, 191–216. [Google Scholar] [CrossRef]
- Valcke, E.; Cremers, A. Sorption-desorption dynamics of radiocaesium in organic matter soils. Sci. Total Environ. 1994, 157, 275–283. [Google Scholar] [CrossRef]




| Soil Physiochemical Properties | 137Cs Retention (%) | |||
|---|---|---|---|---|
| Oak-L | Oak-F | Cedar-L | Cedar-F | |
| Bulk density (kg m−3) | −0.969 * | −0.801 | −0.985 * | −0.834 |
| Mass depth (kg m−2) | −0.990 ** | −0.901 | −0.979 * | −0.936 * |
| pH | −0.944 * | −0.788 | −0.967 * | −0.823 |
| Total carbon (kg m−2) | 0.976 * | 0.925 | 0.889 | 0.956 * |
| Total nitrogen (kg m−2) | 0.987 * | 0.910 | 0.863 | 0.973 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensah, A.D.; Toda, H.; Bellingrath-Kimura, S.D.; Kato, H.; Choi, D. The Distribution and Migration of 137Cs in Oak (Quercus serrata) and Cedar (Cryptomeria japonica) Forest Organic Fractions. Forests 2021, 12, 1045. https://doi.org/10.3390/f12081045
Mensah AD, Toda H, Bellingrath-Kimura SD, Kato H, Choi D. The Distribution and Migration of 137Cs in Oak (Quercus serrata) and Cedar (Cryptomeria japonica) Forest Organic Fractions. Forests. 2021; 12(8):1045. https://doi.org/10.3390/f12081045
Chicago/Turabian StyleMensah, Akwasi Dwira, Hiroto Toda, Sonoko Dorothea Bellingrath-Kimura, Hiroaki Kato, and Dongsu Choi. 2021. "The Distribution and Migration of 137Cs in Oak (Quercus serrata) and Cedar (Cryptomeria japonica) Forest Organic Fractions" Forests 12, no. 8: 1045. https://doi.org/10.3390/f12081045
APA StyleMensah, A. D., Toda, H., Bellingrath-Kimura, S. D., Kato, H., & Choi, D. (2021). The Distribution and Migration of 137Cs in Oak (Quercus serrata) and Cedar (Cryptomeria japonica) Forest Organic Fractions. Forests, 12(8), 1045. https://doi.org/10.3390/f12081045






