Silvicultural Interventions Drive the Changes in Soil Organic Carbon in Romanian Forests According to Two Model Simulations
Abstract
1. Introduction
- (1)
- Does including detailed soil organic carbon dynamic models, i.e., running carbon pools by CBM and chemical compounds by Yasso15, improve the simulations of the initialized C stock compared to measured ones?
- (2)
- Do models perform comparatively on short term assuming the same litterfall dynamic?
- (3)
- How do different harvesting scenarios for Romania’s forest affect the carbon simulations?
2. Materials and Methods
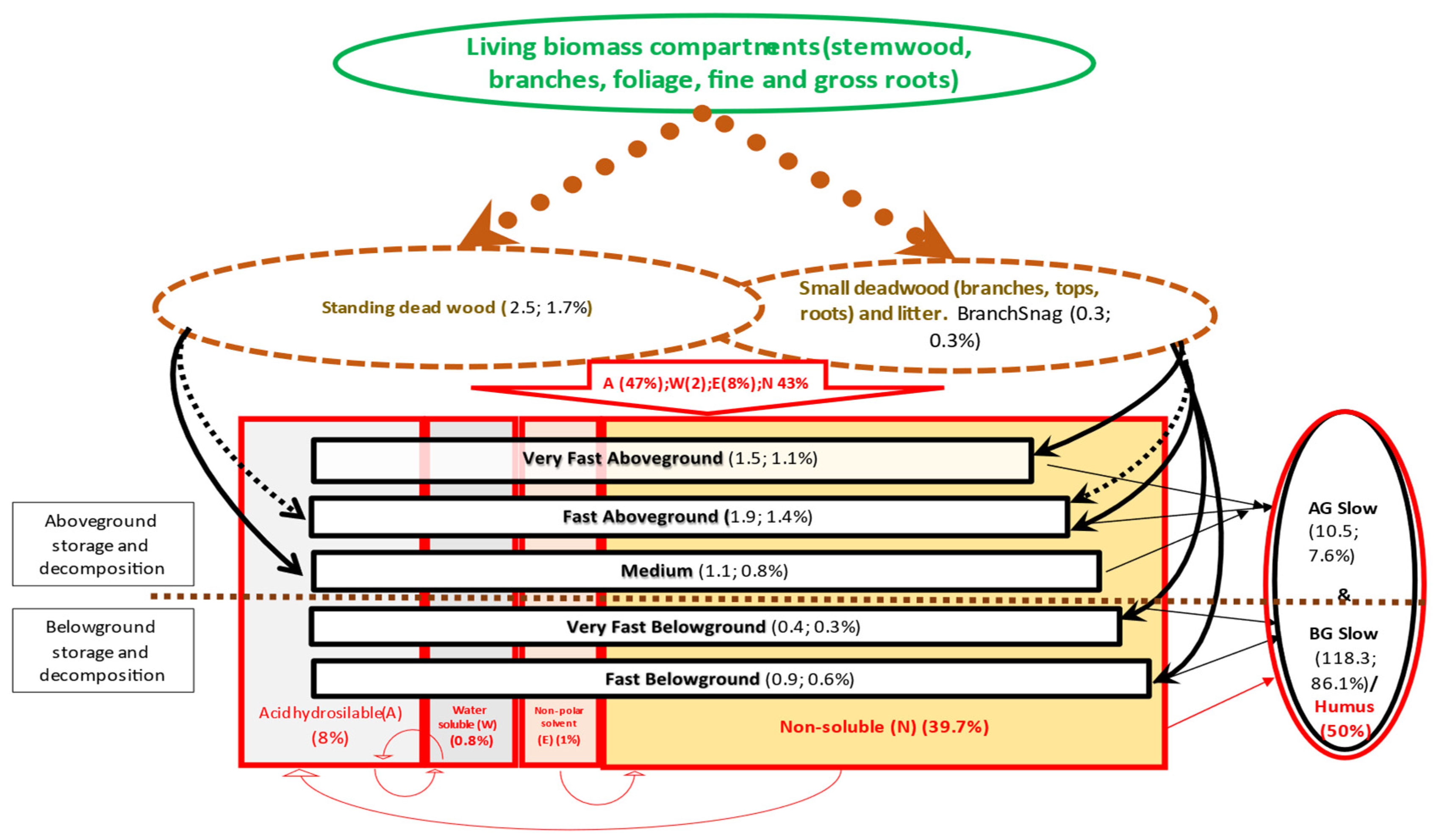
2.1. Description of Soil Modules of CBM-CFS3 and Yasso15
2.2. National Forest Inventory
2.3. Forest Soil Inventory
2.4. Litterfall Estimates
2.5. Harmonization of the Decomposition Process
2.6. Scenarios
2.7. Data Processing
3. Results
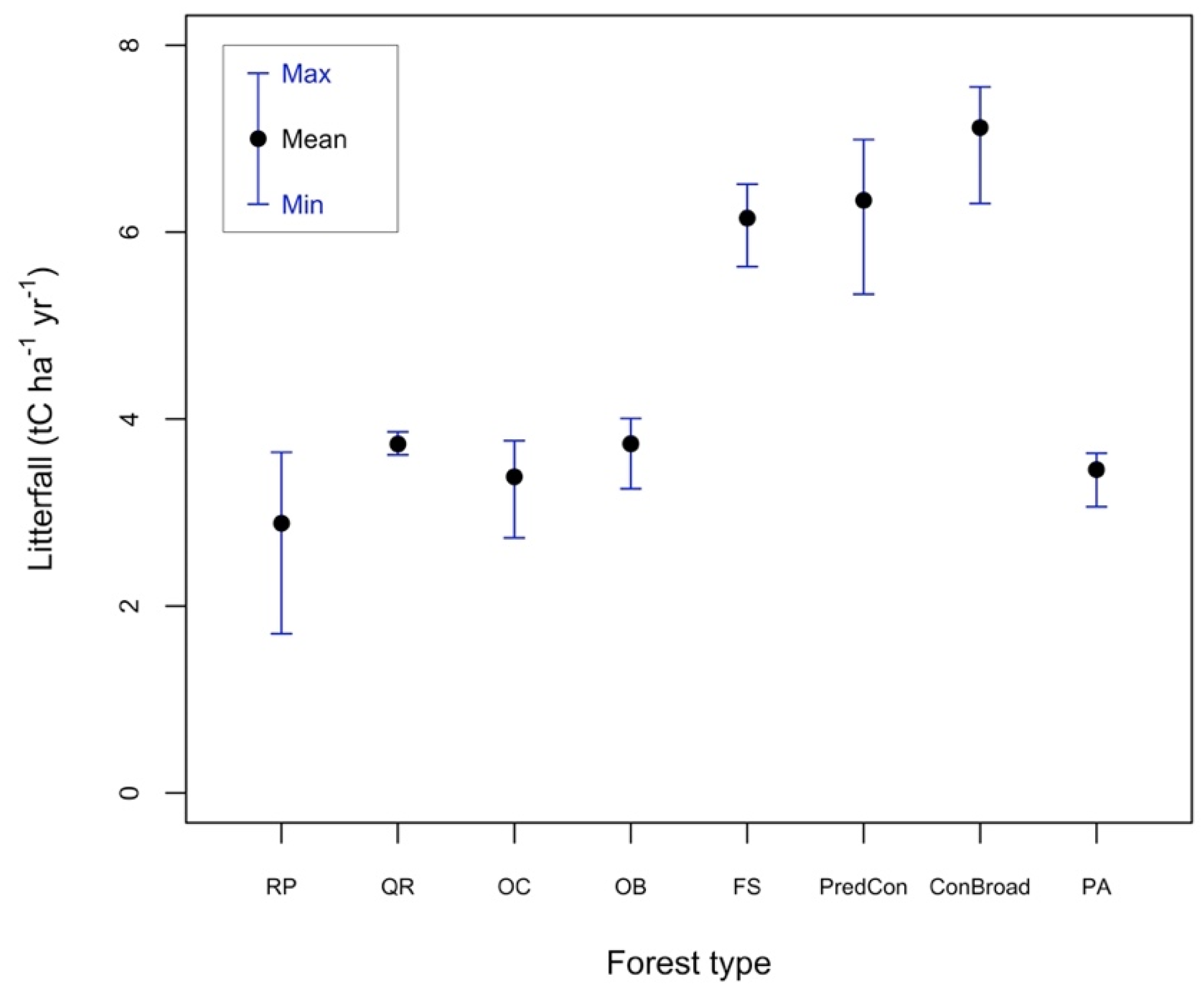
3.1. Litterfall Amounts during Spinoff
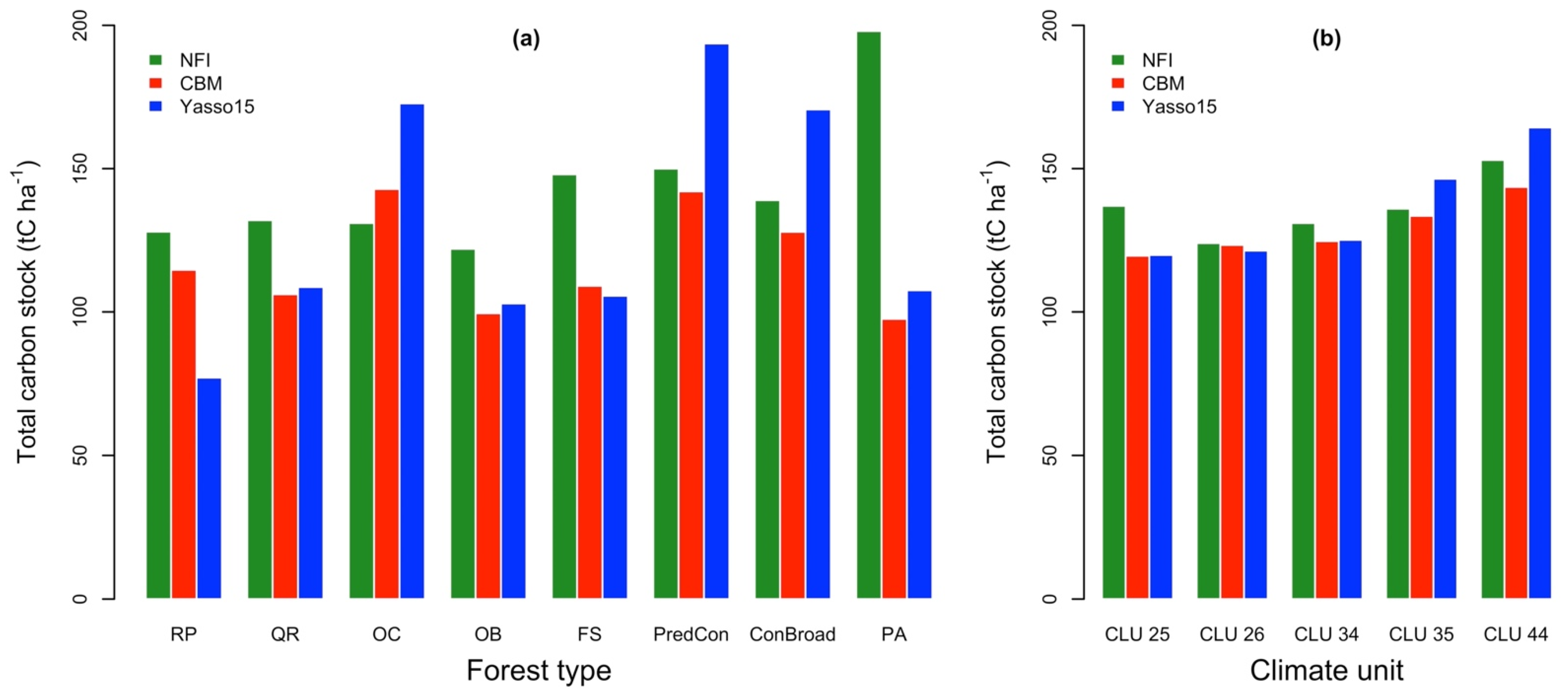
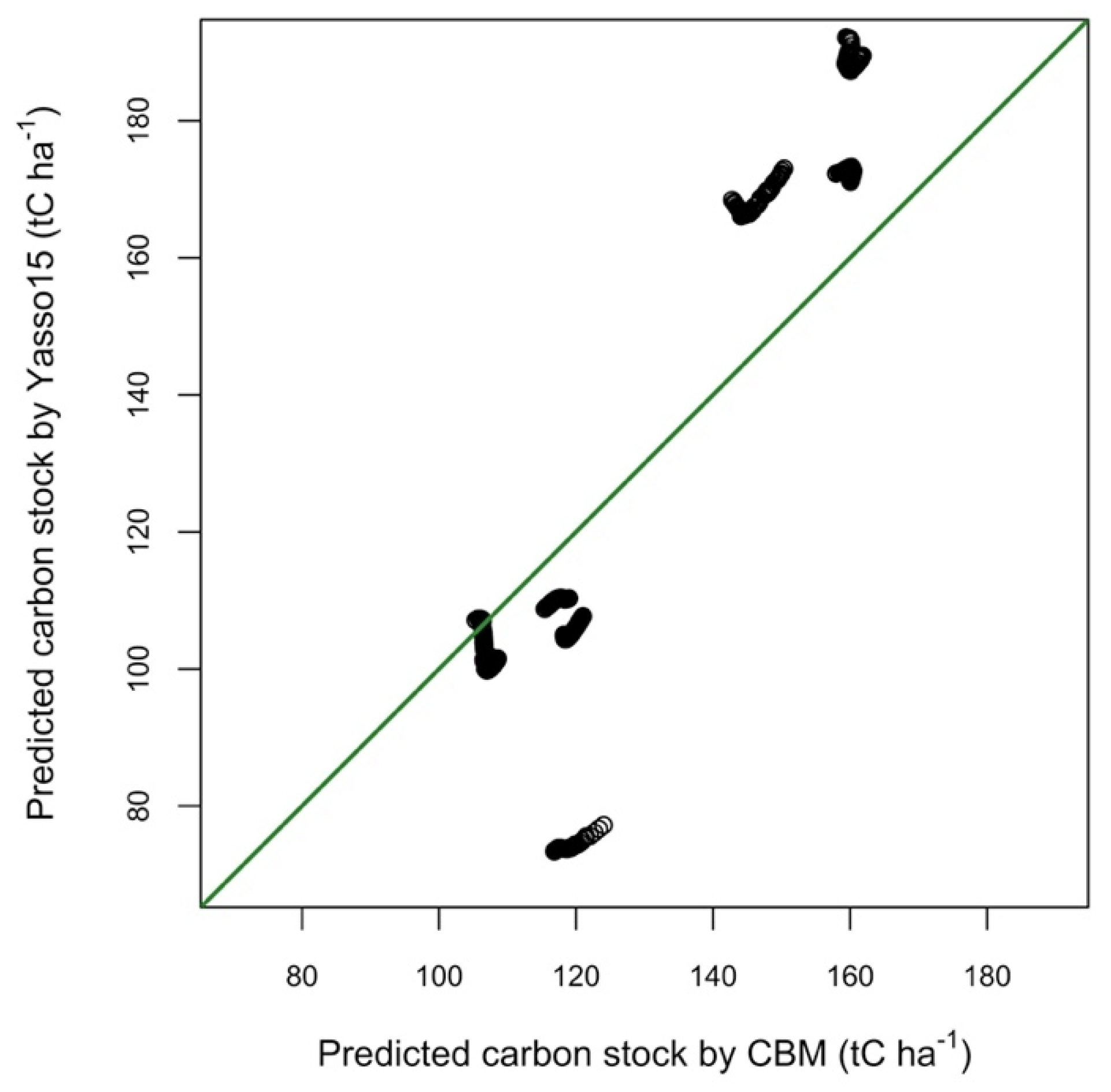
3.2. Model Performance for the Initialization of Total C Stock
3.3. Initialization of C Stocks in the Soil Subpools
3.4. Litterfall Dynamic for the Scenarios
3.5. Projections of Soil Total C Stock and Dynamics of the Annual C Stock Change
3.6. Simulated Soil Carbon Stock Change by Subpools
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Scharlemann, J.; Tanner, E.V.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Piñeiro, G. The Ecology of Soil Carbon: Pools, Vulnerabilities, and Biotic and Abiotic Controls. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 419–445. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.; Shvidenko, A.; Lewis, S.L.; Canadell, J.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Yigini, Y.; Panagos, P. Assessment of soil organic carbon stocks under future climate and land cover changes in Europe. Sci. Total. Environ. 2016, 557–558, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Lugato, E.; Smith, P.; Borrelli, P.; Panagos, P.; Ballabio, C.; Orgiazzi, A.; Fernandez-Ugalde, O.; Montanarella, L.; Jones, A. Soil erosion is unlikely to drive a future carbon sink in Europe. Sci. Adv. 2018, 4, eaau3523. [Google Scholar] [CrossRef]
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- De Vos, B.; Cools, N.; Ilvesniemi, H.; Vesterdal, L.; Vanguelova, E.; Carnicelli, S. Benchmark values for forest soil carbon stocks in Europe: Results from a large scale forest soil survey. Geoderma 2015, 251–252, 33–46. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A.; Vesterdal, L.; Leifeld, J.; Van Wesemael, B.; Schumacher, J.; Gensior, A. Temporal dynamics of soil organic carbon after land-use change in the temperate zone—Carbon response functions as a model approach. Glob. Chang. Biol. 2011, 17, 2415–2427. [Google Scholar] [CrossRef]
- Houghton, R.A. The annual net flux of carbon to the atmosphere from changes in land use 1850–1990. Tellus B Chem. Phys. Meteorol. 1999, 51, 298–313. [Google Scholar] [CrossRef]
- Ghazoul, J.; Burivalova, Z.; Garcia-Ulloa, J.; King, L.A. Conceptualizing Forest Degradation. Trends Ecol. Evol. 2015, 30, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Bernal, B.; Murray, L.T.; Pearson, T.R.H. Global carbon dioxide removal rates from forest landscape restoration activities. Carbon Balance Manag. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- European Commission. Soil: The hidden Part of the Climate Cycle; Publications Office of the European Union: Luxembourg, 2011. [Google Scholar]
- IPCC. Agriculture, Forestry and Other Land Use. In 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Eggleston, H., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; INGES Japan: Hayama, Japan, 2006; Volume 4. [Google Scholar]
- IPCC. 2013 Revised Supplementary Methods and Good Practice Guidance Arising from the Kyoto Protocol; Hiraishi, T., Krug, T., Tanabe, K., Srivastava, N., Baasansuren, J., Fukuda, M., Troxler, T.G., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014. [Google Scholar]
- IPCC. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Calvo Buendia, E., Tanabe, K., Kranjc, A., Baasansuren, J., Fukuda, M., Ngarize, S., Osako, A., Pyrozhenko, Y., Shermanau, P., Federici, S., Eds.; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Didion, M.; Repo, A.; Liski, J.; Forsius, M.; Bierbaumer, M.; Djukic, I. Towards Harmonizing Leaf Litter Decomposition Studies Using Standard Tea Bags—A Field Study and Model Application. Forests 2016, 7, 167. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Jonard, M.; Nicolas, M.; Coomes, D.A.; Caignet, I.; Saenger, A.; Ponette, Q. Forest soils in France are sequestering substantial amounts of carbon. Sci. Total Environ. 2017, 574, 616–628. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Page-Dumroese, D.; Busse, M.; Palik, B.; Zhang, J.; Eaton, B.; Slesak, R.; Tirocke, J.; Kwon, H. Effects of forest harvesting and biomass removal on soil carbon and nitrogen: Two complementary meta-analyses. For. Ecol. Manag. 2021, 485, 118935. [Google Scholar] [CrossRef]
- UNFCCC. UNFCCC The Marrakesh Accords; United Nations: New York, NY, USA, 2002. [Google Scholar]
- UNFCCC. Kyoto Protocol to the United Nations Framework Convention on Climate Change; United Nations: New York, NY, USA, 1998. [Google Scholar]
- United Nations. Paris Agreement; United Nations: New York, NY, USA, 2016. [Google Scholar]
- Jurgensen, M.F.; Page-Dumroese, D.S.; Brown, R.E.; Tirocke, J.M.; Miller, C.A.; Pickens, J.B.; Wang, M. Estimating Carbon and Nitrogen Pools in a Forest Soil: Influence of Soil Bulk Density Methods and Rock Content. Soil Sci. Soc. Am. J. 2017, 81, 1689–1696. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Shi, L.; Wang, X.; Liu, Y.; Mao, R.; Rao, X.; Lin, Y.; Shao, Y.; Li, X.; et al. An alternative approach to reduce algorithm-derived biases in monitoring soil organic carbon changes. Ecol. Evol. 2019, 9, 7586–7596. [Google Scholar] [CrossRef]
- Meeussen, C.; Govaert, S.; Vanneste, T.; Haesen, S.; Van Meerbeek, K.; Bollmann, K.; Brunet, J.; Calders, K.; Cousins, S.A.O.; Diekmann, M.; et al. Drivers of carbon stocks in forest edges across Europe. Sci. Total Environ. 2021, 759, 143497. [Google Scholar] [CrossRef]
- Lacarce, E.; Le Bas, C.; Cousin, J.L.; Pesty, B.; Toutain, B.; Houston Durrant, T.; Montanarella, L. Data management for mon-itoring forest soils in Europe for the Biosoil project. Soil Use Manag. 2009, 25, 57–65. [Google Scholar] [CrossRef]
- Aksoy, E.; Yigini, Y.; Montanarella, L. Combining Soil Databases for Topsoil Organic Carbon Mapping in Europe. PLoS ONE 2016, 11, e0152098. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, P.H.; Loveland, P.J.; Bradley, R.I.; Lark, R.M.; Kirk, G.J.D. Carbon losses from all soils across England and Wales 1978–2003. Nature 2005, 437, 245–248. [Google Scholar] [CrossRef]
- Rantakari, M.M.; Lehtonen, A.; Linkosalo, T.; Tuomi, M.; Tamminen, P.; Heikkinen, J.; Liski, J.; Mäkipää, R.; Ilvesniemi, H.; Sievänen, R. The Yasso07 soil carbon model—Testing against repeated soil carbon inventory. For. Ecol. Manag. 2012, 286, 137–147. [Google Scholar] [CrossRef]
- Callesen, I.; Stupak, I.; Georgiadis, P.; Johannsen, V.K.; Østergaard, H.S.; Vesterdal, L. Soil carbon stock change in the for-ests of Denmark between 1990 and 2008. Geoderma Reg. 2015, 5, 169–180. [Google Scholar] [CrossRef]
- Van Leeuwen, J.P.; Saby, N.P.A.; Jones, A.; Louwagie, G.; Micheli, E.; Rutgers, M.; Schulte, R.P.O.; Spiegel, H.; Toth, G.; Creamer, R.E. Gap assessment in current soil monitoring networks across Europe for measuring soil functions. Environ. Res. Lett. 2017, 12, 124007. [Google Scholar] [CrossRef]
- Pilli, R.; Grassi, G.; Kurz, W.A.; Fiorese, G.; Cescatti, A. The European forest sector: Past and future carbon budget and flux-es under different management scenarios. Biogeosciences 2017, 14, 2387–2405. [Google Scholar] [CrossRef]
- Smyth, C.E.; Xu, Z.; Lemprière, T.C.; Kurz, W.A. Climate change mitigation in British Columbia’s forest sector: GHG re-ductions, costs, and environmental impacts. Carbon Balance Manag. 2020, 15, 1–22. [Google Scholar] [CrossRef]
- Kurz, W.; Dymond, C.; White, T.; Stinson, G.; Shaw, C.; Rampley, G.; Smyth, C.; Simpson, B.; Neilson, E.; Trofymow, J.; et al. CBM-CFS3: A model of carbon-dynamics in forestry and land-use change implementing IPCC standards. Ecol. Model. 2009, 220, 480–504. [Google Scholar] [CrossRef]
- Lehtonen, A.; Linkosalo, T.; Peltoniemi, M.; Sievänen, R.; Mäkipää, R.; Tamminen, P.; Salemaa, M.; Nieminen, T.; Ťupek, B.; Heikkinen, J.; et al. Forest soil carbon stock estimates in a nationwide inventory: Evaluating performance of the ROMULv and Yasso07 models in Finland. Geosci. Model Dev. 2016, 9, 4169–4183. [Google Scholar] [CrossRef]
- Ziche, D.; Grüneberg, E.; Hilbrig, L.; Höhle, J.; Kompa, T.; Liski, J.; Repo, A.; Wellbrock, N. Comparing soil inventory with modelling: Carbon balance in central European forest soils varies among forest types. Sci. Total. Environ. 2019, 647, 1573–1585. [Google Scholar] [CrossRef]
- Dincă, L.C.; Dincă, M.; Vasile, D.; Spârchez, G.; Holonec, L. Calculating organic carbon stock from forest soils. Not. Bot. Hortic. Agrobot. 2015, 43, 568–575. [Google Scholar] [CrossRef]
- Vintilă, R.; Munteanu, I.; Cojocaru, G.; Radnea, C.; Turnea, D.; Curelariu, G.; Nilca, I.; Jalbă, M.; Piciu, I.; Râşnoveanu, I.; et al. The Geographic Information System of Soil Resources of Romania “SIGSTAR-200”: Development and Main Types of Applications. In Proceedings of the XVII National Conference of Soil Science, Timisoara, Romania, 2004; pp. 439–449. [Google Scholar]
- Dincǎ, L.C.; Spârchez, G.; Dincǎ, M.; Blujdea, V.N.B. Organic carbon concentrations and stocks in Romanian mineral forest soils. Ann. For. Res. 2012, 55, 229–241. [Google Scholar]
- Dincǎ, L.; Spârchez, G.; Dincǎ, M. Romanian’s forest soils gis map and database and their ecological implications. Carpathian J. Earth Environ. Sci. 2014, 9, 133–142. [Google Scholar]
- Marin, G.; Bouriaud, O.; Nitu, D.M.; Calota, C.I.; Dumitru, M. Inventarul Forestier National din Romania. Ciclul I (2008–2012); Editura Silvica: Voluntati, Romania, 2019. [Google Scholar]
- Bouriaud, O.; Marin, G.; Hervé, J.-C.; Riedel, T.; Lanz, A. Estimation Methods in the Romanian National Forest Inventory; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2020. [Google Scholar]
- Blujdea, V.N.B. Raport Final la Contractul 88/2014 MMSC Privind Privind Administrarea Sectorului Folosinţa Terenurilor, Schimbarea Folosinţei Terenurilor şi Silvicultură al INEGES (CRF Sector 4) în Acord cu Obligaţiile sub Convenţia Cadru a Naţiunilor Unite Asupra Schim; ICAS: Bucuresti, Romania, 2014. [Google Scholar]
- Tuomi, M.; Laiho, R.; Repo, A.; Liski, J. Wood decomposition model for boreal forests. Ecol. Model. 2011, 222, 709–718. [Google Scholar] [CrossRef]
- Järvenpä;ä;, M.; Repo, A.; Akujä;rvi, A.; Kaasalainen, M.; Liski, J. Soil Carbon Model Yasso15—Bayesian Calibration Using Worldwide Litter Decomposition and Carbon Stock Data. In Geosci. Model Dev.; 2018; Manuscript in preparation. [Google Scholar]
- IFN Rezultate IFN—Ciclul II. Available online: http://roifn.ro/site/rezultate-ifn-2/ (accessed on 16 June 2021).
- ICAS. ICAS Instructiuni Privind Culegerea Datelor de Teren Pentru Inventarul Forestier National; ICAS: Bucuresti, Romania, 2008. [Google Scholar]
- Hengl, T.; De Jesus, J.M.; MacMillan, R.A.; Batjes, N.H.; Heuvelink, G.B.M.; Ribeiro, E.; Samuel-Rosa, A.; Kempen, B.; Leenaars, J.G.; Walsh, M.G.; et al. SoilGrids1km—Global Soil Information Based on Automated Mapping. PLoS ONE 2014, 9, e105992. [Google Scholar] [CrossRef] [PubMed]
- Jandl, R.; Ledermann, T.; Kindermann, G.; Freudenschuss, A.; Gschwantner, T.; Weiss, P. Strategies for Climate-Smart Forest Management in Austria. Forests 2018, 9, 592. [Google Scholar] [CrossRef]
- Birsan, A.; Dumitrescu, M.-V. ROCADA: A gridded daily climatic dataset over Romania (1961–2013) for nine meteorological variables. Nat. Hazards 2015, 78, 1045–1063. [Google Scholar]
- Hălălișan, A.-F.; Nicorescu, A.-I.; Popa, B.; Neykov, N.; Marinescu, V.; Abrudan, I.V. The relationships between forestry sector standardization, market evolution and sustainability approaches in the communist and post-communist economies: The case of Romania. Not. Bot. Hortic. Agrobot. Cluj-Napoca 2020, 48, 1683–1698. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Snäll, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Fröberg, M.; Stendahl, J.; Philipson, C.; et al. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Commun. 2013, 4, 1340. [Google Scholar] [CrossRef]
- Adi, S.H.; Grunwald, S. Integrative environmental modeling of soil carbon fractions based on a new latent variable model approach. Sci. Total. Environ. 2020, 711, 134566. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Zhuang, Q.; He, N. Investigating the spatio-temporal variability of soil organic carbon stocks in different ecosystems of China. Sci. Total. Environ. 2021, 758, 143644. [Google Scholar] [CrossRef] [PubMed]
- Hernández, L.; Jandl, R.; Blujdea, V.N.; Lehtonen, A.; Kriiska, K.; Alberdi, I.; Adermann, V.; Cañellas, I.; Marin, G.; Moreno-Fernández, D.; et al. Towards complete and harmonized assessment of soil carbon stocks and balance in forests: The ability of the Yasso07 model across a wide gradient of climatic and forest conditions in Europe. Sci. Total. Environ. 2017, 599, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- MMAP. Raport Privind Starea Pădurilor României în Anul 2010; Ministerul Mediului, Apelor si Padurilor: Bucuresti, Romania, 2011. [Google Scholar]
- Mayer, M.; Prescott, C.E.; Abaker, W.E.A.; Augusto, L.; Cécillon, L.; Ferreira, G.W.D.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.P.; et al. Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. For. Ecol. Manag. 2020, 466, 118127. [Google Scholar] [CrossRef]
- Achat, D.L.; Fortin, M.; Landmann, G.; Ringeval, B.; Augusto, L. Forest soil carbon is threatened by intensive biomass har-vesting. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kauppi, P.; Hanewinkel, M.; Lundmark, T.; Nabuurs, G.; Peltola, H.; Trasobares, A.; Hetemäki, L. Climate Smart Forestry in Europe; European Forest Institute: Joensuu, Finland, 2018. [Google Scholar]
- Mao, Z.; Derrien, D.; Didion, M.; Liski, J.; Eglin, T.; Nicolas, M.; Jonard, M.; Saint-André, L. Modeling soil organic carbon dynamics in temperate forests with Yasso07. Biogeosciences 2019, 16, 1955–1973. [Google Scholar] [CrossRef]
- Didion, M.; Blujdea, V.; Grassi, G.; Hernández, L.; Jandl, R.; Kriiska, K.; Lehtonen, A.; Saint-André, L. Models for reporting forest litter and soil C pools in national greenhouse gas inventories: Methodological considerations and requirements. Carbon Manag. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Canarache, A. Fizica Solurilor Agricole; Ceres: Bucuresti, Romania, 1990. [Google Scholar]
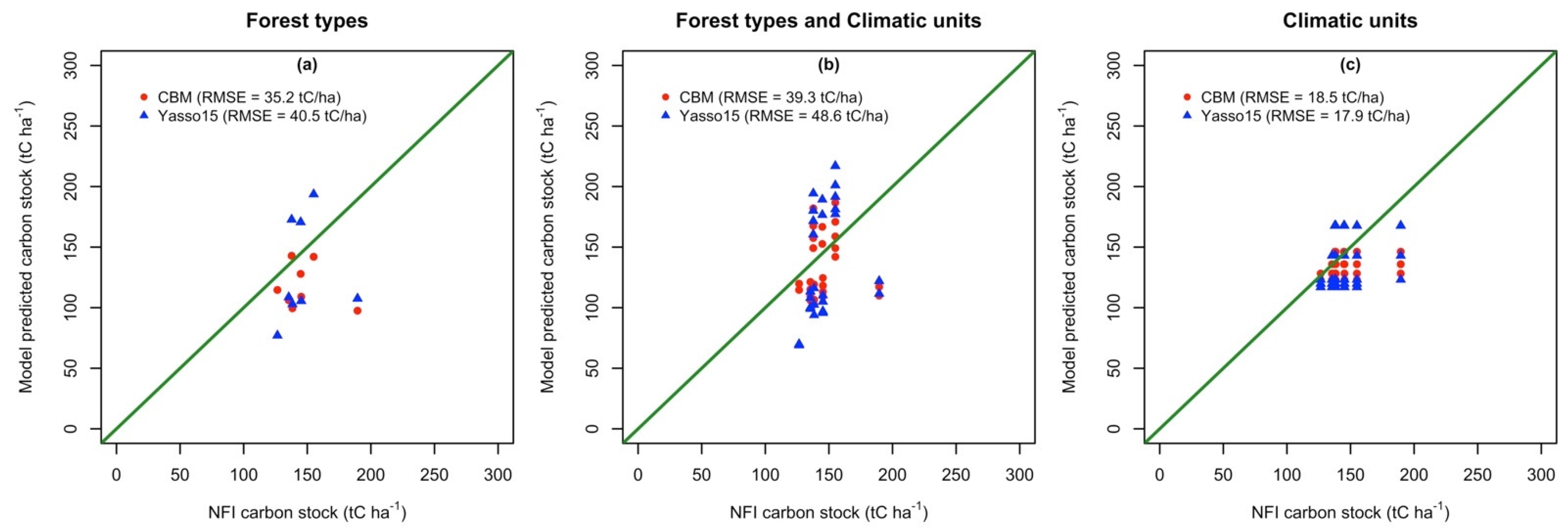
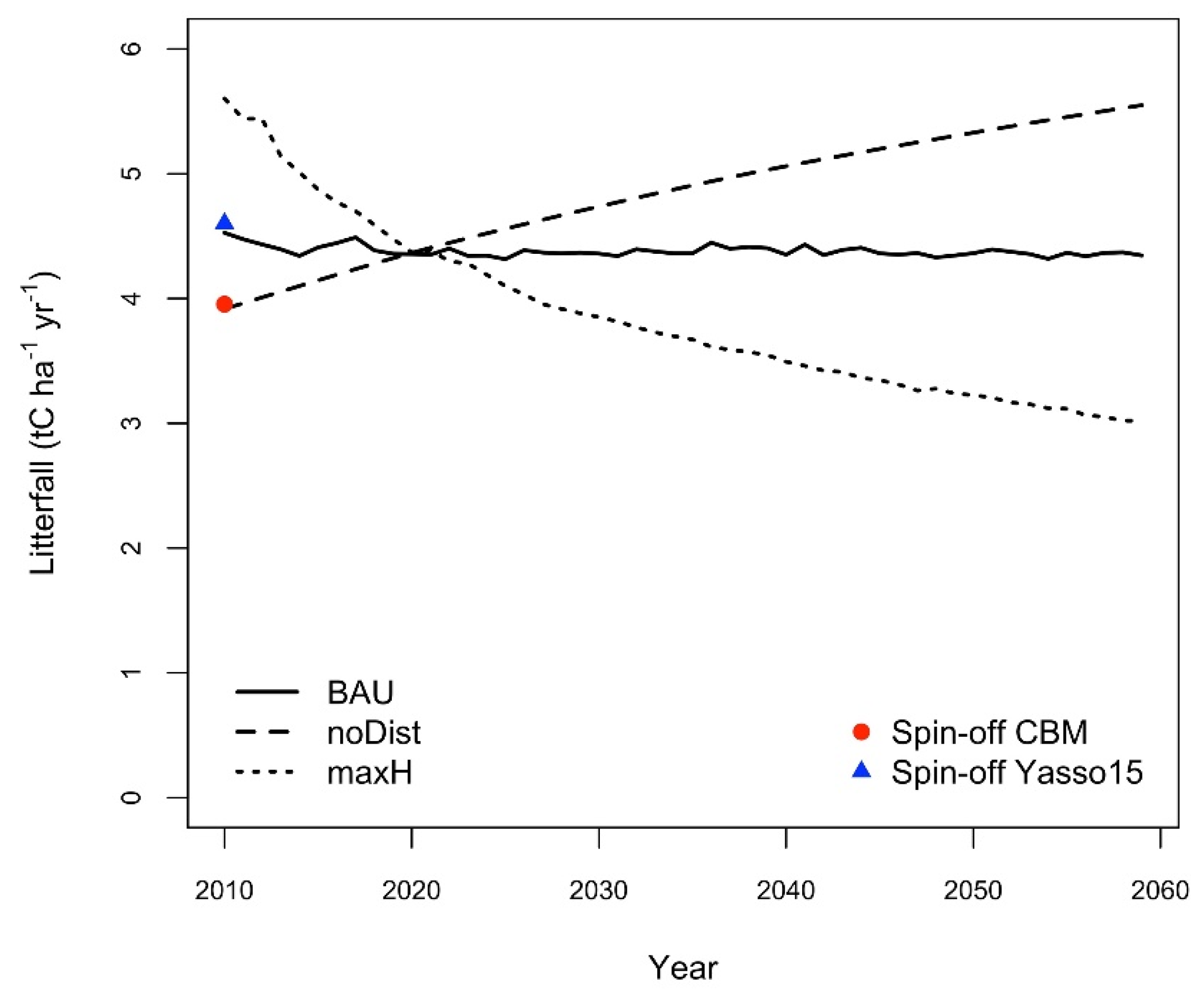

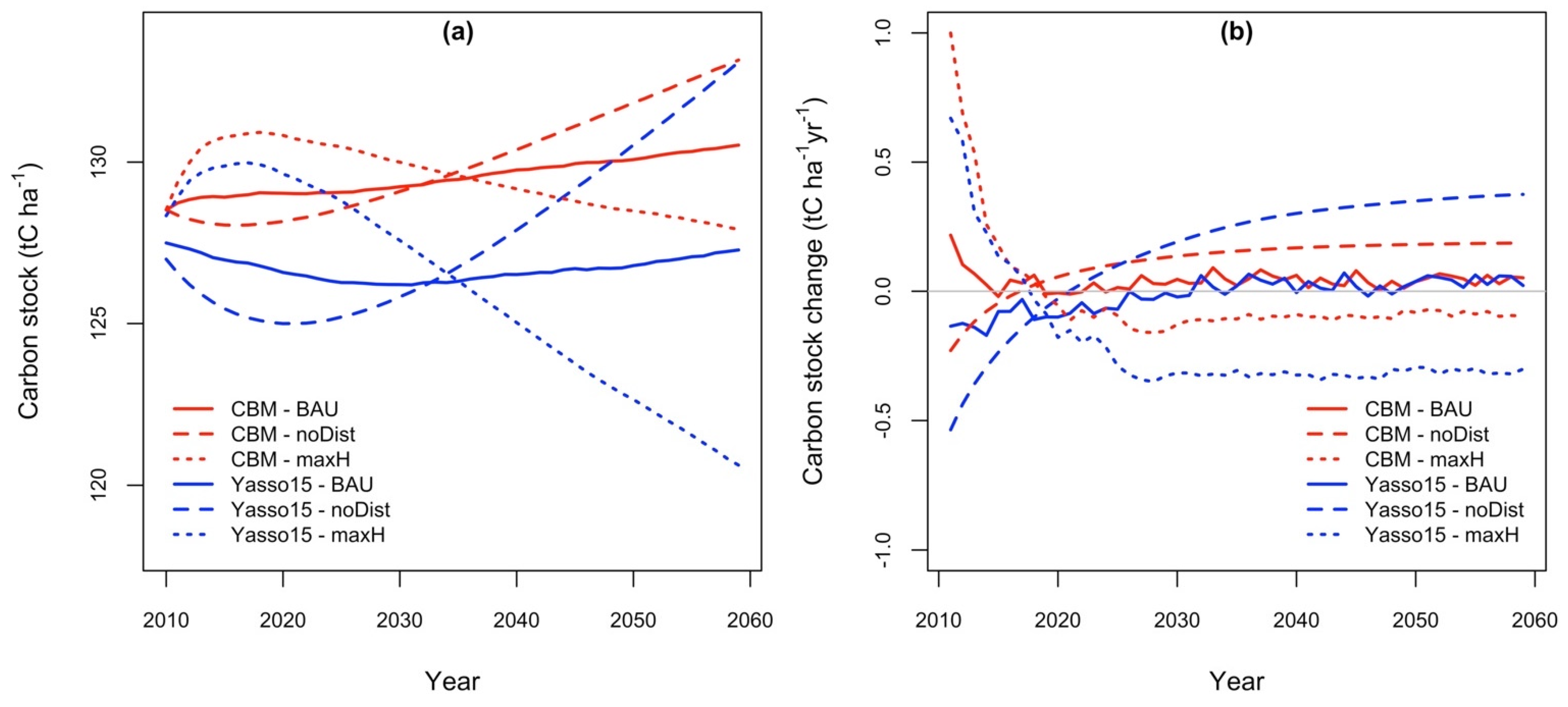
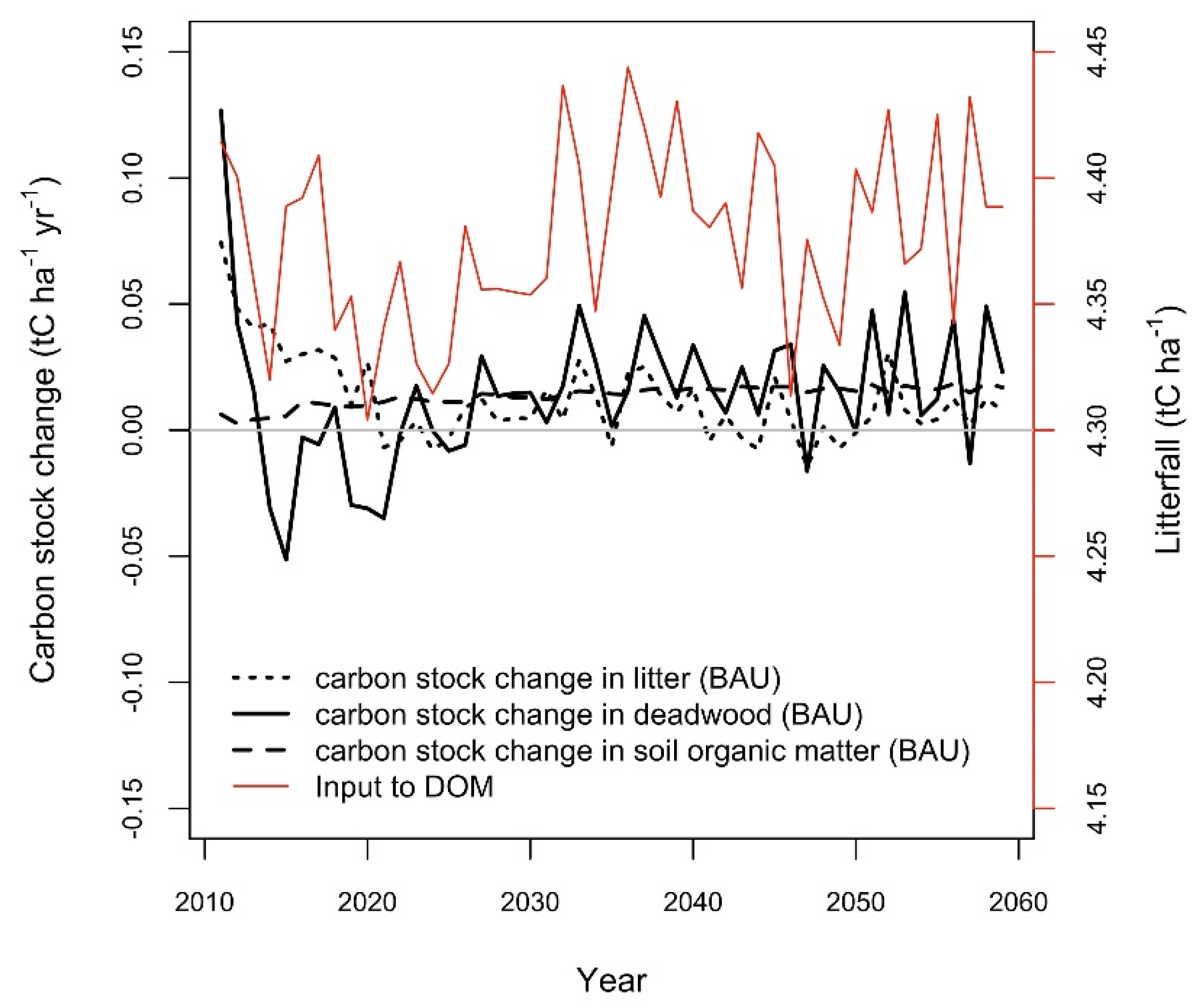
| Abbrev | Forest Types (The Share of Main Tree Species) | Area (ha) |
|---|---|---|
| FS | Fagus sylvatica (>90% beech) | 914,359 |
| PA | Picea abies (>90% Norway spruce) | 674,483 |
| QR | Quercus sp. (all oak species) | 505,508 |
| RP | Robinia pseudoacacia (black locust) | 123,069 |
| OB | Other broadleaved (>90% broadleaved species) | 2,668,032 |
| OC | Other coniferous (including Abies alba, silver fir, >90% coniferous species) | 32,861 |
| ConBroad | Mixed coniferous and broadleaved species | 527,284 |
| PreCon | Predominantly coniferous (>70% coniferous species) | 330,923 |
| CLU Code | Tm | Tmax | Tmin | Tamp | Precipitation |
|---|---|---|---|---|---|
| 44 | 4.7 | 19.3 | −9.6 | 14.4 | 886.3 |
| 35 | 6.7 | 22.0 | −8.4 | 15.2 | 823.1 |
| 34 | 8.3 | 24.2 | −7.4 | 15.8 | 751.7 |
| 26 | 9.8 | 26.2 | −5.7 | 15.9 | 748.7 |
| 25 | 11.0 | 27.7 | −4.6 | 16.2 | 678.2 |
| Source | Pool | PA | ConBroad | FS | QR | OC | OB | PredCon | RP |
|---|---|---|---|---|---|---|---|---|---|
| NFI | SOM | 131.1–195.3 | 103.9–149.2 | 113.1–158 | 101–158.8 | 89.9–139.3 | 117.6–169.9 | 131.4–138 | 120.5–129.9 |
| LT | 8.1 | 4.5 | 4 | 2.9 | 5.2 | 1.6 | 4.7 | 2.2 | |
| DW | 0.6–1.6 | 0.7–1.7 | 0.5–1.2 | 0.1–0.4 | 0.2–2.4 | 0.1–2.3 | 0.5–2.1 | 0.2–1.1 | |
| Total | 135–205 | 108–155 | 117–163 | 104–162 | 95–145 | 119–172 | 137–143 | 123–128 | |
| CBM | SOM | 88.4–100.8 | 124–153.7 | 126.7–151.9 | 96.4–106.3 | 90.4–103.2 | 100–110.3 | 113.8–139.9 | 104.7–111.3 |
| LT | 5.7–11.6 | 14–28 | 11.8–23.6 | 7.2–11.7 | 5.7–11.5 | 6.5–10.8 | 11.5–23.2 | 5.6–8 | |
| DW | 3.4–4.9 | 4–5.1 | 4.4–6.5 | 2.6–3.4 | 3.4–4.7 | 2.6–3.5 | 2.7–3.6 | 4.3–5.5 | |
| Total | 97–117 | 142–187 | 143–182 | 106–121 | 99–119 | 109–125 | 128–167 | 115–125 |
| Scenario | Litterfall Origin | Merchantable Standing Stock | Other Woody Compartments | Foliage | Fine Roots | Coarse Roots |
|---|---|---|---|---|---|---|
| Spin-off | Natural turnovers | 8 (2–17)% | 12 (4–25)% | 38 (3–58)% | 30 (19–52)% | 12 (7–22)% |
| BAU | Natural turnovers Forest operations residues | 6 (2–11)% 15% | 16 (4–40)% 16% | 28 (2–55)% 37% | 36 (17–50)% 13% | 16 (9–33)% 39% |
| maxH | Natural turnovers Forest operations residues | 5 (2–13)% 25% | 19 (1–48)% 29% | 24 (1–50)% 24% | 27 (14–53)% 11% | 25 (12–52)% 45% |
| noDist | Natural turnovers Forest operations residues | 7 (2–11)% NA | 14 (4–24)% NA | 36 (7–59)% NA | 30 (20–52)% NA | 13 (7–21)% NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blujdea, V.N.B.; Viskari, T.; Kulmala, L.; Gârbacea, G.; Dutcă, I.; Miclăuș, M.; Marin, G.; Liski, J. Silvicultural Interventions Drive the Changes in Soil Organic Carbon in Romanian Forests According to Two Model Simulations. Forests 2021, 12, 795. https://doi.org/10.3390/f12060795
Blujdea VNB, Viskari T, Kulmala L, Gârbacea G, Dutcă I, Miclăuș M, Marin G, Liski J. Silvicultural Interventions Drive the Changes in Soil Organic Carbon in Romanian Forests According to Two Model Simulations. Forests. 2021; 12(6):795. https://doi.org/10.3390/f12060795
Chicago/Turabian StyleBlujdea, Viorel N. B., Toni Viskari, Liisa Kulmala, George Gârbacea, Ioan Dutcă, Mihaela Miclăuș, Gheorghe Marin, and Jari Liski. 2021. "Silvicultural Interventions Drive the Changes in Soil Organic Carbon in Romanian Forests According to Two Model Simulations" Forests 12, no. 6: 795. https://doi.org/10.3390/f12060795
APA StyleBlujdea, V. N. B., Viskari, T., Kulmala, L., Gârbacea, G., Dutcă, I., Miclăuș, M., Marin, G., & Liski, J. (2021). Silvicultural Interventions Drive the Changes in Soil Organic Carbon in Romanian Forests According to Two Model Simulations. Forests, 12(6), 795. https://doi.org/10.3390/f12060795






