Abstract
To have a cleaner environment, good well-being, and improve the health of citizens it is necessary to expand green urban and suburban areas using productive and adapted material of tree species. The quality of urban greenery, resistance to negative climate change factors and pollution, as well as efficiency of short-rotation forestry in suburban areas, depends primarily on the selection of hybrids and clones, suitable for the local environmental conditions. We postulate that ecogenetic response, phenotypic plasticity, and genotypic variation of hybrid poplars (Populus L.) grown in plantations are affected not only by the peculiarities of hybrids and clones, but also by environmental conditions of their vegetative propagation. The aim of the present study was to estimate growth and biochemical responses, the phenotypic plasticity, genotypic variation of adaptive traits, and genetically regulated adaptability of Populus hybrids in field trials which may be predisposed by the simulated contrasting temperature conditions at their vegetative propagation phase. The research was performed with the 20 cultivars and experimental clones of one intraspecific cross and four different interspecific hybrids of poplars propagated under six contrasting temperature regimes in phytotron. The results suggest that certain environmental conditions during vegetative propagation not only have a short-term effect on tree viability and growth, but also can help to adapt to climate change conditions and grow successfully in the long-term. It was found that tree growth and biochemical traits (the chlorophyll A and B, pigments content and the chlorophyll A/B ratio) of hybrid poplar clones grown in field trials, as well as their traits’ genetic parameters, were affected by the rooting-growing conditions during vegetative propagation phase. Hybrids P. balsamifera × P. trichocarpa, and P. trichocarpa × P. trichocarpa have shown the most substantial changes of biochemical traits across vegetative propagation treatments in field trial. Rooting-growing conditions during vegetative propagation had also an impact on coefficients of genotypic variation and heritability in hybrid poplar clones when grown in field trials.
1. Introduction
Urban green territories and surrounding forests are of great importance for our quality of life. Urban trees as part of urban forests generate a range of environmental, economic, social, and cultural benefits that contribute to the well-being and health of citizens [1]. Urban trees play an essential role in improving air quality, fixing carbon, and mitigating environmental degradation. In a typical urban environment, citizens are exposed to about 200 air pollutants or classes of air pollutants [2], which cause health problems and increase mortality. The importance of forests as a source of renewable energy, raw materials, and CO2 (carbon dioxide) absorbed from the atmosphere, thus reducing greenhouse gases and climate change, is increasingly emphasized in the world, leading to the establishing of new forests in many countries [3,4,5]. Poplar hybrids are a very fast-growing, ornamental tree; their wood is valuable for paper industry and bioenergy and it is considered a promising tree species both economically and ecologically [6,7]. In terms of biodiversity, poplar is extremely valuable [8,9,10]. Poplars are worldwide tree species with a wide natural range of geographical distribution, thus growing in a variety of ecological conditions [11,12,13]. The distribution range of P. trichocarpa in from north to south is extremely large—it grows in America from California to Alaska. The range of P. deltoides in the Americas is more southern, from the Gulf of Mexico to southwestern Canada. Balsam poplar (P. balsamifera) is adapted to the northern climate—it grows from southeastern Canada to Alaska. Cross-breeding uses Amur/Japanese poplar (P. maximowiczii), native to East Asia and tolerant of drought [14]. Different poplars and their hybrids are successfully planted in parks, along urban and rural road corridors, in other urban territories as protective, recreational greenery all over the world [13,15,16,17]. Poplars are important roadside trees for their rural-urban linking and ecological contributions as well as for their demonstrated natural effect and their visual diversity, effectiveness, and suppleness [15]. Climate change is a growing threat to the sustainability of existing, as well as newly planted, forests, parks, afforested urban areas, etc., and it poses new challenges to for city planners and researchers, forestry, and plant science. With increasing urbanization, we are continuously changing our landscapes and altering ecological processes to make our cities warmer [18] and increasing rainfall runoff [19]. Due to rising temperatures, CO2 concentrations, and increasing precipitation in northern Europe, many deciduous tree species are expected to improve their growth rate, but other consequences of climate change, such as increased frost and heat waves, droughts, hot soils, mild winters, reduced snow cover, and depth of frozen ground, pollutants, etc., may act also as a negative factors both in city parks and forest ecosystems. These factors directly or indirectly causes stress to trees, disturbs their growth rhythm, development, growth or even damages them, induces defoliation, disturbs physiological processes and induces changes in the biochemical response [20,21,22,23,24]. Current environmental changes are much faster than those undergoing severe climate change in the post-glacial period [15,25]. Epigenetic phenomena often occur here—adaptive changes due to changes in environmental conditions or stressors which results from changes in gene expression [26,27,28]. Such severe stress can cause not only a seasonal and physiological changes in species, but through increased natural selection, the genotype of the offspring may be altered and genetic diversity reduced. Phenotypic plasticity shows a trait amplitude of a genotype (clone, family, or populartion) when tested under at least two environmental conditions. Short-term adaptation can be achieved through physiological, phenotypic, and morphological plasticity, but long-term genetic adaptation to large environmental changes and even species evolution can only be ensured by genetic variation and selection. One of the biggest challenges facing the breeding and growing of tree species is that change in tree characteristics and adaptation capacity is difficult to predict under climate change. The significance of epigenetics in tree adaptation has been studied for some time [29,30,31]. Epigenetic processes are known to determine heritable alterations in gene function but do not alter the primary DNA (deoxyribonucleic acid) sequence [32]. DNA methylation is covalently attached to cytosines and, as a result, is inherited through mitosis and/or meiosis [33,34]. Epigenetic can be define as mitotically and/or meiotically heritable variation in phenotype [35]. Earlier environment impact affects the ability of a tree to respond to its current environment, determines its survival or the manifestation of certain properties or attributes [27]. The ability of long-lived plants to adapt to environmental conditions in the context of global warming is crucial to both the conservation of species and ecosystems and the improvement of their certain properties. Photoprotective pigments play an important role in short-term responses to climate stress in plants but knowledge of their role in adaptive processes is lacking.
We hypothesize that certain environmental conditions during vegetative propagation not only have a short-term effect on tree viability or growth, but also help trees to adapt to climate change conditions and grow successfully in the long-term. The aim of the present study therefore was to estimate growth and biochemical responses, the phenotypic plasticity, genotypic variation, and heritability of adaptive traits and genetically regulated adaptability of Populus hybrids grown in field trials, which may be predisposed by simulated contrasting temperature conditions at their vegetative propagation phase.
2. Materials and Methods
2.1. Material
The study was performed on 20 cultivars and experimental clones of intraspecific crosses of poplars (P. trichocarpa (Torr. & Gray.) and four different interspecific hybrids of poplars (P. deltoides L. × P. nigra, P. deltoides × P. trichocarpa, P. maximowiczii A. Henry × P. trichocarpa, and P. balsamifera L. × P. trichocarpa) with distinguished bio-ecological characteristics (Table 1). Aspen (P. tremula L.) was used as control. The clones were selected in clonal collection of hybrid poplars of the LAMMC (Lithuanian Research Centre for Agriculture and Forestry) Institute of Forestry, Kaunas district, central Lithuania. The clonal test plantation was established using vegetatively propagated plants rooted and grown under contrasting environmental conditions simulated in the Phytotron of LAMMC Institute of Forestry. The clonal experimental plantation was established in Jonava forest district of the State Forest Enterprise located in Jonava district, central Lithuania. The location is in the lowlands of central Lithuania. The average annual rainfall is 572 mm, mean temperature is 6.5 °C.

Table 1.
Code list of hybrid poplar clones by crossing types and combination of crossed poplar species (only underlined clones were used in the pigment study).
2.2. Methods
Hybrid poplar clones for the testing in the clonal field trial were vegetatively propagated by rooting of cuttings under different environment conditions (treatments) set in automated Phytotron greenhouse in the middle of April 2018. Cuttings (15–17 cm in length) of each clone were planted into the squared plastic pots (15 × 15 × 20 cm) filled with 3.5 l of peat soil (Klasmann KTS-1), which were placed on irrigation tables. The first buds appeared two weeks later. Each clone was represented by 60–70 ramets. One-third of the ramets were rooted in pots outdoors under natural conditions, one-third in the Phytotron greenhouse, and one-third in Phytotron greenhouse with additional electric heating of pots with substrate from below. During the rooting phase of vegetative propagation, the average air temperature in the greenhouse was 25 °C; outdoors it was 19 °C. The average soil temperature outdoors was 19 °C, in the greenhouse it was 22 °C, and in pots with additional heating it was 24 °C. Air humidity was kept between 65% and 85% using an automated fog sprinkle system. The plants were regularly watered from below by temporary (0.5 h a day) flooding pots on irrigation tables to fully saturate soil and keep the soil moisture at 80–95% of the full moisture capacity (FMC) throughout the experiment. In the middle of the growing season (in July 2018), the growing conditions were changed: half of the ramets sprouted in the greenhouse were moved to grow outdoors, half of the ramets that were rooted outdoors were moved to the greenhouse, heating of the roots was turned off, and they continued to grow in the greenhouse. The rest of them were moved outdoors. These resulted in six temperature treatments/regimes during vegetative propagation: cool rooting and cool growing conditions (CR + CG), cool rooting and warm growing conditions (CR + WG), warm rooting and cool growing conditions (WR + CG), warm rooting and warm growing conditions (WR + WG), hot rooting and cool growing conditions (HR + CG), and hot rooting and warm growing conditions (HR + WG). Aspen was planted to the field trial as a native control tree species.
Next spring (in 2019) the trees were planted in clonal field trial in Jonava forest district. Clonal trial was established in a randomized complete block design. Clones were planted in row plots containing 5 to 10 trees. Trees were planted with 2.6 m spacing between rows and 2 m within rows. In total, over 1000 trees were planted. Each clone was represented by 60–70 plants. Tree height and stem diameter at the root collar were measured—at the beginning of the growing season in 2019 and at the end of growing seasons in 2019 and in 2020. The survival and tree condition were evaluated in September 2019 and 2020 using a rating system from 1 point, meaning the tree is in very bad condition, damaged by wild animals, pests, overshadowed by competing vegetation, and has low vitality, up to 5 points meaning the tree is in very good condition, viable, and undamaged by wild animals or pests. For biochemical analysis, the third leaf from the top of the leading shoot was sampled from 5 trees in each clone in the end of August 2020. The photosynthetic pigments were extracted from 0.05 g of fresh leaf material in 3 mL of N, N′-dimethylformamide (DMF). The light absorption was measured at 480, 664, and 647 nm wavelengths. Chlorophyll a/b ratios and concentrations of chlorophylls a and b and carotenoids were calculated according to Wellburn [36]. All spectrophotometrical measurements were made using spectrophotometer Synergy HT Multi-Mode Microplate Reader (BioTek Instruments, Inc., Bad Friedrichshall, Germany). All data are expressed on a leaf fresh weight basis.
In order to estimate the significance of the effects of various factors—treatments (rooting conditions and growing conditions), blocks, clones and hybrids and their interaction with treatments—the multifactor variance analysis was performed on single-tree data using the MIXED procedure (procedure option—“Covparms“) of the SAS software [37] which is based on mixed model equations (MME) and the restricted maximum likelihood (REML) method. The following linear models were used for the joint analyses (1) and (2) of the treatments and for the separate analyses (3) of an individual treatment:
where yjklmn is an observation on the lth ramet from the nth hybrid in the mth block in the jth rooting and kth growing treatment, ylnjk is an observation on the lth ramet from the nth hybrid in the jkth treatment in the mth block, yilm is an observation on the ith ramet from the ith clone in the mth block, μ is the overall mean, trj is the fixed effect due to the jth rooting treatment, tgk is the fixed effect due to the k th growing treatment, bm is the fixed effect due to the m th block, trj*trk is the fixed effect of jth rooting × kth growing treatments interaction, hn is fixed effect due to nth hybrid, hn × trjk is fixed effect due to nth hybrid × jkth treatment interaction, hn × trj is the fixed effect of interaction of the nth hybrid × jth rooting treatment, hn × tgk is the fixed effect of interaction of the nth hybrid × kth growing treatment, ci is the random effect due to the ith clone, and εijklm, εljnk, and εiklm are the random residuals. The model assumes that the random effects are normally distributed with the expectation of zero and corresponding variances: , , , , and . Assumptions of normal distribution of residuals and variance homogeneity were tested using the GLM and UNIVARIATE procedures of the SAS software [37]. Statistical significance of the effects of fixed factors—treatments, blocks and interactions between treatments and blocks—was estimated by P-test using the MIXED procedure of the SAS software [37]. Z tests were used to determine where random effects were significantly different from zero. Least-squares means estimates were obtained for treatments, as well as for hybrids and clones in each treatment. Statistical significance (at p < 0.05) of differences between least-squares means was tested using t-test, MIXED procedure of the SAS software, SAS Institute Inc.: Cary, NC, USA, 2020, [37].
yjklmn = µ + trj + tgk + trj × tgk + hn + hn × trj + hn × tgk + bm + εjklmn,
ylnjk = µ + hn + trjk + hn × trjk + bm + εljnk,
yim = µ+ ci + bm + εim,
Using statistical model 2, clonal variance components were estimated as:
where is the clonal variance component, is the clonal variance, —is random residual. The variance component of each effect was expressed as a percentage of dispersion of all analyzed (included in the model) random effects. Genetic parameters were estimated using results of variance analysis separately for each treatment. Clonal heritability coefficient on the level of individuals for each trait was calculated by the following formula:
where: is the coefficient of clonal individual heritability, is the clonal variance, and is the phenotypic variance. The standard errors of heritability coefficient under unequal number of trees per family were calculated based on Becker [38]. Clonal heritability coefficient (repeatability) on the level of means was estimated using formula:
where is the clonal heritability coefficient on the level of means, is the clonal variance, is the random variance, and k is the coefficient showing mean number of trees per clone. The errors of heritability coefficients were estimated according to Swiger et al. [39] method modified by Becker [39] for an uneven number of observations. Genotypic variation coefficient in every clonal trial was estimated based on Falconer [40], and Falconer et al. [41].
3. Results and Discussion
3.1. Dependence of Hybrid Populus Growth in Clonal Field Trial on the Vegatative Propagation (Rooting-Growing) Conditions
Analysis of variance (ANOVA) showed that the effect of treatment during vegetative propagation (rooting + growing conditions) in Phytotron greenhouse and the effect of hybrid on tree diameter of outplanted trees in the field trial was significant (p < 0.001, Table 2), as was as their interaction (p < 0.001). The impact of treatments of cuttings in the greenhouse and the effect of hybridity was not significant on tree height (Table 2). Impact of interaction hybrid × treatment conditions was very significant on tree diameter (p < 0.001).

Table 2.
Results of ANOVA (model 2): F-criteria and significance of fixed effects (treatments, hybrids and their interaction) on different traits of Populus hybrids in clonal field trial. Level of significance of effects is denoted by: * 0.01 < p < 0.05; ** 0.001 < p < 0.01; *** p < 0.001.
The highest mean tree height was obtained for trees vegetatively propagated under cool rooting and cool growing (CR + CG) and warm rooting and cool growing (WR + CG) conditions, 1.39 and 1.37 m respectively, and the lowest mean tree height was obtained for trees propagated under warm rooting and warm growing (WR + WG) conditions—1.17 m (Figure 1). The tallest hybrid among all of the trees was P. deltoides × P. trichocarpa. Mean height among all conditions reached 1.28 m which exceeds the average height of the aspen by 121 cm (as a control tree species).
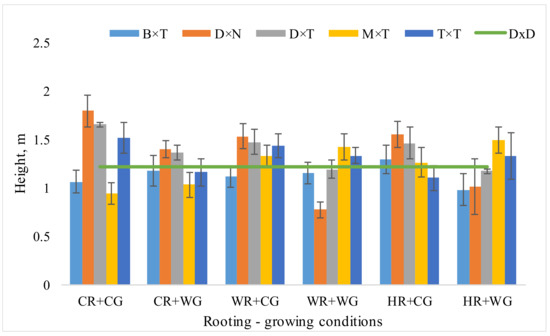
Figure 1.
Mean height (m) of Populus hybrids in field trial in 2020 which were vegetatively propagated under different rooting-growing conditions. Rooting-growing condition abbreviations: cool rooting and cool growing conditions (CR + CG), cool rooting and warm growing conditions (CR + WG), warm rooting and cool growing conditions (WR + CG), warm rooting and warm growing conditions (WR + WG), hot rooting and cool growing conditions (HR + CG), and hot rooting and warm growing conditions (HR + WG). Hybrid type abbreviations: B × T—P. balsamifera × P. trichocarpa, D × N—P. deltoides × P. nigra, D × T—P. deltoides × P. trichocarpa, M × T—P. maximowiczii × P. trichocarpa, T × T—P. trichocarpa × P. trichocarpa, D × D—P. tremula.
After HR + CG treatment, the outplanted P. deltoides × P. trichocarpa and P. deltoides × P. nigra trees grew quite well—after two growing seasons mean height reached 1.46 ± 0.16 and 1.55 ± 0.13 m respectively in 2020. It is well known that air and soil temperatures are higher in urban areas than in rural areas. The soil temperature is important because of its influence on plant growth and fine-root respiration [42,43]. Increased soil temperature very often causes stress and is a limiting factor for growth, so the ability of a tree to reproduce and grow under stressful conditions is important. However, some studies show that some poplar hybrids and clones adapt to this stressor. Some P. deltoides clones that have been planted in urban and rural sites produced twice as much biomass in urban sites comparing to rural sites [16], which is promising for city greeneries in the context of climate change
The best growth of the P. deltoides × P. trichocarpa hybrid resulted from CR + CG vegetative propagation treatment (Figure 1). Large height differences between treatments were obtained for P. deltoides × P. nigra. Interestingly, propagated under cool conditions CR + CG the tree height of this hybrid was 1.79 ± 0.16 m, however under warm conditions of propagation WR + WG it was more than 1 m less—only 0.77 ± 0.08 m. P. deltoides × P. nigra is a hybrid of southern origin. Although P. deltoides naturally grows in moist habitats, it also requires higher air temperatures [44]. P.nigra habitat in Europe does not extend far north, which indicates that cold is a limiting factor [45]. Outplanting from optimal vegetatively propagation conditions to the field could cause high stress for the hybrid and disrupt growth. Two growing seasons were probably not enough to adapt to field conditions. Meanwhile, when P. deltoides × P. nigra was propagated under similar to the field conditions (CR + CG); after outplanting to the field it grew successfully regardless of its southern origin. Raj et al. [46] have found that there is the nursery effect on a stress response in a common controlled environment for three economically important poplar hybrid (P. deltoides × P. nigra, P. deltoides var. occidentalis × P. laurifolia × P. nigra, and P. laurifolia × P. nigra) genotypes. The impact of clone history on subsequent response to stress could have profound implications for natural forests and tree plantations [46]. If the growth of a tree is limited by environmental conditions or stressors, it loses some of its decorative properties and is not suitable for parks or green areas. Disrupted growth rhythm also affects the ecological functions provided by greenery. It was determined that the growth of hybrid poplar (P. nigra × P. nigra) trees in the plantation, i.e., its average height and average diameter, does not depend on the distance to stationary and mobile sources of air pollution and to technogenic toxicants [7], which means that the hybrid is resistant to pollution, and climatic conditions are a major limiting factor.
In our experiment, the response of the hybrids to hot rooting depended on the conditions under which they later grew in phytotron. When vegetatively propagated under HR + WG conditions in field trials, 3 of 5 hybrids grew weaker than these propagated under HR + CG. P. trichocarpa × P. trichocarpa vegetatively propagated under HR + CG demonstrated the lowest mean height among all propagation environments (Figure 1), which may indicate the stress experienced by the hybrid under hot rooting conditions. Better growth of other hybrids may be explained by the heterosis effect, which is usually observed in interspecific crossings. Plants can transduce positive and negative signals among roots and shoots to coordinate growth rate and behavior and adapt to variable environments. When environmental stresses suppress root growth and change root distribution, shoot growth and functions may also be reduced as an effect of root-to-shoot signaling [47]. It was shown that soil heating causes not only shoot and root growth disorders but decreases the transpiration rate and photosynthesis activity, particularly through the effect on photosystem PS II, and lead to earlier crop maturity and poor productivity [8]. Interestingly, P. balsamifera × P. trichocarpa vegetatively propagated under HR + CG conditions demonstrated the highest mean height in field trials among all treatments (Figure 1). This hybrid is characterized by high plasticity level; it shows that trees can respond to and adapt to climate and environmental change in a relatively short time [20].
The best survival rate in field trials after the winter season was observed in most hybrids propagated under HR + CG conditions, reaching 87%, and the lowest was under CR + WG conditions, reaching 73%. (Figure 2).
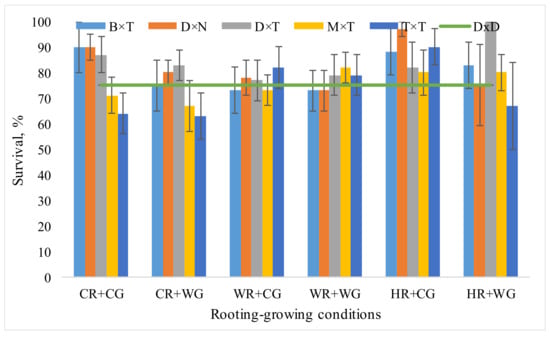
Figure 2.
Survival rate (%) of Populus hybrids in field trials, which in 2020 were vegetatively propagated under different rooting-growing conditions. Rooting-growing condition abbreviations: cool rooting and cool growing conditions (CR + CG), cool rooting and warm growing conditions (CR + WG), warm rooting and cool growing conditions (WR + CG), warm rooting and warm growing conditions (WR + WG), hot rooting and cool growing conditions (HR + CG), and hot rooting and warm growing conditions (HR + WG). Hybrid type abbreviations: B × T—P. balsamifera × P. trichocarpa, D × N—P. deltoides × P. nigra, D × T—P. deltoides × P. trichocarpa, M × T—P. maximowiczii × P. trichocarpa, T × T—P. trichocarpa × P. trichocarpa, D × D—P. tremula.
P. deltoides × P. trichocarpa hybrids was characterized by best survival among all hybrids after two growing seasons. P. deltoides × P. trichocarpa survival when propagated under HR + WG reached 100% (Figure 2). In our previous studies, a large proportion of the P. deltoides × P. trichocarpa seedlings died due to simulated spring frosts [20]. Obviously, at a young age, this hybrid needs warmer air and soil temperatures which leads to better adaptation. P. balsamifera × P. trichocarpa, P. deltoides × P. nigra and P. deltoides × P. trichocarpa demonstrated high survival rate when propagated under CR + CG conditions, while P. maximowiczii × P. trichocarpa and P. trichocarpa × P. trichocarpa hybrids demonstrated rather low survival rate. Meanwhile, under HR + CG propagation conditions P. trichocarpa × P. trichocarpa demonstrated high survival rate (Figure 2). Despite the good survival of the P. trichocarpa × P. trichocarpa hybrid under HR + CG conditions, the mean survival rate was the worst among all conditions of vegetative propagation—74% (Figure 2). The survival rate of aspen as a control tree was 75%.
Interestingly, overall better condition (vitality) of the trees in the clonal trial was obtained in the autumn of 2020, not in 2019. This showed that the trees that successfully overwintered managed to take root perfectly. In 2019 the average score for aspen was 3.51 and it was lowest comparing to other hybrids. After two growing seasons in the field, average condition of hybrids was already assessed at 4.4 points. In general, P. deltoides × P. trichocarpa hybrids were characterized by best tree condition (Figure 3). The best tree condition value when propagated under HR + WG conditions was got in P. deltoides × P. trichocarpa and under HR + CG conditions in P. deltoides × P. nigra, 4.89 and 4.68 respectively (Figure 3). By worst tree condition (vitality) were characterized, P. trichocarpa × P. trichocarpa hybrid when propagated under CR + CG and CR + WG conditions, 3.40 and 3.43 respectively, and P. maximowiczii × P. trichocarpa under CR + WG—3.40 (Figure 3).
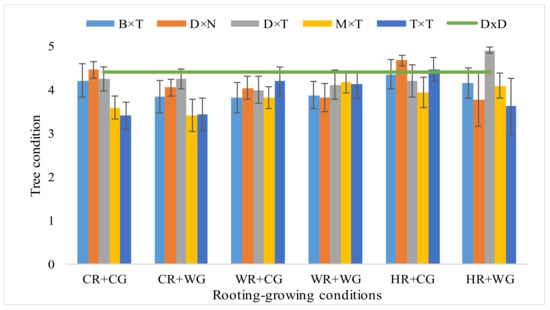
Figure 3.
Tree condition (points) of Populus hybrids in field trial in 2020 which were vegetatively propagated under different rooting-growing conditions. Rooting-growing condition abbreviations: cool rooting and cool growing conditions (CR + CG), cool rooting and warm growing conditions (CR + WG), warm rooting and cool growing conditions (WR + CG), warm rooting and warm growing conditions (WR + WG), hot rooting and cool growing conditions (HR + CG), hot rooting and warm growing conditions (HR + WG). Hybrid type abbreviations: B × T—P. balsamifera × P. trichocarpa, D × N—P. deltoides × P. nigra, D × T—P. deltoides × P. trichocarpa, M × T—P. maximowiczii × P. trichocarpa, T × T—P. trichocarpa × P. trichocarpa, D × D—P. tremula.
3.2. Dependence of Pigment Content in Leaves of Populus in Clonal Field Trial on Different Factors of Vegetative Propagation
3.2.1. Impact of Hybrids and Treatments during Vegetative Propagation on Pigment Content in Hybrid Populus Leaves in Clonal Field Trial
Analysis of variance showed that the effect of plant rooting and growing conditions in the Phytotron greenhouse during vegetative propagation in terms of amount of all pigments and chlorophyll ratio in trees outplanted in field trials was highly significant (p < 0.001) (Table 3). The impact of rooting conditions of cuttings in the greenhouse was highly significant (p < 0.001) on chlorophyll A, carotenoids, and chlorophyll ratio of outplanted trees, but not on chlorophyll B (p = 0.0195) (Table 3). Impact of interaction rooting × growing conditions was also very significant on chlorophyll A, chlorophyll ratio, and carotenoids. This interaction did not affect chlorophyll B content (p = 0.0715; Table 3). The dependence of amount of photosynthetic pigments on stressful environmental conditions was also found in Phytotron studies of different forest tree species [20].

Table 3.
Results of ANOVA (model 1): F-criteria and significance of fixed effects of rooting and growing conditions, hybrid and their interaction during vegetative propagation on different traits of Populus hybrids in clonal field trial. Level of significance of effects is denoted by: * 0.01 < p < 0.05; ***, p < 0.001.
The maximum amount of chlorophyll A in field trials was observed when vegetatively propagated under CR + WG and WR + WG conditions (Figure 4). The highest mean chlorophyll A quantity (1.49 µg/g) was obtained for P. deltoides × P. nigra hybrid. This hybrid had the highest chlorophyll A content in field trial when propagated under CR + WG and WR + CG conditions (Figure 4). Though P. trichocarpa × P. trichocarpa hybrid had lowest mean chlorophyll A quantity (1.25 µg/g), but when propagated under WR + WG conditions in field trials it reached 2.06 µg/g. When propagated under different environmental (rooting-growing) conditions, the P. deltoides × P. trichocarpa hybrid retained a fairly constant amount of chlorophyll A in field trials.
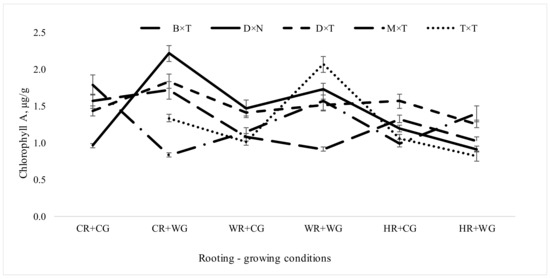
Figure 4.
Chlorophyll A amount (µg/g) in Populus hybrids in field trial which were vegetatively propagated under different rooting-growing conditions Rooting-growing condition abbreviations: cool rooting and cool growing conditions (CR + CG), cool rooting and warm growing conditions (CR + WG), warm rooting and cool growing conditions (WR + CG), warm rooting and warm growing conditions (WR + WG), hot rooting and cool growing conditions (HR + CG), and hot rooting and warm growing conditions (HR + WG). Hybrid type abbreviations: B × T—P. balsamifera × P. trichocarpa, D × N—P. deltoides × P. nigra, D × T—P. deltoides × P. trichocarpa, M × T—P. maximowiczii × P. trichocarpa, T × T—P. trichocarpa × P. trichocarpa, D × D—P. tremula.
The most sensitive to environmental conditions was P. deltoides × P. nigra hybrid showing most variable chlorophyll A quantities in field trials across vegetative propagation treatments. Such changes in chlorophyll quantity are not surprising, as it has been found in other plants that epigenetic regulation plays an important role in chlorophyll biosynthesis and in photosynthesis [48,49]. Changing levels of chlorophyll A due to stressors can negatively affect the process of photosynthesis, which is directly related to plant growth. In our previous studies, this hybrid suffered significant growth losses due to environmental stressors under controlled environmental conditions [20].
The lowest amount of chlorophyll A was found in P. balsamifera × P. trichocarpa hybrid, which were vegetatively propagated under CR + WG conditions, in P. deltoides × P. nigra under CR + CG and HR + WG conditions, and P. maximowiczii × P. trichocarpa under WR + WG conditions. It means that certain conditions at the vegetative propagation phase in certain hybrids may lead to losses in photosynthesis.
When vegetatively propagated under HR + CG and HR + WG conditions (when roots were heated during rooting period) there were almost no significant differences between the hybrids according to chlorophyll A quantity when measured in field trials (except for P. deltoides × P. trichocarpa). The amount of chlorophyll A in aspen used as a control species was 1.5 ± 0.07 µg/g, which is slightly above the total average of the experiment. Under HR + WG treatment P. trichocarpa × P. trichocarpa had the lowest amount of chlorophyll A among all hybrids and treatments. Although light is the most important factor in the synthesis of chlorophyll, temperature and the plant root system are also significant components. The survival and tree condition in 2020 of P. trichocarpa × P. trichocarpa was lower than the average of the experiment (Figure 2 and Figure 3); this indicates a less developed root system, which may have led to adaptation difficulties after outplanting into the field. Only well-developed plant roots will ensure the synthesis of hormones that promote chlorophyll activity. Studies of maples (Acer mono) have shown that N and P application significantly affected plant height, root collar diameter, chlorophyll content, and root morphology [50].
The highest chlorophyll B amount in field trials was found when trees were propagated under HR + WG conditions in P. balsamifera × P. trichocarpa—1.14 µg/g and under CR + WG and WR + WG conditions in P. deltoides × P. nigra, 1.13 and 1.11 µg/g respectively (Figure 5). The maximum value of chlorophyll B resulted from propagating under WG conditions. Different rooting-growing conditions had the greatest effect on the chlorophyll B content in the P. deltoides × P. nigra hybrid. The chlorophyll B amount of the P. deltoides × P. trichocarpa hybrid remained relatively most stable regardless of rooting-growing conditions. The chlorophyll B amount of aspen reached 1.03 ± 0.01 µg/g (Figure 5).
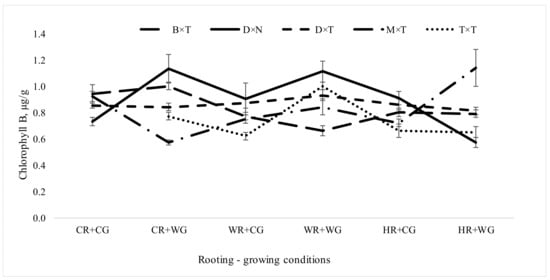
Figure 5.
Chlorophyll B amount (µg/g) in Populus hybrids in field trial which were vegetatively propagated under different rooting-growing conditions. Rooting-growing condition abbreviations: cool rooting and cool growing conditions (CR + CG), cool rooting and warm growing conditions (CR + WG), warm rooting and cool growing conditions (WR + CG), warm rooting and warm growing conditions (WR + WG), hot rooting and cool growing conditions (HR + CG), and hot rooting and warm growing conditions (HR + WG). Hybrid type abbreviations: B × T—P. balsamifera × P. trichocarpa, D × N—P. deltoides × P. nigra, D × T—P. deltoides × P. trichocarpa, M × T—P. maximowiczii × P. trichocarpa, T × T—P. trichocarpa × P. trichocarpa, D × D—P. tremula.
Studies of oaks have shown that the content of chlorophyll in the leaves depends not only on the species or water regime, but also on the places of origin [51]. Stressful conditions such as drought alter the amount and proportions of chlorophyll pigments in walnut leaves as well [18]. In Lee et al.’s study [52], Populus sibirica in the summer drought condition was limited in its growth and assimilation of carbon via the stomatal aperture. Furthermore, Populus sibirica avoided photodamage through a decline in chlorophyll content, which could limit the absorbed energy, and photochemical quenching, which could dissipate the excess energy.
Chlorophyll content can be used as a measurement of the healthiness of a plant’s canopy [53], therefore, higher chlorophyll content found for certain hybrids in our experiment also indicates better plant health. Leaf chlorophyll is a key indicator of leaf greenness, and it is often used to investigate leaf nutrient deficiencies and changes in chlorophyll [54]. Leaf greenery is especially important in creating parks or other green spaces in cities. Canopy chlorophyll content is also an indicator of seasonal carbon uptake in forest ecosystems [55]. Carbon uptake function play a very important role to improving urban air quality, reducing temperature inside the cities and perform other ecological functions/services.
Carotenoids act to protect photosynthetic organisms, including chlorophylls, from the harmful effects of excess exposure to light [56]. The amount of carotenoids usually is 1.5–2 times less than the amount of chlorophyll. In our studies, carotenoid level was also lower than chlorophylls (Figure 6). The maximum value of carotenoids among all hybrids was obtained when vegetatively propagated under CR + WG conditions (Figure 6). Besides, the amount of carotenoids was similar in all hybrids, ranging from 0.37 to 0.9 µg/g, regardless of the vegetative propagation environment. The highest carotenoid content was obtained in the P. deltoides × P. nigra and P. deltoides × P. trichocarpa hybrids, which in its natural range is accustomed to absorb more sunlight from its environment.
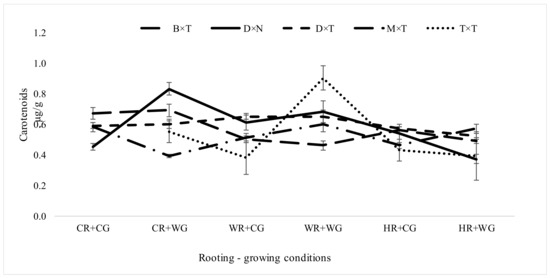
Figure 6.
Carotenoids amount (µg/g) in Populus hybrids in field trial which were vegetatively propagated under different rooting-growing conditions. Rooting-growing condition abbreviations: cool rooting and cool growing conditions (CR + CG), cool rooting and warm growing conditions (CR + WG), warm rooting and cool growing conditions (WR + CG), warm rooting and warm growing conditions (WR + WG), hot rooting and cool growing conditions (HR + CG), and hot rooting and warm growing conditions (HR + WG). Hybrid type abbreviations: B × T—P. balsamifera × P. trichocarpa, D × N—P. deltoides × P. nigra, D × T—P. deltoides × P. trichocarpa, M × T—P. maximowiczii × P. trichocarpa, T × T—P. trichocarpa × P. trichocarpa, D × D—P. tremula.
An increased amount of carotenoids can increase an adaptation to existing conditions because they expand the wavelength range of light that is able to drive photosynthesis [56]. Under different environmental (rooting-growing) conditions, carotenoid amount varied most in P. trichocarpa × P. trichocarpa—from 0.38 to 0.9 µg/g (Figure 6). The first report implicating epigenetic regulatory mechanisms in the control of carotenoid composition was authored by Cazzoneli et al. in 2009 [48]. This study suggests a possible role for epigenetic modification in regulating lutein levels and carotenoid composition. More detailed studies are needed to determine whether carotenoid amounts in trees are determined by epigenetics or by phenotypic plasticity with partial epigenetic aspects and its influences on genetic parameters. Rooting-growing conditions had little effect on carotenoid quantity in P. deltoides × P. trichocarpa hybrid in the field trial. The carotenoids content of aspen reached 0.61 ± 0.02 µg/g. Under HR + CG and HR + WG conditions the carotenoids amount was low and stable among all hybrids. Root heating during the rooting period reduced the amount of carotenoids in the leaves in field trial. This means that certain conditions in certain hybrids may lead to losses of the protective mechanism of photosynthesis. Carotenoids play vital roles in drought stress signaling, neutralizing oxidative stress, and acclimation in plants such as carotenoid-derived phytohormone abscisic acid induces stomatal closure inhibiting transpiration whenever plants experience signals [57].
The maximum concentration of chlorophyll A and B ratio were obtained when vegetatively propagated under CR + WG conditions (Figure 2). P. deltoides × P. trichocarpa were characterized by the highest chlorophyll A and B ratio, while P. maximowiczii × P. trichocarpa had the lowest. The highest chlorophyll A and B ratio in field trial was found when propagated under CR + WG conditions in P. balsamifera × P. trichocarpa—2.18 and under WR + WG conditions in P. trichocarpa × P. trichocarpa—2.06. The chlorophyll A and B ratio of aspen reached 1.45 ± 0.05 µg/g. Plants respond to changing environmental conditions by changes in composition of pigments in the chloroplasts. For proper functioning of the photosynthetic mechanism, the chlorophyll A/B ratio may decrease in leaves, because chlorophyll A has a tendency to degrade faster in stressful conditions than chlorophyll B [58].
Stress-induced changes in the composition of photosynthetic pigments are indicative of plant functioning and short-term or long-term stresses. Changes in the composition of chlorophyll determine the efficiency of photosynthesis and can therefore be used as an indicator of vegetation health and viability, not only in herbaceous plants, vegetables, and moss, but also in trees [59,60].
3.2.2. Changes of Genetic Parameters of Different Traits of Trees in Clonal Field Trial Depending on Rooting-Growing Treatments during Vegetative Propagation in Phytotron Greenhouse
The clonal component of variation, which shows the share of clonal genetic variation in the overall variability of traits, ranged from 27.85% to 65.90% for different traits under different treatments (rooting-growing conditions) (Table 4). The highest clonal variation component, 65.90%, was obtained for chlorophyll B under WR + WG conditions. The amount of pigments in most cases under CR + WG conditions were characterized by a higher clonal variation component than under other conditions. The clonal variation component of chlorophyll A decreased the most: from 64.57% under CR + WG conditions down to 27.85% under HR + WG conditions (0.01 < p < 0.05). The clonal variation component of chlorophyll A and B ratio under WR + CG conditions was only 3.66%, however, it was insignificant.

Table 4.
Trait means and genetic parameters of different traits of Populus hybrids in field trial effected by different rooting-growing treatments: coefficient of genotypic variation (CVG), coefficient of individual heritability (Hi2), clonal mean heritability (Hm2), coefficient of phenotypic variation (CVF). and clonal variance component. Level of significance of effects is denoted by: * 0.01 < p < 0.05.
The highest individual heritability coefficient was obtained for chlorophyll B under CR + WG conditions (Hi2 = 0.66) and the lowest—for chlorophyll A and B ratio under WR + CG treatment (Hi2 = 0.04) and chlorophyll A under HR + WG treatment (Hi2 = 0.28) (Table 4).
As indicated by coefficient of heritability under warm growth conditions (WG), where growing of rooted cuttings was performed inside a greenhouse, more than 60% of this variability in the amounts of chlorophyll A and B and carotenoids was due to genotypic reasons (Table 4). High mean values of heritability coefficient indicate strong genetic control of the trait. The high values of heritability coefficients shows that in this environmental conditions ecological variation of these traits was lower in relation to genotypic variation. A higher coefficient of heritability allows a more precise identification of a genotype by its phenotype. Along with changes in environmental conditions (different treatments), the values of heritability coefficient alter as well. To our knowledge, there are no studies examining the heritability of chlorophyll in trees, but studies of agricultural plants have shown a similar heritability coefficient rate—65.4% in rose [61], 67.9% in okra [62], 87.5% in wheat [63].
The lowest genotypic variation was found for chlorophyll A and B ratio (in WR + CG conditions), –3.74% (Table 4). The highest genotypic variation in field trials was found for chlorophyll B when plants were vegetatively propagated under HR + WG—CVg reached 41.11%, however it decreased more than 3 times under CR + CG conditions to 12.12% (Table 4). Differences in genotypic variation in different environmental conditions are determined by an uneven biochemical processes disruption rate of different clones and their different phenotypic plasticity. Genetic diversity is one of the guarantors of vegetation sustainability, as with high genetic diversity; there are many different gene variants that can recombine into genotypes that are suitable for an ever-changing environment during sexual reproduction, thus guaranteeing the species adaptation and survival. Adaptation to stressful conditions is very important for the establishment of green spaces, such as forest parks in urban environments, where greenery face not only the drought, higher temperatures, soil heating, etc., but also air and soil pollution.
Chlorophyll content is one of the most important physiological traits, as it is closely related to leaf photosynthesis and crop yield potential. It is one of the most important traits in agricultural crop breeding [64]. Above-ground biomass is important for predicting plant growth and development within different ecosystems. Our experiment shows that all genetic parameters in field trials change depending on treatment at the vegetative propagation phase, which most likely is a consequence of the presence of aftereffects in the performance of clones due to phenotypic plasticity, which has a lasting effect even after transplanting into experimental plantations. However, our study did not allow us to distinguish direct epigenetic ‘memory’ effects of different treatments in phytotron on the performance of trees in field trials from ones that resulted from treatment-predisposed different heights and diameters of trees per se.
It has been argued that epigenetic variation in natural populations can be independent from genetic variation [65]. Avramidou et al. [66] found that epigenetic variation is uncoupled from genetic variation in natural populations of Prunus avium. Epigenetics refers to potentially heritable traits that cannot be explained by modifications in the DNA sequence [67].
Raj [46] studied the lasting effect of clone history on current plant performance; cuttings of the same genotype were obtained from different geographic locations and grown under common environmental conditions and the transcriptome response to an important environmental stress, drought, was obtained. Differences in transcript abundance patterns in response to drought were found to be based on differences in geographic origin [51]. This means that the same genetics does not guarantee the same response to stressors.
In our studies, poplars experienced different levels of stress during the vegetative propagation phase (rooting-growing period). The present study shows that biochemical responses (the amount of chlorophyll A and carotenoids) to the environment after outplanting in the field trial were based on conditions during the rooting-growing period—trees kept stress-related memory. Only P. deltoides × P. trichocarpa kept a stable amount of chlorophyll B and carotenoids under different treatments (Figure 6). This corresponds to findings that stress memory determined amount of photosynthetic pigments in seaweed under different levels of thermal stress [66,67,68].
Continuation of these studies is needed in order to clarify how long the phenotypic plasticity effects of different treatments during vegetative propagation on growth of different poplar hybrids and their clones in field trials will last.
4. Conclusions
Our study shows that certain environmental conditions during propagation not only have a short-term effect on tree viability or growth, but can also help the tree to adapt to climate change conditions and grow successfully in the long-term. It was found that tree growth and biochemical traits (the chlorophyll A and B, pigments content and the chlorophyll A/B ratio) of hybrid poplar clones outplanted in field trial, and the traits’ genetic parameters, were affected by the rooting-growing temperature conditions during vegetative propagation phase. The greatest mean height of outplanted trees resulted from WR + CG rooting-growing conditions. P. deltoides × P. trichocarpa were characterized by the greatest mean height among all hybrids. The maximum amount of chlorophyll A resulted from WR + WG conditions, and the maximum amount of chlorophyll B resulted from HR + WG. Significant hybrids by treatment interaction found in ANOVA for growth traits showed that different hybrids outplanted in field trials specifically change their performance in field trials depending on plant rooting-growing temperature conditions in Phytotron during the vegetative propagation phase. Hybrids P. balsamifera × P. trichocarpa, and P. trichocarpa × P. trichocarpa showed the most substantial changes of biochemical traits across vegetative propagation treatments in field trials.
Rooting-growing conditions during vegetative propagation also had an impact on genetic parameters of hybrid poplar clones outplanted and grown in field trials. The highest genotypic variation of chlorophyll A was found for under CR + WG conditions—CVg reached 37.70%—however it decreased under HR + CG conditions to 20.97%. Rooting-growing temperature conditions also had an impact on the heritability of traits. The greatest heritability coefficient for chlorophyll A was obtained under CR + WG treatment 0.65, but under HR + CG it was 0.28. The heritability coefficient for chlorophyll B decreased from 0.66 under CR + WG treatment to 0.30 under CR + WG. Thus, the environmental conditions of vegetative propagation might affect the genetic gain in the breeding of hybrid poplars.
Author Contributions
Conceptualization, A.P. and V.G.-F.; methodology, V.G.-F. and A.P.; software, V.G.-F.; validation, A.P.; formal analysis, V.G.-F.; investigation, V.G.-F.; resources, V.G.-F.; data curation, V.G.-F.; writing—original draft preparation, V.G.-F.; writing—review and editing, A.P.; visualization, V.G.-F.; supervision, A.P.; project administration, V.G.-F.; funding acquisition, V.G.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the European Social Fund under the “Development of Competences of Scientists, other Researchers and Students through Practical Research Activities” project No 09.3.3-LMT-K-712.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This research was supported by the European Social Fund under the “Development of Competences of Scientists, other Researchers and Students through Practical Research Activities” project No 09.3.3-LMT-K-712.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Szczepańska, A.; Senetra, A. Forests as the key component of green belts surrounding urban areas. Balt. For. 2019, 25. [Google Scholar] [CrossRef]
- Sicard, P.; Agathokleous, E.; Araminiene, V.; Carrari, E.; Hoshika, Y.; De Marco, A.; Paoletti, E. Should we see urban trees as effective solutions to reduce increasing ozone levels in cities? Environ. Poll. 2018, 243, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.; Paula, L.; Dodd, T.; Németh, S.; Nanou, C.; Mega, V.; Campos, P. EU ambition to build the world’s leading bioe-conomy—Uncertain times demand innovative and sustainable solutions. New Biotechnol. 2018, 40, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Köhl, M.; Lasco, R.; Cifuentes, M.; Jonsson, Ö.; Korhonen, K.T.; Mundhenk, P.; Navar, J.D.J.; Stinson, G. Changes in forest production, biomass and carbon: Results from the 2015 UN FAO Global Forest Resource Assessment. For. Ecol. Manag. 2015, 352, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Payn, T.; Carnus, J.-M.; Freer-Smith, P.; Kimberley, M.; Kollert, W.; Liu, S.; Orazio, C.; Rodriguez, L.; Silva, L.N.; Wingfield, M.J. Changes in planted forests and future global implications. For. Ecol. Manag. 2015, 352, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Álvarez, P.; Pizarro, C.; Barrio-Anta, M.; Cámara-Obregón, A.; Bueno, J.L.M.; Álvarez, A.; Gutiérrez, I.; Burslem, D.F.R.P. Evaluation of Tree Species for Biomass Energy Production in Northwest Spain. Forests 2018, 9, 160. [Google Scholar] [CrossRef] [Green Version]
- Blonskaya, L.; Sultanova, R.; Muftakhova, S.; Martynova, M.; Konashova, S.; Sabirzyanov, I.; Odintsov, G. Biological indices of Bashkir Lombardy poplar (Populus nigra L. × Populus nigra var. italica Du Roi) in urban landscapes. Bulg. J. Agric. Sci. 2019, 25, 30–36. [Google Scholar]
- Chong, G.W.; Simonson, S.E.; Stohlgren, T.J.; Kalkhan, M.A. Biodiversity: Aspen stands have the lead, but will nonnative species take over. In Proceedings of the Sustaining Aspen in Western Landscapes Symposium, Grand Junction, CO, USA, 13–15 June 2000; p. 261. [Google Scholar]
- Nilsson, S.G.; Hedin, J.; Niklasson, M. Biodiversity and its Assessment in Boreal and Nemoral Forests. Scand. J. For. Res. 2001, 16, 10–26. [Google Scholar] [CrossRef] [Green Version]
- Latva-Karjanmaa, T.; Penttilä, R.; Siitonen, J. The demographic structure of European aspen (Populus tremula) populations in managed and old-growth boreal forests in eastern Finland. Can. J. For. Res. 2007, 37, 1070–1081. [Google Scholar] [CrossRef]
- De Rigo, D.; Enescu, C.M.; Houston Durrant, T.; Caudullo, G. Populus nigra in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; Publications Office of the European Union: 2 Rue Mercier, 2985 Luxembourg, 2016. [Google Scholar]
- Dickmann, D.I. An overview of the genus Populus. In Poplar Culture in North America; Part A; Canadian Science Publishing: Ottawa, ON, Canada, 2001. [Google Scholar]
- Isebrands, J.G.; Richardson, J. (Eds.) Poplars and Willows: Trees for Society and the Environment; CABI: Wallingford, UK, 2014. [Google Scholar]
- Blake, T.J.; Tschaplinski, T.; Eastham, A. Stomatal control of water use efficiency in poplar clones and hybrids. Can. J. Bot. 1984, 62, 1344–1351. [Google Scholar] [CrossRef]
- Eroğlu, E.; Acar, C. A Visual Assessment of Roadside Poplar Plantings in Turkey. J. Agric. Sci. 2018, 24, 185–198. [Google Scholar] [CrossRef]
- Gregg, J.W.; Jones, C.; Dawson, T.E. Urbanization effects on tree growth in the vicinity of New York City. Nat. Cell Biol. 2003, 424, 183–187. [Google Scholar] [CrossRef]
- Robinson, N. The Planting Design Handbook; Routledge: London, UK, 2016. [Google Scholar]
- Liu, B.; Liang, J.; Tang, G.; Wang, X.; Liu, F.; Zhao, D. Drought stress affects on growth, water use efficiency, gas exchange and chlorophyll fluorescence of Juglans rootstocks. Sci. Hortic. 2019, 250, 230–235. [Google Scholar] [CrossRef]
- Elliott, R.M.; Adkins, E.R.; Culligan, P.J.; Palmer, M.I. Stormwater infiltration capacity of street tree pits: Quantifying the influence of different design and management strategies in New York City. Ecol. Eng. 2018, 111, 157–166. [Google Scholar] [CrossRef]
- Gudynaitė-Franckevičienė, V.; Pliura, A.; Suchockas, V. Ecogenetic plasticity and genetic variation in Populus hybrids under the impact of simulated climate change related stressors. Balt. For. 2020, 26. [Google Scholar] [CrossRef]
- Je, S.-M.; Woo, S.Y.; Lee, S.H.; Kwak, M.J.; Lee, T.Y.; Kim, S.H. Combined effect of elevated CO2 concentration and drought on the photosynthetic apparatus and leaf morphology traits in seedlings of yellow poplar. Ecol. Res. 2017, 33, 403–412. [Google Scholar] [CrossRef]
- Pliūra, A.; Jankauskiene, J.; Lygis, V.; Suchockas, V.; Bajerkevičiene, G.; Verbylaite, R. Response of juvenile progeny of seven forest tree species and their populations to simulated climate change-related stressors, heat, elevated humidity and drought. iForest Biogeosc. For. 2018, 11, 374–388. [Google Scholar] [CrossRef] [Green Version]
- Pollastrini, M.; Puletti, N.; Selvi, F.; Iacopetti, G.; Bussotti, F. Widespread Crown Defoliation After a Drought and Heat Wave in the Forests of Tuscany (Central Italy) and Their Recovery—A Case Study from Summer 2017. Front. For. Glob. Chang. 2019, 2. [Google Scholar] [CrossRef] [Green Version]
- Sicard, P.; Augustaitis, A.; Belyazid, S.; Calfapietra, C.; de Marco, A.; Fenn, M.; Bytnerowicz, A.; Grulke, N.; He, S.; Matyssek, R.; et al. Global topics and novel approaches in the study of air pollution, climate change and forest ecosystems. Environ. Pollut. 2016, 213, 977–987. [Google Scholar] [CrossRef]
- Giesecke, T.; Brewer, S.; Finsinger, W.; Leydet, M.; Bradshaw, R.H. Patterns and dynamics of European vegetation change over the last 15,000 years. J. Biogeogr. 2017, 44, 1441–1456. [Google Scholar] [CrossRef] [Green Version]
- Kvaalen, H.; Johnsen, Ö. Timing of bud set in Picea abies is regulated by a memory of temperature during zygotic and somatic embryogenesis. New Phytol. 2007, 177, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Bräutigam, K.; Vining, K.J.; Lafon-Placette, C.; Fossdal, C.G.; Mirouze, M.; Gutiérrez Marcos, J.; Fluch, S.; Fernández Fraga, M.; Guevara, M.Á.; Abarca, D.; et al. Epigenetic regulation of adaptive responses of forest tree species to the environment. Ecol. Evolut. 2013, 3, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.-K. Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 2009, 12, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyko, A.; Kovalchuk, I. Epigenetic control of plant stress response. Environ. Mol. Mutagen. 2008, 49, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Groszmann, M.; Greaves, I.K.; Albertyn, Z.I.; Scofield, G.N.; Peacock, W.J.; Dennis, E.S. Changes in 24-nt siRNA levels in Arabidopsis hybrids suggest an epigenetic contribution to hybrid vigor. Proc. Natl. Acad. Sci. USA 2011, 108, 2617–2622. [Google Scholar] [CrossRef] [Green Version]
- Mirouze, M.; Paszkowski, J. Epigenetic contribution to stress adaptation in plants. Curr. Opin. Plant Biol. 2011, 14, 267–274. [Google Scholar] [CrossRef]
- Bonasio, R.; Tu, S.; Reinberg, D. Molecular Signals of Epigenetic States. Science 2010, 330, 612–616. [Google Scholar] [CrossRef] [Green Version]
- Calarco, J.P.; Borges, F.; Donoghue, M.T.; Van Ex, F.; Jullien, P.E.; Lopes, T.; Gardner, R.; Berger, F.; Feijó, J.; Becker, J.; et al. Reprogramming of DNA Methylation in Pollen Guides Epigenetic Inheritance via Small RNA. Cell 2012, 151, 194–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finnegan, E.J.; Genger, R.K.; Peacock, W.J.; Dennis, E.S. DNA methylation in plants. Ann. Rev. Plant Biol. 1998, 49, 223–247. [Google Scholar] [CrossRef]
- Niederhuth, C.E.; Schmitz, R.J. Covering Your Bases: Inheritance of DNA Methylation in Plant Genomes. Mol. Plant 2014, 7, 472–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- SAS Institute. SAS® 9.4 TS Level 1M4, X64_7PRO Platform; SAS Institute Inc.: Cary, NC, USA, 2020. [Google Scholar]
- Becker, W.A. Manual of Procedures in Quantitative Genetics, 4th ed.; Academic Enterprises: Pullman, WA, USA, 1984; 190p. [Google Scholar]
- Swiger, L.A.; Harvey, W.R.; Everson, D.O.; Gregory, K.E. The Variance of Intraclass Correlation Involving Groups with One Observation. Biometrics 1964, 20, 818. [Google Scholar] [CrossRef] [Green Version]
- Falconer, D.S. Introduction to Quantitative Genetics, 4th ed.; Trends in Genetics; Benjamin-Cummings Publishing: San Francisco, CA, USA, 1996; Volume 12, p. 280. [Google Scholar]
- Falconer, D.S. Introduction to Quantitative Genetics, 3rd ed.; Burnt Mill: Harlow, UK; Wiley: New York, NY, USA, 1989; p. 448. [Google Scholar]
- Ozkan, U.; Gökbulak, F. Effect of vegetation change from forest to herbaceous vegetation cover on soil moisture and temperature regimes and soil water chemistry. Catena 2017, 149, 158–166. [Google Scholar] [CrossRef]
- Tang, C.-S.; Shi, B.; Gao, L.; Daniels, J.L.; Jiang, H.-T.; Liu, C. Urbanization effect on soil temperature in Nanjing, China. Energy Build. 2011, 43, 3090–3098. [Google Scholar] [CrossRef]
- Burns, R.M.; Honkala, B.H. Hardwoods, Silvics of North America; US Department of Agriculture, Forest Service: Washington, DC, USA, 1990; p. 877.
- European Forest Genetic Resources Programme. 2017. Available online: www.euforgen.eu (accessed on 5 March 2021).
- Raj, S. Clone history shapes Populus drought responses. Proc. Natl. Acad. Sci. USA 2011, 108, 12521–12526. [Google Scholar] [CrossRef] [Green Version]
- Novák, V.; Lipiec, J. Water extraction by roots under environmental stresses. In Pollution and Water Resources, Columbia University Seminar Proceedings: Impact of Anthropogenic Activity and Climate Changes on the Environment of Central Europe and USA; Halasi-Kun, J., Stekauerová, V., Fodor, I., Nagy, V., Sinóros-Szabó, B., Lo Pinto, R., Eds.; Columbia University Press: New York, NY, USA, 2012. [Google Scholar]
- Cazzonelli, C.; Cuttriss, A.J.; Cossetto, S.B.; Pye, W.; Crisp, P.; Whelan, J.; Finnegan, E.J.; Turnbull, C.; Pogson, B.J. Regulation of Carotenoid Composition and Shoot Branching in Arabidopsis by a Chromatin Modifying Histone Methyltransferase, SDG8. Plant Cell 2009, 21, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Heimann, L.; Horst, I.; Perduns, R.; Dreesen, B.; Offermann, S.; Peterhansel, C. A Common Histone Modification Code on C4 Genes in Maize and Its Conservation in Sorghum and Setaria italica. Plant Physiol. 2013, 162, 456–469. [Google Scholar] [CrossRef] [Green Version]
- Razaq, M.; Zhang, P.; Shen, H.-L. Salahuddin Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 2017, 12, e0171321. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Valiente, J.A.; Koehler, K.; Cavender-Bares, J. Climatic origins predict variation in photoprotective leaf pigments in response to drought and low temperatures in live oaks (Quercus series Virentes). Tree Physiol. 2015, 35, 521–534. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.; Je, S.; Kwak, M.; Akhmadi, K.; Tumurbaatar, E.; Khaine, I.; Lee, H.; Jang, J.; Kim, H.; Ahn, H.; et al. Physiological responses of Populus sibirica to different irrigation regimes for reforestation in arid area. S. Afr. J. Bot. 2017, 112, 329–335. [Google Scholar] [CrossRef]
- Kamble, P.N.; Giri, S.P.; Mane, R.S.; Tiwana, A. Estimation of chlorophyll content in young and adult leaves of some selected plants. Univ. J. Environ. Res. Technol. 2015, 5, 306–310. [Google Scholar]
- Ali, M.M.; Al-Ani, A.; Eamus, D.; Tan, D.K.Y. Leaf nitrogen determination using non-destructive techniques—A review. J. Plant Nutr. 2017, 40, 928–953. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Froelich, N.J.; Chen, B.; Staebler, R.M. Seasonal controls of canopy chlorophyll content on forest carbon uptake: Implications for GPP modeling. J. Geophys. Res. Biogeosci. 2015, 120, 1576–1586. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and Photosynthesis; Springer: Berlin/Heidelberg, Germany, 2016; Volume 79, pp. 111–139. [Google Scholar]
- Dhami, N.; Cazzonelli, C.I. Environmental impacts on carotenoid metabolism in leaves. Plant Growth Regul. 2020, 92, 455–477. [Google Scholar] [CrossRef]
- Morais, R.R.; Gonçalves, J.F.; Santos, U.M., Jr.; Dünisch, O.; Santos, A.L. Chloroplastid pigment contents and chlorophyll a fluorescence in amazonian tropical three species. Rev. Árvore 2007, 31, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Smith, J.G.; Stringer, P.; Ennos, A.R. Effect of rooting conditions on the growth and cooling ability of Pyrus calleryana. Urban. For. Urban. Green. 2011, 10, 185–192. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F. Light Adaptation and Senescence of the Photosynthetic Apparatus. Changes in Pigment Composition, Chlorophyll Fluorescence Parameters and Photosynthetic Activity. Chlorophyll A Fluoresc. 2007, 19, 713–736. [Google Scholar] [CrossRef]
- Soujanya, P.; Kulkarni, B.S.; Harshavardhan, M. Genetic variability and heritability in rose. J. Pharm. Phytochem. 2019, 8, 2065–2067. [Google Scholar]
- Thulasiram, L.B.; Bhople, S.R.; Srikanth, M.; Nayak, B.R. Genetic variability and heritability studies in okra (Abelmoschus esculentus (L.) Moench). Plant Arch. 2017, 17, 907–910. [Google Scholar]
- Kumar, A.N.U.J.; Gaurav, S.S.; Bahuguba, D.K.; Sharma, P.; Singh, T.; Chand, P. Analysis of variability, heritability and genetic advance for yield and yield related trait in wheat (Triticum aestivum L.) genotypes. Inter. J. Agri. Sci. Res. 2017, 7, 4. [Google Scholar]
- Wang, Q.; Xie, W.; Xing, H.; Yan, J.; Meng, X.; Li, X.; Fu, X.; Xu, J.; Lian, X.; Yu, S.; et al. Genetic Architecture of Natural Variation in Rice Chlorophyll Content Revealed by a Genome-Wide Association Study. Mol. Plant 2015, 8, 946–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bossdorf, O.; Richards, C.; Pigliucci, M. Epigenetics for ecologists. Ecol. Lett. 2007, 11, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Avramidou, E.; Ganopoulos, I.; Doulis, A.G.; Tsaftaris, A.S.; Aravanopoulos, F.A. Beyond population genetics: Natural epigenetic variation in wild cherry (Prunus avium). Tree Genet. Genomes 2015, 11, 1–9. [Google Scholar] [CrossRef]
- Markus, C.; Pecinka, A.; Karan, R.; Barney, J.N.; Merotto, A., Jr. Epigenetic regulation-contribution to herbicide resistance in weeds? Pest. Manag. Sci. 2018, 74, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Kim, M.; Ralph, P.J.; Marín-Guirao, L.; Pernice, M.; Procaccini, G. Stress Memory in Seagrasses: First Insight into the Effects of Thermal Priming and the Role of Epigenetic Modifications. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).