Correlation of Leaf and Root Traits of Two Angiosperm Tree Species in Northeast China under Contrasting Light and Nitrogen Availabilities
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Site
2.2. Experimental Design
2.3. Seedling Biomass above and below Ground
2.4. Leaf Morphology
2.5. Root Morphology
2.6. Soil Sampling and Chemical Analysis
2.7. Statistics
3. Results
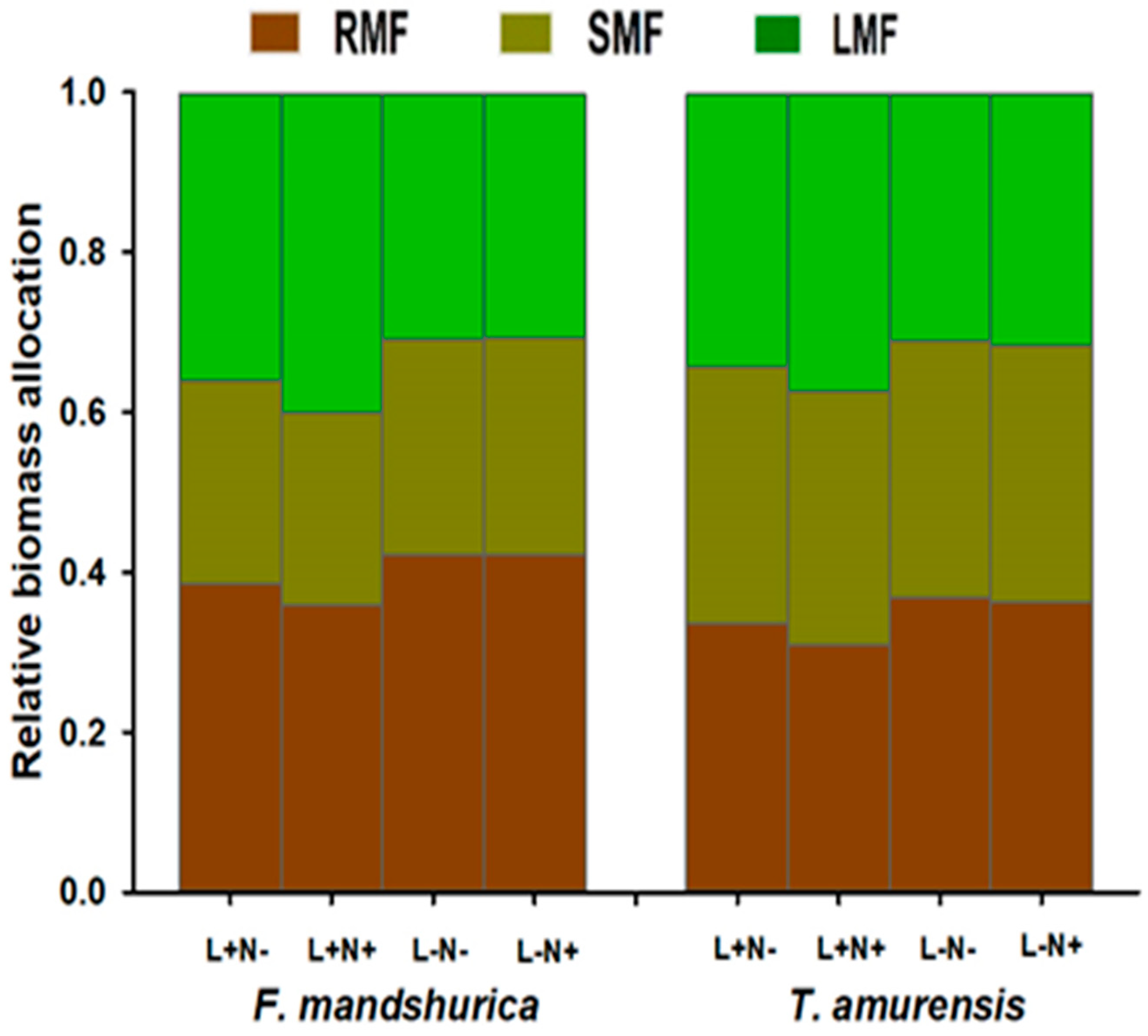
3.1. Seedling Biomass above and below Ground
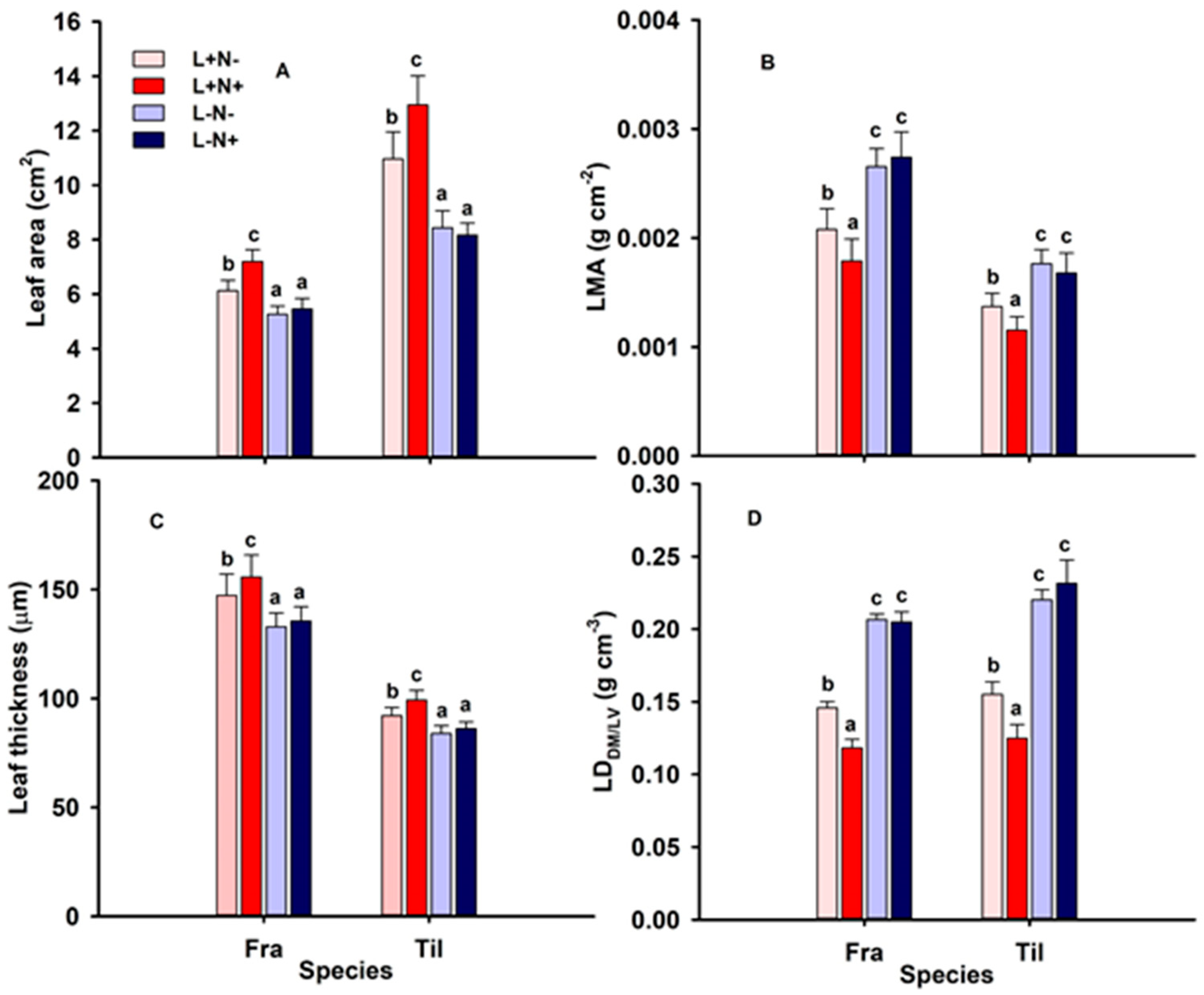
3.2. Leaf Morphology
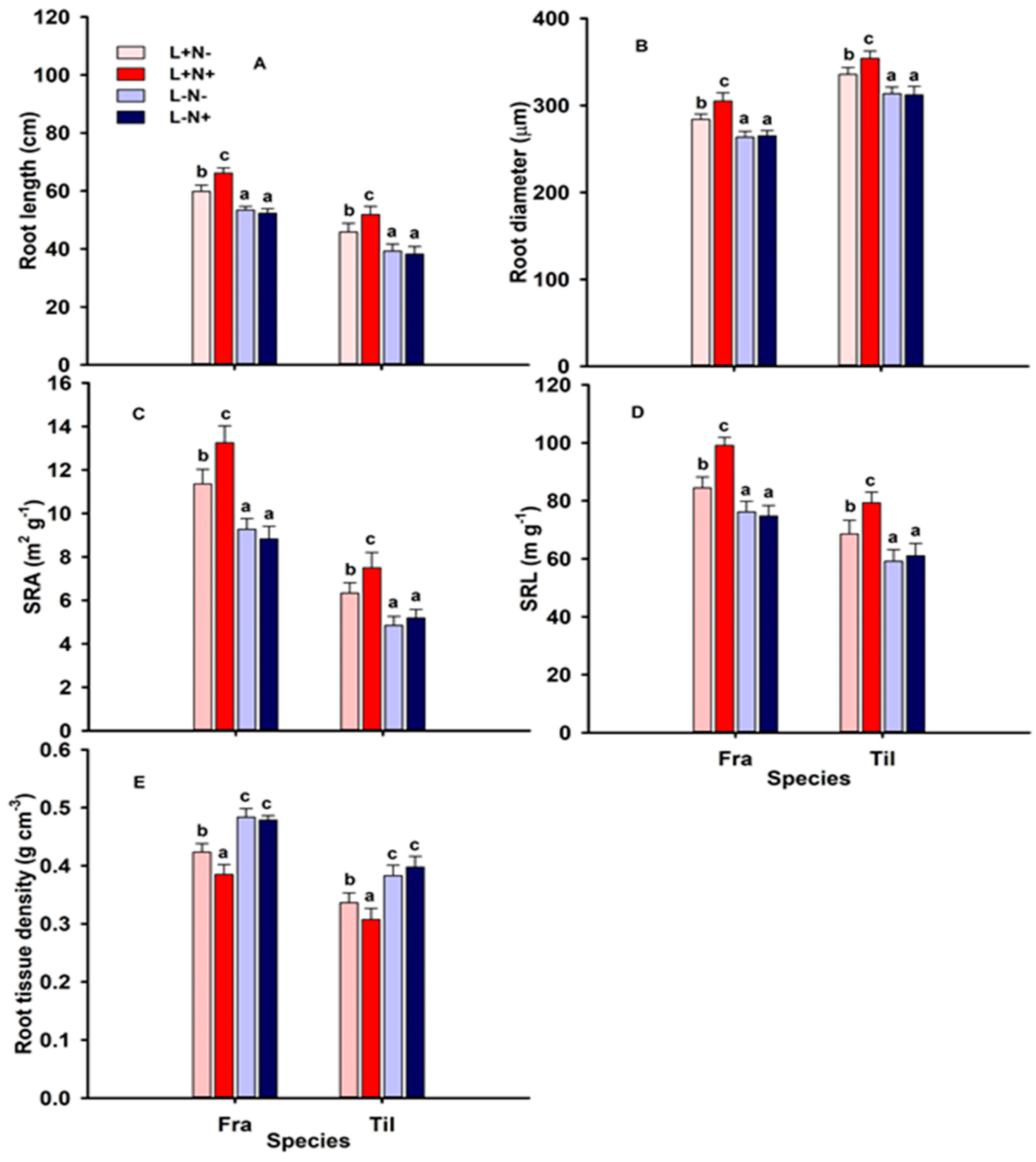
3.3. Root Length, and Morphology of Fine Roots
3.4. Correlation of Leaf and Root Functional Traits with Environmental Parameters
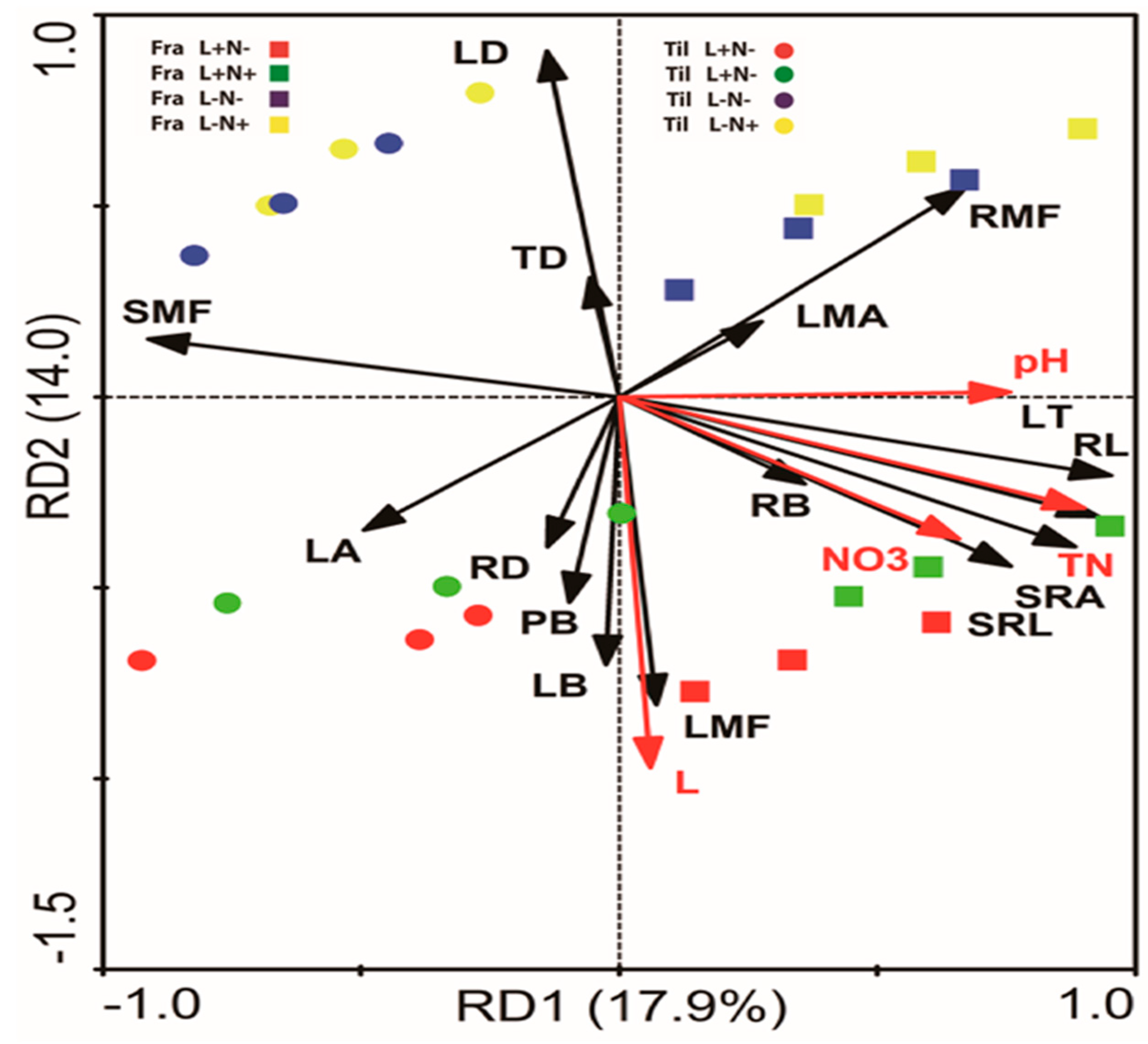
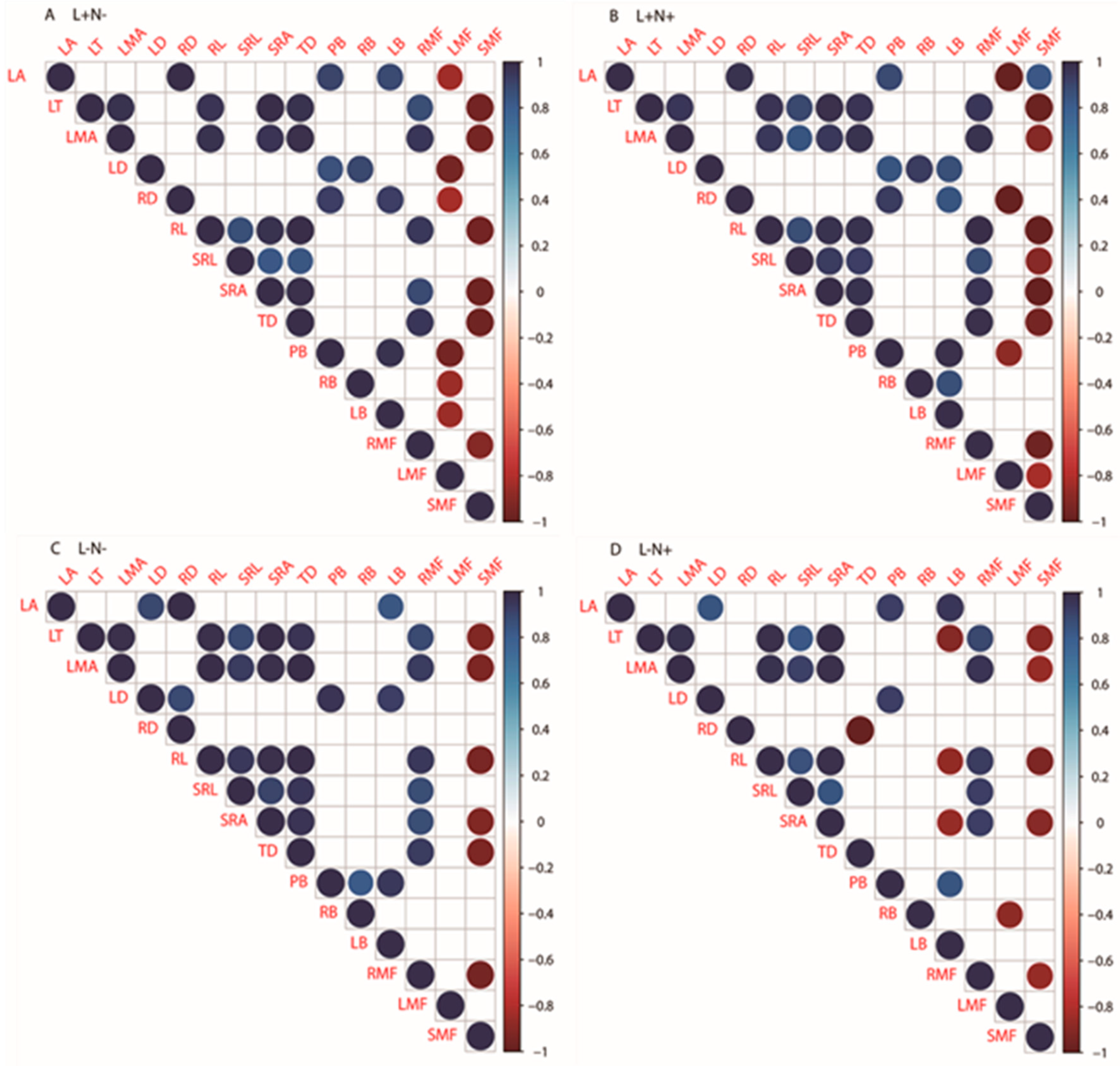
3.5. Interrelation of Leaf and Root Functional Traits
4. Discussion
4.1. Effects of Shade and N Availability on Leaf Traits
4.2. Effects of Shade and N Availability on Fine Root Traits
4.3. Interrelations of Leaf and Root Traits under Different Light Levels and N Availability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valladares, F.; Chico, J.; Aranda, I.; Balaguer, L.; Dizengremel, P.; Manrique, E.; Dreyer, E. Greater high light seedling tolerance of Quercus robur over Fagus sylvatica is linked to a greater physiological plasticity. Trees 2002, 16, 395–403. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade Tolerance, a Key Plant Feature of Complex Nature and Consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Sevillano, I.; Short, I.; Grant, J.; O’Reilly, C. Effects of light availability on morphology, growth and biomass allocation of Fagus sylvatica and Quercus robur seedlings. For. Ecol. Manag. 2016, 374, 11–19. [Google Scholar] [CrossRef]
- Lusk, C. Leaf area accumulation helps juvenile trees tolerate shade in a temperate rainforest. Oecologia 2002, 132, 188–196. [Google Scholar] [CrossRef]
- Lusk, C. Leaf area and growth of juvenile temperate evergreens in low light: Species of contrasting shade tolerance change rank during ontogeny. Funct. Ecol. 2004, 18, 820–828. [Google Scholar] [CrossRef]
- Capers, R.; Chazdon, R.; Brenes, A.; Alvarado, B. Successional dynamics of woody seedling communities in wet tropical secondary forests. J. Ecol. 2005, 93, 1071–1084. [Google Scholar] [CrossRef]
- Myers, J.; Kitajima, K. Carbohydrate storage enhances seedling shade and stress tolerance in a Neotropical forest. Ecology. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Kelly, V.R.; Canham, C.D. Resource heterogeneity in old fields. J. Veg. Sci. 1992, 3, 545–552. [Google Scholar] [CrossRef]
- Canham, C.D.; Finzi, A.C.; Pacala, S.W.; Burbank, D.H. Causes and consequences of resource heterogeneity in forests: Interspecific variation in light transmission by canopy trees. Can. J. For. Res. 1994, 24, 337–349. [Google Scholar] [CrossRef]
- Poorter, L. Light-dependent changes in allocation and their effects on the growth of rain forest tree species. Funct. Ecol. 2001, 15. [Google Scholar] [CrossRef]
- Poorter, L.; Arets, E. Light environment and tree strategies in a Bolivian tropical moist forest: An evaluation of the light partitioning hypothesis. Plant Ecol. 2003, 166, 295–306. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Valladares, F. Tolerance to shade, drought and waterlogging in the temperate dendroflora of the Northern hemisphere: Tradeoffs, phylogenetic signal and implications for niche differentiation. Ecol. Monogr. 2006, 76, 521–547. [Google Scholar] [CrossRef]
- Fownes, J.; Harrington, R. Seedling response to gaps: Separating effects of light and nitrogen. For. Ecol. Manag. 2004, 203, 297–310. [Google Scholar] [CrossRef]
- Saldaña-Acosta, A.; Meave, J.; Sánchez-Velásquez, L. Seedling biomass allocation and vital rates of cloud forest tree species: Responses to light in shade house conditions. For. Ecol. Manag. 2009, 258, 1650–1659. [Google Scholar] [CrossRef]
- Niinemets, Ü. Growth of Young Trees of Acer platanoides and Quercus robur Along a Gap- Understory Continuum: Interrelationships between Allometry, Biomass Partitioning, Nitrogen, and Shade Tolerance. Int. J. Plant Sci. 1998, 159, 318–330. [Google Scholar] [CrossRef]
- Sanford, N.; Harrington, R.; Fownes, J. Survival and growth of native and alien woody seedlings in open and understory environments. For. Ecol. Manag. 2003, 183, 377–385. [Google Scholar] [CrossRef]
- Harrington, R.; Brown, B.; Reich, P. Ecophysiology of exotic and native shrubs in Southern Wisconsin. Oecologia 1989, 80, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L. Growth responses of fifteen rain forest tree species to a light gradient: The relative importance of morphological and physiological traits. Funct. Ecol. 1999, 13. [Google Scholar] [CrossRef]
- Paz, H. Root/Shoot Allocation and Root Architecture in Seedlings: Variation among Forest Sites, Microhabitats, and Ecological Groups. Biotropica 2003, 35, 318–332. [Google Scholar] [CrossRef]
- He, W.; Wu, F.; Yang, W.; Zhang, D.; Xu, Z.; Tan, B.; Zhao, Y.; Justine, M.F. Gap locations influence the release of carbon, nitrogen and phosphorus in two shrub foliar litter in an alpine fir forest. Sci. Rep. 2016, 6, 22014. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, J.; Yang, W.; Yin, R.; Xu, Z.; Liu, Y.; Zhang, L.; Li, H.; You, C. Forest gaps retard carbon and nutrient release from twig litter in alpine forest ecosystems. Eur. J. For. Res. 2020, 139, 53–65. [Google Scholar] [CrossRef]
- Kondori, A.A.; Vajari, K.A.; Feizian, M.; Montagnoli, A.; Di Iorio, A. Gap Size in Hyrcanian Forest Affects the Lignin and N Concentrations of the Oriental Beech (Fagus orientalis Lipsky) Fine Roots but Does Not Change Their Morphological Traits in the Medium Term. Forests 2021, 12, 137. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, F.; Yang, W.; Zhao, Y.; He, W.; Tan, B. Foliar Litter Nitrogen Dynamics as Affected by Forest Gap in the Alpine Forest of Eastern Tibet Plateau. PLoS ONE 2014, 9, e97112. [Google Scholar] [CrossRef]
- Aerts, R.; van Bodegom, P.M.; Cornelissen, J.H. Litter stoichiometric traits of plant species of high-latitude ecosystems show high responsiveness to global change without causing strong variation in litter decomposition. New Phytol. 2012, 196, 181–188. [Google Scholar] [CrossRef]
- Bilbrough, C.; Caldwell, M. The effect of shading and N status on root proliferation in nutrient patches by the perennial grass Agropyron desertorum in the field. Oecologia 1995, 103, 10–16. [Google Scholar] [CrossRef]
- Khan, A.; Sun, J.; Zarif, N.; Khan, K.; Jamil, M.A.; Yang, L.; Clothier, B.; Rewald, B. Effects of Increased N Deposition on Leaf Functional Traits of Four Contrasting Tree Species in Northeast China. Plants 2020, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.; Gurvich, D.; et al. New handbook for standardise measurement of plant functional traits worldwide. Aus. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Denslow, J.; Ellison, A.; Sanford Jr., R. Treefall gap size effects on above- and below-ground processes in a tropical wet forest. J. Ecol. 2001, 86, 597–609. [Google Scholar] [CrossRef]
- Craine, J.; Lee, W. Covariation in leaf and root traits for native and non-native grasses along an altitudinal gradient in New Zealand. Oecologia 2003, 134, 471–478. [Google Scholar] [CrossRef]
- Wahl, S.; Ryser, P. Root tissue structure is linked to ecological strategies of grasses. New Phytol. 2002, 148, 459–471. [Google Scholar] [CrossRef]
- Comas, L.; Bouma, T.; Eissenstat, D. Linking root traits to potential growth rate in six temperate tree species. Oecologia 2002, 132, 34–43. [Google Scholar] [CrossRef]
- Withington, J.; Reich, P.; Oleksyn, J.; Eissenstat, D. Comparison of structure and life span in roots and leaves among temperate trees. Ecol. Monogr. 2006, 76, 381–397. [Google Scholar] [CrossRef]
- Liu, G.; Freschet, G.; Pan, X.; Cornelissen, J.; Li, Y.; Dong, M. Coordinated variation in leaf and root traits across multiple spatial scales in Chinese semi-arid and arid ecosystems. New Phytol. 2010, 188, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Ma, C.; Zhang, Q.; Li, L.; Chen, X.; Zeng, H.; Guo, D. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol. 2014, 203. [Google Scholar] [CrossRef]
- Reich, P. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102. [Google Scholar] [CrossRef]
- Blomberg, S.; Garland, T.; Ives, A. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 2003, 57, 717–745. [Google Scholar] [CrossRef]
- Adair, K.; Lindgreen, S.; Poole, A.; Young, L.; Bernard-Verdier, M.; Wardle, D.; Tylianakis, J. Above and belowground community strategies respond to different global change drivers. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Qi, Y.; Huang, J.-L.; Zhang, S.-B. Correlated evolution of leaf and root anatomic traits in Dendrobium (Orchidaceae). AoB PLANTS 2020, 12, plaa034. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef]
- Morales, A.; Kaiser, E. Photosynthetic Acclimation to Fluctuating Irradiance in Plants. Front. Plant Sci. 2020, 11, 268. [Google Scholar] [CrossRef]
- Pan, Y.P.; Wang, Y.S.; Tang, G.Q.; Wu, D. Wet and dry deposition of atmospheric nitrogen at ten sites in Northern China. Atmos. Chem. Phys. 2012, 12, 6515–6535. [Google Scholar] [CrossRef]
- Gorfer, M.; Mayer, M.; Berger, H.; Rewald, B.; Tallian, C.; Matthews, B.; Sandén, H.; Katzensteiner, K.; Godbold, D.L. High Fungal Diversity but Low Seasonal Dynamics and Ectomycorrhizal Abundance in a Mountain Beech Forest. Microb. Ecol. 2021. [Google Scholar] [CrossRef]
- Poorter, H.; Jagodzinski, A.M.; Ruiz-Peinado, R.; Kuyah, S.; Luo, Y.; Oleksyn, J.; Usoltsev, V.A.; Buckley, T.N.; Reich, P.B.; Sack, L. How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytol. 2015, 208, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Oguchi, R.; Hikosaka, K. Does the change in light acclimation need leaf anatomy? Plant Cell Environ. 2003, 26, 505–512. [Google Scholar] [CrossRef]
- De la Riva, E.G.; Olmo, M.; Poorter, H.; Ubera, J.L.; Villar, R. Leaf Mass per Area (LMA) and Its Relationship with Leaf Structure and Anatomy in 34 Mediterranean Woody Species along a Water Availability Gradient. PLoS ONE 2016, 11, e0148788. [Google Scholar] [CrossRef]
- Coble, A.; Cavaleri, M. Light drives vertical gradients of leaf morphology in a sugar maple (Acer saccharum) forest. Tree Physiol. 2014, 34. [Google Scholar] [CrossRef] [PubMed]
- Pregitzer, K.; Deforest, J.; Burton, A.; Allen, M.; Ruess, R.; Hendrick, R. Fine Root Architecture of Nine North American Trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Rao, C.R. The Use and Interpretation of Principal Component Analysis in Applied Research. Sankhyā IJS 1964, 26, 329–358. [Google Scholar]
- Legendre, P.; Oksanen, J.; ter Braak, C. Testing the significance of canonical axes in redundancy analysis. Meth. Ecol. Evol. 2011, 2, 269–277. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; Stevens, M.; Wagner, H. Community ecology package: Ordination, diversity and dissimilarities. J. Veg. Sci. 2013, 2, 1–8. [Google Scholar]
- Wei, T.; Simko, V.J. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84); R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Gaudio, N.; Gendre, X.; Saudreau, M.; Seigner, V.; Balandier, P. Impact of tree canopy on thermal and radiative microclimates in a mixed temperate forest: A new statistical method to analyse hourly temporal dynamics. Agric. For. Meteor. 2017, 237–238, 71–79. [Google Scholar] [CrossRef]
- Holladay, C.A.; Kwit, C.; Collins, B. Woody regeneration in and around aging southern bottomland hardwood forest gaps: Effects of herbivory and gap size. For. Ecol. Manage 2006, 223, 218–225. [Google Scholar] [CrossRef]
- Kalcsits, L.; Musacchi, S.; Layne, D.R.; Schmidt, T.; Mupambi, G.; Serra, S.; Mendoza, M.; Asteggiano, L.; Jarolmasjed, S.; Sankaran, S. Above and below-ground environmental changes associated with the use of photoselective protective netting to reduce sunburn in apple. Agric. For. Meteor 2017, 237–238, 9–17. [Google Scholar] [CrossRef]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z. Shade netting on subtropical fruit: Effect on environmental conditions, tree physiology and fruit quality. Sci. Hort. 2019, 256, 108556. [Google Scholar] [CrossRef]
- Mayer, M.; Rewald, B.; Matthews, B.; Sandén, H.; Rosinger, C.; Katzensteiner, K.; Gorfer, M.; Berger, H.; Tallian, C.; Berger, T.; et al. Soil fertility relates to fungal-mediated decomposition and organic-matter turnover in a temperate mountain forest. New Phytol. 2021. [Google Scholar] [CrossRef]
- Ataka, M.; Sun, L.; Nakaji, T.; Katayama, A.; Hiura, T. Five-year nitrogen addition affects fine root exudation and its correlation with root respiration in a dominant species, Quercus crispula, of a cool temperate forest, Japan. Tree Physiol. 2020, 40, 367–376. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Z.; Wang, Z.; Chen, Y.; Wen, Z.; Liu, B.; Tigabu, M. Responses of leaf morphology, NSCs contents and C:N:P stoichiometry of Cunninghamia Lanceolata and Schima Superba to shading. BMC Plant Biol. 2020, 20, 354. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.; Adams, C.; Holsinger, K. Intraspecific variation in stomatal traits, leaf traits and physiology reflects adaptation along aridity gradients in a South African shrub. Ann. Bot. 2015, 117. [Google Scholar] [CrossRef]
- Walters, M.; Kruger, E.; Reich, P. Relative growth rate in relation to physiological and morphological traits for northern hardwood tree seedlings: Species, light environment and ontogenetic considerations. Oecologia 1993, 96, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Components of leaf dry mass per area: Thickness and density: Alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol. 1999, 144, 35–47. [Google Scholar] [CrossRef]
- Mao, R.; Zhang, X.-H.; Song, C. Effects of nitrogen addition on plant functional traits in freshwater wetland of Sanjiang Plain, Northeast China. Chin. Geogr. Sci. 2014, 24, 674–681. [Google Scholar] [CrossRef]
- Villar, R.; Ruiz-Robleto, J.; Ubera, J.; Poorter, H. Exploring variation in leaf mass per area (LMA) from leaf to cell: An anatomical analysis of 26 woody species. Am. J. Bot. 2013, 100. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; He, N.; Yu, G. Evaluation of leaf non-structural carbohydrate contents in typical forest ecosystems in northern China. Acta Ecol. Sin. 2016, 36. [Google Scholar] [CrossRef]
- King, D.A. Allocation of above-ground growth is related to light in temperate deciduous saplings. Funct. Ecol. 2003, 17, 482–488. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, C.; Zhou, Z.; Gu, J. Contrasting responses of hydraulic traits between leaf and branch to 16-year nitrogen addition in a larch plantation. For. Ecol. Manag. 2020, 475, 118461. [Google Scholar] [CrossRef]
- Ostonen, I.; Lõhmus, K.; Helmisaari, H.-S.; Truu, J.; Meel, S. Fine root morphological adaptations in Scots pine, Norway spruce and silver birch along a latitudinal gradient in boreal forests. Tree Physiol. 2007, 27, 1627–1634. [Google Scholar] [CrossRef]
- Noguchi, K.; Nagakura, J.; Kaneko, S. Biomass and morphology of fine roots of sugi (Cryptomeria japonica) after 3 years of nitrogen fertilization. Front. Plant Sci. 2013, 4, 347. [Google Scholar] [CrossRef]
- Kou, L.; Guo, D.; Yang, H.; Gao, W.; Li, S. Growth, morphological traits and mycorrhizal colonization of fine roots respond differently to nitrogen addition in a slash pine plantation in subtropical China. Plant Soil 2015, 391, 207–218. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Hoch, G.; Wang, Z.; Gu, J. Linkage of root morphology to anatomy with increasing nitrogen availability in six temperate tree species. Plant Soil 2018, 425, 189–200. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, H.; Ji, L.; Yang, L. Effects of exponential fertilization on growth and root morphology of Tilia amurensis seedlings. J. Nanjing For. Univ. Nat. Sci. Ed. 2019. [Google Scholar] [CrossRef]
- Kramer-Walter, K.; Laughlin, D. Root nutrient concentration and biomass allocation are more plastic than morphological traits in response to nutrient limitation. Plant Soil. 2017, 416. [Google Scholar] [CrossRef]
- Cui, C. Shading reduces exploitation of soil nitrate and phosphate by Agropyron desertorum and Artemisia tridentata from soils with patchy and uniform nutrient distributions. Oecologia 1996, 109, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Eissenstat, D. On the relationship between specific root length and the rate of root proliferation: A field study using citrus rootstocks. New Phytol. 1991, 118, 63–68. [Google Scholar] [CrossRef]
- Gross, J.; Hobbs, N.; Wunder, B. Independent Variables for Predicting Intake Rate of Mammalian Herbivores: Biomass Density, Plant Density, or Bite Size? Oikos 1993, 68, 75–81. [Google Scholar] [CrossRef]
- Shaver, G.; Billings, W. Root Production and Root Turnover in a Wet Tundra Ecosystem, Barrow, Alaska. Ecology 1975, 56, 401. [Google Scholar] [CrossRef]
- Jackson, R.; Caldwell, M. Shading and the capture of localized soil nutrients: Nutrient contents, carbohydrates, and root uptake kinetics of a perennial tussock grass. Oecologia 1992, 91, 457–462. [Google Scholar] [CrossRef]
- Freschet, G.; Pagès, L.; Iversen, C.; Comas, L.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.; et al. A starting guide to root ecology: Strengthening ecological concepts and standardizing root classification, sampling, processing and trait measurements. New Phytol. 2021. [Google Scholar]
- Hernández, E.; Vilagrosa, A.; Pausas, J.; Bellot, J. Morphological traits and water use strategies in seedlings of Mediterranean coexisting species. Plant. Ecol. 2010, 207, 233–244. [Google Scholar] [CrossRef]
- Zadworny, M.; Comas, L.; Eissenstat, D. Linking fine root morphology, hydraulic functioning and shade tolerance of trees. Ann. Bot. 2018, 122. [Google Scholar] [CrossRef]
- Fortunel, C.; Fine, P.; Baraloto, C. Leaf, stem and root tissue strategies across 758 Neotropical tree species. Funct. Ecol. 2012, 26, 1153–1161. [Google Scholar] [CrossRef]
- Baraloto, C.; Rabaud, S.; Molto, Q.; Blanc, L.; Fortunel, C.; Herault, B.; Davila, N.; Mesones, I.; Paredes, M.; Valderrama, E. Disentangling stand and environmental correlates of aboveground biomass in Amazonian forests. Glob. Chang. Biol. 2011, 17, 2677–2688. [Google Scholar] [CrossRef]
- Grime, J.; Thompson, K.; Hunt, R.; Hodgson, J.; Cornelissen, J.; Rorison, I.; Hendry, G.; Ashenden, T.; Askew, A.; Band, S. Integrated Screening Validates Primary Axes of Specialisation in Plants. Oikos 1997, 79, 259–281. [Google Scholar] [CrossRef]
- Wright, I.; Westoby, M. Leaves at low versus high rainfall: Coordination of structure, lifespan and physiology. New Phytol. 2002, 155, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, E.; Lamont, B. Leaf specific mass confounds leaf density and thickness. Oecologia 1991, 88, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Lambers, H.; Evans, J.R. Trait correlation networks: A whole-plant perspective on the recently criticized leaf economic spectrum. New Phytol. 2014, 201, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Alonzo, R.; Paz, H.; Zuluaga, R.C.; Rosell, J.A.; Olson, M.E. Coordinated evolution of leaf and stem economics in tropical dry forest trees. Ecology 2012, 93, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
| Source of Variation | df | TN | NO3 | pH | PB | RB | LB | RMF | LMF | SMF |
|---|---|---|---|---|---|---|---|---|---|---|
| Species (Sp) | 1 | <0.001 | 0.045 | <0.001 | 0.003 | 0.871 | 0.001 | <0.001 | 0.265 | <0.001 |
| Light (L) | 1 | 0.017 | 0.003 | 0.957 | <0.001 | 0.081 | <0.001 | 0.001 | <0.001 | <0.001 |
| Nitrogen (N) | 1 | 0.349 | 0.019 | 0.020 | 0.036 | 0.523 | <0.001 | 0.201 | 0.015 | 0.620 |
| Sp × L | 1 | 0.661 | 0.969 | 0.291 | 0.456 | 0.842 | 0.953 | 0.759 | <0.001 | 0.152 |
| L × N | 1 | 0.908 | 0.649 | 0.905 | 0.048 | 0.496 | <0.001 | 0.285 | 0.032 | 0.604 |
| Source of Variation | df | Leaf Area | LT | LMA | LDDM/LV | |
|---|---|---|---|---|---|---|
| Species (Sp) | 1 | <0.001 | <0.001 | <0.001 | 0.034 | |
| Light (L) | 1 | <0.001 | 0.008 | <0.001 | <0.001 | |
| Nitrogen (N) | 1 | 0.121 | 0.281 | 0.316 | 0.064 | |
| Sp × L | 1 | 0.020 | 0.485 | 0.230 | 0.334 | |
| L × N | 1 | 0.105 | 0.567 | 0.314 | 0.013 | |
| Root length | Root diameter | SRA | SRL | TD | ||
| Species (Sp) | 1 | <0.001 | 0.428 | <0.001 | <0.001 | <0.001 |
| Light (L) | 1 | <0.001 | 0.077 | <0.001 | <0.001 | 0.003 |
| Nitrogen (N) | 1 | 0.138 | 0.099 | 0.091 | 0.069 | 0.226 |
| L × N | 1 | 0.044 | 0.101 | 0.070 | 0.080 | 0.112 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Zarif, N.; Yang, L.; Clothier, B.; Rewald, B. Correlation of Leaf and Root Traits of Two Angiosperm Tree Species in Northeast China under Contrasting Light and Nitrogen Availabilities. Forests 2021, 12, 596. https://doi.org/10.3390/f12050596
Khan A, Zarif N, Yang L, Clothier B, Rewald B. Correlation of Leaf and Root Traits of Two Angiosperm Tree Species in Northeast China under Contrasting Light and Nitrogen Availabilities. Forests. 2021; 12(5):596. https://doi.org/10.3390/f12050596
Chicago/Turabian StyleKhan, Attaullah, Nowsherwan Zarif, Lixue Yang, Brent Clothier, and Boris Rewald. 2021. "Correlation of Leaf and Root Traits of Two Angiosperm Tree Species in Northeast China under Contrasting Light and Nitrogen Availabilities" Forests 12, no. 5: 596. https://doi.org/10.3390/f12050596
APA StyleKhan, A., Zarif, N., Yang, L., Clothier, B., & Rewald, B. (2021). Correlation of Leaf and Root Traits of Two Angiosperm Tree Species in Northeast China under Contrasting Light and Nitrogen Availabilities. Forests, 12(5), 596. https://doi.org/10.3390/f12050596









