Induced Volatile Emissions, Photosynthetic Characteristics, and Pigment Content in Juglans regia Leaves Infected with the Erineum-Forming Mite Aceria erinea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Plant Material
2.2. Foliage Gas-Exchange Measurements
2.3. Leaf Pigment Analysis
2.4. Volatile Sampling and GC–MS Analyses
2.5. Estimations of the Leaf Area and the Degree of Infection
2.6. Statistical Analysis and Data Handling
3. Results
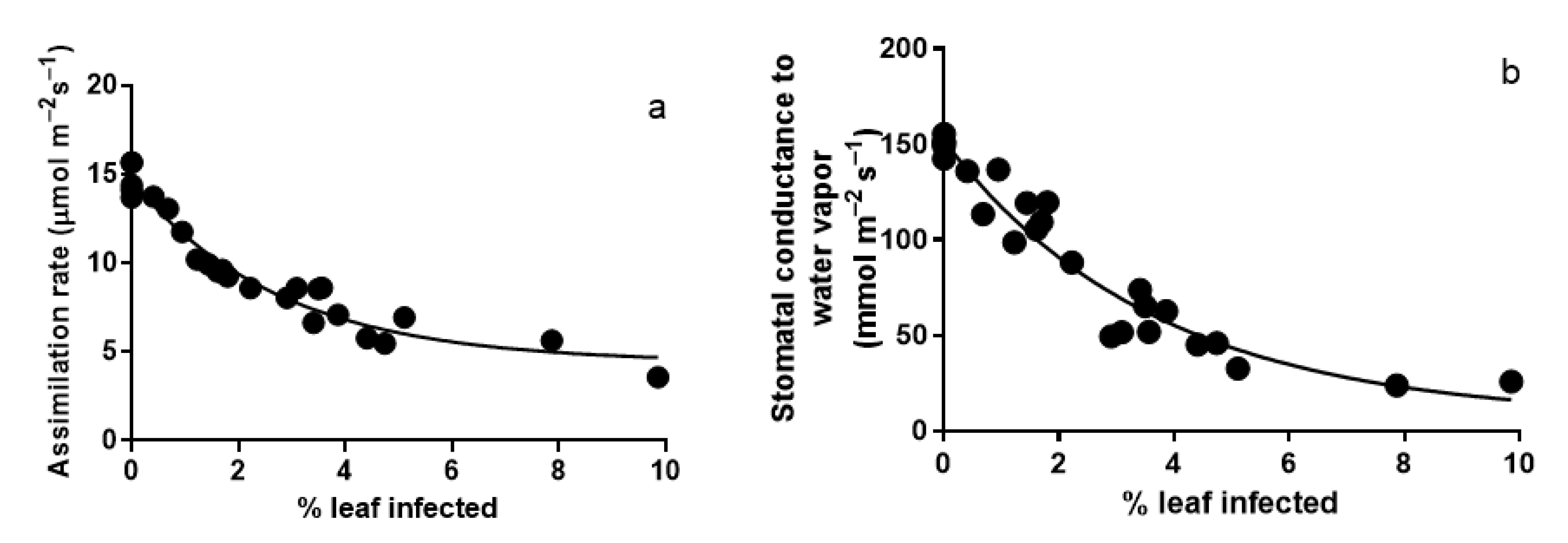
3.1. Photosynthesis Characteristics of Leaves Infected with A. erinea
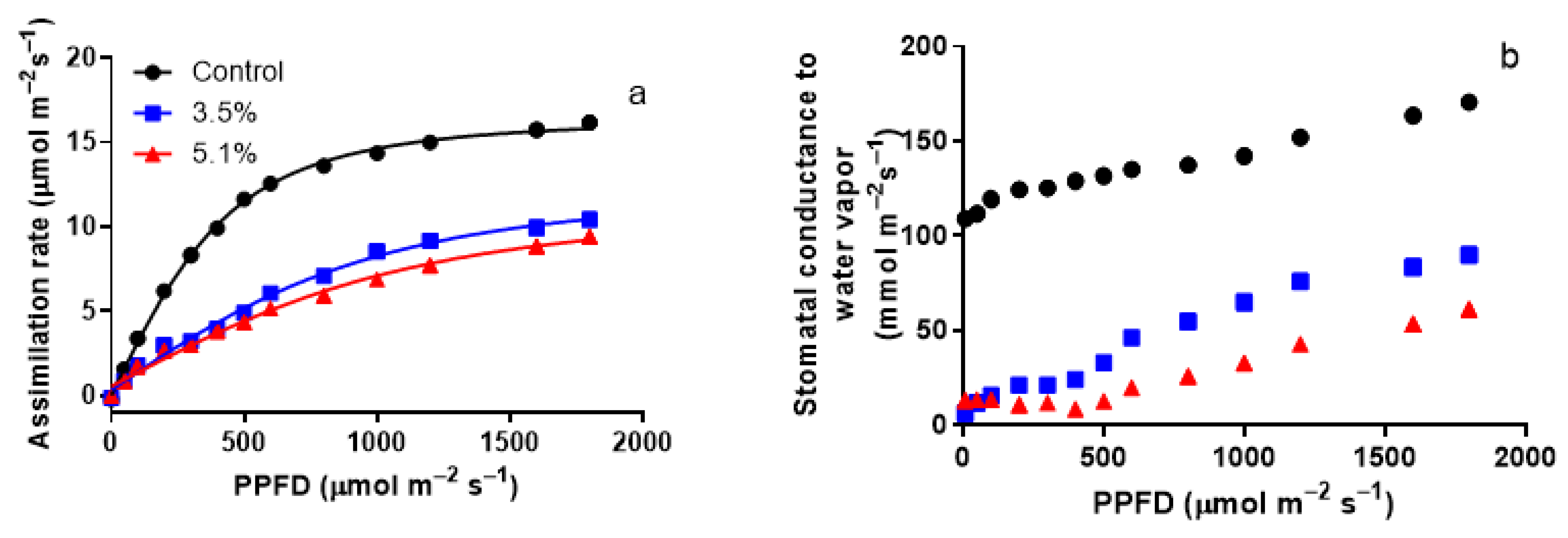
3.2. Modification of Light Responses of Photosynthesis by A. erinea Infection
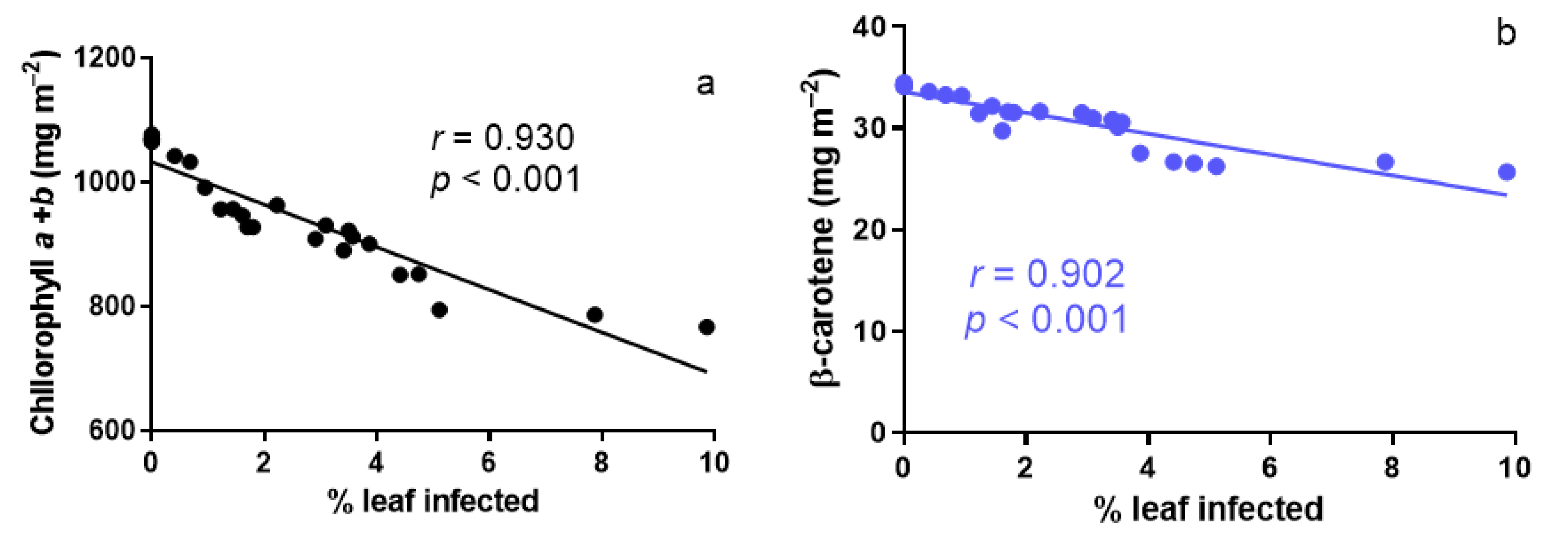
3.3. Responses of Foliage Chlorophylls and β-Carotene Contents to A. erinea Infection
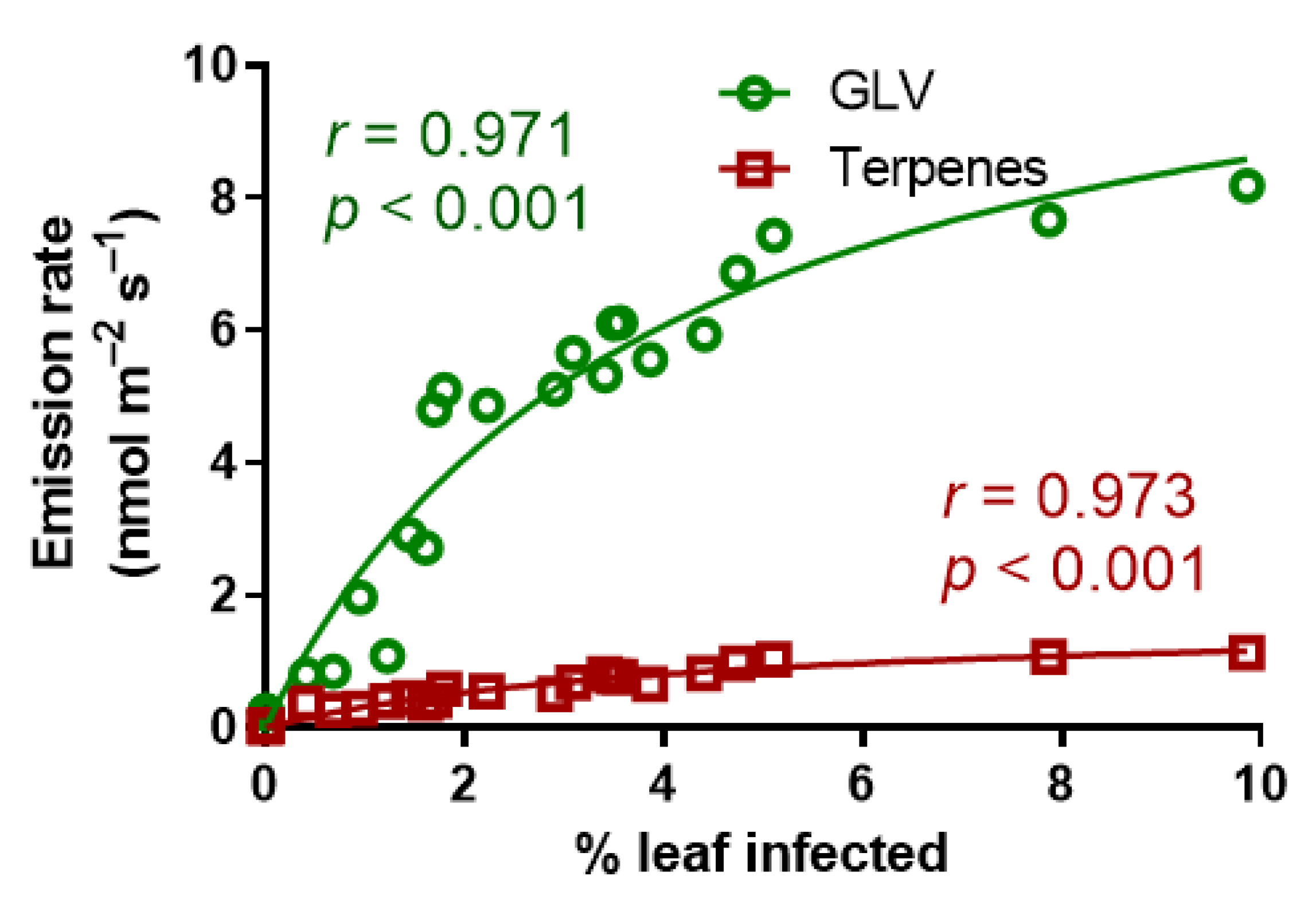
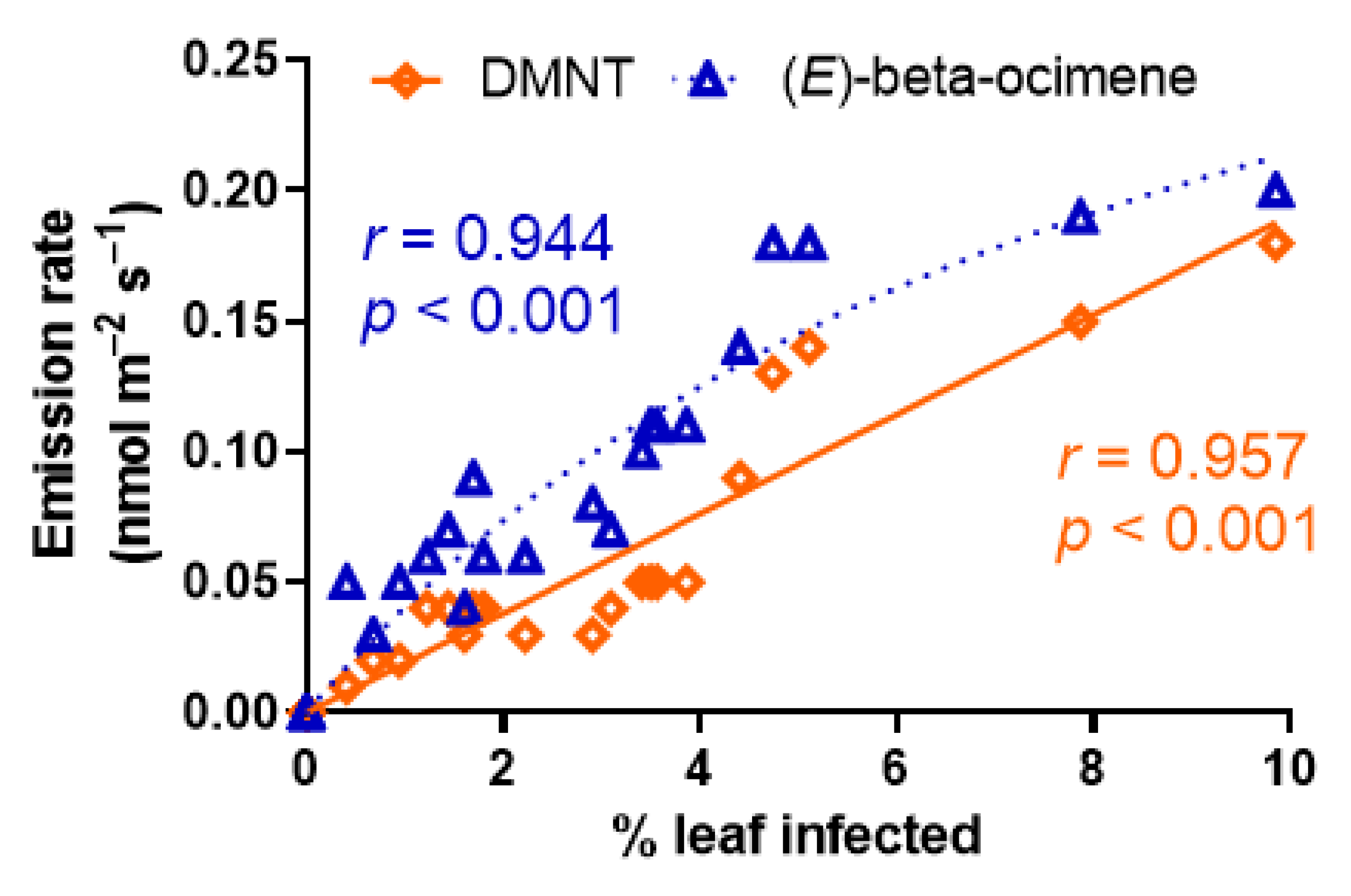
3.4. Effects of A. erinea Infection on Stress-Elicited Volatile Emissions
4. Discussion
4.1. The Influence of Aceria erinea Infection on Photosynthetic Characteristics of Juglans regia
4.2. Emission of Volatile Organic Compounds from Leaves Infected with A. erinea
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bernard, A.; Lheureux, F.; Dirlewanger, E. Walnut: Past and future of genetic improvement. Tree Genet. Genomes 2018, 14, 1. [Google Scholar] [CrossRef]
- Durrant, T.H.; Caudullo, G.; Enescu, C.M.; De Rigo, D.; Tinner, W. Juglans regia in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; Schweizerische Zahnärzte-Gesellschaft SSO: Bern, Switzerland, 2016. [Google Scholar]
- Martínez-García, P.J.; Crepeau, M.; Puiu, D.; Gonzalez-Ibeas, D.; Whalen, J.; Stevens, K.A.; Paul, R.; Butterfield, T.S.; Britton, M.T.; Reagan, R.L.; et al. The walnut (Juglans regia) genome sequence reveals diversity in genes coding for the biosynthesis of non-structural polyphenols. Plant J. 2016, 87, 507–532. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; Hemery, G.; Savill, P. Early growth and form of common walnut (Juglans regia L.) in mixture with tree and shrub nurse species in southern England. Forestry 2008, 81, 631–644. [Google Scholar] [CrossRef]
- Khan, A.; Kundoo, A.A. Pests of Walnut. In Pests and Their Management; Springer: Berlin/Heidelberg, Germany, 2018; pp. 605–647. [Google Scholar]
- Poggetti, L.; Raranciuc, S.; Chiabà, C.; Vischi, M.; Zandigiacomo, P. Altitude affects the distribution and abundance of two non-native insect pests of the common walnut. J. Appl. Entomol. 2019, 143, 527–534. [Google Scholar] [CrossRef]
- Karczmarz, K. Dynamics of population and bionomics of Panaphis juglandis (Goeze, 1778) (Homoptera, Phyllaphididae) on common walnut (Juglans regia L.) in Lublin’s parks and gardens. Acta Sci. Pol. Hortorum Cultus 2012, 11, 53–70. [Google Scholar]
- Cecilio, A.; Ilharco, F. The Control of Walnut Aphid, Chromaphis Juglandicola (Homoptera: Aphidoidea) In Walnut Orchards In Portugal. Acta Hortic. 1997, 442, 399–406. [Google Scholar] [CrossRef]
- McGranahan, G.; Leslie, C. Breeding Walnuts (Juglans regia). In Breeding Plantation Tree Crops: Temperate Species; Gradziel, T.M., Ed.; Springer: New York, NY, USA, 2009; pp. 249–273. [Google Scholar]
- Ahmad–Hosseini, M.; Khanjani, M.; Karamian, R. Resistance of some commercial walnut cultivars and genotypes to Aceria tristriata (Nalepa) (Acari: Eriophyidae) and its correlation with some plant features. Pest Manag. Sci. 2020, 76, 986–995. [Google Scholar] [CrossRef]
- Flechtmann, C.H.W.; Auger, P.; Veraeghe, A.; Cambronne, N.; Kreiter, S. The eriophyoid mites (Acarina) from walnut trees in Grenoble (Isere, France). Acaralogia 2002, 42, 377–388. [Google Scholar]
- Shorthouse, J.D.; Wool, D.; Raman, A. Gall-inducing insects—Nature’s most sophisticated herbivores. Basic Appl. Ecol. 2005, 6, 407–411. [Google Scholar] [CrossRef]
- Dos Santos, C.S.; Varanda, E. Morphological and histochemical study of leaf galls of Tabebuia ochracea (Cham.) Standl. (Bignoniaceae). Phytomorphol. Int. J. Plant Morphol. 2003, 53, 207–214. [Google Scholar]
- Chireceanu, C.; Chiriloaie, A.; Teodoru, A.; Sivu, C. Contribution to knowledge of the gall insects and mites associated with plants in Southern Romania. Sci. Pap. Ser. B Hortic. 2015, 59, 27–36. [Google Scholar]
- Amrine, J.W. 4.1.2 Phyllocoptes fructiphilus and biological control of multiflora rose. In World Crop Pests; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 6, pp. 741–749. [Google Scholar]
- Valenzano, D.; Tumminello, M.T.; Gualandri, V.; De Lillo, E. Morphological and molecular characterization of the Colomerus vitis erineum strain (Trombidiformes: Eriophyidae) from grapevine erinea and buds. Exp. Appl. Acarol. 2020, 80, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Veromann-Jürgenson, L.-L.; Ye, J.; Niinemets, Ü. Oak gall wasp infections of Quercus roburleaves lead to profound modifications in foliage photosynthetic and volatile emission characteristics. Plant Cell Environ. 2017, 41, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Künkler, N.; Brandl, R.; Brändle, M. Changes in Clonal Poplar Leaf Chemistry Caused by Stem Galls Alter Herbivory and Leaf Litter Decomposition. PLoS ONE 2013, 8, e79994. [Google Scholar] [CrossRef][Green Version]
- Biswas, S.M.; Chakraborty, N.; Pal, B. Foliar gall and antioxidant enzyme responses in Alstonia scholaris, r. Br. After Psylloid herbivory—An experimental and statistical analysis. Glob. J. Bot. Sci. 2014, 2, 12–20. [Google Scholar]
- Jiang, Y.; Ye, J.; Veromann-Jürgenson, L.-L.; Niinemets, Ü. Gall- and erineum-forming Eriophyes mites alter photosynthesis and volatile emissions in an infection severity-dependent manner in broad-leaved trees Alnus glutinosa and Tilia cordata. Tree Physiol. 2020. [Google Scholar] [CrossRef]
- Andersen, P.C.; Mizell, R.F. Physiological Effects of Galls Induced by Phylloxera notabilis (Homoptera: Phylloxeridae) on Pecan Foliage. Environ. Entomol. 1987, 16, 264–268. [Google Scholar] [CrossRef]
- Aldea, M.; Hamilton, J.G.; Resti, J.P.; Zangerl, A.R.; Berenbaum, M.R.; Frank, T.D.; DeLucia, E.H. Comparison of photosynthetic damage from arthropod herbivory and pathogen infection in understory hardwood saplings. Oecologia 2006, 149, 221–232. [Google Scholar] [CrossRef]
- Huang, M.-Y.; Chou, H.-M.; Chang, Y.-T.; Yang, C.-M. The number of cecidomyiid insect galls affects the photosynthesis of Machilus thunbergii host leaves. J. Asia Pac. Entomol. 2014, 17, 151–154. [Google Scholar] [CrossRef]
- Welter, S.C. Arthropod Impact on Plant Gas Exchange. In Insect-Plant Interaction, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 135–164. [Google Scholar]
- Ye, J.; Jiang, Y.; Veromann-Jürgenson, L.-L.; Niinemets, Ü. Petiole gall aphid (Pemphigus spyrothecae) infestation of Populus × petrovskiana leaves alters foliage photosynthetic characteristics and leads to enhanced emissions of both constitutive and stress-induced volatiles. Trees 2019, 33, 37–51. [Google Scholar] [CrossRef]
- Fay, P.A.; Hartnett, D.C.; Knapp, A.K. Plant Tolerance of Gall-Insect Attack and Gall-Insect Performance. Ecology 1996, 77, 521–534. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Remmel, T.; Vislap, V.; Niinemets, Ü. Volatile Emissions from Alnus glutionosa Induced by Herbivory are Quantitatively Related to the Extent of Damage. J. Chem. Ecol. 2011, 37, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, L.; Vaartnou, F.; Portillo-Estrada, M.; Niinemets, Ü. Oak powdery mildew (Erysiphe alphitoides)-induced volatile emissions scale with the degree of infection in Quercus robur. Tree Physiol. 2014, 34, 1399–1410. [Google Scholar] [CrossRef]
- Dicke, M.; Baldwin, I. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Van Loon, J.J.A.; Soler, R. Chemical complexity of volatiles from plants induced by multiple attack. Nat. Chem. Biol. 2009, 5, 317–324. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Blande, J.D. Where do herbivore-induced plant volatiles go? Front. Plant Sci. 2013, 4, 185. [Google Scholar] [CrossRef] [PubMed]
- Juráň, S.; Grace, J.; Urban, O. Temporal Changes in Ozone Concentrations and Their Impact on Vegetation. Atmosphere 2021, 12, 82. [Google Scholar] [CrossRef]
- Dulić-Stojanović, Z.; Stevanović, B.M.; Petanović, R. Morfološke i anatomske promene listova oraha izazvane eriofidama Aceria erinea i Aceria tristriata. (Morphological and anatomical alterations of common walnut leaves caused by eriophyids Aceria erinea and A. tristriata). Zaštita Bilja 2001, 52, 99–114. [Google Scholar]
- Karioti, A.; Tooulakou, G.; Bilia, A.R.; Psaras, G.K.; Karabourniotis, G.; Skaltsa, H. Erinea formation on Quercus ilex leaves: Anatomical, physiological and chemical responses of leaf trichomes against mite attack. Phytochemistry 2011, 72, 230–237. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Copolovici, L.; Hüve, K. High within-canopy variation in isoprene emission potentials in temperate trees: Implications for predicting canopy-scale isoprene fluxes. J. Geophys. Res. Space Phys. 2010, 115, 115. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L. Photosynthesis in Relation to Light and Carbon Dioxide. Proc. Natl. Acad. Sci. USA 1936, 22, 504–511. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Sun, Z.; Talts, E. Controls of the quantum yield and saturation light of isoprene emission in different-aged aspen leaves. Plant Cell Environ. 2015, 38, 2707–2720. [Google Scholar] [CrossRef] [PubMed]
- Taschina, M.; Copolovici, D.M.; Bungau, S.; Lupitu, A.I.; Copolovici, L.; Iovan, C. The influence of residual acetaminophen on Phaseolus vulgaris L secondary metabolites. Farmacia 2017, 65, 709–713. [Google Scholar]
- Opris, O.; Copaciu, F.; Soran, M.-L.; Ristoiu, D.; Niinemets, Ü.; Copolovici, L. Influence of nine antibiotics on key secondary metabolites and physiological characteristics in Triticum aestivum: Leaf volatiles as a promising new tool to assess toxicity. Ecotoxicol. Environ. Saf. 2013, 87, 70–79. [Google Scholar] [CrossRef]
- Kännaste, A.; Copolovici, L.; Niinemets, Ü. Gas Chromatography–Mass Spectrometry Method for Determination of Biogenic Volatile Organic Compounds Emitted by Plants. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1153, pp. 161–169. [Google Scholar]
- Copolovici, L.; Timis, D.; Taschina, M.; Copolovici, D.; Cioca, G.; Bungau, S. Diclofenac influence on photosynthetic parameters and volatile organic compounds emission from Phaseolus vulgaris L. plants. Rev. Chim. 2017, 68, 2076–2078. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kuhn, U.; Harley, P.C.; Staudt, M.; Arneth, A.; Cescatti, A.; Ciccioli, P.; Copolovici, L.; Geron, C.; Guenther, A.; et al. Estimations of isoprenoid emission capacity from enclosure studies: Measurements, data processing, quality and standardized measurement protocols. Biogeosciences 2011, 8, 2209–2246. [Google Scholar] [CrossRef]
- Larson, K.C. The impact of two gall-forming arthropods on the photosynthetic rates of their hosts. Oecologia 1998, 115, 161–166. [Google Scholar] [CrossRef]
- Khederi, S.J.; Khanjani, M.; Gholami, M.; De Lillo, E. Impact of the erineum strain of Colomerus vitis (Acari: Eriophyidae) on the development of plants of grapevine cultivars of Iran. Exp. Appl. Acarol. 2018, 74, 347–363. [Google Scholar] [CrossRef]
- Patankar, R.; Thomas, S.C.; Smith, S.M. A gall-inducing arthropod drives declines in canopy tree photosynthesis. Oecologia 2011, 167, 701–709. [Google Scholar] [CrossRef]
- Toome, M.; Randjärv, P.; Copolovici, L.; Niinemets, Ü.; Heinsoo, K.; Luik, A.; Noe, S. Leaf rust induced volatile organic compounds signalling in willow during the infection. Planta 2010, 232, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Haiden, S.A.; Hoffmann, J.H.; Cramer, M.D. Benefits of photosynthesis for insects in galls. Oecologia 2012, 170, 987–997. [Google Scholar] [CrossRef]
- Castro, A.; Oliveira, D.; Moreira, A.; Lemos-Filho, J.; Isaias, R. Source–sink relationship and photosynthesis in the horn-shaped gall and its host plant Copaifera langsdorffii Desf. (Fabaceae). S. Afr. J. Bot. 2012, 83, 121–126. [Google Scholar] [CrossRef]
- Hossain, M.M.; Khalequzzaman, K.M.; Sarkar, M.A.; Sarker, D. Assessment of chlorophyll loss due to infestation of gall mite in bay leaf. Asian J. Appl. Sci. Eng. 2017, 6, 123–128. [Google Scholar]
- Oliveira, D.C.; Moreira, A.S.F.P.; Isaias, R.M.S.; Martini, V.; Rezende, U.C. Sink Status and Photosynthetic Rate of the Leaflet Galls Induced by Bystracoccus mataybae (Eriococcidae) on Matayba guianensis (Sapindaceae). Front. Plant Sci. 2017, 8, 1249. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.G.; Oliveira, D.C.; Moreira, A.S.F.P.; Faria, A.M.; Guedes, L.M.; França, M.G.C.; Álvarez, R.; Isaias, R.M.S. Antioxidant metabolism in galls due to the extended phenotypes of the associated organisms. PLoS ONE 2018, 13, e0205364. [Google Scholar] [CrossRef]
- Larson, K.C.; Whitham, T.G. Competition between gall aphids and natural plant sinks: Plant architecture affects resistance to galling. Oecologia 1997, 109, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, L.; Pag, A.; Kännaste, A.; Bodescu, A.; Tomescu, D.; Copolovici, D.; Soran, M.-L.; Niinemets, Ü. Disproportionate photosynthetic decline and inverse relationship between constitutive and induced volatile emissions upon feeding of Quercus robur leaves by large larvae of gypsy moth (Lymantria dispar). Environ. Exp. Bot. 2017, 138, 184–192. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Remmel, T.; Niinemets, Ü. Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environ. Exp. Bot. 2014, 100, 55–63. [Google Scholar] [CrossRef]
- Li, S.; Harley, P.C.; Niinemets, Ü. Ozone-induced foliar damage and release of stress volatiles is highly dependent on stomatal openness and priming by low-level ozone exposure in Phaseolus vulgaris. Plant Cell Environ. 2017, 40, 1984–2003. [Google Scholar] [CrossRef]
- Andreou, A.; Feussner, I. Lipoxygenases—Structure and reaction mechanism. Phytochemistry 2009, 70, 1504–1510. [Google Scholar] [CrossRef]
- Gigot, C.; Ongena, M.; Fauconnier, M.-L.; Wathelet, J.-P.; Du Jardin, P.; Thonart, P. The lipoxygenase metabolic pathway in plants: Potential for industrial production of natural green leaf volatiles. Biotechnol. Agron. Soc. Environ. 2010, 14, 451–460. [Google Scholar]
- Matsui, K.; Sugimoto, K.; Mano, J.; Ozawa, R.; Takabayashi, J. Differential Metabolisms of Green Leaf Volatiles in Injured and Intact Parts of a Wounded Leaf Meet Distinct Ecophysiological Requirements. PLoS ONE 2012, 7, e36433. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, B.A.; Dar, M.Y.; Wani, B.A.; Shah, W.A.; Bhat, B.A.; Ganai, B.A.; Bhat, K.A.; Anand, R.; Qurishi, M.A. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine 2012, 19, 1185–1190. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Thul, S.T. Phytochemical analysis of the leaf volatile oil of walnut tree (Juglans regia L.) from western Himalaya. Ind. Crop. Prod. 2013, 42, 195–201. [Google Scholar] [CrossRef]
- Faiola, C.; Taipale, D. Impact of insect herbivory on plant stress volatile emissions from trees: A synthesis of quantitative measurements and recommendations for future research. Atmos. Environ. X 2020, 5, 100060. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.; Li, C.; Lee, I.H.; Lam, H.-M.; Chan, T.-F.; Hui, J.H. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef] [PubMed]
- Tak, J.-H.; Isman, M.B. Acaricidal and repellent activity of plant essential oil-derived terpenes and the effect of binary mixtures against Tetranychus urticae Koch (Acari: Tetranychidae). Ind. Crop. Prod. 2017, 108, 786–792. [Google Scholar] [CrossRef]
- Gulati, R. Eco-Friendly Management of Phytophagous Mites. In Integrated Pest Management; Abrol, D.P., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 461–491. [Google Scholar] [CrossRef]
- Mumm, R.; Posthumus, M.A.; Dicke, M. Significance of terpenoids in induced indirect plant defence against herbivorous arthropods. Plant Cell Environ. 2008, 31, 575–585. [Google Scholar] [CrossRef]
- Cui, Y.; Kong, S.; Liu, X.; Liu, S. Comparison of the volatiles composition between healthy and buprestid infected Juglans regia (Juglandaceae). Rev. Colomb. Entomol. 2020, 46, e8649. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popitanu, C.; Lupitu, A.; Copolovici, L.; Bungău, S.; Niinemets, Ü.; Copolovici, D.M. Induced Volatile Emissions, Photosynthetic Characteristics, and Pigment Content in Juglans regia Leaves Infected with the Erineum-Forming Mite Aceria erinea. Forests 2021, 12, 920. https://doi.org/10.3390/f12070920
Popitanu C, Lupitu A, Copolovici L, Bungău S, Niinemets Ü, Copolovici DM. Induced Volatile Emissions, Photosynthetic Characteristics, and Pigment Content in Juglans regia Leaves Infected with the Erineum-Forming Mite Aceria erinea. Forests. 2021; 12(7):920. https://doi.org/10.3390/f12070920
Chicago/Turabian StylePopitanu, Corina, Andreea Lupitu, Lucian Copolovici, Simona Bungău, Ülo Niinemets, and Dana Maria Copolovici. 2021. "Induced Volatile Emissions, Photosynthetic Characteristics, and Pigment Content in Juglans regia Leaves Infected with the Erineum-Forming Mite Aceria erinea" Forests 12, no. 7: 920. https://doi.org/10.3390/f12070920
APA StylePopitanu, C., Lupitu, A., Copolovici, L., Bungău, S., Niinemets, Ü., & Copolovici, D. M. (2021). Induced Volatile Emissions, Photosynthetic Characteristics, and Pigment Content in Juglans regia Leaves Infected with the Erineum-Forming Mite Aceria erinea. Forests, 12(7), 920. https://doi.org/10.3390/f12070920









