Abstract
Understory vegetation plays a crucial role in nutrient turnover and cycling in plantations, but it also competes for nutrients with crop trees when only a single species is present due to its specific nutrient requirements. However, it remains unclear whether this competition can be alleviated when the species richness of understory vegetation increases. In this study, we tested different gradients of understory vegetation species richness, including understory vegetation removal (UR), the retention of a single main understory vegetation species (RS), and the retention of natural diverse understory vegetation (RD) as part of a poplar (Populus deltoides ‘Nanlin-3804′) plantation, to study their effects on poplar growth, and to evaluate nitrogen (N) usage and how this was affected by the interactions between the poplar and understory vegetation. The results showed a generally lower periodic growth, and a significant decline in the foliar chlorophyll content and glutamine synthetase activity of poplar under treatment with RS and RD compared to those under UR treatment conducted in July 2019, which clearly indicated N competition between the understory vegetation and poplar trees. However, no significant difference was detected in the foliar chlorophyll content and glutamine synthetase activity of poplar under RD and RS treatment; only the nitrate reductase activity in poplar leaves under RD treatment declined significantly, by 22.25%, in June 2019. On the contrary, the diameter at breast height (DBH) of the poplar under RD treatment showed an increase of 34.69% from July to August 2019, compared with that under RS treatment. Furthermore, the increase in the species richness of understory vegetation resulted in an increase in the δ15N values in the poplar leaves, which was strongly regulated by the NH4+-N pool in the 10–20 cm soil layer, indicating the effective coordination of N utilization between poplar and understory vegetation when diversified understory plant species were present. These findings demonstrate the essential role of understory vegetation species diversity in alleviating N competition with crop trees, and provide guidance for understory vegetation management in poplar plantations.
1. Introduction
Understory vegetation is a natural and essential component of the plantation ecosystem [1,2]. Gilliam [3] reviewed the ecological significance of understory vegetation, emphasizing the function of understory vegetation in maintaining the biodiversity of forest ecosystems, and also demonstrated that understory vegetation competes with overstory trees for water and nutrients. Therefore, understory vegetation is traditionally removed from or controlled in plantations to eliminate competition [4,5,6] and to facilitate the growth of the crop trees, especially in the early stages [7,8]. However, recent research has revealed that understory vegetation plays crucial roles in the productivity and sustainability of plantation ecosystems by promoting the decomposition of litterfall and nutrient cycling [9,10].
In the last few decades, attention has been directed toward the relationship between biodiversity and ecosystem functions [11,12]. It is hypothesized that species diversity affects the ecosystem function through the “mass effect” and the “diversity effect” [13,14,15,16]. In terms of plantations, studies have paid more attention to the upper crop trees [17,18,19,20] than to the understory vegetation. Under natural conditions, understory vegetation in plantations generally contains diverse plant species, greatly contributing to the maintenance of biodiversity [16,21]. However, few studies have addressed the role of understory vegetation diversity in terms of its nutrient effects. Several recent studies have indicated that diverse understory vegetation can produce diverse litter, thus promoting the litter decomposition process and accordingly increasing the nutrient supply [22,23]. Some research has also shown that diverse understory species could enhance the resilience and stability of forest ecosystems [24,25]. Generally, different understory plants have specific nutrient, water, and space requirements [26,27]. Therefore, compared with an understory comprising a single plant species, diverse understory vegetation should somewhat reduce competition with crop trees [28,29]. Moreover, diverse understory vegetation, given the variety of growth periods associated with its different individual species, should theoretically be able to intercept excess nutrients and store them in biomass over an extended period [30,31], subsequently releasing them upon plant death and decomposition for utilization by trees in the next growing season [32]. However, research on this matter is insufficient, and clarification of the mechanisms of understory vegetation species diversity in plantations awaits.
Poplar (Populus spp.) is an important timber forest species in China; however, severe site degradation and productivity decline, due to short-term harvest rotation and whole-tree harvesting in poplar plantations, have been recently reported [33]. Nitrogen (N) is the most essential nutrient for poplar growth, and its supply is closely related to soil quality and site productivity [34,35]. Accordingly, in poplar plantations, the existence of understory vegetation can improve the soil quality and nutrient supply by promoting nutrient cycling, and its species richness can facilitate the alleviation of nutrient competition between poplar and understory vegetation. However, few studies have evaluated the effects of an increase in the species richness of understory vegetation on the N utilization of crop trees in plantations. Theoretically, the utilization of N varies considerably among co-existent plants due to differences in root distribution and preferences for specific N forms [36,37], which can be somewhat distinguished through the fractionation of natural N isotopes according to abundance [38,39,40]. Therefore, we established a gradient of understory vegetation species richness (UVSR) in a poplar plantation to determine its effect on the growth and N metabolism of poplar trees, as well as the N competition between poplar trees and understory vegetation. Specifically, we attempted to answer the following two questions:
- (1)
- How does the presence of understory vegetation affect the growth and N utilization of poplar trees?
- (2)
- Does an increase in UVSR alleviate N competition with poplar trees and maintain poplar growth?
We attempt to devise an appropriate and relevant approach to understory vegetation management so as to ensure the sustainability of the poplar plantation.
2. Materials and Methods
2.1. Study Site Description and Experimental Design
The study site was Malanghu Forest Farm, Sihong County, Jiangsu Province, China (33°32′ N, 118°36′ E). The mean annual air temperature and precipitation are 14.6 °C and 893.9 mm, respectively. The soil at this site is a clay loam derived from lacustrine fine sediments of Hongze Lake. The site has moderate soil fertility and quality with a pH value of 7.03, bulk density of 1.33 g cm−3, and organic carbon and total N contents of 20.23 g kg−1 and 2.03 g kg−1, respectively, in the 0–10 cm soil layer.
The studied poplar plantations were set up in March 2016 using one-year-old rooted cuttings of poplar clone ‘Nanlin-3804′ (P. deltoides ‘Nanlin-3804′). Clone ‘Nanlin-3804′ is the fast-growing male poplar clone, with good wood quality for plywood and pulp production. The planting spacing was 6 m × 6 m. According to a previous investigation of the plantation in August 2017, the mean tree height and diameter at breast height (DBH) of the poplar trees are 7.59 ± 0.43 m and 9.33 ± 0.12 cm, respectively, and the main understory plant species are barnyard grass (Echinochloa crusgalli), Setcreasea purpurea, Rhizoma cyperi, and Ammannia coccinea, representing approximately 60%, 20%, 10%, and 10% of the total biomass of the understory vegetation, respectively.
A gradient of UVSR was set up in August 2017 based on the previous investigation. The following three forms of understory species richness treatment were designed: understory vegetation removal (UR), retention of a single main understory plant species (RS), and retention of naturally diverse understory vegetation (RD). A randomized block design was employed the experiments, with three rectangular blocks formed by three rows and 7 trees per row for each plot. For the UR treatment, after removing all the understory vegetation, the surface was covered with black weed barrier fabric to control the re-growth of the understory plants while ensuring air and water permeability. For the RS treatment, the dominant barnyard grass was retained by removing all the other understory species and appropriate reseeding was carried out to ensure its coverage. For the RD treatment, the understory vegetation was kept intact to remain its natural richness. The understory vegetation was subsequently managed monthly according to the design.
2.2. Tree Growth Monitoring
To avoid an edge effect, the five poplar trees in the center of each plot were used for measuring tree height and DBH growth. Tree height was measured via the trigonometric method using a Blume Leiss hypsometer every two months from June to October 2018 and from April to August 2019; DBH was measured monthly at breast height (1.3 m) using the dendrometer band method [41,42] during the same monitoring period.
2.3. Poplar Leaf, Understory Vegetation, and Soil Sampling and Preparation
The same five central poplar trees in each plot were used to determine foliar N metabolism and natural δ15N value. Leaf samples were collected monthly from the upper 30% of the crown of each tree at four locations from May to August 2019. The samples were transported in a cool box at 4 °C to the laboratory. The samples from all the trees in each plot were mixed in equal parts, and this mixed sample was then divided into two parts. One part was dried at 60 °C for 24 h, ground to fine powder, and then sieved through a 75-μm mesh for the analysis of total N and δ15N value. The other was stored at 4 °C before the determination of chlorophyll content and enzyme activities related to N metabolism.
Five 1 m × 1 m subplots were randomly established in each plot of the RS and RD treatments. All the aboveground understory vegetation in each subplot was harvested three times in April, June and August 2019. The harvested biomass was brought back to the laboratory, weighed after oven-drying, and ground for the analysis of total N content. Moreover, the biomass of the barnyard grass that was sampled in June and August 2019 was used for the analysis of δ15N value.
In June and August 2019, after harvesting all the aboveground understory vegetation, mineral soil samples from the 0–5, 5–10, and 10–20 cm soil layers were collected in each subplot. The samples were transported under cool conditions to the laboratory; after sieving through a 2-mm mesh, the soil samples were stored at 4 °C for the subsequent extraction of NH4+-N and NO3−-N and the analysis of the natural abundance of 15N.
2.4. Measurement of Poplar Leaf, Understory Vegetation, and Soil Variables
The total N contents of the poplar leaves and the understory vegetation were determined with an elemental analyzer (Vario MACRO Cube, Elementar Company, Langenselbold, Germany). The natural 15N abundance of poplar leaf and barnyard grass was determined using a stable isotope ratio mass spectrometer (Delta V Advantage, Thermo Scientific, Waltham, MA, USA).
The chlorophyll of the poplar leaves was extracted by soaking the fresh leaf sample in 80% acetone in darkness for 24 h. Then, the absorbance of the extraction determined at 646 nm and 663 nm via a spectrophotometer (Analytik Jena AG, Jena, Germany) was used to calculate the chlorophyll content [43].
The activities of glutamine synthetase (GS) and nitrate reductase (NR) of the poplar leaf were analyzed to evaluate the relative importance of NH4+-N and NO3−-N in the poplar when undergoing different treatments. GS activity was determined via the method described by Aldarini et al. [44] and Ferreira et al. [45]. Fresh poplar leaf powder grounded in liquid nitrogen with quartz sand was extracted with Tris-HCl buffer (0.05 mol L−1, pH 7.4) for 1 h; after centrifugation at 3000 rpm for 25 min at 4 °C, the supernatant was incubated with the reaction solution (40 mmol L−1 ATP, 80 mmol L−1 MgSO4, 20 mmol L−1 sodium glutamate, 20 mmol L−1 cysteine, 2 mmol L−1 EGTA and 80 mmol L−1 hydroxylamine-HCl) for 30 min at 37 °C, then the stop solution (0.2 mol L−1 TCA, 0.4 mol L−1 FeCl3 and 0.6 mol L−1 HCl) was added to halt the reaction. The absorbance of the supernatant was determined at 540 nm via a spectrophotometer (Analytik Jena AG, Jena, Germany). A paired determination procedure with no hydroxylamine HCl in the reaction solution was used as the blank. The GS activity was calculated as the difference between the test and the blank, with A540 mg−1 protein h−1 as the unit.
The NR activity was determined in vivo according to the method of Radin [46], with minor modifications. The freshly chopped poplar leaf sample (~Ø 2.5 mm discs) was immersed in the reaction solution (0.1 mol L−1 KNO3, 0.1 mol L−1 KH2PO4 and 3% 1-propanol, adjusted to pH 7.5) via vacuum infiltration, and incubated for 1 h at 30 °C in the dark. Then, the reaction was stopped by the addition of 30% trichloroacetic acid solution. Nitrite (NO2−) was determined colorimetrically via diazotization and absorbance at 540 nm [47]. A paired determination procedure with no KNO3 in the reaction solution was used as the blank. The NR activity was calculated as the difference between the test and blank, with mg NO2− g−1 h−1 as the unit.
Soil inorganic N (NH4+-N and NO3−-N) was extracted from the fresh soil with a 2 mol L−1 KCl solution at a 1:5 (w/v) soil-to-solution ratio by shaking for 1 h (180 r min−1). After suction filtration, the NH4+-N concentration was determined via the indophenol blue method [48] and the NO3−-N concentration was determined via the dual-wavelength ultraviolet spectrophotometry method [49].
The δ15N values of soil inorganic N were measured using a modified ammonia diffusion protocol [50]. The soil extract with inorganic N (50 mL) was transferred into a glass media bottle (250 mL). An acidified GF/D filter disk (Whatman # 1823010, Sigma-Aldrich, St. Louis, MO, USA) enclosed in a 10-μm pore-size polytetrafluoroethylene (PTFE) membrane (Millipore LCWP 02500, Merck Millipore, Burlington, MS, USA) was floated on the solution as an ammonia trap. The bottle was closed tightly after approximately 0.1 g of MgO was added to adjust the pH of the solution to 11, and it was then shaken on a thermostat bath oscillator (30–35 °C) for 5 days to ensure the complete diffusion of NH3 into the acidified trap. The end of NH3 diffusion was confirmed via an ammonia nitrogen test. The filter pack was removed from the diffusion bottle and placed in a desiccator with silica gel and an open container of concentrated sulfuric acid to dry for 2–3 days. Then, a new trap and approximately 1.5 g of Devarda’s alloy were added to the bottle, and the bottle was immediately closed and shaken for another 5 days to convert NO3−-N to NH4+-N, which then diffused into the acidified trap. After drying, the PTFE membrane was separated, and the acidified filter disk was sent for consequent analysis of δ15N. δ15N isotopic analysis was carried out using a stable isotope ratio mass spectrometer (Delta V Advantage, Thermo Scientific, USA).
2.5. Statistical Analyses
All values reported in this paper are presented as arithmetic mean ± standard error. One-way analysis of variance (ANOVA) of the SPSS 21.0 statistical software program (SPSS, Chicago, IL, USA) was used to assess the differences among understory vegetation treatments. Duncan’s test was used to differentiate means. Stepwise multiple linear regression analyses were adopted to elucidate the N utilization strategy of poplar and barnyard grass for different inorganic N forms and soil layers using the SPSS 21.0 statistical software program. All the figures were drawn via SigmaPlot 14 software (Systat Software, Chicago, IL, USA).
3. Results
3.1. Impact of UVSR on Poplar Growth
The increments in the tree height and DBH of the poplar were generally lower under the RS and RD treatments than under the UR treatment (Figure 1). From June to August, the poplar tree height increment was 20.29% to 36.30% lower, and the DBH increment was 13.89% to 28.70% lower under the RS and RD treatment compared to the UR treatment (p < 0.05), in 2018 and 2019, respectively; however, when comparing the RD and RS treatments, there was no significant difference in the periodic increments in the poplar tree height and the DBH, except for a significant increase in the DBH of 34.69% during the period from July to August 2019 (Figure 1). In general, the annual increments in poplar tree height and DBH showed no significant differences between the three understory vegetation treatments (Figure 1).
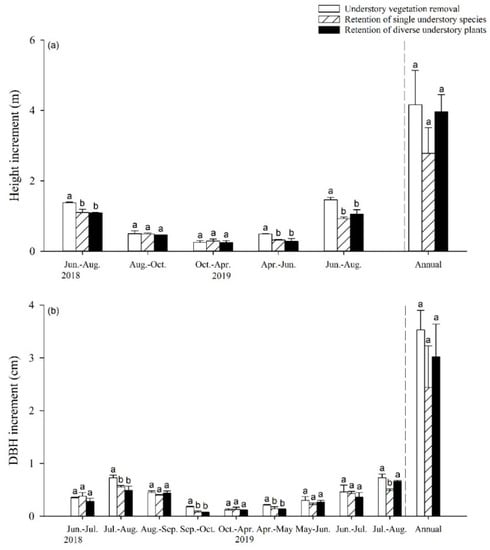
Figure 1.
Increment of poplar tree height (a) and DBH (b) with increase in understory vegetation species richness. The annual increment was determined by summing the increments of poplar tree height and DBH from June 2018 to May 2019. Different letters indicate significant differences between treatments at p < 0.05.
3.2. Impact of UVSR on Poplar Foliar N and Chlorophyll Content and Enzyme Activity
The foliar N and chlorophyll contents of the poplar showed no significant differences between the three understory treatments in different growth periods, except for the chlorophyll content in July (Figure 2). In July, the foliar chlorophyll content was 12.96% and 18.62% lower under the RS and RD treatments than under the UR treatment (p < 0.05), respectively; however, there was no significant difference between the RS and RD treatments (Figure 2b).
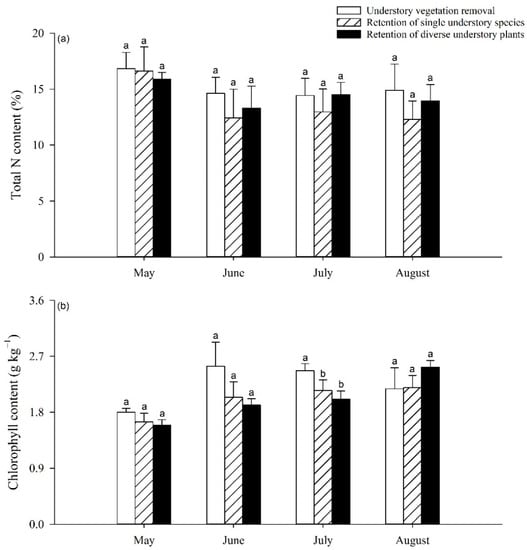
Figure 2.
Variation in foliar N content (a) and chlorophyll content (b) of poplar with increase in understory vegetation species richness. Different letters indicate significant differences between treatments at p < 0.05.
The activities of GS and NR in poplar leaves increased most significantly after May 2019, at which point significant differences among the three understory treatments began to appear (Figure 3). Significant decreases in GS activity were detected in July and August 2019; this activity was 16.86% to 22.27% lower under RS and RD treatments, respectively, than under UR treatment (p < 0.05). However, no significant difference was found between the RS and RD treatments (Figure 3a), while for NR activity, a significant difference was only found in June 2019, with decreases of 27.68% and 22.25% under the RD treatment compared to the UR and RS treatments, respectively (Figure 3b).
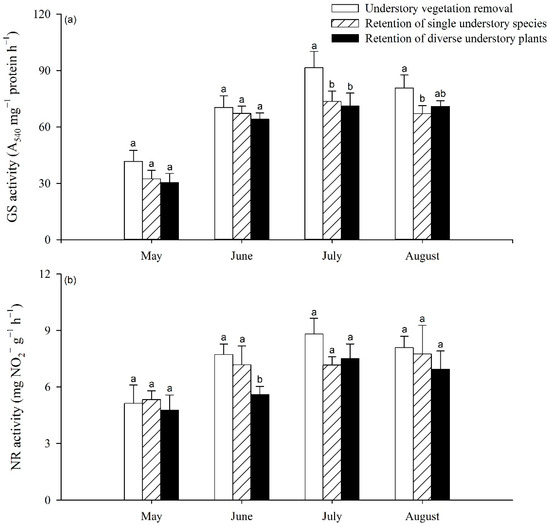
Figure 3.
Variation in poplar foliar GS (a) and NR (b) activity with increase in understory vegetation species richness. Different letters indicate significant differences between treatments at p < 0.05.
3.3. Biomass and N Accumulation in Understory Vegetation
No understory vegetation was found throughout the whole investigation period when using the UR treatment (Figure 4a). Furthermore, no understory vegetation was found in April under the RS treatment, while appreciable levels of biomass and N accumulation were found during this period under the RD treatment (Figure 4). Moreover, the levels of biomass and N accumulation in the understory vegetation were significantly higher under the RD treatment than under the RS treatment in June and August (p < 0.05), with an increase of 28.78% and 34.63% in biomass, and 64.47% and 89.84% in N accumulation (Figure 4).
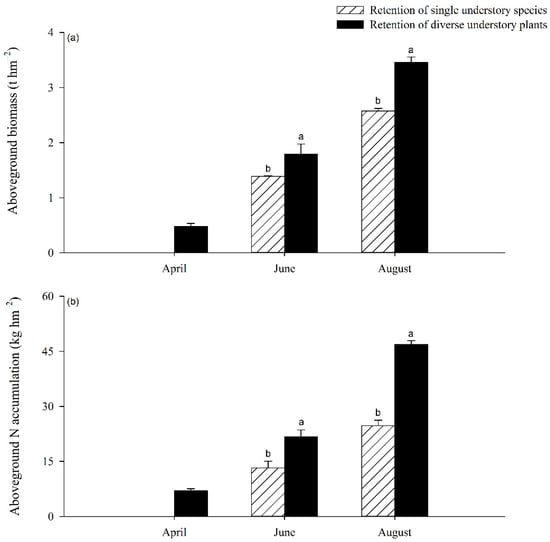
Figure 4.
Aboveground biomass (a) and N accumulation (b) in the understory vegetation under RS and RD treatments. Different letters indicate significant differences between treatments at p < 0.05.
3.4. Linking δ15N of Plants and Soil Inorganic N Pool in Response to UVSR
The UVSR showed a significant impact on the δ15N value of the poplar leaves (Figure 5). The value of poplar foliar δ15N displayed no significant differences when under the RS treatment compared to RD, while both of these values were significantly higher than when under the UR treatment (p < 0.05) in August 2019. Apart from this, there was no significant difference in poplar foliar δ15N in June among the three understory treatments (Figure 5a). As for the δ15N values of barnyard grass, no significant differences were found between the RS and RD treatments between June and August 2019 (Figure 5b).
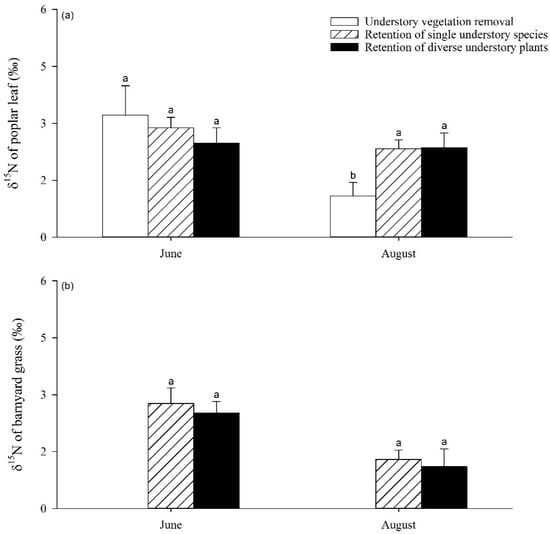
Figure 5.
Variation in δ15N values of poplar leaf (a) and barnyard grass (b) with increases in understory vegetation species richness. Different letters indicate significant differences between treatments at p < 0.05.
The soil inorganic N was dominated by NO3−-N in both June and August (Table 1). The soil NH4+-N content was generally higher under the RS and RD treatments than under the UR treatment, especially in June when the difference was significant (Table 1). Different patterns arose in the soil NO3−-N content, with no significant differences in the 0–5 cm soil layer among the three understory treatments in June and August, while significantly lower values arose in the 5–10 and 10–20 cm soil layers under both the RS and RD treatments compared to the UR treatment (Table 1). However, no significant difference was found between the RS and RD treatments, except for a significantly lower NH4+-N content in the 10–20 cm soil layer in June under the RD treatment compared to the RS treatment (Table 1).

Table 1.
Variation in inorganic N content and δ15N value with increase in understory vegetation species richness. UR, RS and RD refer to the treatments of understory vegetation removal, the retention of a single main understory plant species, and the retention of natural diverse understory vegetation, respectively. Lowercase letters indicate significant differences in inorganic N content and δ15N value between treatments (p < 0.05).
The δ15N value of the soil inorganic N was higher in June than in August, and the δ15N value of the soil NH4+-N was higher than that of NO3−-N in both June and August (Table 1). The δ15N values of the soil NH4+-N in both the 0–5 and 5–10 cm soil layers were generally lower under the RS and RD treatments than under the UR treatment in June, but there was no significant difference among the three understory treatments in the three soil layers in August (Table 1). The δ15N values of the soil NO3−-N were generally lower under the RS and RD treatments than under the UR treatment in both the 0–5 and 5–10 cm soil layers in June, but they were higher under the RS and RD treatments than under the UR treatment in the 10–20 cm soil layer in August (Table 1). However, there was generally no significant difference between the RS and RD treatments, except for the significantly higher δ15N value of the soil NO3−-N in the 0–5 cm layer in June under the RD treatment compared to the RS treatment, and the significantly lower δ15N value of the soil NO3−-N in the 5–10 cm soil layer in June under the RD treatment compared to the RS treatment (p < 0.05).
Stepwise multiple linear regression was performed to evaluate the continuity in δ15N between the plants and soil inorganic N source. The results (Table 2) showed that, in June, the δ15N of the poplar under the UR treatment and that of the barnyard grass under the RS and RD treatment were strongly regulated by NO3−-N in the 10–20 cm soil layer, and then by NH4+-N in the 10–20 cm soil layer; the δ15N of the poplar under the RS treatment was regulated in a coordinated manner by both NH4+-N and NO3−-N in the 10–20 cm soil layer, while the δ15N of the poplar under the RD treatment was strongly regulated by NH4+-N, and then by NO3−-N in the 10–20 cm soil layer. As in August, the relationship showed obvious changes. The δ15N of the poplar under the UR treatment and that of the barnyard grass under the RS and RD treatment were primarily regulated by NO3−-N in the 10–20 cm soil layer, while the δ15N of the poplar under the RS and RD treatment was primarily regulated by NH4+-N in the 10–20 and in 0–5 cm soil layers, respectively.

Table 2.
Summary of stepwise regressions between δ15N values in plants with δ15N values of inorganic N. UR, RS and RD refer to the treatments of understory vegetation removal, the retention of a single main understory plant species, and the retention of naturally diverse understory vegetation, respectively. x1, x2 and x3 refer to the δ15N values of NH4+-N in the 0–5, 5–10, and 10–20 cm soil layers, and x4, x5 and x6 refer to the δ15N values of NO3−-N in the 0–5, 5–10, and 10–20 cm soil layers, respectively. The symbol “-” means that the independent variable was not incorporated into the regression model.
4. Discussion
The slightly smaller increments in the poplar tree height and DBH, accompanying the reductions in the chlorophyll content and N-related enzyme activity in the poplar leaf under the RS and RD treatment compared to the UR treatment, especially during the main understory vegetation growing season, suggest N competition between understory vegetation and poplar trees. This finding is consistent with several previous reports [51,52], implying that understory vegetation influences crop tree growth and needs to be scientifically managed [53,54].
Our results have also revealed that the poplar growth under the RD treatment is comparable to that under the UR treatment, but is generally higher than under the RS treatment, indicating that UVSR could alleviate competition, as mentioned above, rather than only increasing the biodiversity in plantations [21,55]. Elliott et al. [16] reported that increases in herbaceous organic material could stimulate soil N mineralization and benefit the N condition for the overstory trees. The aboveground biomass of the understory vegetation was significantly higher under the RD treatment than under the RS treatment. The large biomass of the understory vegetation produces more plant litter, which, in turn, contributes to the increase in soil decomposers, litter decomposition, and nutrient cycling [56,57]. This might explain why UVSR alleviated N competition between the understory vegetation and crop trees. Moreover, many studies have confirmed that mixed litter from diverse plant species could increase the complexity of food resources for soil microorganisms and sustain diverse soil microflora, thus promoting microbial activity, and accelerating litter decomposition and nutrient release [58,59,60,61,62]. In our study, UVSR produced more diverse litter under the RD treatment than under the RS treatment, which could be conducive to litter decomposition and nutrient cycling in the poplar plantations. Besides this, given that different understory species displayed unique rhythms of annual growth and nutrient utilization under the RD treatment, excess nutrients in a certain season could be intercepted by and stored in the biomass of specific understory species, thereby reducing the extent of nutrient loss from the plantation system. As such, increases in UVSR could alleviate N competition and facilitate poplar growth to a certain extent in poplar plantations.
The δ15N value of the soil NH4+-N was generally higher than that of NO3−-N due to the fractionation of N isotopes with N transformational processes in the soil [63]. The poplar foliar δ15N values suggest that the existence and diversity of the understory vegetation might have changed the N utilization strategy of poplar. The NH4+-N in the shallower soil layer accounted for the greater variation in poplar leaf δ15N under the RD treatment compared to the RS treatment. The vertical fine root distribution in the diverse understory vegetation may be more complicated than that in the single species of understory vegetation [64,65]. In addition, different plants have varying preferences for different N species [66,67], meaning that the N in the shallow soil layer has a greater impact on poplar N utilization than that in the deep soil layer. Many studies have shown that the nutrient content, soil microbial activity and extracellular enzyme activity decrease with soil depth [68,69,70], while at the same time, the nutrients in the litter are primarily released into the surface soil [71,72]. Therefore, as compared with the RS treatment, the RD treatment can improve the N utilization of poplar trees.
Recently, more studies have reported the importance of understory vegetation in sustaining soil nutrient conditions in orchards [73] or plantations [74,75], and the effective management of understory vegetation has been studied in more detail [76,77]. However, in addition to the maintenance of biodiversity [78,79], the species richness of understory vegetation has received little attention for its benefits in alleviating nutrient competition and improving overstory tree growth. In order to verify this effect, though, corresponding experiments must be carried out in other plantations and for much longer periods. From the perspective of the diversity management of understory vegetation, several studies in grasslands have shown that proper mowing, such as mowing at intervals, could increase plant biodiversity [80,81,82]. Therefore, it would be worthwhile to study whether the diversity of understory vegetation could be increased by proper mowing, and whether this is beneficial to the nutrient conditions and tree growth in plantations. Such future studies could provide important information facilitating plantation owners in formulating understory vegetation management strategies to ensure better plantation productivity.
5. Conclusions
Understory vegetation removal has been previously shown to be beneficial to the growth and N utilization of poplars in early plantation stages when compared with understory vegetation retention. However, our results show that the growth values of the poplars under treatment with the retention of diverse understory vegetation were close to those for the poplars under treatment with understory vegetation removal. Although the retention of understory vegetation reduced the activities of the enzymes involved in N conversion in poplar leaves, the retention of diverse understory vegetation gave rise to an effective coordination pattern of nitrogen utilization between the poplar and understory vegetation. From the perspective of soil fertility and productivity maintenance, the retention of diverse understory vegetation is an economical and effective method of understory vegetation management for poplar plantations.
Author Contributions
Y.T. and J.Z. were mainly responsible for the conceptualization, methodology used, data evaluation, data validation, and formal analysis. Investigations and data curation were conducted by all authors. The original draft of this article was prepared by Y.T. and J.Z. who were also responsible for the review and editing process of this article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China, grant number 2016YFD0600402.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Xiaowei E, Ting She and Wenqi Pan for their assistance in the laboratory work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nilsson, M.C.; Wardle, D.A. Understory vegetation as a forest ecosystem driver: Evidence from the northern Swedish boreal forest. Front. Ecol. Environ. 2005, 3, 421–428. [Google Scholar] [CrossRef]
- Pablo, C.P.; Piotr, T.; Nicholas, C.C.; Ángela, R.L. Characterizing understory vegetation in Mediterranean forests using full-waveform airborne laser scanning data. Remote Sens. Environ. 2018, 217, 400–413. [Google Scholar]
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. BioScience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Messier, C.; Coll, L.; Poitras-Larivière, A.P.; Jacques Brisson, N.B. Resource and non-resource root competition effects of grasses on early-versus late-successional trees. J. Ecol. 2009, 97, 548–554. [Google Scholar] [CrossRef]
- Lecerf, A.; Evangelista, C.; Cucherousset, J.; Boiché, A. Riparian overstory-understory interactions and their potential implications for forest-stream linkages. For. Ecol. Manag. 2016, 367, 112–119. [Google Scholar] [CrossRef]
- Liao, Y.C.; Fan, H.B.; Wei, X.H.; Wu, J.P.; Duan, H.L.; Fu, X.L.; Liu, W.F.; Wang, H.M.; Zhan, X.W.; Tang, P.; et al. Competition increased fine root biomass in Chinese fir (Cunninghamia lanceolata) plantations in subtropical China. For. Ecol. Manag. 2019, 435, 151–157. [Google Scholar] [CrossRef]
- Giuggiola, A.; Zweifel, R.; Feichtinger, L.; Vollenweider, P.; Bugmann, H.; Haeni, M.; Rigling, A. Competition for water in a xeric forest ecosystem-Effects of understory removal on soil micro-climate, growth and physiology of dominant Scots pine trees. For. Ecol. Manag. 2018, 409, 241–249. [Google Scholar] [CrossRef]
- Osburn, E.D.; Elliottt, K.J.; Knoepp, J.D.; Miniat, C.F.; Barrett, J.E. Soil microbial response to Rhododendron understory removal in southern Appalachian forests: Effects on extracellular enzymes. Soil Biol. Biochem. 2018, 127, 50–59. [Google Scholar] [CrossRef]
- Zhang, J.J.; Li, Y.F.; Chang, S.X.; Jiang, P.K.; Zhou, G.M.; Liu, J.; Wu, J.S.; Shen, Z.M. Understory vegetation management affected greenhouse gas emissions and labile organic carbon pools in an intensively managed Chinese chestnut plantation. Plant Soil 2014, 376, 363–375. [Google Scholar] [CrossRef]
- Gutierrez-Coarite, R.; Mollinedo, J.; Cho, A.; Wright, M.G. Canopy management of macadamia trees and understory plant diversification to reduce macadamia felted coccid (Eriococcus ironsidei) populations. Crop Prot. 2018, 113, 75–83. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A.; Zackrisson, O. Effects of species and functional group loss on island ecosystem properties. Nature 2005, 435, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Benefits of plant diversity to ecosystem: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; de Bello, F.; Quétier, F.; Grigulis, K.; Robson, M. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef]
- Swenson, N.G. Plant functional diversity: Organism traits, community structure, and ecosystem properties. Ecology 2016, 97, 3556–3558. [Google Scholar] [CrossRef]
- Elliott, K.J.; Vose, J.M.; Knoepp, J.D.; Clinton, B.D.; Kloeppel, B.D. Functional role of the herbaceous layer in eastern deciduous forest ecosystems. Ecosystems 2015, 18, 221–236. [Google Scholar] [CrossRef]
- Potvin, C.; Gotelli, N.J. Biodiversity enhances individual performance but does not affect survivorship in tropical trees. Ecol. Lett. 2008, 11, 217–223. [Google Scholar] [CrossRef]
- Archaux, F.; Chevalier, R.; Berthelot, A. Towards practices favourable to plant diversity in hybrid poplar plantations. For. Ecol. Manag. 2010, 259, 2410–2417. [Google Scholar] [CrossRef]
- Paquette, A.; Messier, C. The effect of biodiversity on tree productivity: From temperate to boreal forests. Glob. Ecol. Biogeogr. 2011, 20, 170–180. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Castro-Izaguirre, N.; Baruffol, M.; Brezzi, M.; Lang, A.N.; Li, Y.; Hardtle, W.; Oheimb, G.; Yang, X.; et al. Impacts of species richness on productivity in a large-scale subtropical forest experiment. Science 2018, 362, 80–83. [Google Scholar] [CrossRef]
- McEwan, R.W.; Muller, R.N. Dynamics, diversity, and resource gradient relationships in the herbaceous layer of an old-growth Appalachian forest. Plant Ecol. 2011, 212, 1179–1191. [Google Scholar] [CrossRef]
- Chomel, M.; Guittonny-Larchevêque, M.; DesRochers, A.; Baldy, V. Effect of mixing herbaceous litter with tree litters on decomposition and N release in boreal plantations. Plant Soil 2016, 398, 229–241. [Google Scholar] [CrossRef]
- Santonja, M.; Rancon, A.; Fromin, N.; Baldy, V.; Hättenschwiler, S.; Fernandez, C.; Montès, N.; Mirleau, P. Plant litter diversity increases microbial abundance, fungal diversity, and carbon and nitrogen cycling in a Mediterranean shrubland. Soil Biol. Biochem. 2017, 111, 124–134. [Google Scholar] [CrossRef]
- Gavinet, J.; Prévosto, B.; Fernandez, C. Do shrubs facilitate oak seedling establishment in Mediterranean pine forest understory? For. Ecol. Manag. 2016, 381, 289–296. [Google Scholar] [CrossRef]
- Pennekamp, F.; Pontarp, M.; Tabi, A.; Altermatt, F.; Alther, R.; Choffat, Y.; Fronhofer, E.A.; Ganesanandamoorthy, P.; Garnier, A.; Griffiths, J.I.; et al. Biodiversity increases and decreases ecosystem stability. Nature 2018, 563, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Kawarasaki, S.; Hori, Y. Flowering phenology of understory herbaceous species in a cool temperate deciduous forest in Ogawa forest reserve, central Japan. J. Plant Res. 2001, 114, 19–23. [Google Scholar] [CrossRef]
- Gherardi, L.A.; Sala, O.E.; Yahdjian, L. Preference for different inorganic nitrogen forms among plant functional types and species of the Patagonian steppe. Oecologia 2013, 173, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved-A critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Matonis, M.S.; Binkley, D. Not just about the trees: Key role of mosaic-meadows in restoration of ponderosa pine ecosystems. For. Ecol. Manag. 2018, 411, 120–131. [Google Scholar] [CrossRef]
- Gallart, M.; Love, J.; Meason, D.F.; Coker, G.; Clinton, P.W.; Xue, J.M.; Jameson, P.E.; Klápště, J.; Turnbull, M.H. Field-scale variability in site conditions explain phenotypic plasticity in response to nitrogen source in Pinus radiata D. Don. Plant Soil 2019, 443, 353–368. [Google Scholar] [CrossRef]
- Tateno, R.; Nakayama, M.; Yano, M.; Fukuzawa, K.; Inagaki, Y.; Koba, K.; Ugawa, S. Nitrogen source utilization in co-existing canopy tree and dwarf bamboo in a northern hardwood forest in Japan. TreesStruct. Funct. 2020, 34, 1047–1057. [Google Scholar] [CrossRef]
- Qiao, Y.F.; Miao, S.J.; Silva, L.C.R.; Horwath, W.R. Understory species regulate litter decomposition and accumulation of C and N in forest soils: A long-term dual-isotope experiment. For. Ecol. Manag. 2014, 329, 318–327. [Google Scholar] [CrossRef]
- Ge, X.M.; Tian, Y.; Tang, L.Z. Nutrient distribution indicated whole-tree harvesting as a possible factor restricting the sustainable productivity of a poplar plantation system in China. PLoS ONE 2015, 10, e0125303. [Google Scholar] [CrossRef]
- Sui, J.; Ji, C.; Wang, X.; Liu, Z.; Sa, R.; Hu, Y.; Wang, C.; Li, Q.; Liu, X. A plant growth-promoting bacterium alters the microbial community of continuous cropping poplar trees’ rhizosphere. J. Appl. Microbiol. 2019, 126, 1209–1220. [Google Scholar] [CrossRef]
- Ventura, M.; Panzacchi, P.; Muzzi, E.; Magnani, F.; Tonon, G. Carbon balance and soil carbon input in a poplar short rotation coppice plantation as affected by nitrogen and wood ash application. New For. 2019, 50, 969–990. [Google Scholar] [CrossRef]
- Tanaka-Oda, A.; Kenzo, T.; Inoue, Y.; Yano, M.; Koba, K.; Ichie, T. Variation in leaf and soil 15N in diverse tree species in a lowland dipterocarp rainforest, Malaysia. Trees Struct. Funct. 2016, 30, 509–522. [Google Scholar] [CrossRef]
- Debiasi, T.V.; Calzavara, A.K.; da Silva, L.M.I.; da Silva, J.G.; Bianchini, E.; Pimenta, J.A.; Stolf-Moreira, R.; Aidar, M.P.M.; Sodek, L.; Oliveira, H.C. Nitrogen metabolism of Neotropical tree seedlings with contrasting ecological characteristics. Acta. Physiol. Plant. 2019, 41, 131. [Google Scholar] [CrossRef]
- Muruganandam, S.; Israel, D.W.; Robarge, W.P. Nitrogen transformations and microbial communities in soil aggregates from three tillage systems. Soil Sci. Soc. Am. J. 2010, 74, 120–129. [Google Scholar] [CrossRef]
- Craine, J.M.; Brookshire, E.N.J.; Cramer, M.D.; Hasselquist, N.J.; Koba, K.; Marin-Spiotta, E.; Wang, L.X. Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 2015, 396, 1–26. [Google Scholar] [CrossRef]
- Philben, M.; Billings, S.A.; Edwards, K.A.; Podrebarac, F.A.; van Biesen, G.; Ziegler, S.E. Amino acid delta 15N indicates lack of N isotope fractionation during soil organic nitrogen decomposition. Biogeochemistry 2018, 138, 69–83. [Google Scholar] [CrossRef]
- Ohashi, Y.; Sahri, M.H.; Yoshizawa, N.; Itoh, T. Annual rhythm of xylem growth in rubberwood (Hevea brasiliensis) trees grown in Malaysia. Holzforschung 2001, 55, 151–154. [Google Scholar] [CrossRef]
- Just, M.G.; Frank, S.D. Evaluation of an easy-to-install, low-cost dendrometer band for citizen-science tree research. J. For. 2019, 117, 317–322. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Gitelson, A.; Lang, M. Non-destructive determination of chlorophyll content of leaves of a green and an aurea mutant of tobacco by reflectance measurements. Plant Physiol. 1996, 148, 483–493. [Google Scholar] [CrossRef]
- Aldarini, N.; Alhasawi, A.A.; Thomas, S.C.; Appanna, V.D. The role of glutamine synthetase in energy production and glutamine metabolism during oxidative stress. Antonie Leeuwenhoek 2017, 110, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.J.; Vale, D.; Cunha, L.; Melo, P. Role of the C-terminal extension peptide of plastid located glutamine synthetase from Medicago truncatula: Crucial for enzyme activity and needless for protein import into the plastids. Plant Physiol. Biochem. 2017, 111, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Radin, J.W. In vivo assay of nitrate reductase in cotton leaf discs. Plant Physiol. 1973, 51, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Maevskaya, S.N.; Egorova, E.A.; Bukhov, N.G. Effect of elevated temperature on nitrite and nitrate reduction in leaves and intact chloroplasts. Russ. J. Plant Physiol. 2003, 50, 599–603. [Google Scholar] [CrossRef]
- Gao, W.; Yang, J.; Ren, S.R.; Liu, H.L. The trend of soil organic carbon, total nitrogen, and wheat and maize productivity under different long-term fertilizations in the upland fluvo-aquic soil of North China. Nutr. Cycl. Agroecosyst. 2015, 103, 61–73. [Google Scholar] [CrossRef]
- Norman, R.J.; Edberg, J.C.; Stucki, J.W. Determination of nitrate in soil extracts by dual-wavelength ultraviolet spectrophotometry. Soil Sci. Soc. Am. J. 1985, 49, 1182–1185. [Google Scholar] [CrossRef]
- Griesheim, K.L.; Mulvaney, R.L. Improving the accuracy of diffusion for inorganic 15N analyses of soil extracts and water. Commun. Soil Sci. Plant Anal. 2019, 50, 1161–1169. [Google Scholar] [CrossRef]
- Matsushima, M.; Choi, W.J.; Chang, S.X. White spruce foliar δ13C and δ15N indicate changed soil N availability by understory removal and N fertilization in a 13-year-old boreal plantation. Plant Soil 2012, 361, 375–384. [Google Scholar] [CrossRef]
- Littke, K.M.; Harrington, T.B.; Slesak, R.A.; Holub, S.M.; Hatten, J.A.; Gallo, A.C.; Littke, W.R.; Harrison, R.B.; Turnblom, E.C. Impacts of organic matter removal and vegetation control on nutrition and growth of Douglas-fir at three Pacific Northwestern Long-Term Soil Productivity sites. For. Ecol. Manag. 2020, 468, 118176. [Google Scholar] [CrossRef]
- Dubbert, M.; Piayda, A.; Cuntz, M.; Correia, A.C.; Silva, F.C.E.; Pereira, J.S.; Werner, C. Stable oxygen isotope and flux partitioning demonstrates understory of an oak savanna contributes up to half of ecosystem carbon and water exchange. Front. Plant Sci. 2014, 5, 530. [Google Scholar] [CrossRef]
- Mazzochini, G.G.; Camargo, J.L.C. Understory plant interactions along a successional gradient in central Amazon. Plant Soil 2020, 450, 81–92. [Google Scholar] [CrossRef]
- Gilliam, F. The Herbaceous Layer in Forests of Eastern North America; Oxford Scholarship Online; Oxford University: Oxford, UK, 2014; pp. 1–24. [Google Scholar]
- De Long, J.R.; Dorrepaal, E.; Kardol, P.; Nilsson, M.C.; Teuber, L.M.; Wardle, D.A. Understory plant functional groups and litter species identity are stronger drivers of litter decomposition than warming along a boreal forest post-fire successional gradient. Soil Biol. Biochem. 2016, 98, 159–170. [Google Scholar] [CrossRef]
- Trentini, C.P.; Villagra, M.; Pámies, D.G.; Laborde, V.B.; Bedano, J.C.; Campanello, P.I. Effect of nitrogen addition and litter removal on understory vegetation, soil mesofauna, and litter decomposition in loblolly pine plantations in subtropical Argentina. For. Ecol. Manag. 2018, 429, 133–142. [Google Scholar] [CrossRef]
- Wardle, D.A.; Nilsson, M.C.; Zackrisson, O.; Gallet, C. Determinants of litter mixing effects in a Swedish boreal forest. Soil Biol. Biochem. 2003, 35, 827–835. [Google Scholar] [CrossRef]
- Pei, Z.Q.; Leppert, K.N.; Eichenberg, D.; Bruelheide, H.; Niklaus, P.A.; Buscot, F.; Gutknecht, J.L.M. Leaf litter diversity alters microbial activity, microbial abundances, and nutrient cycling in a subtropical forest ecosystem. Biogeochemistry 2017, 134, 163–181. [Google Scholar] [CrossRef]
- Zimonick, B.J.; Simard, S.W.; Roach, W.J. Selective removal of paper birch increases growth of juvenile Douglas-fir while minimizing impacts on the plant community. Scand. J. For. Res. 2017, 32, 708–716. [Google Scholar] [CrossRef]
- Otsing, E.; Barantal, S.; Anslan, S.; Koricheva, J.; Tedersoo, L. Litter species richness and composition effects on fungal richness and community structure in decomposing foliar and root litter. Soil Biol. Biochem. 2018, 125, 328–339. [Google Scholar] [CrossRef]
- Xiao, W.Y.; Chen, H.Y.H.; Kumar, P.; Chen, C.; Guan, Q.W. Multiple interactions between tree composition and diversity and microbial diversity underly litter decomposition. Geoderma 2019, 341, 161–171. [Google Scholar] [CrossRef]
- Jones, A.R.; Dalal, R.C. Enrichment of natural 15N abundance during soil N losses under 20 years of continuous cereal cropping. Sci. Total Environ. 2017, 574, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Li, F.L.; McCormack, M.L.; Liu, X.; Hu, H.; Feng, D.F.; Bao, W.K. Vertical fine-root distributions in five subalpine forest types shifts with soil properties across environmental gradients. Plant Soil 2020, 456, 129–143. [Google Scholar] [CrossRef]
- Zhang, C.; Stratópoulos, L.M.F.; Xu, C.; Pretzsch, H.; Rötzer, T. Development of fine root biomass of two contrasting urban tree cultivars in response to drought stress. Forests 2020, 11, 108. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Zhao, X.Q.; Chen, Y.L.; Zhang, L.Y.; Shen, R.F. Case of a stronger capability of maize seedlings to use ammonium being responsible for the higher 15N recovery efficiency of ammonium compared with nitrate. Plant Soil 2019, 440, 293–309. [Google Scholar] [CrossRef]
- Iqbal, A.; Dong, Q.; Wang, X.R.; Gui, H.P.; Zhang, H.H.; Pang, N.C.; Zhang, X.L.; Song, M.Z. Nitrogen preference and genetic variation of cotton genotypes for nitrogen use efficiency. J. Sci. Food Agric. 2020, 100, 2761–2773. [Google Scholar] [CrossRef]
- Veres, Z.; Kotroczó, Z.; Fekete, I.; Tóth, J.A.; Lajtha, K.; Townsend, K.; Tóthmérész, B. Soil extracellular enzyme activities are sensitive indicators of detrital inputs and carbon availability. Appl. Soil Ecol. 2015, 92, 18–23. [Google Scholar] [CrossRef]
- Yan, H.M.; Yang, F.; Gao, J.M.; Peng, Z.H.; Chen, W.M. Subsoil microbial community responses to air exposure and legume growth depend on soil properties across different depths. Sci. Rep. 2019, 9, 18536. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Z.; Bao, X.L.; Yang, Y.L.; Zhao, Y.; Liang, C.; Xie, H.T. Comparison of soil phosphorus and phosphatase activity under long-term no-tillage and maize residue management. Plant Soil Environ. 2019, 65, 408–415. [Google Scholar] [CrossRef]
- Barel, J.M.; Kuyper, T.W.; de Boer, W.; De Deyn, G.B. Plant presence reduces root and shoot litter decomposition rates of crops and wild relatives. Plant Soil 2019, 438, 313–327. [Google Scholar] [CrossRef]
- Chen, F.S.; Wang, G.G.; Fang, X.M.; Wan, S.Z.; Zhang, Y.; Liang, C. Nitrogen deposition effect on forest litter decomposition is interactively regulated by endogenous litter quality and exogenous resource supply. Plant Soil 2019, 437, 413–426. [Google Scholar] [CrossRef]
- Midwood, A.J.; Hannam, K.D.; Forge, T.A.; Neilsen, D.; Emde, D.; Jones, M.D. Importance of drive-row vegetation for soil carbon storage in woody perennial crops: A regional study. Geoderma 2020, 377, 114591. [Google Scholar] [CrossRef]
- Rivaie, A.A. The effects of understory vegetation on P availability in Pinus radiata forest stands: A review. J. For. Res. 2014, 25, 489–500. [Google Scholar] [CrossRef]
- Kitagawa, R.; Ueno, M.; Masaki, T. Initial effects of thinning and concomitant disturbance on the understory woody community in Japanese cedar plantation. J. For. Res. 2018, 23, 120–128. [Google Scholar] [CrossRef]
- Casals, P.; Valor, T.; Besalu, A.; Molina-Terren, D. Understory fuel load and structure eight to nine years after prescribed burning in Mediterranean pine forests. For. Ecol. Manag. 2016, 362, 156–168. [Google Scholar] [CrossRef]
- Dagan, U.; Izhaki, I. Understory vegetation in planted pine forests governs bird community composition and diversity in the eastern Mediterranean region. For. Ecosyst. 2019, 6, 29. [Google Scholar] [CrossRef]
- Duguid, M.C.; Frey, B.R.; Ellum, D.S.; Kelty, M.; Ashton, M.S. The influence of ground disturbance and gap position on understory plant diversity in upland forests of southern New England. For. Ecol. Manag. 2013, 303, 148–159. [Google Scholar] [CrossRef]
- De Stefano, A.; Blazier, M.A.; Comer, C.E.; Dean, T.J.; Wigley, T.B. Understory vegetation richness and diversity of Eucalyptus benthamii and Pinus elliottii plantations in the mid-south US. For. Sci. 2020, 66, 66–81. [Google Scholar]
- Grime, J.; Mackey, J.M.L.; Hillier, S.H.; Read, D.J. Floristic diversity in a model system using experimental microcosms. Nature 1987, 328, 420–422. [Google Scholar] [CrossRef]
- Hansson, M.; Fogelfors, H. Management of a semi-natural grassland; results from a 15-year-old experiment in southern Sweden. J. Veg. Sci. 2000, 11, 31–38. [Google Scholar] [CrossRef]
- Antonsen, H.; Olsson, P. Relative importance of burning, mowing and species translocation in the restoration of a former boreal hayfield: Responses of plant diversity and the microbial community. J. Appl. Ecol. 2005, 42, 337–347. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).