The Use of Subsidence to Estimate Carbon Loss from Deforested and Drained Tropical Peatlands in Indonesia
Abstract
1. Introduction
2. Methods
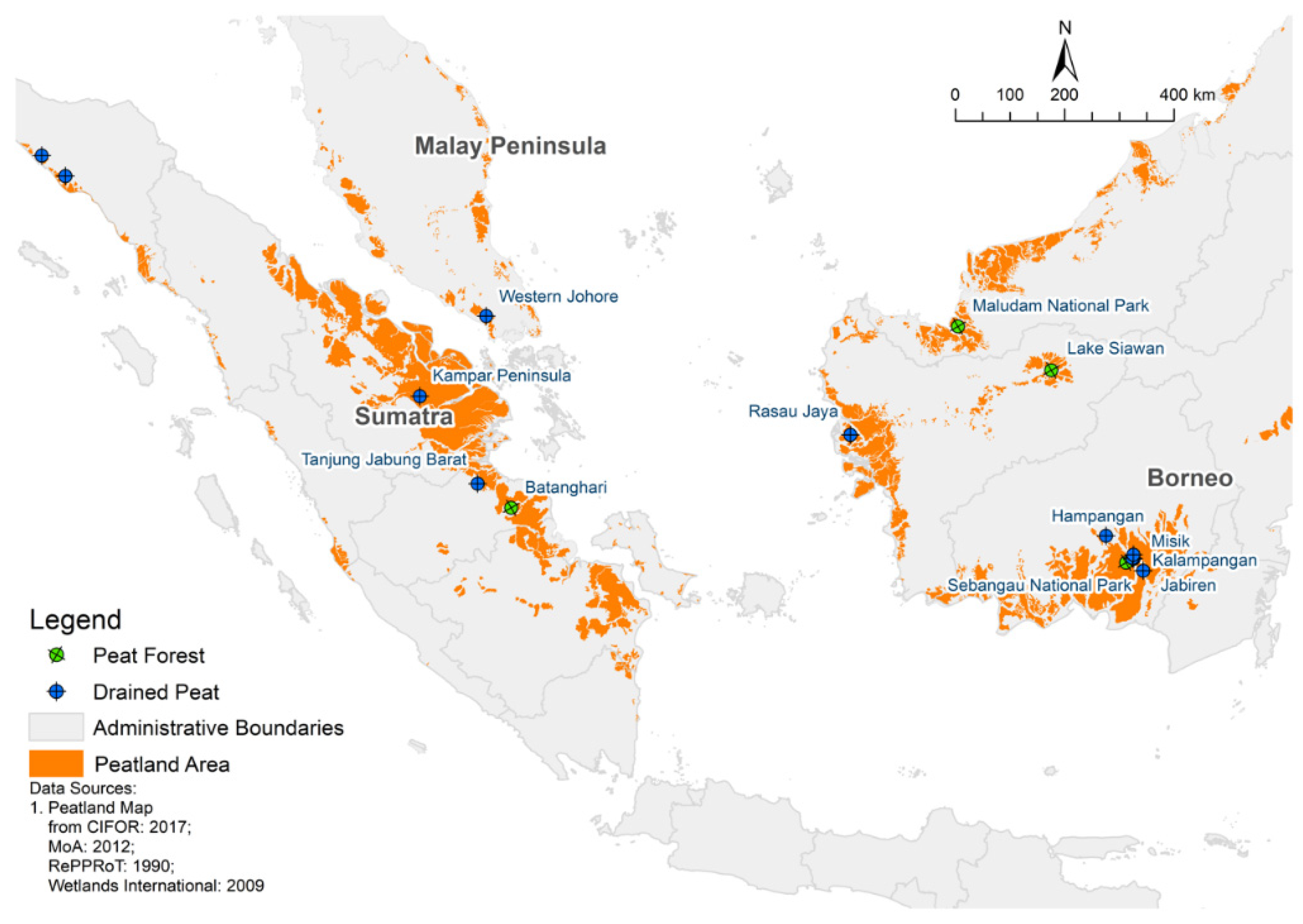
3. Distribution, Vegetation Formation and Uniqueness of Tropical Peat
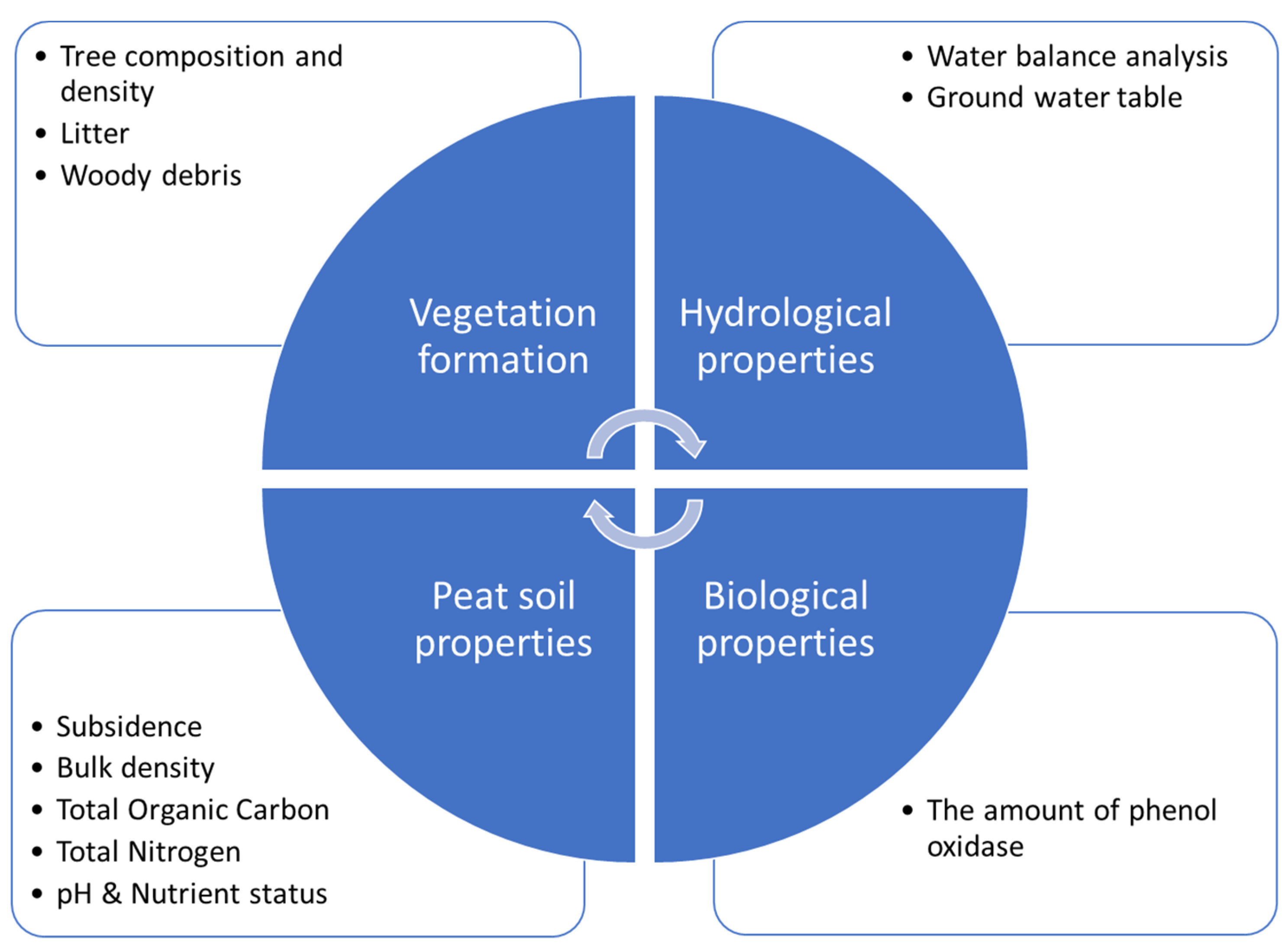
4. Selected Peat Properties Affecting Carbon Stock
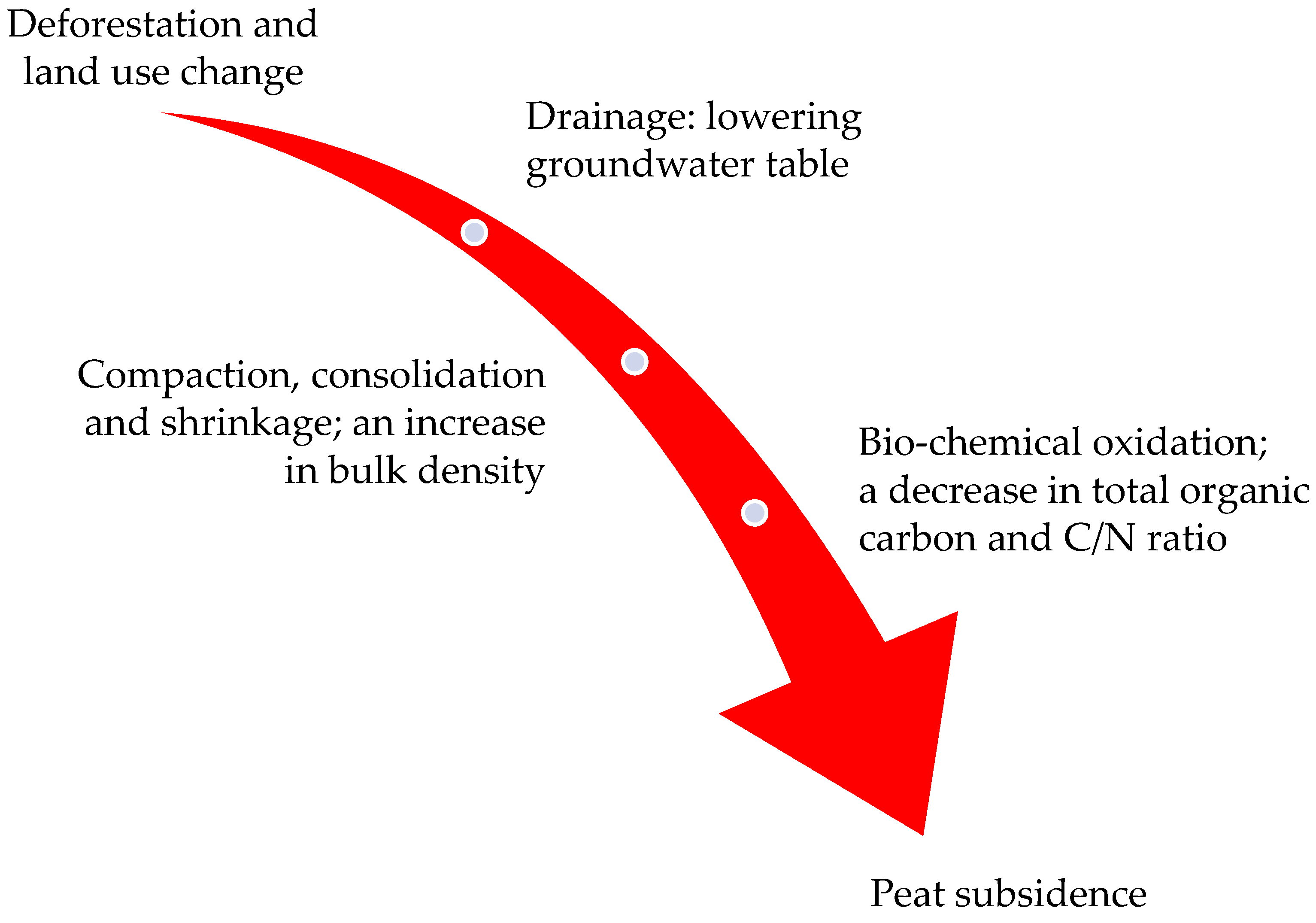
5. Peat Degradation
6. Subsidence
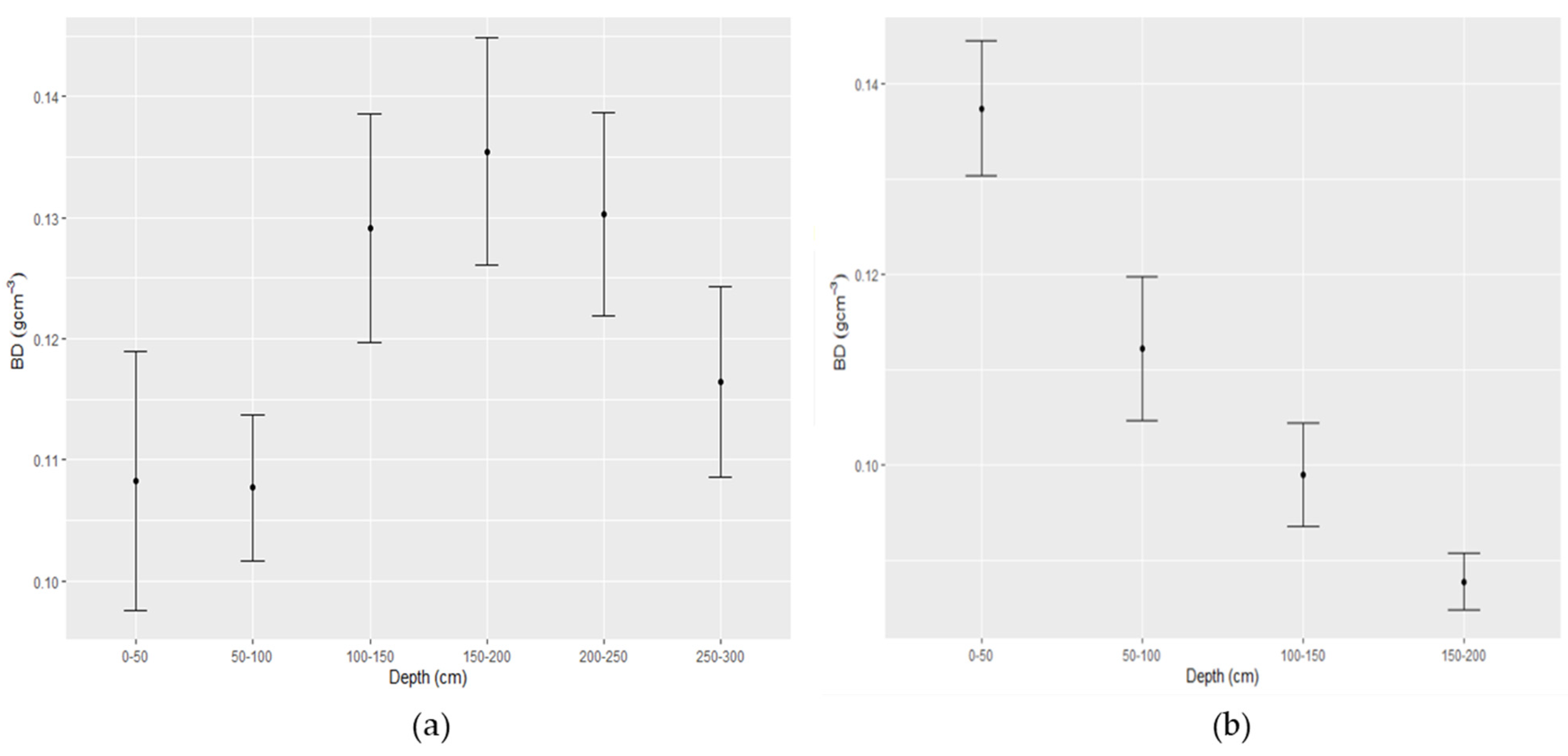
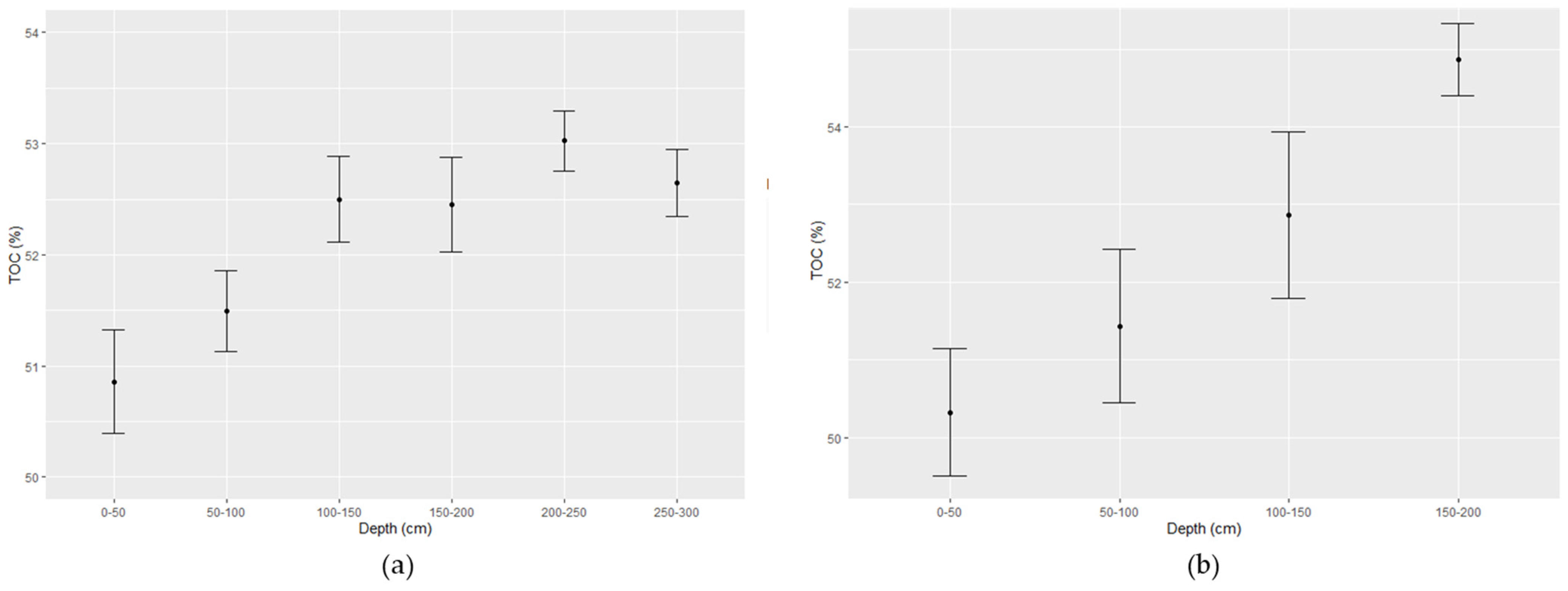
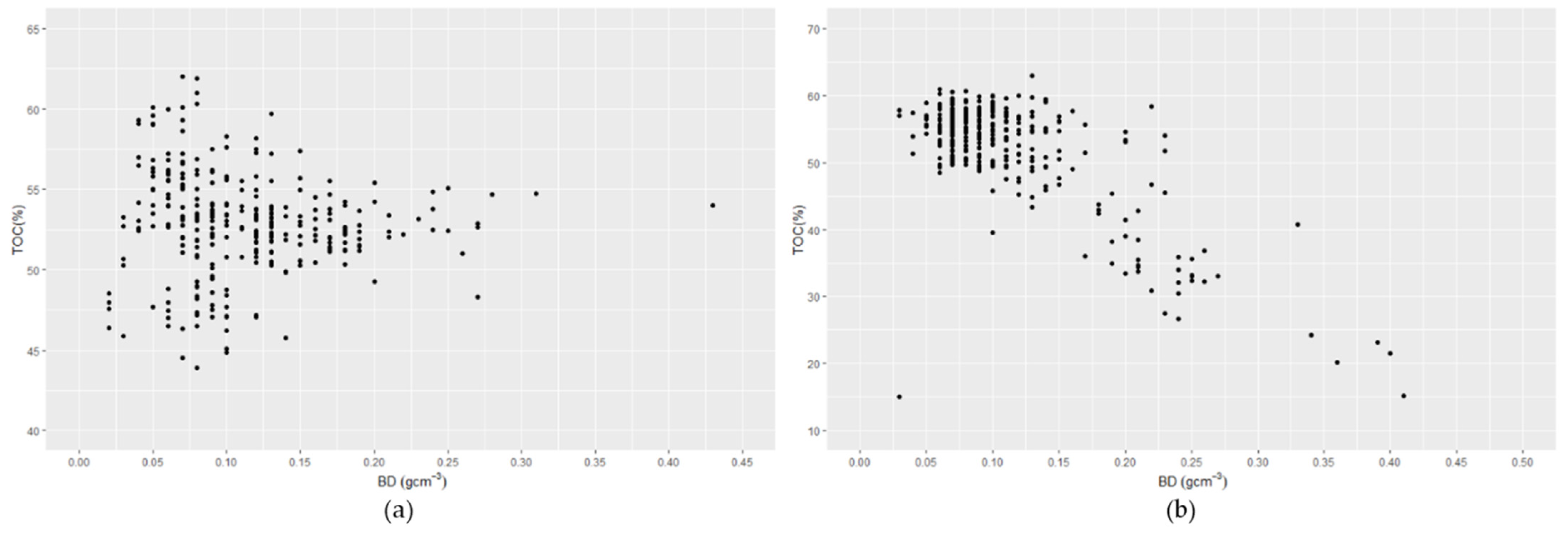
7. Profiles of Bulk Density and Total Organic Carbon
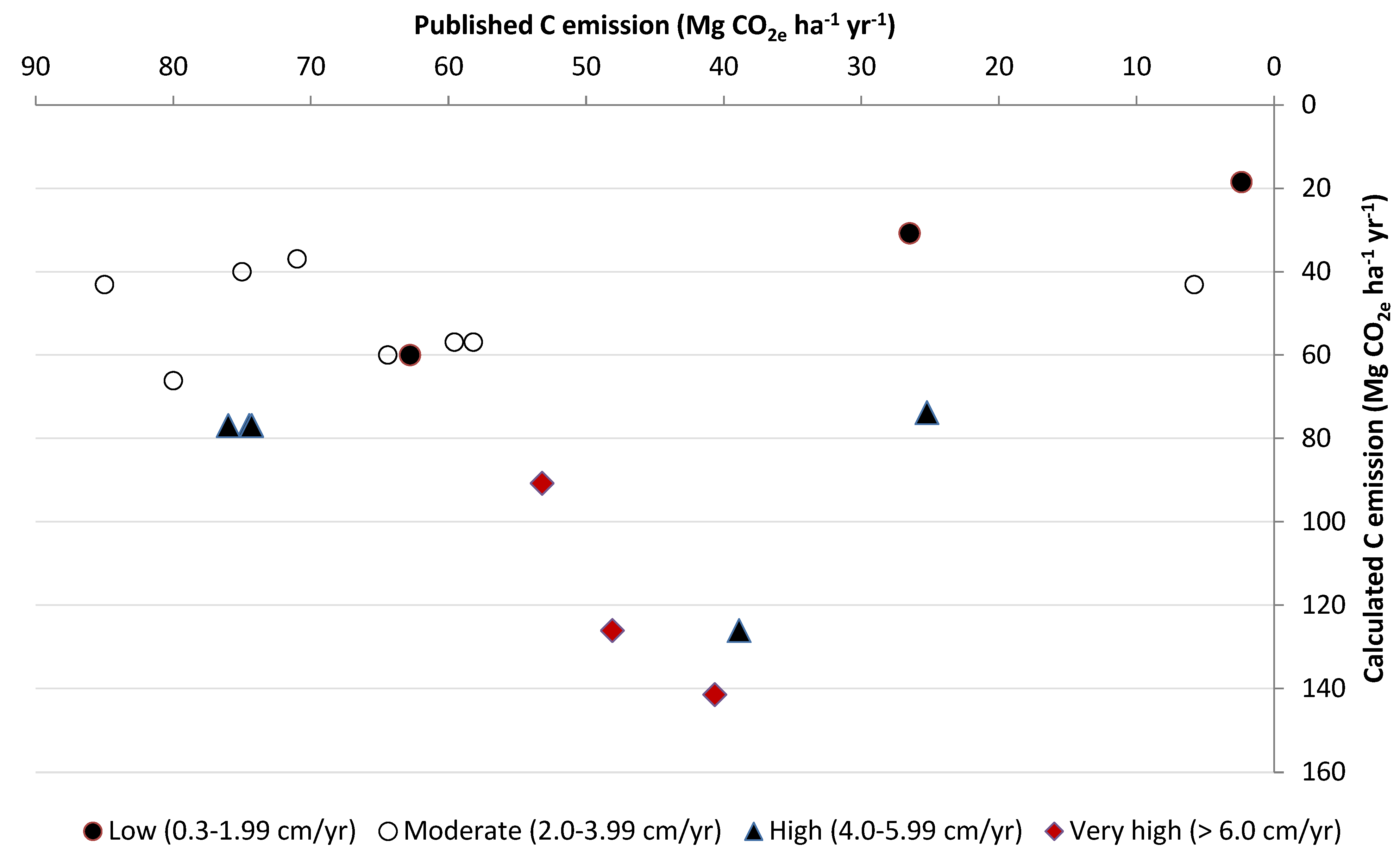
8. Carbon Loss Estimate
| No | Subsidence Class | Subsidence Rate (cm yr−1) | Period of Drainage (yrs) | C Emission (Mg CO2e ha−1 yr−1) | Land Use | Location | Reference | |
|---|---|---|---|---|---|---|---|---|
| Published C Emission | This Study | |||||||
| 1 | Low (0.3–1.99 cm yr−1) | 1.10 | 15.00 | 4.34 | 16.91 | Community oil palm plantation | Aceh Province | [97] |
| 2 | Low (0.3–1.99 cm yr−1) | 1.20 | 15.00 | 2.39 | 18.45 | Community rubber plantation | Aceh Province | [97] |
| 3 | Low (0.3–1.99 cm yr−1) | 2.00 | 20.00 | 26.50 | 30.74 | Agriculture | Western Johor, Malaysia | [8] |
| 4 | Low (0.3–1.99 cm yr−1) | 0.40 | 36.00 | - | 6.15 | Agriculture | Kalampangan, Central Kalimantan | [98] |
| 5 | Low (0.3–1.99 cm yr−1) | 0.36 | 12.00 | - | 5.53 | Community oil palm plantation | Hampangan, Central Kalimantan | [98] |
| 6 | Moderate (2.0–3.99 cm yr−1) | 3.90 | 5.00 | 62.81 | 59.95 | Oil palm | Indonesia | [10] |
| 7 | Moderate (2.0–3.99 cm yr−1) | 3.70 | 19.00 | 59.58 | 56.88 | Oil palm | Indonesia | [10] |
| 8 | Moderate (2.0–3.99 cm yr−1) | 3.90 | 12.00 | 64.42 | 59.95 | Oil palm | Kampar Peninsula, Riau, Sumatra | [99] |
| 9 | Moderate (2.0–3.99 cm yr−1) | 3.70 | 12.00 | 58.19 | 56.88 | Oil palm | Kampar Peninsula, Riau, Sumatra | [99] |
| 10 | Moderate (2.0–3.99 cm yr−1) | 2.80 | 15.00 | 5.82 | 43.04 | Community rubber plantation | Aceh Province | [97] |
| 11 | Moderate (2.0–3.99 cm yr−1) | 2.60 | 30.00 | 75.00 | 39.97 | Community rubber plantation | Tanjung Jabung Barat, Jambi Province | [72] |
| 12 | Moderate (2.0–3.99 cm yr−1) | 2.40 | 20.00 | 71.00 | 36.89 | Mixed agriculture | Tanjung Jabung Barat, Jambi Province | [72] |
| 13 | Moderate (2.0–3.99 cm yr−1) | 2.80 | 40.00 | 85.00 | 43.04 | Mixed agriculture | Tanjung Jabung Barat, Jambi Province | [72] |
| 14 | Moderate (2.0–3.99 cm yr−1) | 2.20 | 10.00 | - | 33.82 | All agricultural drained peat | Southeast Asia | [63] |
| 15 | Moderate (2.0–3.99 cm yr−1) | 3.21 | 6.00 | - | 49.34 | Agriculture | Misik, Central Kalimantan | [98] |
| 16 | High (4.0–5.99 cm yr−1) | 4.30 | 24.00 | 80.00 | 66.10 | Acacia plantation | Riau, Sumatra | [64] |
| 17 | High (4.0–5.99 cm yr−1) | 5.00 | 6.00 | 74.48 | 76.86 | Acacia plantation | Indonesia | [10] |
| 18 | High (4.0–5.99 cm yr−1) | 5.00 | 14.00 | 74.30 | 76.86 | Acacia plantation | Kampar Peninsula, Riau, Sumatra | [99] |
| 19 | High (4.0–5.99 cm yr−1) | 4.80 | 15.00 | 25.23 | 73.79 | Community oil palm plantation | Aceh Province | [97] |
| 20 | High (4.0–5.99 cm yr−1) | 5.90 | 20.00 | 53.20 | 90.69 | Community rubber plantation | Central Kalimantan | [56] |
| 21 | High (4.0–5.99 cm yr−1) | 5.00 | 25.00 | 76.00 | 76.86 | Acacia and oil palm | Riau and Jambi | [99] |
| 22 | High (4.0–5.99 cm yr−1) | 5.27 | 6.00 | - | 81.01 | Community oil palm plantation | Misik, Central Kalimantan | [98] |
| 23 | Very High (>6.0 cm yr−1) | 8.20 | 10.00 | 38.88 | 126.05 | Community oil palm plantation | Aceh Province | [97] |
| 24 | Very High (>6.0 cm yr−1) | 9.20 | 10.00 | 40.64 | 141.42 | Community oil palm plantation | Aceh Province | [97] |
| 25 | Very High (>6.0 cm yr−1) | 8.20 | 1.00 | 48.09 | 126.05 | Community oil palm plantation | Aceh Province | [97] |
| Min | 0.36 | 1.00 | 2.39 | 5.53 | ||||
| Max | 9.20 | 40.00 | 85.00 | 141.42 | ||||
| Mean | 3.89 | 15.92 | 51.29 | 59.73 | ||||
9. Assessment of Peat Subsidence and C Emission
10. Policy Implication
11. Conclusion and Recommendation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooijer, A.; Page, S.; Navratil, P.; Vernimmen, R.; van der Vat, M.; Tansey, K.; Konecny, K.; Siegert, F.; Ballhorn, U.; Mawdsley, N. Carbon Emissions from Drained and Degraded Peatland in Indonesia and Emission Factors for Measurement, Reporting and Verification (MRV) of Peatland Greenhouse Gas Emissions—A Summary of KFCP Research Results for Practioners; IAFCP: Jakarta, Indonesia, 2014. [Google Scholar]
- Jauhiainen, J.; Hooijer, A.; Page, S.E. Carbon dioxide emissions from an Acacia plantation on peatland in Sumatra, Indonesia. Biogeosciences 2012, 9, 617–630. [Google Scholar] [CrossRef]
- Miettinen, J.; Wang, J.; Hooijer, A.; Liew, S. Peatland Conversion and Degradation Processes in Insular Southeast Asia: A Case Study in Jambi, Indonesia. Land Degrad. Dev. 2013, 24, 334–341. [Google Scholar] [CrossRef]
- Hooijer, A.; Page, S.; Jauhiainen, J.; Lee, W.A.; Lu, X.X.; Idris, A.B.; Anshari, G. Subsidence and carbon loss in drained tropical peatlands. Biogeosciences 2012, 9, 1053–1071. [Google Scholar] [CrossRef]
- Mishra, S.R.; Lee, W.A.; Hooijer, A.; Reuben, S.; Sudiana, I.M.; Idris, A.; Swarup, S. Microbial and metabolic profiling reveal strong influence of water table and land-use patterns on classification of degraded tropical peatlands. Biogeosciences 2014, 11, 1727–1741. [Google Scholar] [CrossRef]
- Agus, F.; Henson, I.E.; Sahardjo, B.H.; Harris, N.; van Noordwijk, M.; Killeen, T. Review of Emission Factors for Assessment of CO2 Emission from Land Use Change to Oil Palm in Southeast Asia, Kualalumpur. 2013. Available online: http://www.rspo.org/file/GHGWG2/3_review_of_emission_factors_Agus_et_al.pdf (accessed on 16 April 2019).
- Regina, K.; Sheehy, J.; Myllys, M. Mitigating greenhouse gas fluxes from cultivated organic soils with raised water table. Mitig. Adapt. Strat. Glob. Chang. 2014, 20, 1529–1544. [Google Scholar] [CrossRef]
- Wösten, J.; Ismail, A.; van Wijk, A. Peat subsidence and its practical implications: A case study in Malaysia. Geoderma 1997, 78, 25–36. [Google Scholar] [CrossRef]
- Wösten, J.H.M.; Berg, J.V.D.; Van Eijk, P.; Gevers, G.J.M.; Giesen, W.B.J.T.; Hooijer, A.; Idris, A.; Leenman, P.H.; Rais, D.S.; Siderius, C.; et al. Interrelationships between Hydrology and Ecology in Fire Degraded Tropical Peat Swamp Forests. Int. J. Water Resour. Dev. 2006, 22, 157–174. [Google Scholar] [CrossRef]
- Couwenberg, J.; Hooijer, A. Towards Robust Subsidence-Based Soil Carbon Emission Factors for Peat Soils in SouthEast ASIA, with Special Reference to Oil Palm Plantations. Mires Peat 2013, 12, 1–13. Available online: http://pixelrauschen.de/wbmp/media/map12/map_12_01.pdf (accessed on 7 April 2015).
- Gusmayanti, E.; Anshari, G.; Pramulya, M.; Ruliyansyah, A. CO2 fluxes from drained tropical peatland used for oil palm plantation in relation to peat characteristics and crop age after planting. Biodiversitas J. Biol. Divers. 2019, 20, 1650–1657. [Google Scholar] [CrossRef]
- Nurzakiah, S.; Wakhid, N.; Hairani, A. Carbon dioxide emission and peat hydrophobicity in tidal peatlands. SAINS TANAH-J. Soil Sci. Agroclimatol. 2020, 17, 71–77. [Google Scholar] [CrossRef]
- Kimura, S.D.; Melling, L.; Goh, K.J. Influence of soil aggregate size on greenhouse gas emission and uptake rate from tropical peat soil in forest and different oil palm development years. Geoderma 2012, 185–186, 1–5. [Google Scholar] [CrossRef]
- Girkin, N.; Turner, B.L.; Ostle, N.; Craigon, J.; Sjögersten, S. Root exudate analogues accelerate CO2 and CH4 production in tropical peat. Soil Biol. Biochem. 2018, 117, 48–55. [Google Scholar] [CrossRef]
- Bader, C.; Müller, M.; Schulin, R.; Leifeld, J. Peat decomposability in managed organic soils in relation to land use, organic matter composition and temperature. Biogeosciences 2018, 15, 703–719. [Google Scholar] [CrossRef]
- Bourgeau-Chavez, L.L.; Endres, S.L.; Graham, J.A.; Hribljan, J.A.; Chimner, R.A.; Lillieskov, E.A.; Battaglia, M.J. Mapping peatlands in boreal and tropical ecoregions. In Comprehensive Remote Sensing, 1st ed.; Liang, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 6, pp. 24–44. [Google Scholar]
- Xu, J.; Morris, P.J.; Liu, J.; Holden, J. PEATMAP: Refining estimates of global peatland distribution based on a meta-analysis. Catena 2018, 160, 134–140. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2014. [Google Scholar]
- Householder, J.; Wittmann, F.; Tobler, M.; Janovec, J. Montane bias in lowland Amazonian peatlands: Plant assembly on heterogeneous landscapes and potential significance to palynological inference. Palaeogeogr. Palaeoclim. Palaeoecol. 2015, 423, 138–148. [Google Scholar] [CrossRef]
- Hope, G.S. Peat in the Mountains of New Guinea. Mires Peat 2015, 15, 1–21. [Google Scholar]
- Andriesse, J.P. Nature and Management of Tropical Peat Soils; FAO Soil Bulletin 59: Rome, Italy, 1988. [Google Scholar]
- Page, S.; Rieley, J.O.; Banks, C.J. Global and regional importance of the tropical peatland carbon pool. Glob. Chang. Biol. 2011, 17, 798–818. [Google Scholar] [CrossRef]
- Rieley, J.; Page, S. Tropical Peatland of the World, in Tropical Peatland Ecosystems; Osaki, M., Tsuji, N., Eds.; Springer: Tokyo, Japan, 2016. [Google Scholar]
- Dargie, G.C.; Lawson, I.T.; Rayden, T.J.; Miles, L.; Mitchard, E.T.A.; Page, S.E.; Bocko, Y.E.; Ifo, S.A.; Lewis, S.L. Congo Basin peatlands: Threats and conservation priorities. Mitig. Adapt. Strat. Glob. Chang. 2019, 24, 669–686. [Google Scholar] [CrossRef]
- Page, S.E.; Banks, C.J.; Rieley, J.O. Tropical Peatlands: Distribution, Extent and Carbon Storage—Uncertainties and Knowledge Gaps. Peatl. Int. 2007, 2, 26–27. [Google Scholar]
- Gumbricht, T.; Roman-Cuesta, R.M.; Verchot, L.; Herold, M.; Wittmann, F.; Householder, E.; Herold, N.; Murdiyarso, D. An expert system model for mapping tropical wetlands and peatlands reveals South America as the largest contributor. Glob. Chang. Biol. 2017, 23, 3581–3599. [Google Scholar] [CrossRef]
- Lampela, M.; Jauhiainen, J.; Kämäri, I.; Koskinen, M.; Tanhuanpää, T.; Valkeapää, A.; Vasander, H. Ground surface microtopography and vegetation patterns in a tropical peat swamp forest. Catena 2016, 139, 127–136. [Google Scholar] [CrossRef]
- Lampela, M.; Jauhiainen, J.; Vasander, H. Surface peat structure and chemistry in a tropical peat swamp forest. Plant Soil 2014, 382, 329–347. [Google Scholar] [CrossRef]
- Zweig, C.L.; Newman, S.; Saunders, C.J.; Sklar, F.H.; Kitchens, W.M. Deviations on a theme: Peat patterning in sub-tropical landscapes. Ecol. Model. 2018, 371, 25–36. [Google Scholar] [CrossRef]
- Anderson, J.A.R. The Flora of the Peat Swamp Forest of Sarawak and Brunei, including a Catalogue of all Recorded Species of Flowering Plants, Ferns, and Fern Allies; Singapore Gardens Bulletin: Singapore, 1963; Volume 20, pp. 131–228. [Google Scholar]
- Anderson, J.; Muller, J. Palynological study of a holocene peat and a miocene coal deposit from NW Borneo. Rev. Palaeobot. Palynol. 1975, 19, 291–351. [Google Scholar] [CrossRef]
- Page, S.E.; Rieley, J.O.; Shotyk, Ø.W.; Weiss, D. Interdependence of peat and vegetation in a tropical peat swamp forest. Philos. Trans. R. Soc. B Biol. Sci. 1999, 354, 1885–1897. [Google Scholar] [CrossRef]
- Kuniyasu, M.M.; Tetsuya, S.B. Environments and People of Sumatran Peat Swamp Forests I: Distribution and Typology of Vegetation. Jpn. J. Southeast Asian Stud. 2002, 40, 74–86. [Google Scholar]
- Gallego-Sala, A.V.; Charman, D.J.; Brewer, S.; Page, S.E.; Prentice, I.C.; Friedlingstein, P.; Moreton, S.; Amesbury, M.J.; Beilman, D.W.; Björck, S.; et al. Latitudinal limits to the predicted increase of the peatland carbon sink with warming. Nat. Clim. Chang. 2018, 8, 907–913. [Google Scholar] [CrossRef]
- Brady, M.A. Organic Matter Dynamis of Coastal Peat Deposits in Sumatra; British Columbia University: Vancouver, BC, Canada, 1997. [Google Scholar]
- Houghton, R.A.; House, J.I.; Pongratz, J.; Van Der Werf, G.R.; DeFries, R.S.; Hansen, M.C.; Le Quéré, C.; Ramankutty, N. Carbon emissions from land use and land-cover change. Biogeosciences 2012, 9, 5125–5142. [Google Scholar] [CrossRef]
- Loisel, J.; Gallego-Sala, A.V.; Amesbury, M.J.; Magnan, G.; Anshari, G.; Beilman, D.W.; Benavides, J.C.; Blewett, J.; Camill, P.; Charman, D.J.; et al. Expert assessment of future vulnerability of the global peatland carbon sink. Nat. Clim. Chang. 2021, 11, 70–77. [Google Scholar] [CrossRef]
- Hilasvuori, E.; Akujärvi, A.; Fritze, H.; Karhu, K.; Laiho, R.; Mäkiranta, P.; Oinonen, M.; Palonen, V.; Vanhala, P.; Liski, J. Temperature sensitivity of decomposition in a peat profile. Soil Biol. Biochem. 2013, 67, 47–54. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; Natural Resources Conservation Service: Washington, DC, USA, 2014; pp. 167–172.
- Subardja, D.; Ritung, S.; Anda, M.; Sukarman, E.S.; Subandiono, R.E. Petunjuk Teknis Klasifikasi Tanah Nasional, Edisi ke-2.: Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian, Badan Penelitian dan Pengembangan Pertanian; Balai Penelitian Tanah: Bogor, Indonesia, 2014. [Google Scholar]
- Horák-Terra, I.; Cortizas, A.M.; de Camargo, P.B.; Silva, A.C.; Vidal-Torrado, P. Characterization of properties and main processes related to the genesis and evolution of tropical mountain mires from Serra do Espinhaço Meridional, Minas Gerais, Brazil. Geoderma 2014, 232–234, 183–197. [Google Scholar] [CrossRef]
- Könönen, M.; Jauhiainen, J.; Laiho, R.; Kusin, K.; Vasander, H. Physical and chemical properties of tropical peat under stabilised land uses. Mires Peat 2015, 16, 1–13. [Google Scholar]
- Anshari, G.; Afifudin, M.; Nuriman, M.; Gusmayanti, E.; Arianie, L.; Susana, R.; Nusantara, R.W.; Sugardjito, J.; Rafiastanto, A. Drainage and land use impacts on changes in selected peat properties and peat degradation in West Kalimantan Province, Indonesia. Biogeosciences 2010, 7, 3403–3419. [Google Scholar] [CrossRef]
- Murtilakso, K.; Sabiham, S.; Sutandi, A.; Sutarta, E.S. Hydrophobicity of Tropical Peat Soil from an Oil Palm Plantation in North Sumatra. J. Agron. 2016, 15, 114–121. [Google Scholar] [CrossRef][Green Version]
- Sangok, F.E.; Maie, N.; Melling, L.; Watanabe, A. Evaluation on the decomposability of tropical forest peat soils after conversion to an oil palm plantation. Sci. Total. Environ. 2017, 587–588, 381–388. [Google Scholar] [CrossRef]
- Warren, M.W.; Kauffman, J.B.; Murdiyarso, D.; Anshari, G.; Hergoualc’h, K.; Kurnianto, S.; Purbopuspito, J.; Gusmayanti, E.; Afifudin, M.; Rahajoe, J.; et al. A cost-efficient method to assess carbon stocks in tropical peat soil. Biogeosciences 2012, 9, 4477–4485. [Google Scholar] [CrossRef]
- Nurulita, Y.; Adetutu, E.M.; Kadali, K.K.; Zul, D.; Mansur, A.A.; Ball, A.S. The assessment of the impact of oil palm and rubber plantations on the biotic and abiotic properties of tropical peat swamp soil in Indonesia. Int. J. Agric. Sustain. 2015, 13, 150–166. [Google Scholar] [CrossRef]
- Tonks, A.J.; Aplin, P.; Beriro, D.J.; Cooper, H.; Evers, S.; Vane, C.H.; Sjögersten, S. Impacts of conversion of tropical peat swamp forest to oil palm plantation on peat organic chemistry, physical properties and carbon stocks. Geoderma 2017, 289, 36–45. [Google Scholar] [CrossRef]
- Rixen, T.; Baum, A.; Wit, F.; Samiaji, J. Carbon Leaching from Tropical Peat Soils and Consequences for Carbon Balances. Front. Earth Sci. 2016, 4. [Google Scholar] [CrossRef]
- Muller, D.N.; Warneke, T.; Rixen, T.; Muller, M.; Jamahari, S.; Denis, N.; Mujahid, A.; Notholt, J. Lateral carbon fluxes and CO2 outgassing from a tropical peat-draining river. Biogeosciences 2015, 12, 5967–5979. [Google Scholar] [CrossRef]
- Gandois, L.; Teisserenc, R.; Cobb, A.; Chieng, H.; Lim, L.; Kamariah, A.; Hoyt, A.; Harvey, C. Origin, composition, and transformation of dissolved organic matter in tropical peatlands. Geochim. Cosmochim. Acta 2014, 137, 35–47. [Google Scholar] [CrossRef]
- Gandois, L.; Hoyt, A.M.; Mounier, S.; Le Roux, G.; Harvey, C.F.; Claustres, A.; Nuriman, M.; Anshari, G. From canals to the coast: Dissolved organic matter and trace metal composition in rivers draining degraded tropical peatlands in Indonesia. Biogeosciences 2020, 17, 1897–1909. [Google Scholar] [CrossRef]
- Anshari, G.Z. Circularity and Singularity of Tropical Peat Swamp Forest Ecosystems. In Tropical Peatland Eco-Management; Osaki, M., Tsuji, N., Rieley, J., Foead, N., Eds.; Springer: Tokyo, Japan, 2021. [Google Scholar]
- Yule, C.M.; Lim, Y.Y.; Lim, T.Y. Recycling of phenolic compounds in Borneo’s tropical peat swamp forests. Carbon Balance Manag. 2018, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Yule, C.M.; Lim, Y.Y.; Lim, T.Y. Degradation of Tropical Malaysian Peatlands Decreases Levels of Phenolics in Soil and in Leaves of Macaranga pruinosa. Front. Earth Sci. 2016, 4. [Google Scholar] [CrossRef]
- Wakhid, N.; Hirano, T.; Okimoto, Y.; Nurzakiah, S.; Nursyamsi, D. Soil carbon dioxide emissions from a rubber plantation on tropical peat. Sci. Total. Environ. 2017, 581–582, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Hooijer, A.; Silvius, M.; Wosten, H.; Page, S. PEAT-CO2, Assessment of CO2 Emissions from Drained Peatland in SE Asia; Delft Hydraulic: Delft, The Netherlands, 2006. [Google Scholar]
- Wösten, J.H.M.; Ritzema, H.P. Land and water management options for peatland development in Sarawak. Int. Peat J. 2015, 11, 59–66. [Google Scholar]
- Wösten, J.; Clymans, E.; Page, S.; Rieley, J.; Limin, S. Peat–water interrelationships in a tropical peatland ecosystem in Southeast Asia. Catena 2008, 73, 212–224. [Google Scholar] [CrossRef]
- Stephens, J.C. Peat and muck drainage problems. J. Irrig. Drain. Div. 1969, 2, 285–305. [Google Scholar] [CrossRef]
- Ishikura, K.; Hirano, T.; Okimoto, Y.; Hirata, R.; Kiew, F.; Melling, L.; Aeries, E.B.; Lo, K.S.; Musin, K.K.; Waili, J.W.; et al. Soil carbon dioxide emissions due to oxidative peat decomposition in an oil palm plantation on tropical peat. Agric. Ecosyst. Environ. 2018, 254, 202–212. [Google Scholar] [CrossRef]
- Miettinen, J.; Hooijer, A.; Vernimmen, R.; Liew, S.C.; Page, S.E. From carbon sink to carbon source: Extensive peat oxidation in insular Southeast Asia since 1990. Environ. Res. Lett. 2017, 12, 024014. [Google Scholar] [CrossRef]
- Hoyt, A.M.; Chaussard, E.; Seppalainen, S.S.; Harvey, C.F. Widespread subsidence and carbon emissions across Southeast Asian peatlands. Nat. Geosci. 2020, 13, 435–440. [Google Scholar] [CrossRef]
- Evans, C.D.; Williamson, J.M.; Kacaribu, F.; Irawan, D.; Suardiwerianto, Y.; Hidayat, M.F.; Laurén, A.; Page, S.E. Rates and spatial variability of peat subsidence in Acacia plantation and forest landscapes in Sumatra, Indonesia. Geoderma 2019, 338, 410–421. [Google Scholar] [CrossRef]
- Joosten, H.; Clarke, D. Wise Use of Mires and Peatlands; International Mire Conservation Group and International Peat Society: Devon, UK, 2002. [Google Scholar]
- Khakim, M.Y.N.; Bama, A.A.; Yustian, I.; Poerwono, P.; Tsuji, T.; Matsuoka, T. Peatland subsidence and vegetation cover degradation as impacts of the 2015 El niño event revealed by Sentinel-1A SAR data. Int. J. Appl. Earth Obs. Geoinf. 2020, 84, 101953. [Google Scholar] [CrossRef]
- Darmawan, S.M.; Putro, D.; Baskoro, T.; Nugroho, B. Indications of Compaction in Relation To Subsidence on Peatlands, in Peat in balance. In Proceedings of the 14th International Peat Congress, Stockholm, Sweden, 3–8 June 2012; pp. 115–123. [Google Scholar]
- Kool, D.M.; Buurman, P.; Hoekman, D.H. Oxidation and compaction of a collapsed peat dome in Central Kalimantan. Geoderma 2006, 137, 217–225. [Google Scholar] [CrossRef]
- Carlson, K.M.; Goodman, L.K.; May-Tobin, C.C. Modeling relationships between water table depth and peat soil carbon loss in Southeast Asian plantations. Environ. Res. Lett. 2015, 10, 074006. [Google Scholar] [CrossRef]
- IPCC. 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands Task Force on National Greenhouse Gas Inventories; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Jamaludin, J.; Gusmayanti, E.; Anshari, G.Z. Emisi Karbon Dioksida (CO2) dari Pertanian Skala Kecil di Lahan Gambut. J. Ilmu Lingkung. 2020, 18, 582–588. [Google Scholar] [CrossRef]
- Khasanah, N.; Van Noordwijk, M. Subsidence and carbon dioxide emissions in a smallholder peatland mosaic in Sumatra, Indonesia. Mitig. Adapt. Strat. Glob. Chang. 2019, 24, 147–163. [Google Scholar] [CrossRef]
- Leifeld, J.; Steffens, M.; Galego-Sala, A. Sensitivity of peatland carbon loss to organic matter quality. Geophys. Res. Lett. 2012, 39, 1–6. [Google Scholar] [CrossRef]
- Girkin, N.T.; Vane, C.H.; Cooper, H.V.; Moss-Hayes, V.; Craigon, J.; Turner, B.L.; Ostle, N.; Sjögersten, S. Spatial variability of organic matter properties determines methane fluxes in a tropical forested peatland. Biogeochemistry 2019, 142, 231–245. [Google Scholar] [CrossRef]
- Minick, K.J.; Kelley, A.M.; Miao, G.; Li, X.; Noormets, A.; Mitra, B.; King, J.S. Microtopography Alters Hydrology, Phenol Oxidase Activity and Nutrient Availability in Organic Soils of a Coastal Freshwater Forested Wetland. Wetlands 2018, 39, 263–273. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Takahashi, H.; Heikkinen, J.E.P.; Martikainen, P.J.; Vasander, H. Carbon fluxes from a tropical peat swamp forest floor. Glob. Chang. Biol. 2005, 11, 1788–1797. [Google Scholar] [CrossRef]
- Lilleskov, E.; McCullough, K.; Hergoualc’h, K.; Torres, D.D.C.; Chimner, R.; Murdiyarso, D.; Kolka, R.; Bourgeau-Chavez, L.; Hribljan, J.; Pasquel, J.D.A.; et al. Is Indonesian peatland loss a cautionary tale for Peru? A two-country comparison of the magnitude and causes of tropical peatland degradation. Mitig. Adapt. Strat. Glob. Chang. 2019, 24, 591–623. [Google Scholar] [CrossRef]
- Kareksela, S.; Haapalehto, T.; Juutinen, R.; Matilainen, R.; Tahvanainen, T.; Kotiaho, J.S. Fighting carbon loss of degraded peatlands by jump-starting ecosystem functioning with ecological restoration. Sci. Total Environ. 2015, 537, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Murdiyarso, D.; Hergoualc’h, K.; Verchot, L.V. Opportunities for reducing greenhouse gas emissions in tropical peatlands. Proc. Natl. Acad. Sci. USA 2010, 107, 19655–19660. [Google Scholar] [CrossRef]
- Loisel, J.; Yu, Z.; Beilman, D.W.; Camill, P.; Alm, J.; Amesbury, M.J.; Anderson, D.; Andersson, S.; Bochicchio, C.; Barber, K.; et al. A database and synthesis of northern peatland soil properties and Holocene carbon and nitrogen accumulation. Holocene 2014, 24, 1028–1042. [Google Scholar] [CrossRef]
- Hanson, P.J.; Griffiths, N.A.; Iversen, C.M.; Norby, R.J.; Sebestyen, S.D.; Phillips, J.R.; Chanton, J.P.; Kolka, R.K.; Malhotra, A.; Oleheiser, K.C.; et al. Rapid Net Carbon Loss From a Whole-Ecosystem Warmed Peatland. AGU Adv. 2020, 1, 1–18. [Google Scholar] [CrossRef]
- Wilson, R.M.; Hopple, A.M.; Tfaily, M.M.; Sebestyen, S.; Schadt, C.W.; Pfeifer-Meister, L.; Medvedeff, C.; McFarlane, K.J.; Kostka, J.E.; Kolton, M.; et al. Stability of peatland carbon to rising temperatures. Nat. Commun. 2016, 7, 13723. [Google Scholar] [CrossRef]
- Yu, Z.; Loisel, J.; Turetsky, M.R.; Cai, S.; Zhao, Y.; Frolking, S.; Macdonald, G.M.; Bubier, J.L. Evidence for elevated emissions from high-latitude wetlands contributing to high atmospheric CH4concentration in the early Holocene. Glob. Biogeochem. Cycles 2013, 27, 131–140. [Google Scholar] [CrossRef]
- Ishikura, K.; Yamada, H.; Toma, Y.; Takakai, F.; Morishita, T.; Darung, U.; Limin, A.; Limin, S.H.; Hatano, R. Effect of groundwater level fluctuation on soil respiration rate of tropical peatland in Central Kalimantan, Indonesia. Soil Sci. Plant Nutr. 2016, 63, 1–13. [Google Scholar] [CrossRef]
- Konecny, K.; Ballhorn, U.; Navratil, P.; Jubanski, J.; Page, S.E.; Tansey, K.; Hooijer, A.; Vernimmen, R.; Siegert, F. Variable carbon losses from recurrent fires in drained tropical peatlands. Glob. Chang. Biol. 2016, 22, 1469–1480. [Google Scholar] [CrossRef]
- Astiani, D.; Curran, L.; Burhanuddin; Taherzadeh, M.; Mujiman; Hatta, M.; Pamungkas, W.; Gusmayanti, E. Fire-driven biomass and peat carbon losses and post-fire soil co2 emission in a west Kalimantan Peatland Forest. J. Trop. For. Sci. 2018, 30, 570–575. [Google Scholar] [CrossRef]
- Lupascu, M.; Akhtar, H.; Smith, T.E.L.; Sukri, R.S. Post-Fire Carbon Emissions from Degraded Tropical Peat Swamp Forests in Brunei, EGU General Assembly. 2020. Available online: https://meetingorganizer.copernicus.org/EGU2020/EGU2020-6337.html (accessed on 22 May 2020).
- Hooijer, A.; Page, S.; Canadell, J.; Silvius, M.; Kwadijk, J.; Wösten, H.; Jauhiainen, J. Current and future CO2 emissions from drained peatlands in Southeast Asia. Biogeosciences 2010, 7, 1505–1514. [Google Scholar] [CrossRef]
- Cooper, H.V.; Vane, C.H.; Evers, S.; Aplin, P.; Girkin, N.T.; Sjögersten, S. From peat swamp forest to oil palm plantations: The stability of tropical peatland carbon. Geoderma 2019, 342, 109–117. [Google Scholar] [CrossRef]
- Itoh, M.; Okimoto, Y.; Hirano, T.; Kusin, K. Factors affecting oxidative peat decomposition due to land use in tropical peat swamp forests in Indonesia. Sci. Total. Environ. 2017, 609, 906–915. [Google Scholar] [CrossRef]
- Evers, S.; Yule, C.M.; Padfield, R.; O’Reilly, P.; Varkkey, H. Keep wetlands wet: The myth of sustainable development of tropical peatlands—Implications for policies and management. Glob. Chang. Biol. 2017, 23, 534–549. [Google Scholar] [CrossRef]
- Joosten, H.; Tapio-Biström, M.-L.; Tol, S. Peatlands—Guidance for Climate Change Mitigation through Conservation, Rehabilitation and Sustainable Use; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Günther, A.; Barthelmes, A.; Huth, V.; Joosten, H.; Jurasinski, G.; Koebsch, F.; Couwenberg, J. Prompt rewetting of drained peatlands reduces climate warming despite methane emissions. Nat. Commun. 2020, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Adhi, Y.A.; Anwar, S.; Tarigan, S.D.; Sahari, B. Relationship between groundwater level and water content in oil palm plantation on drained peatland in Siak, Riau Province, Indonesia, Pertanika. J. Trop. Agric. Sci. 2020, 43, 45–427. [Google Scholar]
- Novita, N. Carbon Stocks and Soil Greenhouse Gas Emissions Associated with Forest Conversion to Oil Palm Plantations in Tanjung Puting Tropical Peatlands, Indonesia; Oregon State University: Corvallis, OR, USA, 2016. [Google Scholar]
- Dadap, N.C.; Hoyt, A.M.; Cobb, A.R.; Oner, D.; Kozinski, M.; Fua, P.V.; Rao, K.; Harvey, C.F.; Konings, A.G. Drainage Canals in Southeast Asian Peatlands Increase Carbon Emissions. AGU Adv. 2021, 2, 1–14. [Google Scholar] [CrossRef]
- Hooijer, A.; Vernimmen, R.; Mawdsley, N.; Page, S.; Mulyadi, D.; Visser, M. Assessment of Impacts of Plantation Drainage on the Kampar Peninsula Peatland, Riau; Wetlands International: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Maswar, M. Kajian Cadangan Karbon Pada Lahan Gambut Tropical Yang Didrainase Untuk Tanaman Tahunan. Ph.D. Thesis, Bogor Agricultural University, Bogor, Indonesia, 2011. [Google Scholar]
- Evans, C.D.; Callaghan, N.; Jaya, A.; Grinham, A.; Sjogersten, S.; Page, S.E.; Harrison, M.E.; Kusin, K.; Kho, L.K.; Ledger, M.; et al. A Novel Low-Cost, High-Resolution Camera System for Measuring Peat Subsidence and Water Table Dynamics. Front. Environ. Sci. 2021, 9, 1–18. [Google Scholar] [CrossRef]
- Drexler, J.Z.; De Fontaine, C.S.; Deverel, S.J. The legacy of wetland drainage on the remaining peat in the Sacramento—San Joaquin Delta, California, USA. Wetlands 2009, 29, 372–386. [Google Scholar] [CrossRef]
- Erkens, G.; Van Der Meulen, M.J.; Middelkoop, H. Double trouble: Subsidence and CO2 respiration due to 1,000 years of Dutch coastal peatlands cultivation. Hydrogeol. J. 2016, 24, 551–568. [Google Scholar] [CrossRef]
- Gora, E.M.; Lucas, J.M.; Yanoviak, S.P. Microbial Composition and Wood Decomposition Rates Vary with Microclimate from the Ground to the Canopy in a Tropical Forest. Ecosystems 2019, 22, 1206–1219. [Google Scholar] [CrossRef]
- Neller, J.R. Oxidation loss of Lowmoor peat in fields with different water tables. Soil Sci. 1944, 58, 195–204. [Google Scholar] [CrossRef]
- Fenner, N.; Freeman, C. Drought-induced carbon loss in peatlands. Nat. Geosci. 2011, 4, 895–900. [Google Scholar] [CrossRef]
- Freeman, C.; Ostle, N.; Fenner, N.; Kang, H. A regulatory role for phenol oxidase during decomposition in peatlands. Soil Biol. Biochem. 2004, 36, 1663–1667. [Google Scholar] [CrossRef]
- Kleber, M. What is recalcitrant soil organic matter? Environ. Chem. 2010, 7, 320–332. [Google Scholar] [CrossRef]

| Subsidence | Category | Impact |
|---|---|---|
| Physical | Compaction, consolidation and shrinkage | Bulk density increase, and decrease in volumetric water content |
| Biological | Microbial oxidation/decomposition | Carbon emission/loss to atmosphere and water |
| Thermal | Smoldering fire | Haze pollution and carbon loss to atmosphere |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anshari, G.Z.; Gusmayanti, E.; Novita, N. The Use of Subsidence to Estimate Carbon Loss from Deforested and Drained Tropical Peatlands in Indonesia. Forests 2021, 12, 732. https://doi.org/10.3390/f12060732
Anshari GZ, Gusmayanti E, Novita N. The Use of Subsidence to Estimate Carbon Loss from Deforested and Drained Tropical Peatlands in Indonesia. Forests. 2021; 12(6):732. https://doi.org/10.3390/f12060732
Chicago/Turabian StyleAnshari, Gusti Z., Evi Gusmayanti, and Nisa Novita. 2021. "The Use of Subsidence to Estimate Carbon Loss from Deforested and Drained Tropical Peatlands in Indonesia" Forests 12, no. 6: 732. https://doi.org/10.3390/f12060732
APA StyleAnshari, G. Z., Gusmayanti, E., & Novita, N. (2021). The Use of Subsidence to Estimate Carbon Loss from Deforested and Drained Tropical Peatlands in Indonesia. Forests, 12(6), 732. https://doi.org/10.3390/f12060732






