Abstract
During a survey of diseased plants on Wando Island, Korea from May to June 2020, a severe leaf spot disease was observed in the upper leaves of Japanese bay tree (Machilus thunbergii). Early symptoms were light blackish spots on the leaf surface and enlargement of older spots. Dry leaf spots surrounded with deep black margins were common throughout the plants. Symptomatic leaf samples were collected, and the causal pathogen was isolated on potato dextrose agar (PDA). Three fungal isolates (CMML20-1, CMML20-3, and CMML20-4) were cultured on PDA for morphological characterization at 25 °C in the darkness. Fungal colonies were circular, fast-growing, olivaceous to dark grey, and with abundant aerial mycelium. Sporulation was induced in 14 h-10 h light-dark conditions, and the conidia were single-celled, thin-walled with a smooth surface, ellipsoid with round apices, and measuring 17.5–20.5 (avg. 17.5) μm × 7.5–10.0 (7.9) μm. The morphological characteristics resembled those typical for Neofusicoccum parvum. Molecular identification was confirmed by partially sequencing the internal transcribed spacer (ITS) region and the translation elongation factor 1-α (EF1-α) genes. Pathogenicity tests were conducted on detached leaves and whole plants of M. thunbergii. High disease prevalence was observed, and Koch postulates were fulfilled. This is the first worldwide report of N. parvum causing leaf spots on Machilus thunbergii.
1. Introduction
Machilus thunbergii Sieb. & Zucc., commonly known as the Japanese bay tree, is a member of the Laurel family (Lauraceae), a diverse and widespread group found throughout tropical and subtropical forest areas of the world, especially in southern Korea, Japan, the Bonin Islands, the Ryukyus, Taiwan, and the warm temperate zones of China and the Philippines [1,2,3]. It is a broad-leaved evergreen tree with the potential to grow 30 m tall. Parts of M. thunbergii plants have been used as a traditional medicine to treat edema, abdominal pain, and abdominal distension in Korea, China, and Japan [4,5]. Due to the destruction of natural resources and the ecological environment, M. thunbergii has been listed as a key wild plant protected nationwide in China [1]. Although plant pathogens commonly cause diseases in this plant, few fungal pathogens have been reported for this plant in Japan, China, Korea, and Taiwan. The fungi isolated from Korea were Endophyllum machili (Henn.) F. Stevens, Phomopsis sp., PhytophthoMra cinnamomi Rands, and Glomerella cingulata (Stoneman) Spauld. & H. Schrenk [6].
Neofusicoccum species, commonly cause diseases in woody plants worldwide [7]. N. parvum (Pennycook & Samuels) [8] infects a wide range of host plants and can induce disease in plants [9]. Sakalidis et al. [7] collected, identified, and examined a total of 169 N. parvum from Australia, Chile, Colombia, Hawaii, Indonesia, New Zealand, and South Africa from different host plants including Eucalyptus obliqua L’Hér., Syzgium cordatum Hochst. ex Krauss, Actinidia sp. Lindl., Araucaria sp. Juss., Cinnamomum camphora (L.) J. Presl., Malus sylvestris (L.) Mill., Mangifera indica L., Populus nigra(L.), Ribis sp., and Tibouchina lepidota Baill. The pathogen has been isolated from many other plants such as pome and stone fruit trees [10], blueberries [11], grapevines [12], Island pines [13], and peaches [14]. Their study confirmed that the fungus is widely distributed. In the present study, it was observed that the pathogen severely infects the leaves of M. thunbergii plants in the forest areas of Wando Island in Korea.
Neofusicoccum parvum has been reported as the causal agent of branch cankers, dieback, leaf spots, shoot blight, fruit rot, and trunk diseases [6,15]. Although N. parvum has been associated with a number of plants and causing serious diseases, there has been no detailed investigation of the importance of this pathogen in M. thunbergii. The present study aimed to detect the disease, characterize the causal pathogen, and test and compare the pathogenicity using different inoculation methods.
2. Materials and Methods
2.1. Fungal Isolation
During May to June 2020, a severe leaf spot disease was observed on the Japanese bay tree (M. thunbergii) on Wando island of the Republic of Korea (latitude 34°22′16.9′′ N, length 126°38′31.6′′ E). The island is in the southwestern part of the country and is famous for its many species of forest trees. The disease was observed on the natural vegetation of the plants and severe disease was prevalent throughout the island. Leaf spots leading to necrosis appeared on the foliage and lesions developed on the margin or in the center of the leaves with a chlorotic halo. The spots were common in the early stage of disease formation; spots enlarged with age throughout the leaves and defoliation followed by interrupted plant growth was common (Figure 1A,D). Infected leaves were collected in plastic polyethylene bags, brought to the laboratory, and stored in a refrigerator prior to the isolation of the pathogen. For isolation, the surfaces of the diseased leaves were sterilized with 1% NaOCl solution for 1 min, rinsed with sterilized distilled water three times, and then air-dried on filter paper in a laminar airflow chamber. Each leaf was then cut into small pieces with a sterile scalpel and placed 5 to 6 segments onto potato dextrose agar (PDA) supplemented with 50 μg/mL of streptomycin and ampicillin (MB cell, Seoul, Korea). After incubation at 25 °C for 3–10 days, individual hyphal tips of the developing fungal colonies were placed onto PDA and further incubated for 5–10 days for culture purity. Fungi with similar colony morphology were obtained. Three representative isolates were selected, assigned identification numbers (CMML20-1, CMML20-3, and CMML20-4), and preserved in the Molecular Microbiology Lab., Chonnam National University, Gwangju, Republic of Korea. The fungal isolates were preserved in 20% glycerol stock solution at −80 °C and PDA slant tubes at 4 °C.
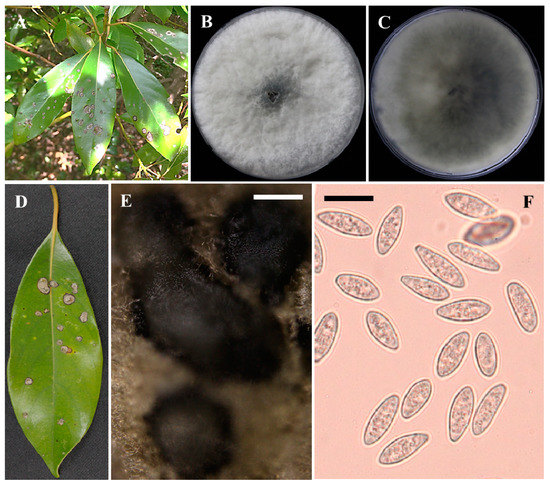
Figure 1.
The symptoms of leaves of Japanese bay tree and the morphology of Neofusicoccum parvum. Symptoms occurring naturally on leaves of Machilus thunbergii on Wando Island, Korea during May to June 2020 (A,D). Obverse and reverse colony morphology on PDA at 25 °C for 7 days (B,C). Pycnidia and conidia induced to form under 14 h–10 h light dark conditions (E,F). Scale bars E = 100 μm and F = 25 μm.
2.2. Morphology
For colony morphology, the fungus (CMML20-1) was cultured on PDA at 25 °C in the dark for 7 days (Figure 1B). For microscopic observation, 5–7-day-old mycelia, were scratched off and incubated under NUV (near-ultraviolet) light in 14 h–10 h light-dark conditions for 3–5 days [16]. The size (n = 30) and shape of the conidia were determined under a microscope (Olympus, Tokyo, Japan).
2.3. DNA Extraction, PCR Amplification and Sequencing
To confirm the identity of the fungus, total genomic DNA was extracted directly from the mycelia grown on PDA using the CTAB DNA extraction method [17]. Two gene regions, namely the internal transcribed spacer (ITS) and elongation factor 1- alpha (EF1-α) were amplified using the primer pairs ITS1 (TCC GTA GGT GAA CCT GCG G)/ITS4 (TCC GCT TAT TGA TAT GC) [18] and EF1-728F (CAT CGA GAA GTT CGA GAA GG)/EF1-986R (TAC TTG AAG GAA CCC TTA CC) [19]. PCR amplification was carried out using a GeneAmp PCR system 2700 (Applied Biosystems, Massachusetts, USA) in a 25μL reaction volume containing 0.25 μL of Takara Ex Taq® DNA polymerase (5U/μL), 2.5 μL of 10X Ex Taq buffer, 2 μL of dNTP mixture (2.5 mM each), 1 μL of each primer (10 pmoles/μL), 1 μL template DNA solution (100 ng/μL), and up to 25 μL of sterilized distilled water. PCR amplification was carried out under the following conditions: initial denaturation at 95 °C for 1 min, followed by 35 cycles of denaturation at 95 °C for 15 s, annealing at 50 °C for ITS, 55 °C for EF1-α for 20 s, extension at 72 °C for 1min, and a final extension at 72 °C for 2 min. The PCR products were cleaned up using an ExoSAP-IT Kit (Applied Biosystems, Massachusetts, USA) according to the manufacturer’s instructions. Purified double stranded PCR products were directly sequenced with BigDye terminator cycle sequencing kits by a commercial sequencing service provider (Macrogen, Daejeon, Korea) in both directions. Gel electrophoresis and data collections were performed on an ABI prism 310 genetic analyzer (Applied Biosystems, CA, USA).
2.4. Molecular Phylogeny
Sequences of the isolates were subjected to Basic Local Alignment Search Tool (BLASTN) searches using the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov, accessed on 23 February 2021) to obtain sequence similarity. Closely related sequences were obtained from GenBank for phylogenetic analysis (Table 1) and were adjusted manually using the MEGA X program [20]. A maximum parsimony (MP) phylogenetic tree was constructed for the combined datasets of the ITS and EF1-α gene sequences.

Table 1.
Neofusicoccum species with their GenBank accession numbers used for phylogenetic analysis.
2.5. Pathogenicity
Pathogenicity tests were artificially performed on detached leaves and whole plants. For the detached leaf assay, the leaves were surface sterilized with 1% NaOCl for 3 min, and then washed with distilled water three times. The dried leaves were inoculated with agar plugs and spore suspension methods. The fungus was grown on PDA for 5 days at 25 °C and 5 mm agar plugs were taken and placed onto the adaxial and abaxial sides of leaves with wounds; non-mycelium plugs served as a control [21,22]. Spores were collected, counted using a hemocytometer, and adjusted to 1 × 105 spores/mL. Then, 20 µL of spore suspensions were wound-inoculated to the adaxial and abaxial sides of the leaves, and non-spore distilled water served as a control. The inoculated leaves were placed onto filter paper in a 90 mm petri dish containing sealed water agar (2%) supplemented with rifampicin (50 g/mL) [21]. The leaves were incubated at 25 °C for 21 days. Onset of the disease was observed after 3 days and disease severity was calculated by measuring the lengths of the lesions. In each replication, three to four leaves were used by cutting into two parts and the pathogen was inoculated into these separate parts. A total of six to eight lesions were measured to assess disease severity and the average lesion lengths were used to prepare graphs. For the whole plant assay, the fungus was grown on PDB at 25 °C for 5 days and mycelia were collected. One gram of mycelia was grinded in 30 mL sterilized distilled water. The grinded mycelia were sprayed onto the 4-year-old whole plants and incubated at 25 ± 2 °C in a culture room; control plants were sprayed with sterilized distilled water [23]. The plants were kept in high humidity conditions in a covered plastic container for 3–4 days at 25 °C. The cover was then removed, and the plants were transferred to a culture room maintained at a temperature of 25 ± 2 °C.
3. Results
3.1. Fungus Isolation and Morphological Characterization
These isolates were fast-growing and formed many aerial mycelia which were initially white but turned to gray to black over time. The reverse of the colonies was white, which also became greyish to black with aging (Figure 1C). The conidial size ranged from 17.5–25.0 (avg. 20.8) μm × 7.5–10.0 (7.9) μm. Pycnidia were produced in PDA, and the size varied from 370.6–908.9 × 234.5–506.4 μm (Figure 1E,F). Morphologically, the fungus was identical to the descriptions previously outlined for N. parvum [15,24,25].
3.2. Molecular Phylogeny
Sequences were submitted to GenBank for assigning accession numbers (Table 1). BLASTN analysis of the sequences indicated that the sequences from the present study matched well with the reliable reference sequences of Neofusicoccum parvum and showed 100% sequence similarity in ITS (strains UY1267, UY1366, and UY754), and 100% in EF1-α gene (strains EFA 470, CMW26844, and CBS 110301). On the basis of the sequences of these genes, a combined phylogenetic tree was constructed which revealed that the present study isolates (CMML20-1, CMML20-3, and CMML20-4) produced a single clade with the reliable reference strains of N. parvum (CMW 9080, CERC 3503 and CBS 123652) supported by a high bootstrap value (Figure 2). Combined phylogenetic analysis confirmed the isolate identification as N. parvum.
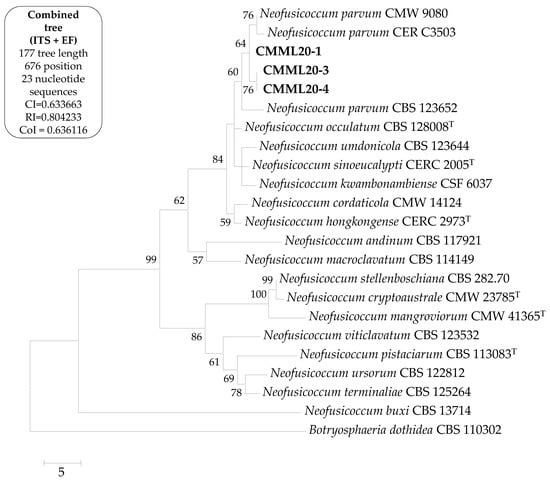
Figure 2.
A maximum parsimonious tree of the combined datasets of the internal transcribed spacer (ITS) and elongation factor 1-α (EF1-α) sequences of the present study isolates and their relatives from GenBank. The consistency index is 0.633663; the retention index is 0.804233; and the composite index is 0.636116 (0.509613) for all sites and parsimony-informative sites. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. Bootstrap values >50% are indicated above the branches. This analysis involved 23 nucleotide sequences. All positions containing gaps and missing data were eliminated. There was a total of 676 positions in the final dataset. Evolutionary analyses were conducted in MEGA X. The present study isolates are marked in bold; ‘T’ indicates type strain.
3.3. Pathogenicity Tests
It was found that disease initiation was rapid and produced significant disease in each replicate after 5 days (Figure 3A–H). Results revealed that the disease was severe in all the cases and inoculation of plugs from the fungal colony caused severe symptoms than the inoculation of spore suspensions. However, there were differences in disease severity between the adaxial and abaxial sides of the leaves. On the adaxial sides, the average lesion length was 29.8, 33.9, and 27.9 mm in water agar, filter paper (plug), and filter paper spore suspensions, respectively; on the other hand, the average lesion length was 10.3, 26.5, and 12.9 mm, respectively, on the abaxial sides. The average lesion lengths on the adaxial side of the leaves in all inoculation methods were higher than the average lengths on the abaxial side, but statistical analysis showed that only the average length between the adaxial and abaxial sides using the water agar plug (WAP) method were statistically significant (p < 0.05) (Figure 4). For the whole plant assay, after 5 days of incubation, the disease was observed to be more severe in the upper leaves of the tree, and the disease occurred more slowly in the lower leaves. The leaf spots disease developed and expanded from 2 to 5 days (Figure 3I–L). The symptoms caused by the artificially inoculated pathogens were similar to those observed naturally and the control plants remained healthy. The requirements of Koch’s postulates were fulfilled. The fungus was constantly isolated from the lesions of detached leaves as well as the leaves of artificially inoculated whole plants and was identified as N. parvum on the basis both cultural and conidial morphology [25].
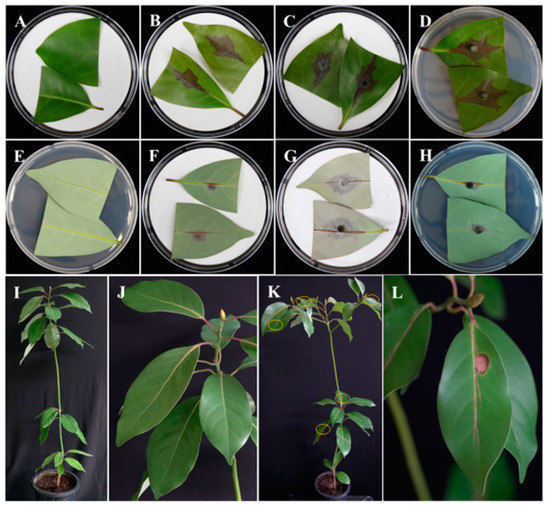
Figure 3.
Pathogenicity tests of Neofusicoccum parvum on the detached leaf and whole plants of Machilus thunbergii. Detached leaf pathogenicity of N. parvum; Control adaxial (A), abaxial sides (E). Fungus inoculated on adaxial and abaxial sides of the leaves by filter paper spore suspensions (FPS) (B,F), filter paper plug (FPP) (C,G), or water agar plug (WAP) (D,H) methods, and cultured at 25 °C for 5 days in moistened conditions. Pathogenicity of whole plants 12 days after inoculation in culture room conditions; control sprayed with distilled water—whole plant (left) and larger view (I,J); N. parvum sprayed on leaves—whole plant (K) and infected leaves in large view (L). Circle indicates symptomatic leaves (K).
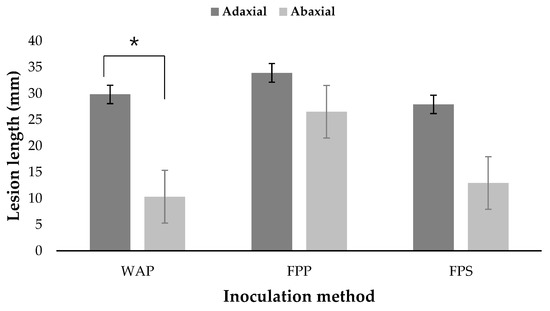
Figure 4.
Pathogenicity of detached leaves using fungal plug and spore suspensions methods. Fungal inoculations were performed on adaxial and abaxial sides of the leaves. WAP, FPP, and FPS represent the water agar plug, filter paper plug, and filter paper spore suspensions, respectively. * indicates a statistically significant difference between the average lesion lengths on adaxial and abaxial sides of the leaves by Student’s t-test (p < 0.05).
4. Discussion
The Japanese bay tree is one of the most important forest trees in Japan, Korea, and China. This plant is prevalent in coastal forest areas in Korea [1,2]. The bark and roots of this plant are using as a traditional medicine in continental regions, while the wood is used as mosquito repellent incense [26]. Lignin and alkaloids have been isolated from this plant which contains health-beneficial properties [5,26].
Plant diseases are common to all plants, including Japanese bay trees. To date, little research has been employed to investigate the occurrence and characterization of diseases in this plant. Few plant pathogens except N. parvum have been reported for this plant in China, Japan, Korea, and Taiwan; but this pathogen is common in many other plants [6]. The pathological characterization of this plant is little known in Korea. However, to save this plant in the oriental region, it is necessary to know the pathogens associated with it.
During our study of forest pathology, we observed a serious disease occurring in the leaves of Machilus thunbergii plants. The pathogen was identified through morphological and molecular approaches by following methods applied earlier [27,28,29,30,31]. Leaf spot disease caused by the fungal pathogen Neofusicoccum parvum has been found in other forest plants, including eucalyptus in Spain, blueberry in Korea, cannabis in Italy, and pine in Australia [13,27,28,29]. Studies suggest that this pathogen may be a latent pathogen and ubiquitous, being distributed across six continents in 29 countries and 90 plant species. However, the disease has never been observed in M. thunbergii in any country in the world. The pathogen has been reported from blueberry plants and walnut trees in Korea. The presence of this fungal species in Korea represents a new threat to M. thunbergii in forest areas of tropical and subtropical regions where climatic conditions favor the development of the disease. In addition, woody trees under environmental stress or having incurred mechanical injuries in natural calamities like strong winds, typhoons, and cyclones are more vulnerable to this pathogen [13]. Further investigation is required to determine factors promoting diseases associated with Neofusicoccum parvum. This could inform strategies to prevent and manage diseases caused by this fungus not only in Machilus thunbergii but also in other plants and crops.
Author Contributions
Conceptualization, H.S.; methodology, H.S., N.C.P., and S.C.; software, S.C. and N.C.P.; formal analysis, S.C.; data curation, S.C.; writing—original draft preparation, S.C. and N.C.P.; review and editing, S.C., N.C.P., K.-H.L., H.-J.K., and H.S.; supervision, H.S., H.-J.K.; funding acquisition, K.-H.L., H.S., and H.-J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Korea Forest Service (Grant No. 2020183C10-2022-AA02) and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through the Useful Agricultural Life Resources Industry Technology Development Program funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (120042-02), Republic of Korea.
Data Availability Statement
The gene sequences have been deposited in GenBank under the accession numbers MW142215, MW535296 & MW535297 for ITS and MW142216, MW535298 & MW535299 for EF1-alpha.
Acknowledgments
The authors give very special thanks to Jongseok Park, Jeollanamdo Wando Arboretum, Wando 59105, Republic of Korea for providing the plants for pathogenicity testing.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Ren, Q.; Wu, D.; Wu, C.; Wang, Z.; Jiao, J.; Jiang, B.; Zhu, J.; Huang, Y.; Li, T.; Yuan, W. Modeling the potential distribution of Machilus thunbergii; under the climate change patterns in China. OJF 2020, 10, 217–231. [Google Scholar] [CrossRef]
- Wu, S.H.; Hwang, C.Y.; Lin, T.P.; Chung, J.D.; Cheng, Y.P.; Hwang, S.Y. Contrasting phylogeographical patterns of two closely related species, Machilus thunbergii and Machilus kusanoi (Lauraceae), in Taiwan. J. Biogeogr. 2006, 33, 936–947. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Flora of China. In Menispermaceae through Capparaceae; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Missouri Botanical Garden Press: St. Louis, MO, USA, 2008. [Google Scholar]
- Li, G.; Ju, H.K.; Chang, H.W.; Jahng, Y.; Lee, S.H.; Son, J.K. Melanin biosynthesis inhibitors from the bark of Machilus thunbergii. Biol. Pharm. Bull. 2003, 26, 1039–1041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimomura, H.; Sashida, Y.; Oohara, M. Lignans from Machilus thunbergii. Phytochemestry 1987, 3, 1513–1515. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, Systematic Mycology and Microbiology Laboratory; USDA: Washington, DC, USA, 2018. [Google Scholar]
- Sakalidis, M.L.; Slippers, B.; Wingfield, B.D.; Hardy, G.S.J.; Burgess, T.I. The Challenge of understanding the origin, pathways and extent of fungal invasions: Global populations of the Neofusicoccum parvum-N. ribis species complex. Divers. Distrib. 2013, 19, 873–883. [Google Scholar] [CrossRef]
- Pennycook, S.R.; Samuels, G.J. Botryosphaeria and Fusicoccum species associated with ripe fruit rot of Actinidia deliciosa (Kiwifruit) in New Zealand. Mycotaxon 1985, 24, 445–458. [Google Scholar]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Slippers, B.; Smit, W.A.; Crous, P.W.; Coutinho, T.A.; Wingfield, B.D.; Wingfield, M.J. Taxonomy, phylogeny and identification of Botryosphaeriaceae associated with pome and stone fruit trees in South Africa and other regions of the world. Plant Pathol. 2007, 56, 128–139. [Google Scholar] [CrossRef]
- Espinoza, J.G.; Briceño, E.X.; Chávez, E.R.; Úrbez-Torres, J.R.; Latorre, B.A. Neofusicoccum spp. associated with stem canker and dieback of blueberry in Chile. Plant Dis. 2009, 93, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Laveau, C.; Letouze, A.; Louvet, G.; Bastien, S.; Guérin-Dubrana, L. Differential aggressiveness of fungi implicated in esca and associated diseases of grapevine in France. Phytopathol. Mediterr. 2009, 48, 15. [Google Scholar]
- Golzar, H.; Burgess, T.I. Neofusicoccum Parvum, a causal agent associated with cankers and decline of Norfolk Island pine in Australia. Australas. Plant Pathol. 2011, 40, 484–489. [Google Scholar] [CrossRef]
- Thomidis, T.; Michailides, T.J.; Exadaktylou, E. Neofusicoccum parvum associated with fruit rot and shoot blight of peaches in Greece. Eur. J. Plant Pathol. 2011, 131, 661–668. [Google Scholar] [CrossRef]
- Vakalounakis, D.J.; Ntougias, S.; Kavroulakis, N.; Protopapadakis, E. Neofusicoccum parvum and Diaporthe foeniculina associated with twig and shoot blight and branch canker of citrus in Greece. J. Phytopathol. 2019, 167, 527–537. [Google Scholar] [CrossRef]
- Chilvers, M.I.; Jones, S.; Meleca, J.; Peever, T.L.; Pethybridge, S.J.; Hay, F.S. Characterization of mating type genes supports the hypothesis that Stagonosporopsis chrysanthemi is homothallic and provides evidence that Stagonosporopsis tanaceti is heterothallic. Curr. Genet. 2014, 60, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Cubero, O.F.; Crespo, A.; Fatehi, J.; Bridge, P.D. DNA Extraction and PCR amplification method suitable for fresh, herbarium-stored, lichenized, and other fungi. Plant. Syst. Evol. 1999, 216, 243–249. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, A.M., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- O’Donnell, K. Molecular phylogeny of the Nectria haematococca-Fusarium solani species complex. Mycologia 2000, 92, 919–938. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.C.; Nam, S.S.; Kachroo, A.; Kim, Y.H.; Yang, J.W. Characterization and pathogenicity of sweet potato (Ipomoea batatas) black rot caused by Ceratocystis fimbriata in Korea. Eur. J. Plant Pathol. 2018, 152, 833–840. [Google Scholar] [CrossRef]
- Yang, J.W.; Nam, S.S.; Lee, H.U.; Choi, K.H.; Hwang, S.G.; Paul, N.C. Fusarium root rot caused by Fusarium solani on sweet potato (Ipomoea batatas) in South Korea. Can. J. Plant Pathol. 2018, 40, 90–95. [Google Scholar] [CrossRef]
- Choi, Y.P.; Paul, N.C.; Lee, H.B.; Yu, S.H. First record of Alternaria simsimi causing leaf spot on sesame (Sesamum indicum L.) in Korea. Mycobiology 2014, 42, 405–408. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef] [PubMed]
- Slippers, B.; Crous, P.W.; Denman, S.; Coutinho, T.A.; Wingfield, B.D.; Wingfield, M.J. Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 2004, 96, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Karikome, H.; Mimaki, Y.; Sashida, Y. A Butanolide and phenolics from Machilus thunbergii. Phytochemistry 1991, 30, 315–319. [Google Scholar] [CrossRef]
- Iturritxa, E.; Slippers, B.; Mesanza, N.; Wingfield, M.J. First report of Neofusicoccum parvum causing canker and die-back of eucalyptus in Spain. Australas. Plant Dis. Notes 2011, 6, 57–59. [Google Scholar] [CrossRef]
- Choi, I.Y.; Sharma, P.K.; Cheong, S.S. First report of Neofusicoccum parvum associated with bark dieback of blueberry in Korea. Plant Pathol. J. 2012, 28, 217. [Google Scholar] [CrossRef]
- Alberti, I.; Prodi, A.; Nipoti, P.; Grassi, G. First report of Neofusicoccum parvum causing stem and branch canker on Cannabis Sativa in Italy. J Plant. Dis. Prot. 2018, 125, 511–513. [Google Scholar] [CrossRef]
- Lopes, U.P.; Zambolim, L.; Pinho, D.B.; Barros, A.V.; Costa, H.; Pereira, O.L. Postharvest rot and mummification of strawberry fruits caused by Neofusicoccum parvum and N. kwambonambiense in Brazil. Trop. Plant Pathol. 2014, 39, 178–183. [Google Scholar] [CrossRef]
- Manca, D.; Bregant, C.; Maddau, L.; Pinna, C.; Montecchio, L.; Linaldeddu, B. First report of canker and dieback caused by Neofusicoccum parvum and Diplodia olivarum on oleaster in Italy. Ital. J. Mycol. 2020, 85–91. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).