Similarities and Differences among Soil Fungal Assemblages in Managed Forests and Formerly Managed Forest Reserves
Abstract
1. Introduction
- The species richness of soil fungal communities (i.e., the total number of species present in an area or a habitat; quantitative structure) would not differ between forest reserves and managed forests.
- The species composition and abundance of soil fungal communities (qualitative structure) would differ between forest reserves and managed forests owing to differences in local niches generated by each management strategy (host tree characteristics, soil characteristics, and local climatic constraints) which may influence fungal diversity.
- 3
- Different environmental factors (e.g., soil mineral and organic chemistry, tree species composition, dead wood volume) arising from different forest strategies (managed forests vs. reserves) will, in a distinctive way (beneficial or restrictive), influence ECM and SAP soil fungal assemblages.
2. Materials and Methods
2.1. Study Sites
2.2. Sampling Processing and Background Data
2.3. Bioinformatics and Statistics
3. Results
3.1. Sequencing Output and General Description of Soil Fungi
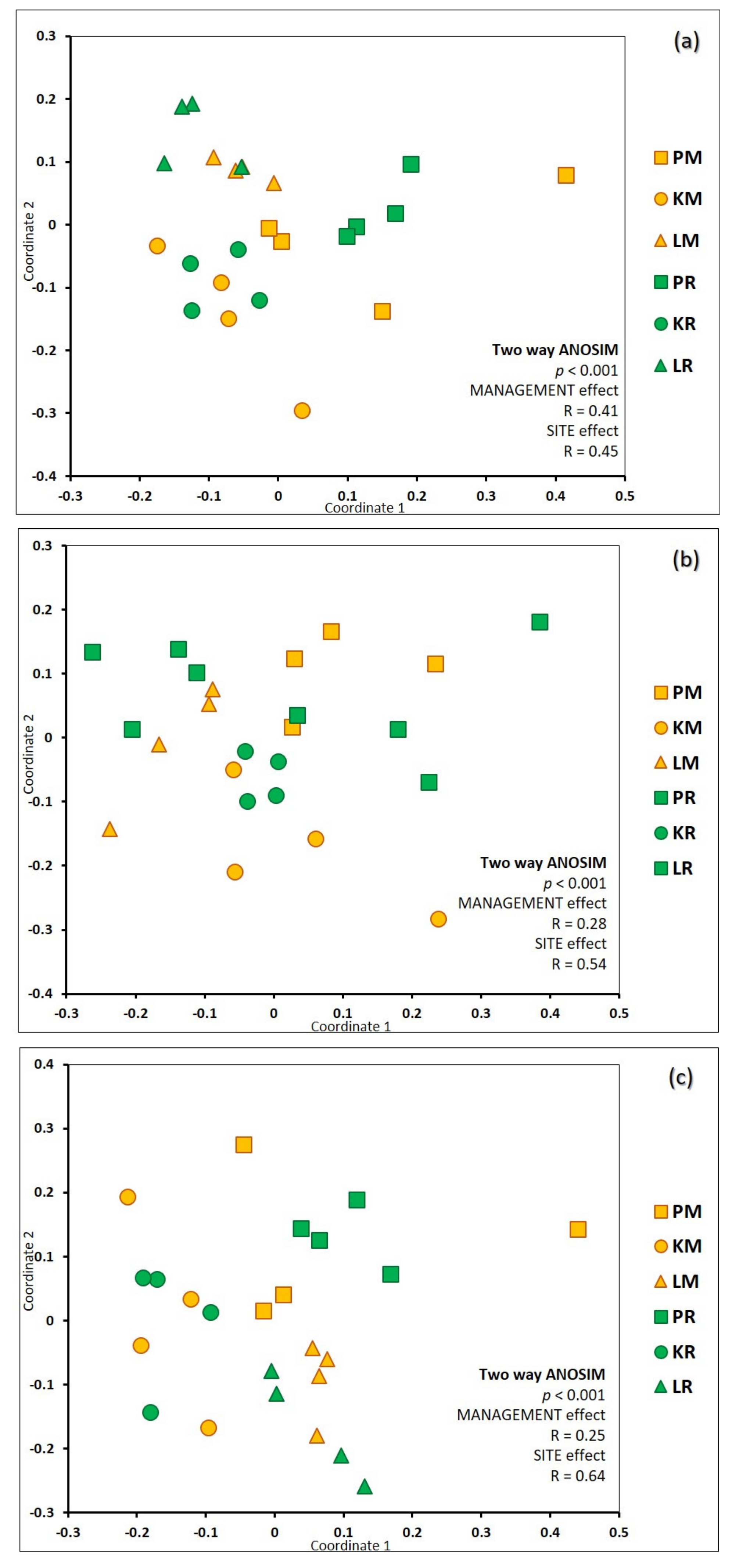
3.2. Richness and Species Composition of Soil Fungal OTUs
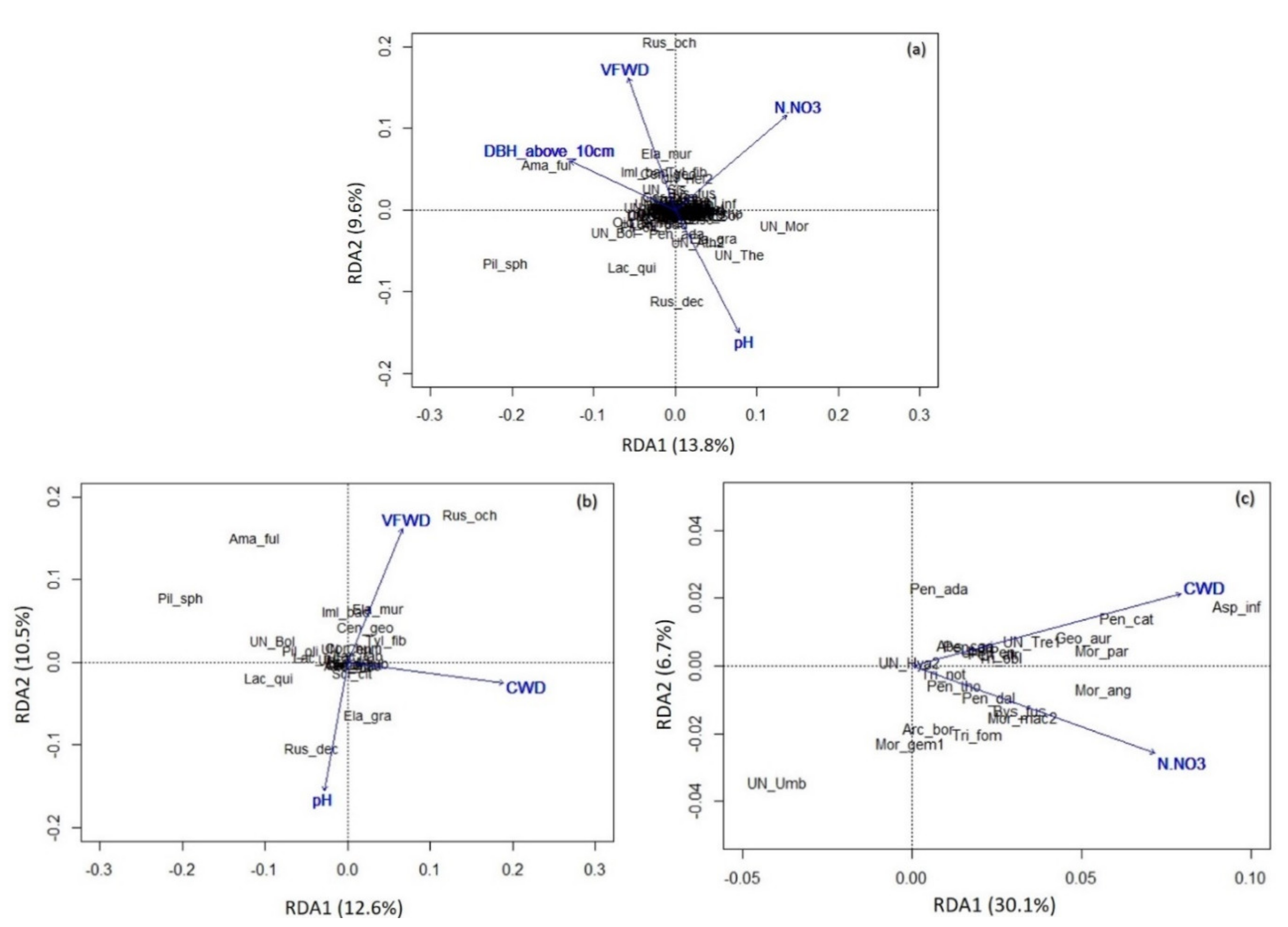
3.3. Impact of Environmental Variables on Soil Fungal Diversity
- (a)
- Total soil fungal community: The first axis explains 13.8% of the variability, the second explains 9.6% of the variability.
- (b)
- Ectomycorrhizal fungal communities: The first axis explains 12.6% of the variability, the second explains 10.5% of the variability.
- (c)
- Saprotrophic fungal communities: The first axis explains 30.1% of the variability, the second explains 6.7% of variability
4. Discussion
4.1. Overall Taxa Richness and Diversity of Trophic Groups
4.2. Environmental Drivers
4.3. The Red-Listed Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawksworth, D.L.; Lücking, R. Fungal Diversity Revisited: 2.2 to 3.8 Million Species. In The Fungal Kingdom; ASM Press: Washington, DC, USA, 2017; pp. 79–95. [Google Scholar]
- Larsen, B.B.; Miller, E.C.; Rhodes, M.K.; Wiens, J.J. Inordinate Fondness Multiplied and Redistributed: The Number of Species on Earth and the New Pie of Life. Q. Rev. Biol. 2017, 92, 229–265. [Google Scholar] [CrossRef]
- Mueller, G.M.; Schmit, J.P. Fungal biodiversity: What do we know? What can we predict? Biodivers. Conserv. 2007, 16, 1–5. [Google Scholar] [CrossRef]
- Taylor, D.L.; Hollingsworth, T.N.; McFarland, J.W.; Lennon, N.J.; Nusbaum, C.; Ruess, R.W. A first comprehensive census of fungi in soil reveals both hyperdiversity and fine-scale niche partitioning. Ecol. Monogr. 2014, 84, 3–20. [Google Scholar] [CrossRef]
- Anderson, I.C.; Genney, D.R.; Alexander, I.J. Fine-scale diversity and distribution of ectomycorrhizal fungal mycelium in a Scots pine forest. New Phytol. 2014, 201, 1423–1430. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, A.; Genney, D.R.; Heilmann-Clausen, J. Developing a comprehensive strategy for fungal conservation in Europe: Current status and future needs. Fungal Ecol. 2010, 3, 50–64. [Google Scholar] [CrossRef]
- Boddy, L.; Büntgen, U.; Egli, S.; Gange, A.C.; Heegaard, E.; Kirk, P.M.; Mohammad, A.; Kauserud, H. Climate variation effects on fungal fruiting. Fungal Ecol. 2014, 10, 20–33. [Google Scholar] [CrossRef]
- Heilmann-Clausen, J.; Barron, E.S.; Boddy, L.; Dahlberg, A.; Griffith, G.W.; Nordén, J.; Ovaskainen, O.; Perini, C.; Senn-Irlet, B.; Halme, P. A fungal perspective on conservation biology. Conserv. Biol. 2015, 29, 61–68. [Google Scholar] [CrossRef]
- Willis, K.J. State of the World’s Fungi 2018; Kew Publishing: Richmond, UK, 2018; ISBN 9781842466780. [Google Scholar]
- Tedersoo, L.; Smith, M.E. Lineages of ectomycorrhizal fungi revisited: Foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biol. Rev. 2013, 27, 83–99. [Google Scholar] [CrossRef]
- Smith, S.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press (Elsevier): London, UK, 2008; ISBN 9780123705266. [Google Scholar]
- Courty, P.-E.; Buée, M.; Diedhiou, A.G.; Frey-Klett, P.; Le Tacon, F.; Rineau, F.; Turpault, M.-P.; Uroz, S.; Garbaye, J. The role of ectomycorrhizal communities in forest ecosystem processes: New perspectives and emerging concepts. Soil Biol. Biochem. 2010, 42, 679–698. [Google Scholar] [CrossRef]
- Dickie, I.A.; Alexander, I.; Lennon, S.; Öpik, M.; Selosse, M.-A.; van der Heijden, M.G.A.; Martin, F.M. Evolving insights to understanding mycorrhizas. New Phytol. 2015, 205, 1369–1374. [Google Scholar] [CrossRef]
- Holah, J.C.; Wilson, M.V.; Hansen, E.M. Effects of a native forest pathogen, Phellinus weirii, on Douglas-fir forest composition in western Oregon. Can. J. For. Res. 1993, 23, 2473–2480. [Google Scholar] [CrossRef]
- Hennon, P.E. Are heart rot fungi major factors of disturbance in gap-dynamic forests? Northwest. Sci. 1995, 69, 284–293. [Google Scholar]
- Baldrian, P.; Voříšková, J.; Dobiášová, P.; Merhautová, V.; Lisá, L.; Valášková, V. Production of extracellular enzymes and degradation of biopolymers by saprotrophic microfungi from the upper layers of forest soil. Plant. Soil 2011, 338, 111–125. [Google Scholar] [CrossRef]
- Lodge, D.J. Factors related to diversity of decomposer fungi in tropical forests. Biodivers. Conserv. 1997, 6, 681–688. [Google Scholar] [CrossRef]
- Vašutová, M.; Mleczko, P.; López-García, A.; Maček, I.; Boros, G.; Ševčík, J.; Fujii, S.; Hackenberger, D.; Tuf, I.H.; Hornung, E.; et al. Taxi drivers: The role of animals in transporting mycorrhizal fungi. Mycorrhiza 2019, 29, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Paillet, Y.; Berges, L.; Hjältén, J.; Ódor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.-J.; De Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity Differences between Managed and Unmanaged Forests: Meta-Analysis of Species Richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef]
- Goldmann, K.; Schöning, I.; Buscot, F.; Wubet, T. Forest management type influences diversity and community composition of soil fungi across temperate forest ecosystems. Front. Microbiol. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tomao, A.; Antonio Bonet, J.; Castaño, C.; De-Miguel, S. How does forest management affect fungal diversity and community composition? Current knowledge and future perspectives for the conservation of forest fungi. For. Ecol. Manag. 2020, 457, 117678. [Google Scholar] [CrossRef]
- Kyaschenko, J.; Clemmensen, K.E.; Hagenbo, A.; Karltun, E.; Lindahl, B.D. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. ISME J. 2017, 11, 863–874. [Google Scholar] [CrossRef]
- Parladé, J.; Queralt, M.; Pera, J.; Bonet, J.A.; Castaño, C.; Martínez-Peña, F.; Piñol, J.; Senar, M.A.; De Miguel, A.M. Temporal dynamics of soil fungal communities after partial and total clear-cutting in a managed Pinus sylvestris stand. For. Ecol. Manag. 2019, 449, 117456. [Google Scholar] [CrossRef]
- Castaño, C.; Alday, J.G.; Lindahl, B.D.; Martínez de Aragón, J.; De-Miguel, S.; Colinas, C.; Parladé, J.; Pera, J.; Bonet, J.A. Lack of thinning effects over inter-annual changes in soil fungal community’ and diversity in a Mediterranean pine forest. For. Ecol. Manag. 2018, 424, 420–427. [Google Scholar] [CrossRef]
- Leski, T.; Rudawska, M.; Kujawska, M.; Stasińska, M.; Janowski, D.; Karliński, L.; Wilgan, R. Both forest reserves and managed forests help maintain ectomycorrhizal fungal diversity. Biol. Conserv. 2019, 238, 108206. [Google Scholar] [CrossRef]
- Spake, R.; van der Linde, S.; Newton, A.C.; Suz, L.M.; Bidartondo, M.I.; Doncaster, C.P. Similar biodiversity of ectomycorrhizal fungi in set-aside plantations and ancient old-growth broadleaved forests. Biol. Conserv. 2016, 194, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Carron, A.I.; Garibaldi, L.A.; Marquez, S.; Fontenla, S. The soil fungal community of native woodland in Andean Patagonian forest: A case study considering experimental forest management and seasonal effects. For. Ecol. Manag. 2020, 461, 117955. [Google Scholar] [CrossRef]
- Lynikienė, J.; Marčiulynienė, D.; Marčiulynas, A.; Gedminas, A.; Vaičiukynė, M.; Menkis, A. Managed and Unmanaged Pinus sylvestris Forest Stands Harbour Similar Diversity and Composition of the Phyllosphere and Soil Fungi. Microorganisms 2020, 8, 259. [Google Scholar] [CrossRef]
- Heilmann-Clausen, J.; Adamčík, S.; Bässler, C.; Halme, P.; Krisai-Greilhuber, I.; Holec, J. State of the art and future directions for mycological research in old-growth forests. Fungal Ecol. 2017, 27, 141–144. [Google Scholar] [CrossRef]
- Forest Europe, 2015: State of Europe’s Forests 2015. Available online: https://foresteurope.org/state-europes-forests-2015-report/.2015 (accessed on 8 February 2021).
- Humphrey, J.W. Benefits to biodiversity from developing old-growth conditions in British upland spruce plantations: A review and recommendations. Forestry 2005, 78, 33–53. [Google Scholar] [CrossRef]
- Deal, R.L. Management strategies to increase stand structural diversity and enhance biodiversity in coastal rainforests of Alaska. Biol. Conserv. 2007, 137, 520–532. [Google Scholar] [CrossRef]
- Bauhus, J.; Puettmann, K.; Messier, C. Silviculture for old-growth attributes. For. Ecol. Manag. 2009, 258, 525–537. [Google Scholar] [CrossRef]
- Spake, R.; Ezard, T.H.G.; Martin, P.A.; Newton, A.C.; Doncaster, C.P. A meta-analysis of functional group responses to forest recovery outside of the tropics. Conserv. Biol. 2015, 29, 1695–1703. [Google Scholar] [CrossRef]
- Dvořák, D.; Vašutová, M.; Hofmeister, J.; Beran, M.; Hošek, J.; Běťák, J.; Burel, J.; Deckerová, H. Macrofungal diversity patterns in central European forests affirm the key importance of old-growth forests. Fungal Ecol. 2017, 27, 145–154. [Google Scholar] [CrossRef]
- Goicoechea, N.; Closa, I.; de Miguel, A.M. Ectomycorrhizal communities within beech (Fagus sylvatica L.) forests that naturally regenerate from clear-cutting in northern Spain. New For. 2009, 38, 157–175. [Google Scholar] [CrossRef]
- Abrego, N.; Salcedo, I. Variety of woody debris as the factor influencing wood-inhabiting fungal richness and assemblages: Is it a question of quantity or quality? For. Ecol. Manag. 2013, 291, 377–385. [Google Scholar] [CrossRef]
- Juutilainen, K.; Mönkkönen, M.; Kotiranta, H.; Halme, P. The effects of forest management on wood-inhabiting fungi occupying dead wood of different diameter fractions. For. Ecol. Manag. 2014, 313, 283–291. [Google Scholar] [CrossRef]
- Lelli, C.; Bruun, H.H.; Chiarucci, A.; Donati, D.; Frascaroli, F.; Fritz, Ö.; Goldberg, I.; Nascimbene, J.; Tøttrup, A.P.; Rahbek, C.; et al. Biodiversity response to forest structure and management: Comparing species richness, conservation relevant species and functional diversity as metrics in forest conservation. For. Ecol. Manag. 2019, 432, 707–717. [Google Scholar] [CrossRef]
- Aguirre-Calderón, O.A. Forest management in the XXI Century. Madera y Bosques 2015, 21, 17–28. [Google Scholar]
- Bengtsson, J.; Nilsson, S.G.; Franc, A.; Menozzi, P. Biodiversity, disturbances, ecosystem function and management of european forests. For. Ecol. Manag. 2000, 132, 39–50. [Google Scholar] [CrossRef]
- Bartniczak, B.; Raszkowski, A. Sustainable forest management in Poland. Manag. Environ. Qual. An. Int. J. 2018, 29, 666–677. [Google Scholar] [CrossRef]
- Gömöryová, E.; Ujházy, K.; Martinák, M.; Gömöry, D. Soil microbial community response to variation in vegetation and abiotic environment in a temperate old-growth forest. Appl. Soil Ecol. 2013, 68, 10–19. [Google Scholar] [CrossRef]
- Buée, M.; Reich, M.; Murat, C.; Morin, E.; Nilsson, R.H.; Uroz, S.; Martin, F. 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol. 2009, 184, 449–456. [Google Scholar] [CrossRef]
- Rykowski, K. Forest biological diversity conservation as an element of sustainable forest management-policy and practice in Poland. Unasylva 2002, 53, 16–24. [Google Scholar]
- Rudawska, M.; Wilgan, R.; Janowski, D.; Iwański, M.; Leski, T. Shifts in taxonomical and functional structure of ectomycorrhizal fungal community of Scots pine (Pinus sylvestris L.) underpinned by partner tree ageing. Pedobiologia (Jena) 2018, 71, 20–30. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR-Protocols and Applications: A Laboratory Manual; Innis, M.A., Gelfand, H., Sninsky, J.S., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Aronesty, E. ea-utils: Command-Line Tools for Processing Biological Sequencing Data. Available online: https://expressionanalysis.github.io/ea-utils/ (accessed on 8 February 2021).
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Colwell, R.K.; EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 9 and Earlier. User’s Guide and Application. 2013. Available online: http://purl.oclc.org/estimates (accessed on 8 February 2021).
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; Del Rio, T.G.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef]
- R Core Computing Team. R: A Language and Environment for Statistical Computing; R Found. Stat. Comput: Vienna, Austria, 2019. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package Version 2.4-2, Community Ecol. Package. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 8 February 2021).
- Naimi, B. Package “usdm” Uncertainty Analysis for Species Distribution Models. Available online: https://cran.r-project.org/web/packages/usdm/index.html (accessed on 8 February 2021).
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Wojewoda, W.; Ławrynowicz, M. Red list of the macrofungi in Poland. In Red List of Plants and Fungi in Poland; Mirek, Z., Zarzycki, K., Wojewoda, W., Szeląg, Z., Eds.; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2006; pp. 53–70. [Google Scholar]
- Vašutová, M.; Edwards-Jonášová, M.; Baldrian, P.; Čermák, M.; Cudlín, P. Distinct environmental variables drive the community composition of mycorrhizal and saprotrophic fungi at the alpine treeline ecotone. Fungal Ecol. 2017, 27, 116–124. [Google Scholar] [CrossRef]
- Bässler, C.; Ernst, R.; Cadotte, M.; Heibl, C.; Müller, J. Near-to-nature logging influences fungal community assembly processes in a temperate forest. J. Appl. Ecol. 2014, 51, 939–948. [Google Scholar] [CrossRef]
- Sitzia, T.; Campagnaro, T.; Dainese, M.; Cassol, M.; Cortivo, M.; Gatti, E.; Padovan, F.; Sommacal, M.; Nascimbene, J. Contrasting multi-taxa diversity patterns between abandoned and non-intensively managed forests in the southern Dolomites. iForest Biogeosci. For. 2017, 10, 845–850. [Google Scholar] [CrossRef]
- Leidinger, J.; Weisser, W.W.; Kienlein, S.; Blaschke, M.; Jung, K.; Kozak, J.; Fischer, A.; Mosandl, R.; Michler, B.; Ehrhardt, M.; et al. Formerly managed forest reserves complement integrative management for biodiversity conservation in temperate European forests. Biol. Conserv. 2020, 242, 108437. [Google Scholar] [CrossRef]
- Chen, J.; Xu, H.; He, D.; Li, Y.; Luo, T.; Yang, H.; Lin, M. Historical logging alters soil fungal community composition and network in a tropical rainforest. For. Ecol. Manag. 2019, 433, 228–239. [Google Scholar] [CrossRef]
- Paillet, Y.; Pernot, C.; Boulanger, V.; Debaive, N.; Fuhr, M.; Gilg, O.; Gosselin, F. Quantifying the recovery of old-growth attributes in forest reserves: A first reference for France. For. Ecol. Manag. 2015, 346, 51–64. [Google Scholar] [CrossRef]
- Vořiškova, J.; Brabcová, V.; Cajthaml, T.; Baldrian, P. Seasonal dynamics of fungal communities in a temperate oak forest soil. New Phytol. 2014, 201, 269–278. [Google Scholar] [CrossRef]
- Větrovský, T.; Kohout, P.; Kopecký, M.; Machac, A.; Man, M.; Bahnmann, B.D.; Brabcová, V.; Choi, J.; Meszárošová, L.; Human, Z.R.; et al. A meta-analysis of global fungal distribution reveals climate-driven patterns. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Wubet, T.; Trogisch, S.; Both, S.; Scholten, T.; Bruelheide, H.; Buscot, F. Forest Age and Plant Species Composition Determine the Soil Fungal Community Composition in a Chinese Subtropical Forest. PLoS ONE 2013, 8, 66829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wubet, T.; Christ, S.; Schöning, I.; Boch, S.; Gawlich, M.; Schnabel, B.; Fischer, M.; Buscot, F. Differences in Soil Fungal Communities between European Beech (Fagus sylvatica L.) Dominated Forests Are Related to Soil and Understory Vegetation. PLoS ONE 2012, 7, e47500. [Google Scholar] [CrossRef]
- James, T.Y.; Kauff, F.; Schoch, C.L.; Matheny, P.B.; Hofstetter, V.; Cox, C.J.; Celio, G.; Gueidan, C.; Fraker, E.; Miadlikowska, J.; et al. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 2006, 443, 818–822. [Google Scholar] [CrossRef]
- Bonfante, P.; Venice, F. Mucoromycota: Going to the roots of plant-interacting fungi. Fungal Biol. Rev. 2020, 34, 100–113. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Nilsson, R.H.; Tedersoo, L.; Abarenkov, K.; Carlsen, T.; Kjøller, R.; Kõljalg, U.; Pennanen, T.; Rosendahl, S.; Stenlid, J.; et al. Fungal community analysis by high-throughput sequencing of amplified markers—a user’s guide. New Phytol. 2013, 199, 288–299. [Google Scholar] [CrossRef]
- Tedersoo, L.; Anslan, S.; Bahram, M.; Põlme, S.; Riit, T.; Liiv, I.; Kõljalg, U.; Kisand, V.; Nilsson, R.H.; Hildebrand, F.; et al. Shotgun metagenomes and multiple primer pair-barcode combinations of amplicons reveal biases in metabarcoding analyses of fungi. MycoKeys 2015, 10, 1–43. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and Associated Fungi Drive Long-Term Carbon Sequestration in Boreal Forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Talbot, J.M.; Martin, F.; Kohler, A.; Henrissat, B.; Peay, K.G. Functional guild classification predicts the enzymatic role of fungi in litter and soil biogeochemistry. Soil Biol. Biochem. 2015, 88, 441–456. [Google Scholar] [CrossRef]
- Peay, K.G.; Baraloto, C.; Fine, P.V.A. Strong coupling of plant and fungal community structure across western Amazonian rainforests. ISME J. 2013, 7, 1852–1861. [Google Scholar] [CrossRef]
- Baptista, P.; Reis, F.; Pereira, E.; Tavares, R.M.; Santos, P.M.; Richard, F.; Selosse, M.A.; Lino-Neto, T. Soil DNA pyrosequencing and fruitbody surveys reveal contrasting diversity for various fungal ecological guilds in chestnut orchards. Environ. Microbiol. Rep. 2015, 7, 946–954. [Google Scholar] [CrossRef]
- Jumpponen, A.; Brown, S.; Trappe, J.; Cázares, E.; Strömmer, R. Analyses of Sporocarps, Morphotyped Ectomycorrhizae, Environmental ITS and LSU Sequences Identify Common Genera that Occur at a Periglacial Site. J. Fungi 2015, 1, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Ramette, A. Multivariate analyses in microbial ecology. FEMS Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Goldmann, K.; Schröter, K.; Pena, R.; Schöning, I.; Schrumpf, M.; Buscot, F.; Polle, A.; Wubet, T. Divergent habitat filtering of root and soil fungal communities in temperate beech forests. Sci. Rep. 2016, 6, 31439. [Google Scholar] [CrossRef]
- Powell, J.R.; Karunaratne, S.; Campbell, C.D.; Yao, H.; Robinson, L.; Singh, B.K. Deterministic processes vary during community assembly for ecologically dissimilar taxa. Nat. Commun. 2015, 6, 8444. [Google Scholar] [CrossRef]
- Bauman, D.; Raspé, O.; Meerts, P.; Degreef, J.; Muledi, J.I.; Drouet, T. Multiscale assemblage of an ectomycorrhizal fungal community: The influence of host functional traits and soil properties in a 10-ha miombo forest. FEMS Microbiol. Ecol. 2016, 92, 1–14. [Google Scholar] [CrossRef]
- Parladé, J.; Martínez-Peña, F.; Pera, J. Effects of forest management and climatic variables on the mycelium dynamics and sporocarp production of the ectomycorrhizal fungus Boletus edulis. For. Ecol. Manag. 2017, 390, 73–79. [Google Scholar] [CrossRef]
- Kohout, P.; Charvátová, M.; Štursová, M.; Mašínová, T.; Tomšovský, M.; Baldrian, P. Clearcutting alters decomposition processes and initiates complex restructuring of fungal communities in soil and tree roots. ISME J. 2018, 12, 692–703. [Google Scholar] [CrossRef]
- Hartmann, M.; Howes, C.G.; VanInsberghe, D.; Yu, H.; Bachar, D.; Christen, R.; Henrik Nilsson, R.; Hallam, S.J.; Mohn, W.W. Significant and persistent impact of timber harvesting on soil microbial communities in Northern coniferous forests. ISME J. 2012, 6, 2199–2218. [Google Scholar] [CrossRef]
- Varenius, K.; Kårén, O.; Lindahl, B.; Dahlberg, A. Long-term effects of tree harvesting on ectomycorrhizal fungal communities in boreal Scots pine forests. For. Ecol. Manag. 2016, 380, 41–49. [Google Scholar] [CrossRef]
- Kyaschenko, J.; Clemmensen, K.E.; Karltun, E.; Lindahl, B.D. Below-ground organic matter accumulation along a boreal forest fertility gradient relates to guild interaction within fungal communities. Ecol. Lett. 2017, 20, 1546–1555. [Google Scholar] [CrossRef]
- Baldrian, P.; Merhautová, V.; Cajthaml, T.; Petránková, M.; Šnajdr, J. Small-scale distribution of extracellular enzymes, fungal, and bacterial biomass in Quercus petraea forest topsoil. Biol. Fertil. Soils 2010, 46, 717–726. [Google Scholar] [CrossRef]
- Rosling, A.; Timling, I.; Taylor, D.L. Archaeorhizomycetes: Patterns of Distribution and Abundance in Soil. In Genomics of Soil- and Plant-Associated Fungi. Soil Biology; Horwitz, B., Mukherjee, P., Mukherjee, M., Kubicek, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 36, pp. 333–349. [Google Scholar]
- Rosling, A.; Cox, F.; Cruz-Martinez, K.; Ihrmark, K.; Grelet, G.A.; Lindahl, B.D.; Menkis, A.; James, T.Y. Archaeorhizomycetes: Unearthing an ancient class of ubiquitous soil fungi. Science 2011, 333, 876–879. [Google Scholar] [CrossRef]
- Menkis, A.; Urbina, H.; James, T.Y.; Rosling, A. Archaeorhizomyces borealis sp. nov. and a sequence-based classification of related soil fungal species. Fungal Biol. 2014, 118, 943–955. [Google Scholar] [CrossRef]
- Pinto-Figueroa, E.A.; Seddon, E.; Yashiro, E.; Buri, A.; Niculita-Hirzel, H.; van der Meer, J.R.; Guisan, A. Archaeorhizomycetes Spatial Distribution in Soils Along Wide Elevational and Environmental Gradients Reveal Co-abundance Patterns With Other Fungal Saprobes and Potential Weathering Capacities. Front. Microbiol. 2019, 10, 656. [Google Scholar] [CrossRef]
- Bahnmann, B.; Mašínová, T.; Halvorsen, R.; Davey, M.L.; Sedlák, P.; Tomšovský, M.; Baldrian, P. Effects of oak, beech and spruce on the distribution and community structure of fungi in litter and soils across a temperate forest. Soil Biol. Biochem. 2018, 119, 162–173. [Google Scholar] [CrossRef]
- Schröter, K.; Wemheuer, B.; Pena, R.; Schöning, I.; Ehbrecht, M.; Schall, P.; Ammer, C.; Daniel, R.; Polle, A. Assembly processes of trophic guilds in the root mycobiome of temperate forests. Mol. Ecol. 2019, 28, 348–364. [Google Scholar] [CrossRef]
- Jonsson, U.; Jung, T.; Sonesson, K.; Rosengren, U. Relationships between health of Quercus robur, occurrence of Phytophthora species and site conditions in southern Sweden. Plant. Pathol. 2005, 54, 502–511. [Google Scholar] [CrossRef]
- Anderson, I.C.; Campbell, C.D.; Prosser, J.I. Diversity of fungi in organic soils under a moorland—Scots pine (Pinus sylvestris L.) gradient. Environ. Microbiol. 2003, 5, 1121–1132. [Google Scholar] [CrossRef]
- Chavarriaga, D.; Bodles, W.J.A.; Leifert, C.; Belbahri, L.; Woodward, S. Phytophthora cinnamomi and other fine root pathogens in north temperate pine forests. FEMS Microbiol. Lett. 2007, 276, 67–74. [Google Scholar] [CrossRef]
- Stirling, G.R. Biological Control. of Plant.-Parasitic Nematodes: Soil Ecosystem Management in Sustainable Agriculture; Stirling, G.R., Ed.; CABI: Wallingford, UK, 2014; ISBN 9781780644158. [Google Scholar]
- Brabcová, V.; Nováková, M.; Davidová, A.; Baldrian, P. Dead fungal mycelium in forest soil represents a decomposition hotspot and a habitat for a specific microbial community. New Phytol. 2016, 210, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Kivlin, S.N.; Hawkes, C.V.; Treseder, K.K. Global diversity and distribution of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2011, 43, 2294–2303. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Fahey, T.J.; Horton, T.R.; Lovett, G.M. Belowground ectomycorrhizal fungal community change over a nitrogen deposition gradient in Alaska. Ecology 2002, 83, 104–115. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.T.; Bowker, M. ssA.; Wilson, J.A.; Miller, R.M. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc. Natl. Acad. Sci. USA 2010, 107, 2093–2098. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Kivlin, S.N.; Rocca, J.D.; Huguet, V.; Thomsen, M.A.; Suttle, K.B. Fungal community responses to precipitation. Glob. Chang. Biol. 2011, 17, 1637–1645. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, N.-F.; Liu, H.-Y.; Zhang, Y.-Q.; Yu, L.-Y. Soil pH is a Key Determinant of Soil Fungal Community Composition in the Ny-Ålesund Region, Svalbard (High Arctic). Front. Microbiol. 2016, 7, 227. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Hobbie, E.A.; Horton, T.R. Conservation of ectomycorrhizal fungi: Exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal Ecol. 2011, 4, 174–183. [Google Scholar] [CrossRef]
- Kjøller, R.; Nilsson, L.-O.; Hansen, K.; Schmidt, I.K.; Vesterdal, L.; Gundersen, P. Dramatic changes in ectomycorrhizal community composition, root tip abundance and mycelial production along a stand-scale nitrogen deposition gradient. New Phytol. 2012, 194, 278–286. [Google Scholar] [CrossRef]
- Choma, M.; Rappe-George, M.O.; Bárta, J.; Čapek, P.; Kaštovská, E.; Gärdenäs, A.I.; Šantrůčková, H. Recovery of the ectomycorrhizal community after termination of long-term nitrogen fertilisation of a boreal Norway spruce forest. Fungal Ecol. 2017, 29, 116–122. [Google Scholar] [CrossRef]
- Morrison, E.W.; Frey, S.D.; Sadowsky, J.J.; van Diepen, L.T.A.; Thomas, W.K.; Pringle, A. Chronic nitrogen additions fundamentally restructure the soil fungal community in a temperate forest. Fungal Ecol. 2016, 23, 48–57. [Google Scholar] [CrossRef]
- Tahovská, K.; Choma, M.; Kaštovská, E.; Oulehle, F.; Bárta, J.; Šantrůčková, H.; Moldan, F. Positive response of soil microbes to long-term nitrogen input in spruce forest: Results from Gårdsjön whole-catchment N-addition experiment. Soil Biol. Biochem. 2020, 143, 107732. [Google Scholar] [CrossRef]
- Walker, J.K.M.; Ward, V.; Paterson, C.; Jones, M.D. Coarse woody debris retention in subalpine clearcuts affects ectomycorrhizal root tip community structure within fifteen years of harvest. Appl. Soil Ecol. 2012, 60, 5–15. [Google Scholar] [CrossRef]
- Tedersoo, L.; Nilsson, R.H.; Abarenkov, K.; Jairus, T.; Sadam, A.; Saar, I.; Bahram, M.; Bechem, E.; Chuyong, G.; Kõljalg, U. 454 Pyrosequencing and Sanger sequencing of tropical mycorrhizal fungi provide similar results but reveal substantial methodological biases. New Phytol. 2010, 188, 291–301. [Google Scholar] [CrossRef]
- Geml, J.; Gravendeel, B.; van der Gaag, K.J.; Neilen, M.; Lammers, Y.; Raes, N.; Semenova, T.A.; de Knijff, P.; Noordeloos, M.E. The Contribution of DNA Metabarcoding to Fungal Conservation: Diversity Assessment, Habitat Partitioning and Mapping Red-Listed Fungi in Protected Coastal Salix repens Communities in the Netherlands. PLoS ONE 2014, 9, e99852. [Google Scholar] [CrossRef]
- Geml, J.; Timling, I.; Robinson, C.H.; Lennon, N.; Nusbaum, H.C.; Brochmann, C.; Noordeloos, M.E.; Taylor, D.L. An arctic community of symbiotic fungi assembled by long-distance dispersers: Phylogenetic diversity of ectomycorrhizal basidiomycetes in Svalbard based on soil and sporocarp DNA. J. Biogeogr. 2012, 39, 74–88. [Google Scholar] [CrossRef]
- Geml, J.; Pastor, N.; Fernandez, L.; Pacheco, S.; Semenova, T.A.; Becerra, A.G.; Wicaksono, C.Y.; Nouhra, E.R. Large-scale fungal diversity assessment in the Andean Yungas forests reveals strong community turnover among forest types along an altitudinal gradient. Mol. Ecol. 2014, 23, 2452–2472. [Google Scholar] [CrossRef]
- Horton, T.R.; Bruns, T.D. The molecular revolution in ectomycorrhizal ecology: Peeking into the black-box. Mol. Ecol. 2001, 10, 1855–1871. [Google Scholar] [CrossRef] [PubMed]
- Ovaskainen, O.; Schigel, D.; Ali-Kovero, H.; Auvinen, P.; Paulin, L.; Nordén, B.; Nordén, J. Combining high-throughput sequencing with fruit body surveys reveals contrasting life-history strategies in fungi. ISME J. 2013, 7, 1696–1709. [Google Scholar] [CrossRef]
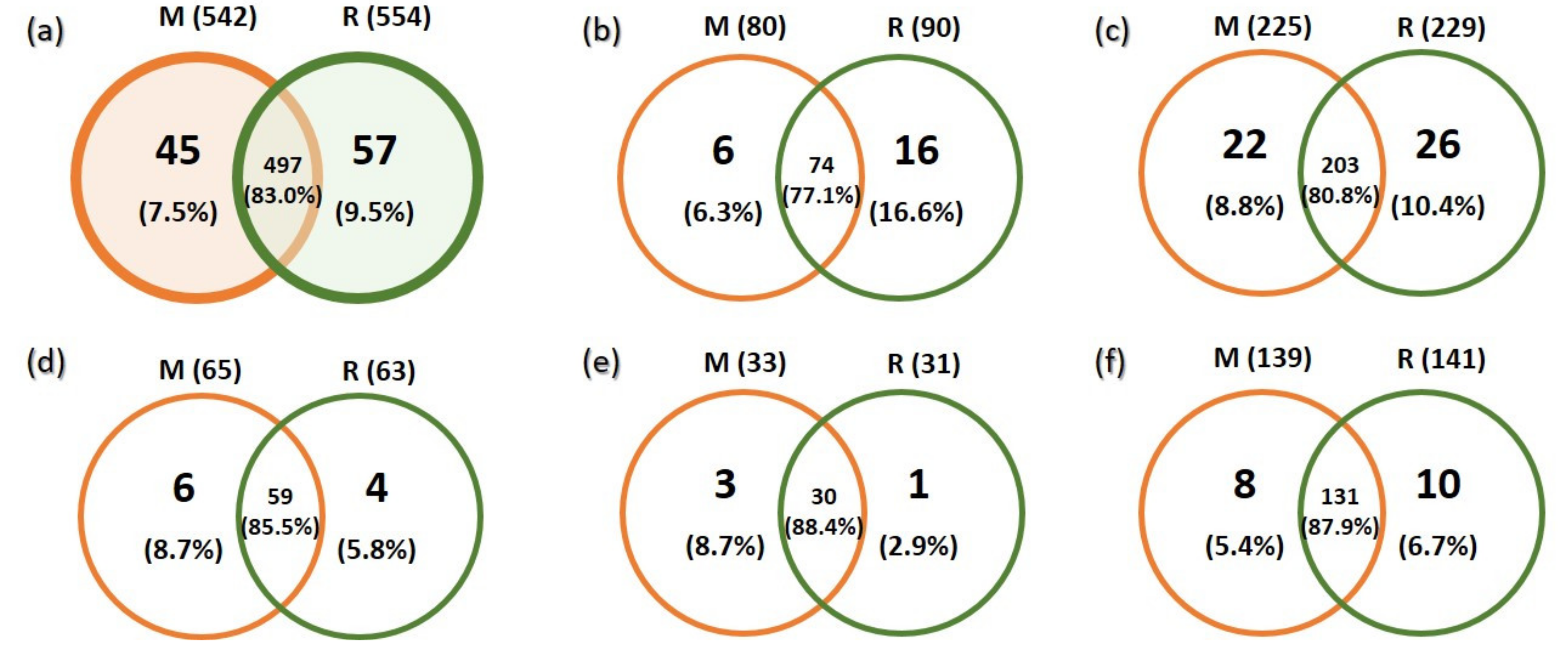
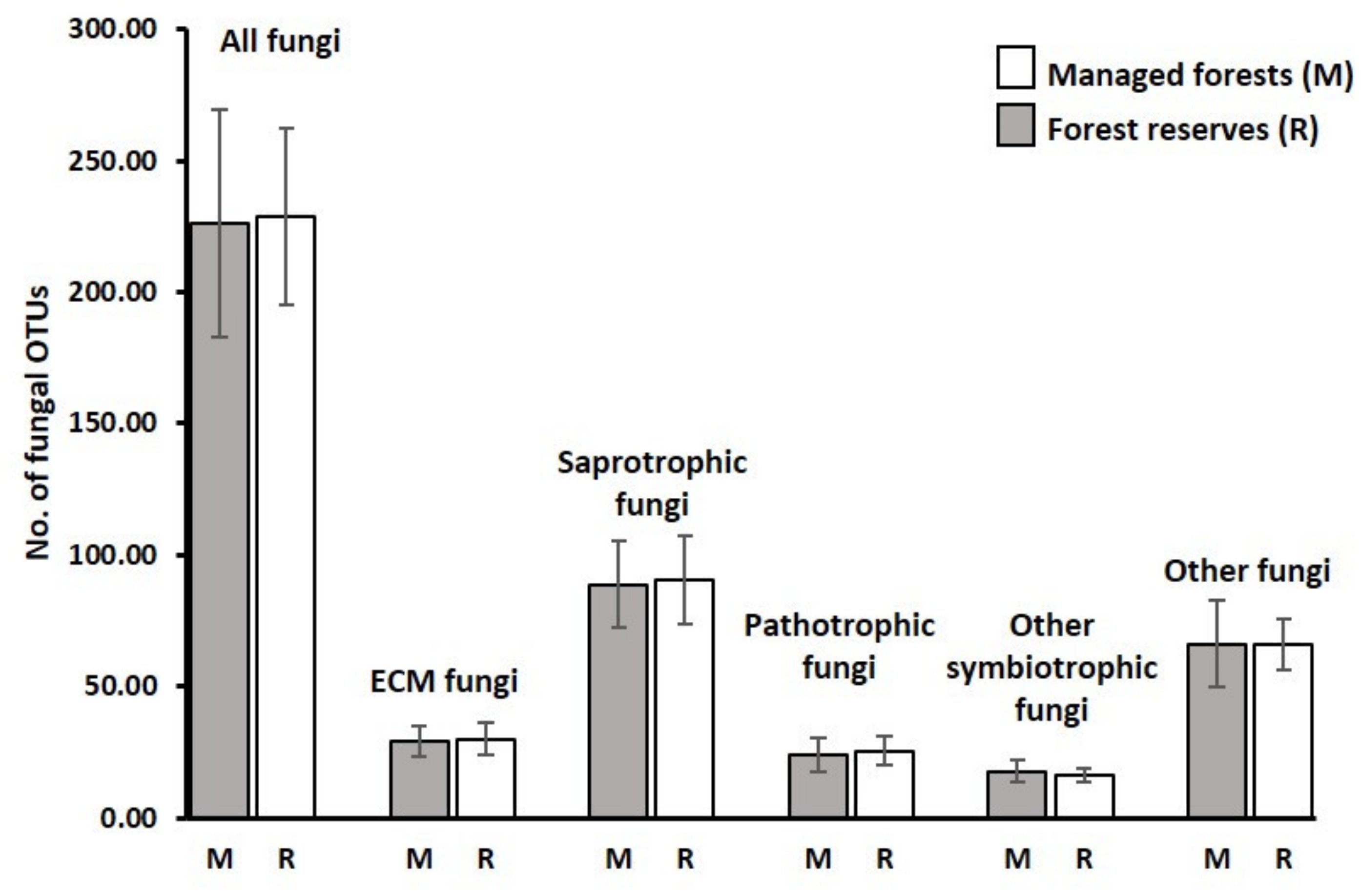
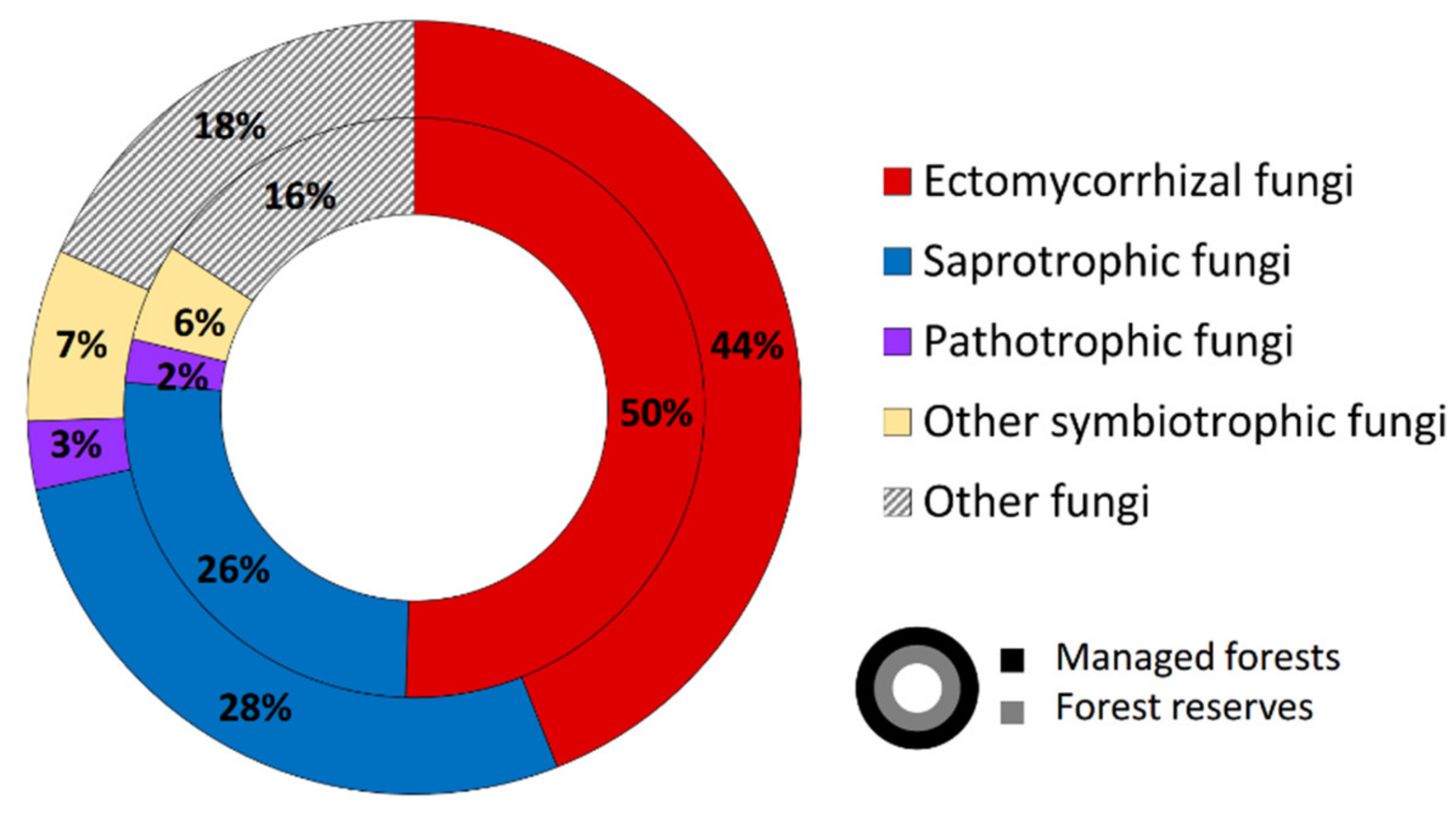
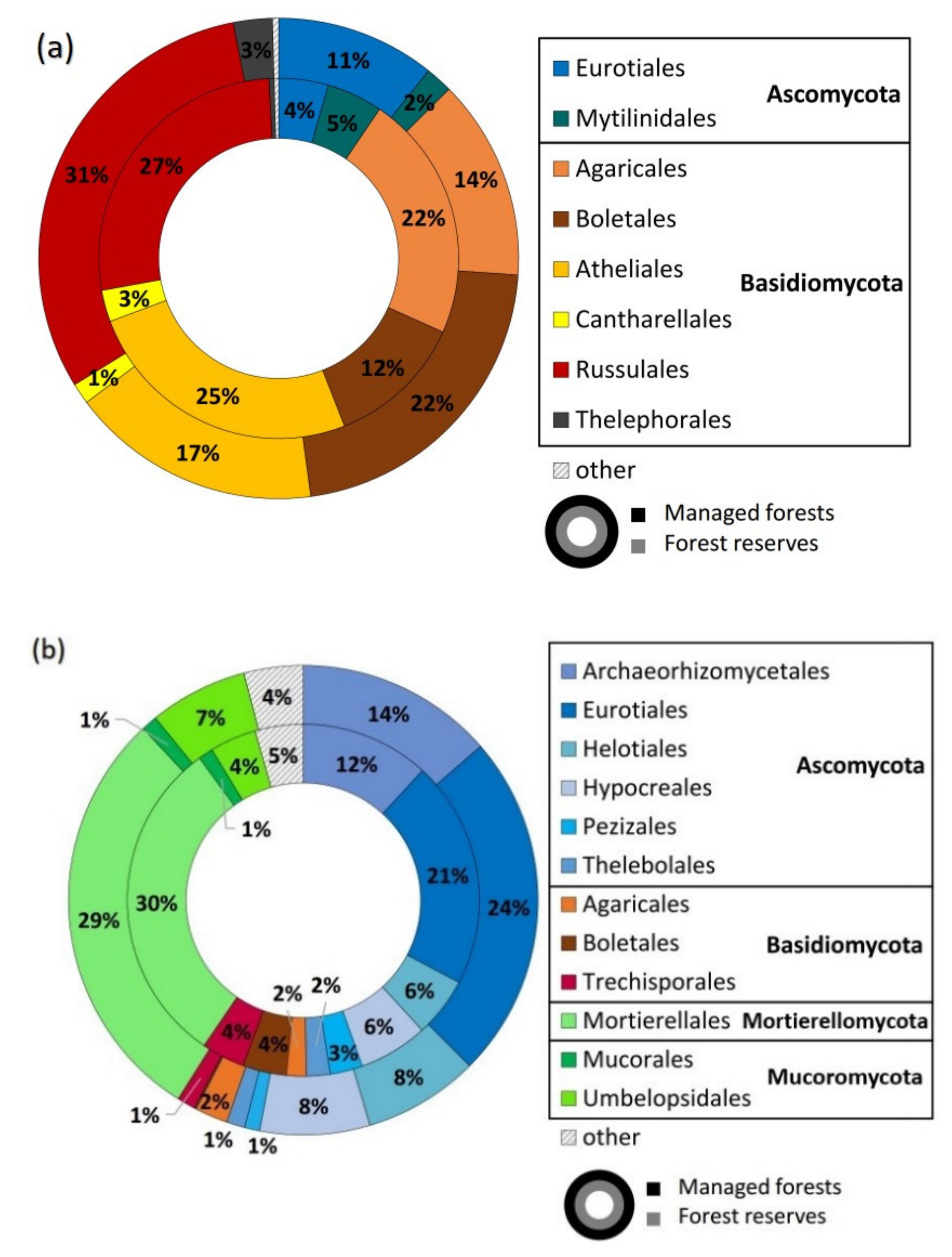
| Environmental Variables | All Fungi | Ectomycorrhizal Fungi | Saprotrophic Fungi | |||||
|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | |||
| OTU richness | Tree cover | No. of tree species | 0.41 | 0.048 | 0.51 | 0.012 | 0.40 | 0.054 |
| No. of ECM tree species | 0.43 | 0.036 | 0.49 | 0.016 | 0.42 | 0.041 | ||
| No. of trees DBH < 10 cm * | 0.45 | 0.026 | 0.47 | 0.022 | 0.38 | 0.064 | ||
| No. of trees DBH > 10 cm * | 0.25 | 0.248 | 0.37 | 0.076 | 0.07 | 0.745 | ||
| TBA of Norway spruce (m2/400 m2) | −0.45 | 0.026 | −0.49 | 0.014 | 0.33 | 0.112 | ||
| TBA of Scots pine (m2/400 m2) | 0.32 | 0.130 | 0.21 | 0.331 | 0.25 | 0.237 | ||
| Forest floor | Organic layer thickness (cm) * | 0.46 | 0.025 | 0.28 | 0.193 | 0.48 | 0.018 | |
| Shannon Index | Tree cover | No. of ECM tree species | −0.14 | 0.522 | 0.07 | 0.747 | 0.21 | 0.330 |
| TBA of Norway spruce (m2/400 m2) | 0.51 | 0.012 | 0.17 | 0.430 | −0.10 | 0.644 | ||
| Total tree basal area (m2/400 m2) | 0.26 | 0.218 | 0.20 | 0.354 | 0.33 | 0.111 | ||
| Forest floor | Organic layer thickness (cm) * | 0.11 | 0.623 | 0.13 | 0.557 | 0.30 | 0.157 | |
| Volume of CWD (m3/400 m2) | 0.42 | 0.042 | 0.29 | 0.166 | 0.24 | 0.261 | ||
| Soil | Total soil C content (%) | 0.59 | 0.002 | 0.57 | 0.004 | 0.21 | 0.317 | |
| Soil organic C content(%) | 0.59 | 0.002 | 0.57 | 0.004 | 0.21 | 0.317 | ||
| Total soil N content (%) | 0.64 | 0.001 | 0.60 | 0.002 | 0.31 | 0.135 | ||
| Soil NO3 content (mg/kg) | 0.52 | 0.009 | 0.35 | 0.097 | 0.31 | 0.140 | ||
| Soil NH4 content (mg/kg) | 0.55 | 0.005 | 0.33 | 0.113 | 0.15 | 0.486 | ||
| Trophic Group | Significant Environmental Variables | df | AIC | F | Pr(>F) | VIF |
|---|---|---|---|---|---|---|
| All fungi | N-NO3 (mg/kg) | 1 | −20.610 | 3.3725 | 0.001 | 7.608 |
| Volume of VFWD (m3/400 m2) | 1 | −21.304 | 2.4945 | 0.001 | 7.317 | |
| No. of trees DBH > 10 cm | 1 | −21.863 | 2.2500 | 0.002 | 9.910 | |
| pH H20 | 1 | −21.936 | 1.7141 | 0.030 | 2.524 | |
| Ectomycorrhizal fungi | Volume of CWD (m3/400 m2) | 1 | −14.027 | 3.0291 | 0.001 | 9.496 |
| pH H20 | 1 | −14.279 | 2.0661 | 0.018 | 2.319 | |
| Volume of VFWD (m3/400 m2) | 1 | −14.441 | 1.8859 | 0.023 | 7.792 | |
| Saprotrophic fungi | Volume of CWD (m3/400 m2) | 1 | −39.014 | 5.5971 | 0.001 | 9.216 |
| N-NO3 (mg/kg) | 1 | −39.322 | 2.1202 | 0.026 | 7.633 |
| Species | Red-List Category * | Trophic Group | Frequency (No of Plots) | |
|---|---|---|---|---|
| M | R | |||
| Tylospora fibrillosa | E | ECM | 3 | 7 |
| Hydnellum concrescens | E | ECM | 2 | 1 |
| Asterodon ferruginosus | E | Saprotroph | 1 | 3 |
| Boidinia furfuracea | E | Saprotroph | 2 | 4 |
| Hygrocybe intermedia | E | Saprotroph | 4 | 8 |
| Lepiota grangei | E | Saprotroph | 9 | 9 |
| Cortinarius malachius | R | ECM | 1 | 3 |
| Cortinarius biformis | R | ECM | 4 | 6 |
| Russula caerulea | R | ECM | 0 | 1 |
| Entoloma rhodocylix | R | Saprotroph | 7 | 9 |
| Postia ptychogaster | R | Saprotroph | 3 | 1 |
| Serpula himantioides | R | Saprotroph | 2 | 3 |
| Botryobasidium obtusisporum | R | Saprotroph | 2 | 2 |
| Chlorencoelia versiformis | R | Saprotroph | 4 | 3 |
| Galerina sphagnorum | R | Saprotroph | 6 | 7 |
| Hygrocybe coccinea | R | Saprotroph | 2 | 1 |
| Hygrocybe quieta | R | Saprotroph | 2 | 3 |
| Entoloma juncinum | R | Saprotroph | 2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kujawska, M.B.; Rudawska, M.; Wilgan, R.; Leski, T. Similarities and Differences among Soil Fungal Assemblages in Managed Forests and Formerly Managed Forest Reserves. Forests 2021, 12, 353. https://doi.org/10.3390/f12030353
Kujawska MB, Rudawska M, Wilgan R, Leski T. Similarities and Differences among Soil Fungal Assemblages in Managed Forests and Formerly Managed Forest Reserves. Forests. 2021; 12(3):353. https://doi.org/10.3390/f12030353
Chicago/Turabian StyleKujawska, Marta Brygida, Maria Rudawska, Robin Wilgan, and Tomasz Leski. 2021. "Similarities and Differences among Soil Fungal Assemblages in Managed Forests and Formerly Managed Forest Reserves" Forests 12, no. 3: 353. https://doi.org/10.3390/f12030353
APA StyleKujawska, M. B., Rudawska, M., Wilgan, R., & Leski, T. (2021). Similarities and Differences among Soil Fungal Assemblages in Managed Forests and Formerly Managed Forest Reserves. Forests, 12(3), 353. https://doi.org/10.3390/f12030353








