Effects of Plant Growth Regulators on the Rapid Propagation System of Broussonetia papyrifera L. Vent Explants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
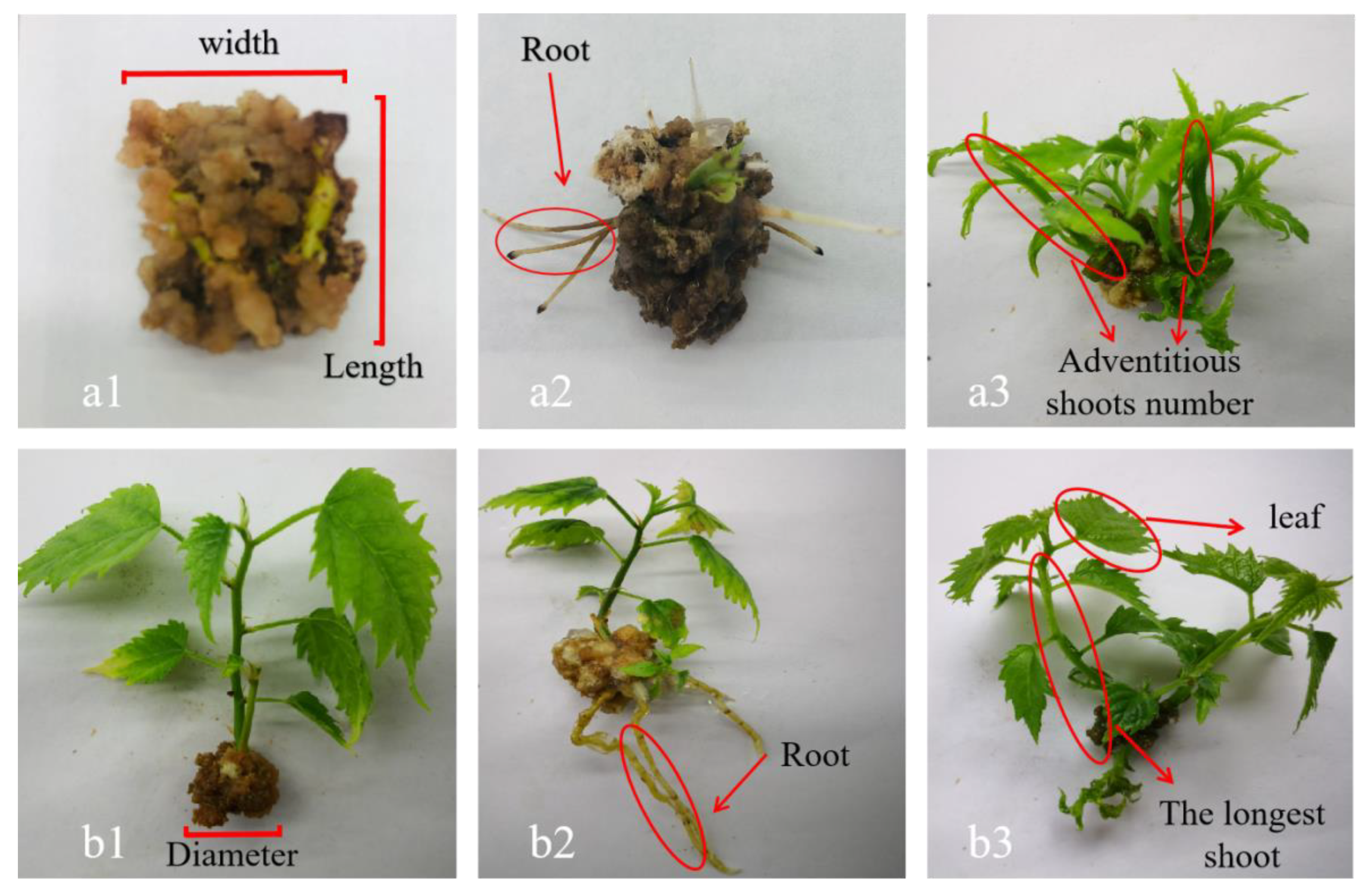
2.3. Determination of Morphological Index
2.4. Acclimatizing and Transplanting of Tissue Culture Plantlets
2.5. Statistical Analysis
3. Results
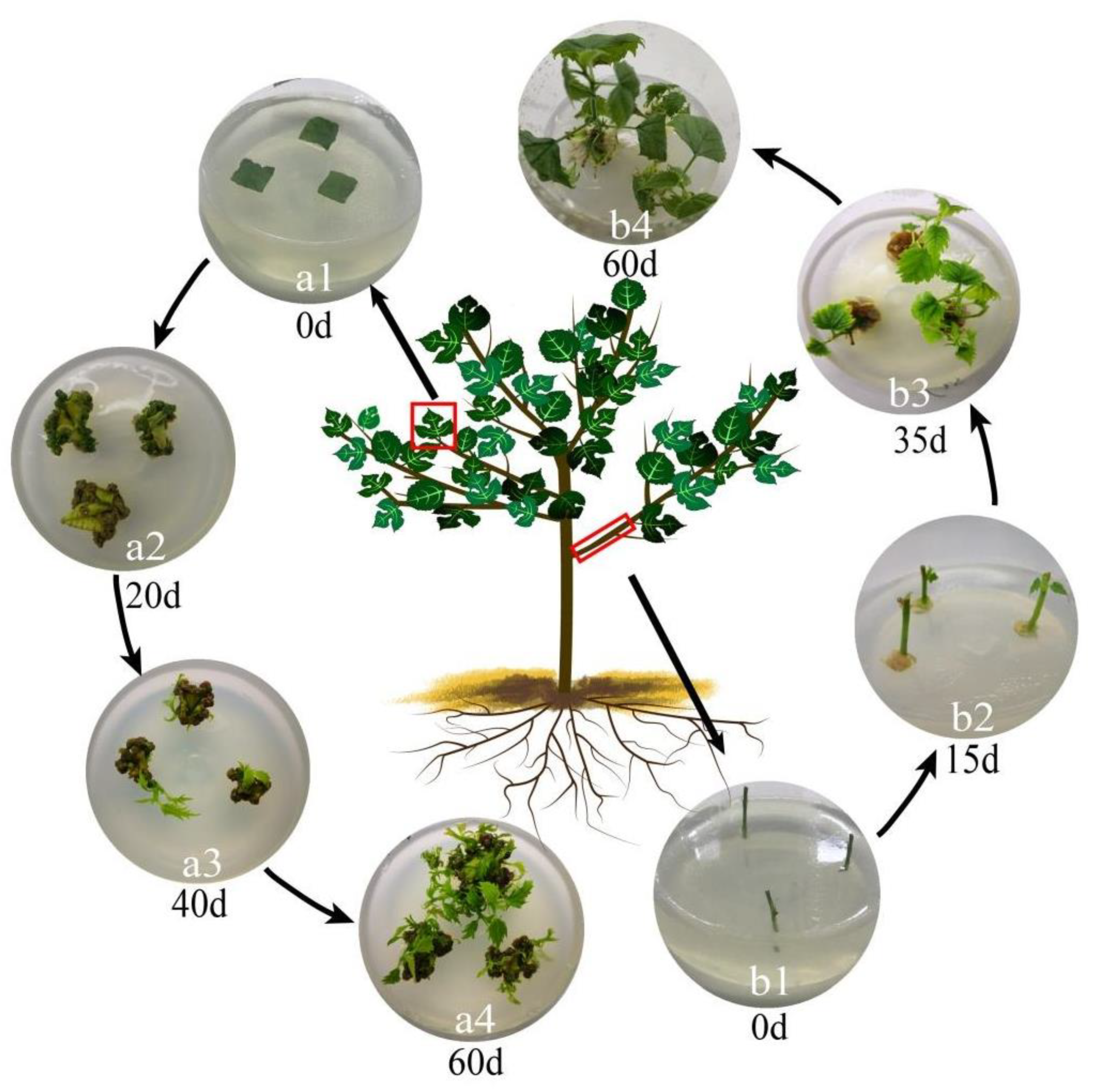
3.1. Growth of Wild B. papyrifera Explants
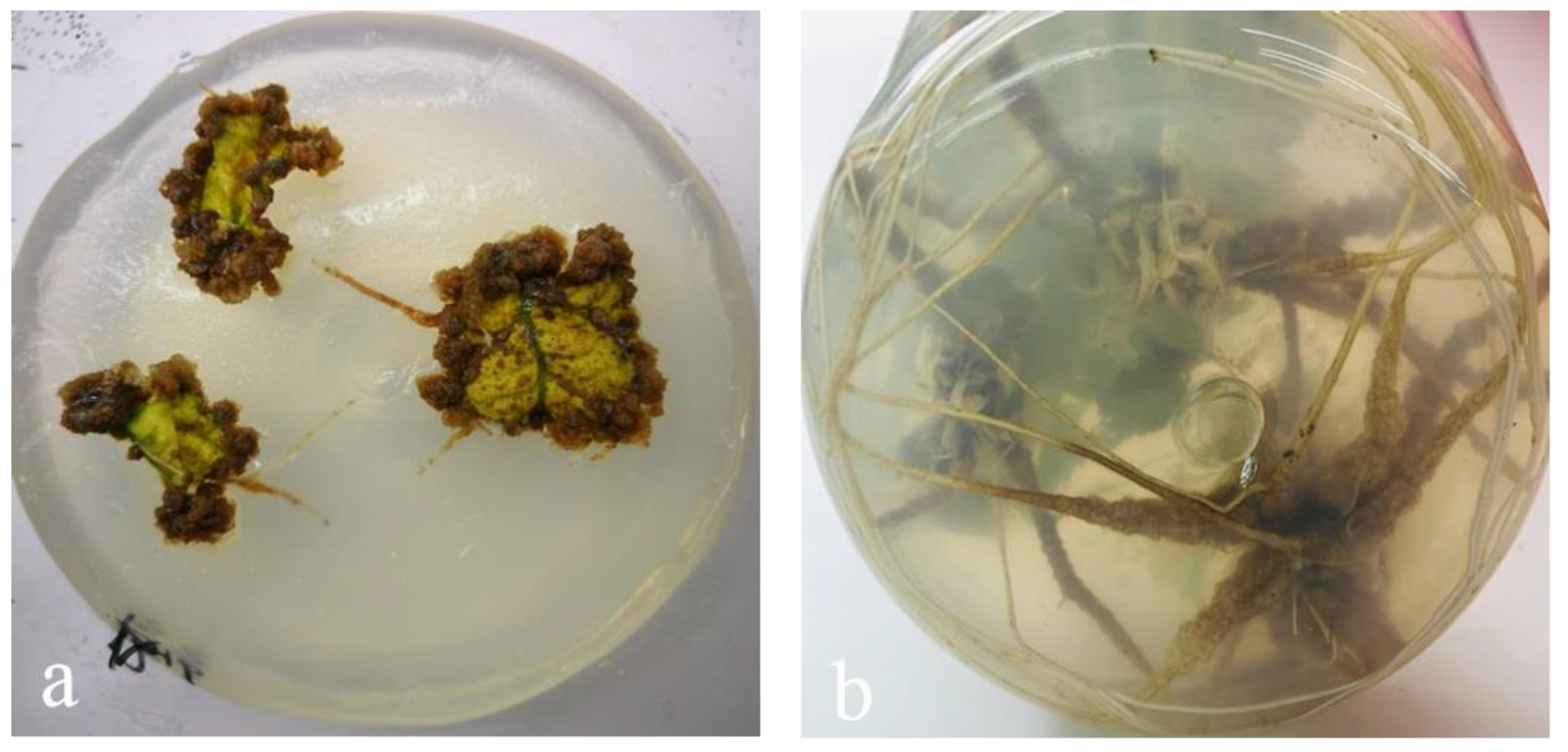
3.2. Induction of Explant Callus
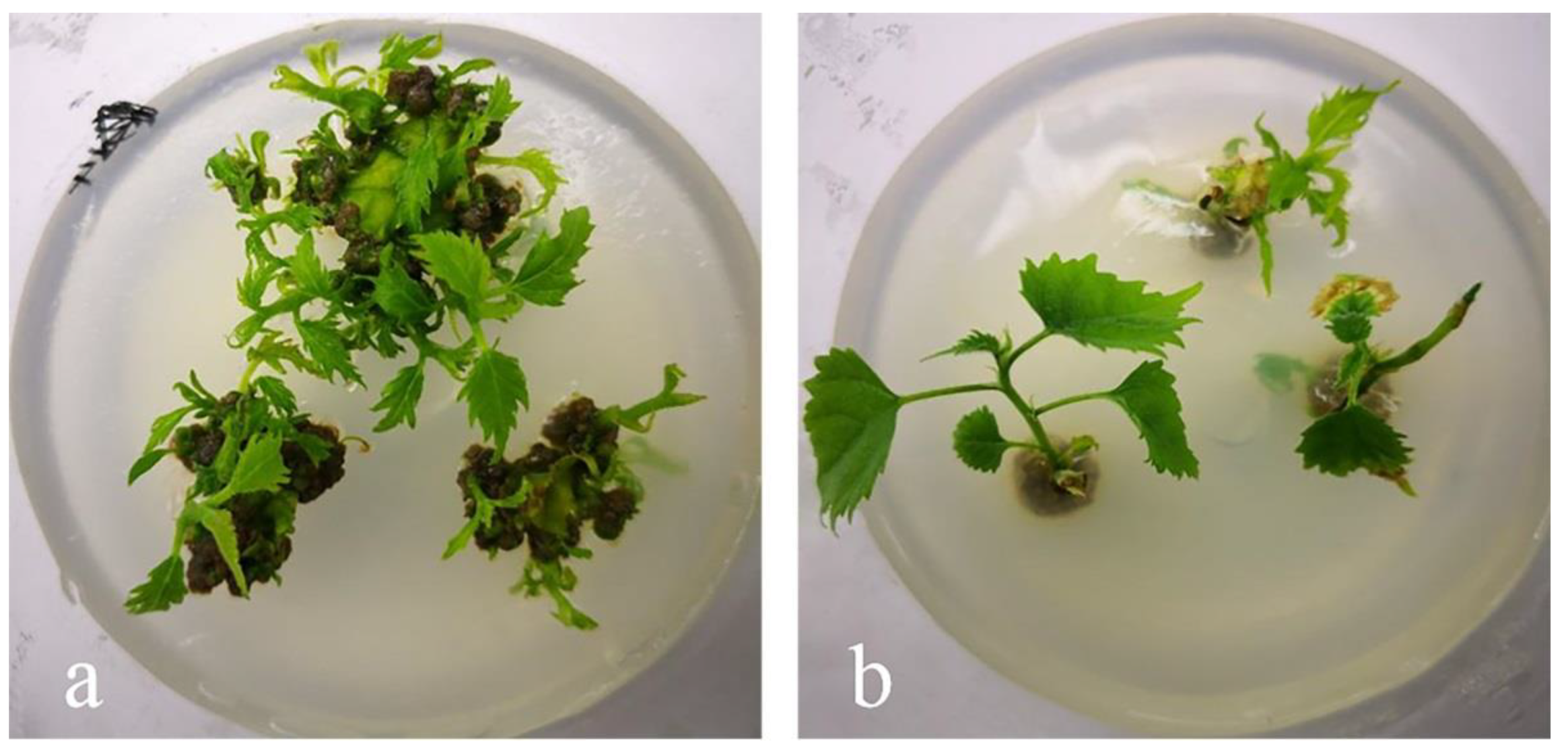
3.3. Induction of Explants Adventitious Buds
3.4. Induction of Explants Rooting
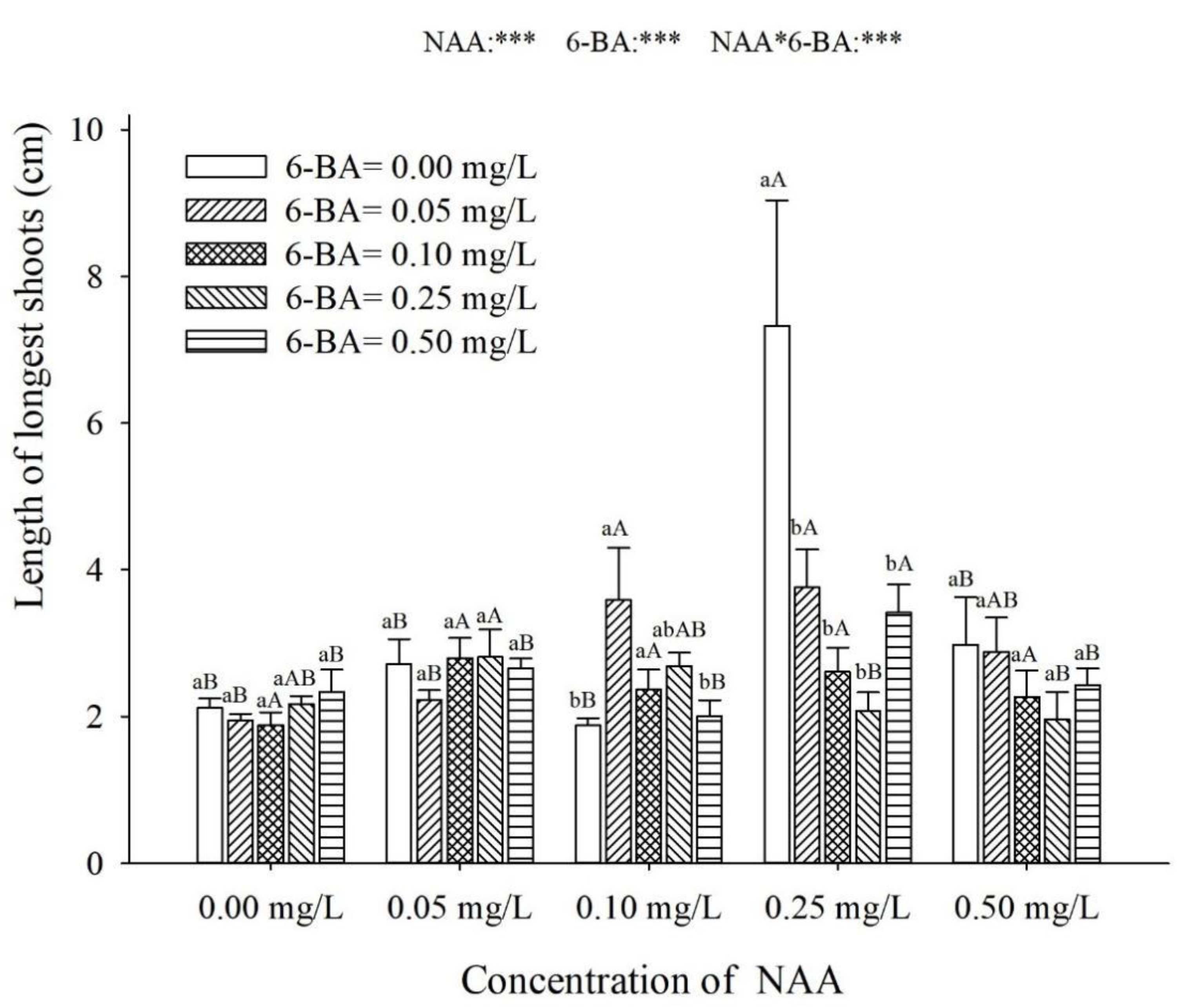
3.5. The Number of Leaves, the Longest Shoot and the Longest Root of the Shoot Explant
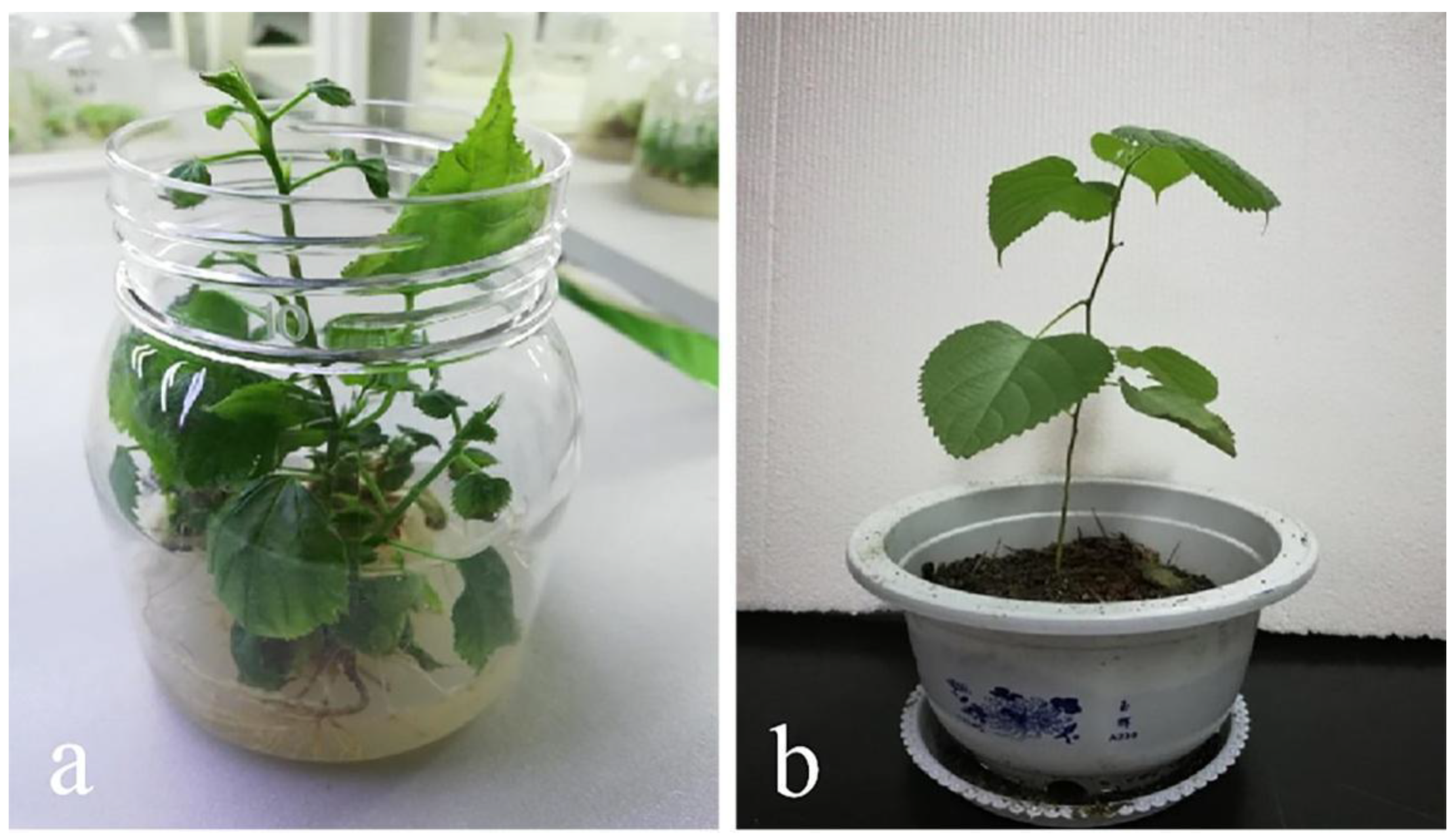
3.6. Transplanting Condition of Tissue Culture Plantlets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yavuz, D.O. Optimization of regeneration conditions and in vitro propagation of Sideritis Stricta Boiss & Heldr. Int. J. Biol. Macromol. 2016, 90, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Kadhimi, A.; Alhasnawi, A.N.; Mohamad, A.; Yusoff, W.M.W.; Zain, C.R.B.C.M. Tissue culture and some of the factors affecting them and the micropropagation of strawberry. Life Sci. J. 2014, 11. [Google Scholar] [CrossRef]
- Twaij, B.M.; Jazar, Z.H.; Hasan, M.N. Trends in the use of tissue culture, applications and future aspects. Int. J. Plant Biol. 2020, 11. [Google Scholar] [CrossRef]
- Baday, S.J.S. Plant Growth regulators importance and effects: A review. Asian J. Microbiol. Biotechnol. 2019, 5, 1–12. [Google Scholar]
- Fajinmi, O.O.; Amoo, S.O.; Finnie, J.F.; Van Staden, J. Optimization of in vitro propagation of Coleonema album, a highly utilized medicinal and ornamental plant. S. Afr. J. Bot. 2014, 94, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Leva, A.; Rinaldi, L.M.R. Recent Advances in Plant in Vitro Culture, 1st ed.; Janeza Trdine: Rijeka, Croatia, 2012; Chapter 1. [Google Scholar]
- Kang, L.; Zheng, K.; Xie, Y.; Deng, Y.; Yu, Y.; Zhu, M.; Xi, R.; Deng, X. Efficient Tissue Culture Protocol for Magnolia lucida (Magnoliaceae) and Confirmation of Genetic Stability of the Regenerated Plants. Plants 2020, 9, 997. [Google Scholar] [CrossRef]
- Yu, L.; Li, X.; Tian, H.; Liu, H.; Xiao, Y.; Liang, N.; Zhao, X.; Zhan, Y. Effects of Hormones and Epigenetic Regulation on the Callus and Adventitious Bud Induction of Fraxinus mandshurica Rupr. Forests 2020, 11, 590. [Google Scholar] [CrossRef]
- Shahsavari, E.; Maheran, A.A.; Siti, A.; Akmar, N.; Hanafi, M.M. The effect of plant growth regulators on optimization of tissue culture system in Malaysian upland rice. Afr. J. Biotechnol. 2010, 9, 2089–2094. [Google Scholar] [CrossRef]
- Milan, M.; Jakub, T.; Veronika, L.; Daniela, N.; Michal, O.; Jaromír, M.; Karel, S. Anti-inflammatory and antioxidant properties of chemical constituents of Broussonetia papyrifera. Bioorg. Chem. 2020, 104. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, C.; Li, P.; Wang, J.; Xing, D.; Wang, B. Photosynthetic characteristics involved in adaptability to Karst soil and alien invasion of paper mulberry (Broussonetia papyrifera (L.) Vent.) in comparison with mulberry (Morus alba L.). Photosynihetica 2009, 47, 155–160. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Xu, Z.; Huang, H.; Zhou, J.; Yang, G. Morphological and Physiological Changes of Broussonetia papyrifera Seedlings in Cadmium Contaminated Soil. Plants 2020, 9, 1698. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, Y.; Xu, Z.; Zhang, W.; Jiang, K. Physiological responses of Broussonetia papyrifera to manganese stress, a candidate plant for phytoremediation. Ecotoxicol. Environ. Saf. 2019, 181, 18–25. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, G.; Dong, M.; Wu, L.; Zhang, W.; Zhao, Y. The complete chloroplast genome of an economic and ecological plant, paper mulberry (Broussonetia kazinoki × Broussonetia papyrifera). Mitochondrial DNA Part B 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Gegenzhula; Wang, S.; Bai, Y. Research Progress of Tissue Culture of Broussonetia papyrifera. J. Inner Mongolia For. Sci. Technol. 2020, 46, 56–59. [Google Scholar]
- Zhang, J.; Lin, M.; Chen, H.; Chen, X. Agrobacterium tumefaciens-mediated transformation of drumstick (Moringa oleifera Lam.). Biotechnol. Biotechnol. Equip. 2017, 31, 1126–1131. [Google Scholar] [CrossRef]
- Yan, H.; Gao, Z.; Chen, T.; Cao, L.; Wang, P. Callus Induction of Hybrid Broussonetia papyrifera Leaves. Henan Sci. 2020, 38, 1776–1780. [Google Scholar]
- Zhi, S.; Shi, L. Effects of Different Medium on Rooting of Broussonetia papyrifera (L.) Vent. Tissue Culture Seedlings. J. Shanxi Agric. Sci. 2020, 48, 1207–1210. [Google Scholar]
- Tian, R.; Huang, Y.; Lu, S.; Xu, A. Establishment of tissue culture system of hybrid Broussonetia papyrifera (L.) Vent. Hubei Agric. Sci. 2019, 58, 120–123. [Google Scholar] [CrossRef]
- Wei, H.; Liu, Z.; Ma, L.; Wan, W.; Sheng, D. Callus induction and plantlet regeneration from leaves of hybrid Broussonetia papyrifera. J. Beijing For. Univ. 2010, 32, 116–120. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. Arevised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gaspar, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.M.; Thorpe, T.A. Plant hormones and plant growth regulators in plant tissue culture. Vitr. Cell. Dev. Biol. Plant 1996, 32. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, L.; Ren, M.; Tian, S. Establishment of Tissue Culture and Rapid Propagation System of Hybrid Broussonetia papyrifera. Shanxi Agric. Sci. 2016, 44, 1073–1076. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Tao, X.; Sheng, M.; Zhang, J.; Zhanng, T.; Liu, H. Establishment of Tissue Culture and Rapid Propagation System of Hybrid Broussonetia papyrifera Leaves. Guizhou Agric. Sci. 2018, 46, 46–48. [Google Scholar]
- Zhang, W.; Zhang, C.; Yang, J.; Liu, Y.; Li, R.; Liu, S.; Wang, Z. Effects of different explants and hormones on the induction of loose callus of North American begonia. J. Tianjin Agric. Univ. 2020, 27, 31–34. [Google Scholar] [CrossRef]
- Azizan, M.N.A.; Zakaria, N.L.; RISDA. The Effect of BAP and NAA Treatment on Micropropagation of Cucumis sativus.L. Int. J. Sci. Res. (IJSR) 2017, 6. [Google Scholar] [CrossRef]
- He, B.; Shen, P.; Zheng, H.; Chu, Y.; Guan, J.; Li, L. Effects of different hormones on germination and callus induction of hemp seeds. IOP Conf. Ser. Earth Environ. Sci. 2020, 526, 012043. [Google Scholar] [CrossRef]
- Fu, Y.; Qin, M.; Chen, Y.; Huang, M.; Xu, W.; Lei, M.; Yang, L. A Method of Callus Induction and Proliferation for Lilium lancifolium Thunb. Mol. Plant Breed. 2020, 1–19. Available online: https://kns.cnki.net/kcms/detail/46.1068.S.20201210.1342.008.html (accessed on 11 December 2020).
- Vakhariya, M.R.R.; Shah, R.R. Over Review on Plant Tissue Culture. J. Trend Sci. Res. Dev. 2019, 4, 469–473. [Google Scholar]
- Malik, K.; Birla, D.; Yadav, H.; Sainger, M.; Chaudhary, D.; Jaiwal, P.K. Evaluation of carbon sources, gelling agents, growth hormones and additives for efficient callus induction and plant regeneration in Indian wheat (Triticum aestivum L.) genotypes using mature embryos. J. Crop Sci. Biotechnol. 2017, 20, 185–192. [Google Scholar] [CrossRef]
- Bell, R.L.; Srinivasan, C.; Lomberk, D. Effect of nutrient media on axillary shoot proliferation and preconditioning for adventitious shoot regeneration of pears. In Vitro Cell. Dev. Biol. Plant 2009, 45. [Google Scholar] [CrossRef]
- Fan, B.; He, R.; Shang, Y.; Xu, L.; Wang, N.; Gao, H.; Liu, X.; Wang, Z. System construction of virus-free and rapid-propagation technology of Baodi garlic (Allium sativum L.). Sci. Hortic. 2017, 225, 498–504. [Google Scholar] [CrossRef]
- Wang, F.; Chai, C.; Wei, Q.; Wei, X. Tissue Culture of Endangered Psammophyte Ammopiptanthus mongolicus. Chin. Agric. Sci. Bull. 2016, 32, 32–36. [Google Scholar]
- Xu, S.; Li, Y.; Ming, A.; Yan, R.; Gao, X.; Zhang, J.; Kang, W. Study on Virus-free and Rapid Propagation Technology of Artemisia selengensis sp. in Yangxin County. Agric. Biotechnol. 2020, 9, 70–72+78. [Google Scholar] [CrossRef]
- Vaiciukyne, M.; Ziauka, J.; Zukiene, R.; Vertelkaite, L.; Kuusiene, S. Abscisic acid promotes root system development in birch tissue culture: A comparison to aspen culture and conventional rooting-related growth regulators. Physiol. Plant. 2019, 165, 114–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfred, U.J.; Olasan, O.J.; Iheukwumere, C.C.; Aguoru, C.U.; Ameh, S.J. Micro Propagation of Pterocarpus santalinoides Using Three Different Growth Media. J. Adv. Biol. Biotechnol. 2019, 1–9. [Google Scholar] [CrossRef]
- Zhang, G.; Shi, X.; Pan, H.; Dai, T. Study on Callus Induction and Tissue Culture Regeneration In Vitro of Dracocephalum Rupestre. Bot. Res. 2019, 8, 525–532. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Y.; Gui, S.; Li, Y.; Nie, G. Study on inducing roots from Polygonatum cyrtonema plantlets. Nonwood For. Res. 2015, 33, 102–105. [Google Scholar] [CrossRef]
- Cui, S.; Ren, Y.; Hao, Y.; Zhang, J.; Chen, Z.; Zou, J.; Zhou, W.; Chen, X. An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants. Open Life Sci. 2020, 15, 318–325. [Google Scholar] [CrossRef]
- Hu, Y.; Shi, L.; Liu, D.; Gong, S.; Wei, M.; Sun, J. Effects of different perlite additions on physical and chemical properties of sewage sludge compost and growth of Tagetes patula. Chin. J. Appl. Ecol. 2014, 25, 1949–1954. [Google Scholar] [CrossRef]
| Group | Plant Growth Regulators | |
|---|---|---|
| NAA (mg/L) | 6-BA (mg/L) | |
| 1 | 0.00 | 0.00 |
| 2 | 0.00 | 0.05 |
| 3 | 0.00 | 0.10 |
| 4 | 0.00 | 0.25 |
| 5 | 0.00 | 0.50 |
| 6 | 0.05 | 0.00 |
| 7 | 0.05 | 0.05 |
| 8 | 0.05 | 0.10 |
| 9 | 0.05 | 0.25 |
| 10 | 0.05 | 0.50 |
| 11 | 0.10 | 0.00 |
| 12 | 0.10 | 0.05 |
| 13 | 0.10 | 0.10 |
| 14 | 0.10 | 0.25 |
| 15 | 0.10 | 0.50 |
| 16 | 0.25 | 0.00 |
| 17 | 0.25 | 0.05 |
| 18 | 0.25 | 0.10 |
| 19 | 0.25 | 0.25 |
| 20 | 0.25 | 0.50 |
| 21 | 0.50 | 0.00 |
| 22 | 0.50 | 0.05 |
| 23 | 0.50 | 0.10 |
| 24 | 0.50 | 0.25 |
| 25 | 0.50 | 0.50 |
| 6-BA (mg/L) | NAA (mg/L) | ||||
|---|---|---|---|---|---|
| 0.00 | 0.05 | 0.10 | 0.25 | 0.50 | |
| 0.00 | 1.00 b D | 1.57 ± 0.13 bc C | 1.76 ± 0.17 b BC | 2.06 ± 0.08 a A | 1.82 ± 0.08 b BC |
| 0.05 | 1.15 ± 0.08 b B | 1.83 ± 0.15 ab A | 1.62 ± 0.17 b AB | 2.07 ± 0.26 a A | 1.93 ± 0.18 b A |
| 0.10 | 1.94 ± 0.19 a AB | 2.31 ± 0.24 a A | 2.00 ± 0.15 ab AB | 1.75 ± 0.16 a B | 2.23 ± 0.18 a AB |
| 0.25 | 1.93 ± 0.19 a AB | 1.27 ± 0.10 c B | 2.18 ± 0.23 ab AB | 2.28 ± 0.33 a AB | 2.73 ± 0.30 a A |
| 0.50 | 1.19 ± 0.08 b B | 1.99 ± 0.16 ab AB | 2.43 ± 0.20 a A | 2.13 ± 0.26 a A | 2.70 ± 0.30 a A |
| 6-BA (mg/L) | NAA (mg/L) | ||||
|---|---|---|---|---|---|
| 0.00 | 0.05 | 0.10 | 0.25 | 0.50 | |
| 0.00 | 0.29 ± 0.04 b C | 0.55 ± 0.08 b BC | 0.58 ± 0.03 b BC | 0.84 ± 0.12 b B | 1.10 ± 0.15 a A |
| 0.05 | 0.32 ± 0.06 b C | 0.95 ± 0.08 a B | 1.00 ± 0.17 a B | 1.08 ± 0.04 ab AB | 1.27 ± 0.09 a A |
| 0.10 | 0.22 ± 0.04 b B | 0.97 ± 0.15 a A | 1.07 ± 0.08 a A | 1.19 ± 0.07 a A | 0.99 ± 0.20 a A |
| 0.25 | 0.49 ± 0.05 a C | 0.98 ± 0.10 a AB | 1.04 ± 0.11 a AB | 0.83 ± 0.12 ab B | 1.17 ± 0.19 a A |
| 0.50 | 0.60 ± 0.11 a B | 0.91 ± 0.13 a AB | 1.04 ± 0.07 a AB | 1.30 ± 0.15 a A | 1.10 ± 0.15 a AB |
| 6-BA (mg/L) | NAA (mg/L) | ||||
|---|---|---|---|---|---|
| 0.00 | 0.05 | 0.10 | 0.25 | 0.50 | |
| 0.00 | 0.00 b A | 0.00 b A | 0.00 c A | 0.00 b A | 0.00 c A |
| 0.05 | 0.00 b B | 0.00 b B | 0.00 c B | 0.33 ± 0.33 ab AB | 1.33 ± 0.5 b A |
| 0.10 | 0.4 ± 0.34 b A | 0.43 ± 0.34 b A | 0.22 ± 0.15 c A | 0.67 ± 0.29 ab A | 0.43 ± 0.30 bc A |
| 0.25 | 1.80 ± 0.67 a B | 3.67 ± 1.34 a AB | 4.39 ± 1.25 a A | 2.44 ± 1.36 a AB | 2.43 ± 1.62 b AB |
| 0.50 | 0.00 b C | 3.00 ± 0.87 a B | 1.50 ± 0.40 b B | 0.00 b C | 10.14 ± 1.82 a A |
| 6-BA (mg/L) | NAA (mg/L) | ||||
|---|---|---|---|---|---|
| 0.00 | 0.05 | 0.10 | 0.25 | 0.50 | |
| 0.00 | 1.00 c B | 1.2 ± 0.11 b A | 1.00 b B | 1.00 b A | 1.38 ± 0.18 a A |
| 0.05 | 1.00 c C | 1.58 ± 0.34 b B | 1.75 ± 0.37 a AB | 2.44 ± 1.49 a A | 1.44 ± 0.24 a B |
| 0.10 | 1.40 ± 0.24 b A | 2.17 ± 0.67 b A | 2.28 ± 0.42 a A | 1.93 ± 0.37 ab A | 1.57 ± 0.30 a A |
| 0.25 | 1.40 ± 0.16 b AB | 4.00 ± 1.03 ab A | 4.15 ± 1.12 a A | 1.56 ± 0.34 ab AB | 1.14 ± 0.14 a B |
| 0.50 | 2.11 ± 0.26 a AB | 5.33 ± 1.19 a A | 1.57 ± 0.17 a B | 1.11 ± 0.11 b B | 2.00 ± 0.69 a AB |
| 16-BA (mg/L) | NAA (mg/L) | ||||
|---|---|---|---|---|---|
| 0 | 0.05 | 0.1 | 0.25 | 0.5 | |
| 0.00 | 0.00 a B | 0.00 a B | 0.10 ± 0.10 ab AB | 1.71 ± 1.04 a A | 0.63 ± 0.50 a AB |
| 0.05 | 0.00 a B | 0.00 a B | 1.25 ± 0.56 a A | 0.00 b B | 0.67 ± 0.55 a AB |
| 0.10 | 0.00 a B | 0.00 a B | 0.00 b AB | 0.00 b B | 0.27 ± 0.18 a A |
| 0.25 | 0.00 a A | 0.08 a A | 0.00 b A | 0.00 b A | 0.00 a A |
| 0.50 | 0.00 a B | 0.00 a B | 0.00 b B | 1.11 ± 0.26 a A | 0.00 a B |
| 6-BA (mg/L) | NAA (mg/L) | ||||
|---|---|---|---|---|---|
| 0.00 | 0.05 | 0.10 | 0.25 | 0.50 | |
| 0.00 | 0.08 ± 0.08 a B | 0.13 ± 0.09 a B | 1.10 ± 0.77 a B | 13.86 ± 3.49 a A | 0.50 ± 0.50 a B |
| 0.05 | 0.00 a B | 0.33 ± 0.33 a AB | 1.25 ± 0.65 a A | 0.78 ± 0.40 b A | 0.22 ± 0.15 a AB |
| 0.10 | 0.00 a B | 0.00 a B | 0.00 b B | 0.80 ± 0.73 b AB | 2.00 ± 1.31 a A |
| 0.25 | 0.00 a A | 0.00 a A | 0.00 b A | 0.00 b A | 0.00 a A |
| 0.50 | 0.00 a A | 0.00 a A | 0.00 b A | 0.89 ± 0.68 b A | 0.00 a A |
| 6-BA (mg/L) | NAA (mg/L) | ||||
|---|---|---|---|---|---|
| 0.00 | 0.05 | 0.10 | 0.25 | 0.50 | |
| 0.00 | 2.08 ± 0.40 b C | 4.67 ± 0.80 b AB | 3.50 ± 0.40 b B | 5.86 ± 1.22 a A | 5.00 ± 0.66 a AB |
| 0.05 | 2.58 ± 0.53 b B | 4.42 ± 0.84 b AB | 7.00 ± 2.47 ab A | 11.33 ± 4.12 a A | 6.89 ± 1.63 a A |
| 0.10 | 3.27 ± 1.37 b B | 8.75 ± 3.42 ab A | 6.44 ± 1.12 ab A | 6.53 ± 2.60 a A | 2.57 ± 1.11 a B |
| 0.25 | 4.20 ± 0.76 ab A | 15.11 ± 7.22 ab A | 13.08 ± 2.83 a A | 3.44 ± 0.85 a A | 3.14 ± 1.16 a A |
| 0.50 | 8.11 ± 2.15 a AB | 19.60 ± 4.27 a A | 3.86 ± 0.85 b B | 4.33 ± 0.67 a B | 4.00 ± 0.87 a B |
| 6-BA (mg/L) | NAA (mg/L) | ||||
|---|---|---|---|---|---|
| 0.00 | 0.05 | 0.10 | 0.25 | 0.50 | |
| 0.00 | 0.02 ± 0.02 a B | 0.63 ± 0.44 a B | 0.10 ± 0.07 b B | 2.67 ± 0.74 a A | 0.28 ± 0.28 a B |
| 0.05 | 0.00 a B | 0.04 ± 0.04 a B | 1.09 ± 0.62 a A | 0.92 ± 0.55 b AB | 0.32 ± 0.23 a AB |
| 0.10 | 0.00 a B | 0.00 a B | 0.00 b B | 0.29 ± 0.23 b AB | 0.50 ± 0.33 a A |
| 0.25 | 0.00 a A | 0.00 a A | 0.00 b A | 0.00 b A | 0.00 a A |
| 0.50 | 0.00 a B | 0.00 a B | 0.00 b B | 0.69 ± 0.48 b A | 0.00 a B |
| Treatment Group | Transplanting Substrate | Number of Surviving Plants | Survival Rate (%) |
|---|---|---|---|
| 1 | River sand | 18.67 ± 1.15 c | 62.2 ± 3.8 c |
| 2 | Campus red loam | 24.33 ± 1.53 b | 81.1 ± 5.1 b |
| 3 | a mixture of soil: perlite: vermiculite (20:1:1) | 27.33 ± 0.57 a | 91.1 ± 1.9 a |
| Types of B. papyrifera | Explants | Time of Each Stage | Optimal Combination of Plant Growth Regulators | References |
|---|---|---|---|---|
| Hybrid | Leaves | Callus appeared in 15 d | Rooting: MS + 2.50 mg/L 6-BA + 2.00 mg/L NAA | [17] |
| Hybrid | Shoots | Statistics of callus on the 30th d | Callus induction: MS + 0.50 mg/L IBA + 2.00 mg/L 6-BA | [19] |
| Statistics of adventitious buds on the 30th d | Adventitious buds induction: MS + 1.50 mg/L IBA + 2.00 mg/L 6-BA | |||
| Rooting in 15 d | Rooting: 1/2 MS + 0.80 mg/L IBA | |||
| Hybrid | Leaves | Adventitious buds began to appear after two weeks | Adventitious buds induction: MS + 2.00 mg/L 6-BA + 0.10 mg/L NAA | [20] |
| Hybrid | Leaves | Callus appeared in 12 d | Callus induction: MS + 1.50 mg/L 6-BA + 1.00 mg/L NAA | [23] |
| Rooting in 15 d | Rooting: 1/2 MS + 0.50 mg/L 6-BA + 1.00 mg/L NAA | |||
| Hybrid | Leaves | Callus appeared in 20 d and Shoot-buds appeared in 30 d | Callus induction and Adventitious buds induction: MS + 2.00 mg/L 6-BA + 0.10 mg/L NAA | [24] |
| Statistics of rooting on the 30th d | Rooting: B5 + 0.01 mg/L 6-BA + 0.30 mg/L NAA + 0.50 mg/L IBA | |||
| Wild | Shoots | Statistics of rooting on the 35th d | Rooting: 1/2 MS + 0.10 mg/L NAA | [18] |
| Wild | Leaves | Adventitious buds appeared in 3 weeks | Adventitious buds induction: MS + 2.00 mg/L 6-BA + 0.05 mg/L IBA | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Liu, Y.; Wu, L.; Zhao, Y.; Zhang, W.; Yang, G.; Xu, Z. Effects of Plant Growth Regulators on the Rapid Propagation System of Broussonetia papyrifera L. Vent Explants. Forests 2021, 12, 874. https://doi.org/10.3390/f12070874
Zhou J, Liu Y, Wu L, Zhao Y, Zhang W, Yang G, Xu Z. Effects of Plant Growth Regulators on the Rapid Propagation System of Broussonetia papyrifera L. Vent Explants. Forests. 2021; 12(7):874. https://doi.org/10.3390/f12070874
Chicago/Turabian StyleZhou, Jiakang, Yang Liu, Liang Wu, Yunlin Zhao, Wan Zhang, Guiyan Yang, and Zhenggang Xu. 2021. "Effects of Plant Growth Regulators on the Rapid Propagation System of Broussonetia papyrifera L. Vent Explants" Forests 12, no. 7: 874. https://doi.org/10.3390/f12070874
APA StyleZhou, J., Liu, Y., Wu, L., Zhao, Y., Zhang, W., Yang, G., & Xu, Z. (2021). Effects of Plant Growth Regulators on the Rapid Propagation System of Broussonetia papyrifera L. Vent Explants. Forests, 12(7), 874. https://doi.org/10.3390/f12070874







