The Effect of Nitrogen Fertilization on Tree Growth, Soil Organic Carbon and Nitrogen Leaching—A Modeling Study in a Steep Nitrogen Deposition Gradient in Sweden
Abstract
1. Introduction
- N fertilization gives no or little increase in tree growth at sites with high historical N deposition while it increases tree growth at sites with low N deposition
- N fertilization gives no or little increase in SOC at sites with high historical N deposition while it increases SOC at sites with low N deposition,
- N fertilization increases N leaching with largest effects at sites with historically high N deposition.
2. Materials and Methods
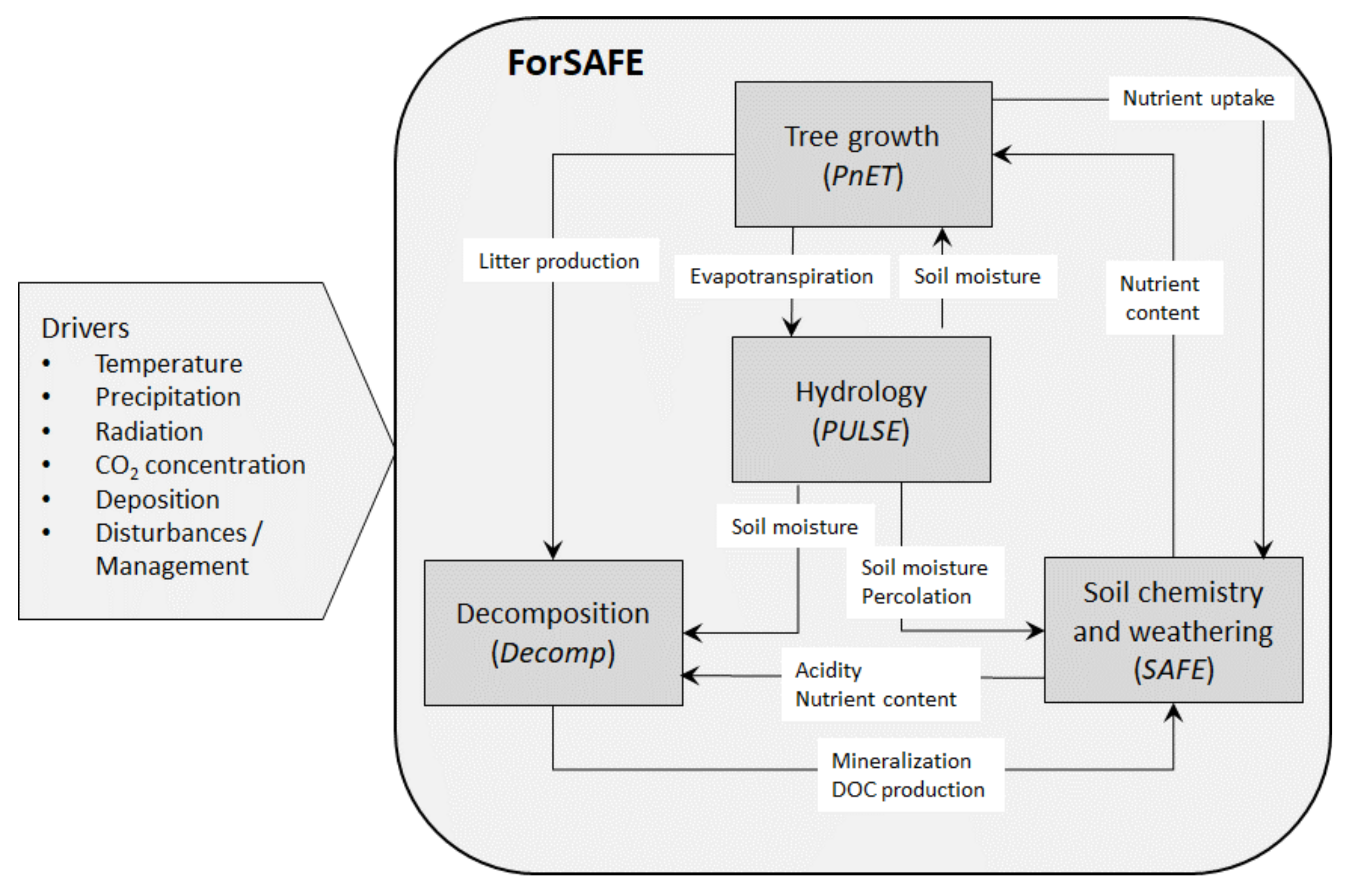
2.1. The ForSAFE Model
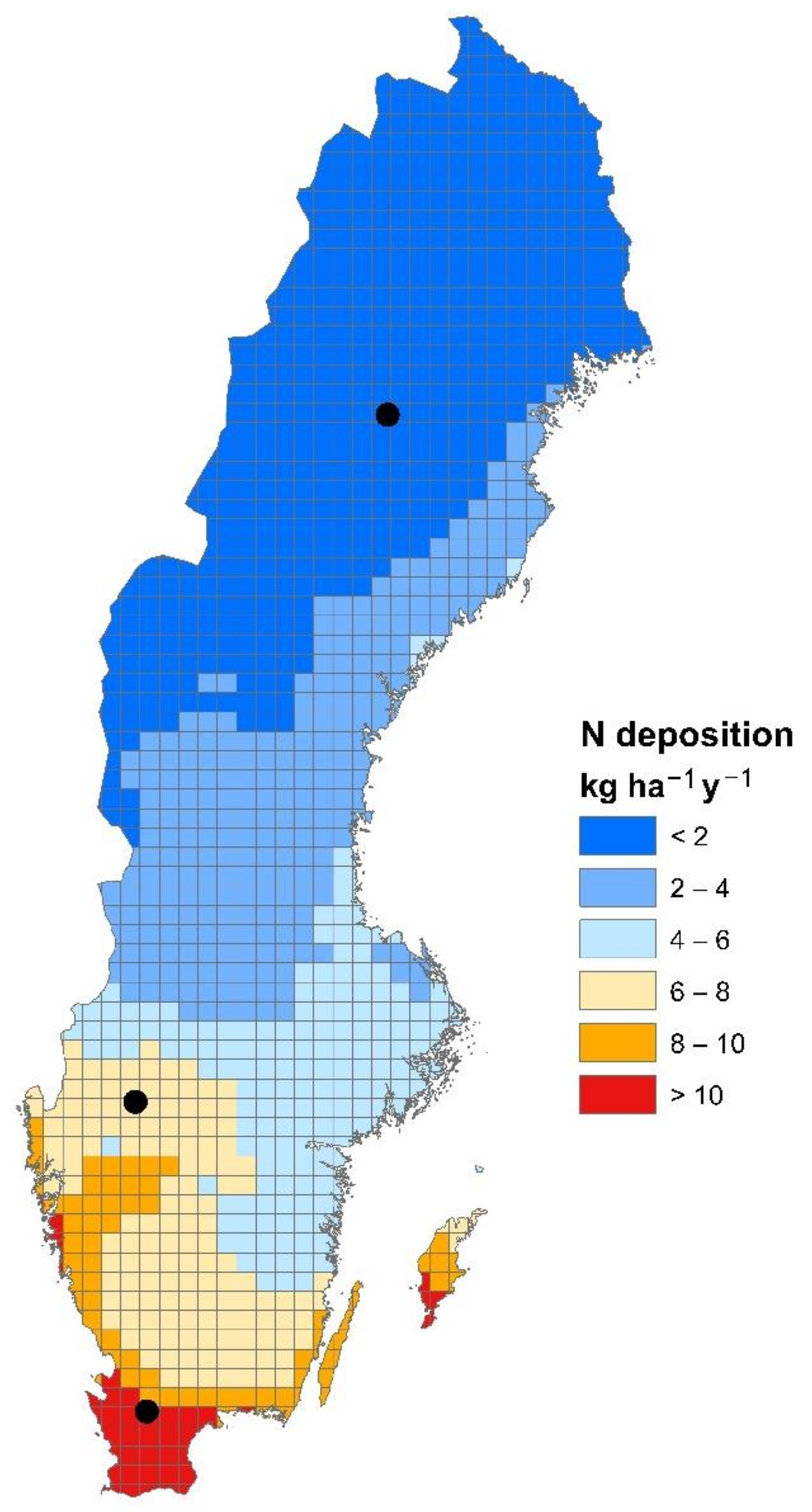
2.2. Study Sites
2.3. Input Data
2.3.1. Climate
2.3.2. Atmospheric Deposition and Nitrogen Fixation
2.3.3. Soil Data
2.3.4. Tree Species and Management
2.4. Model Initialization
2.5. Statistical Metrics
3. Results and Discussion
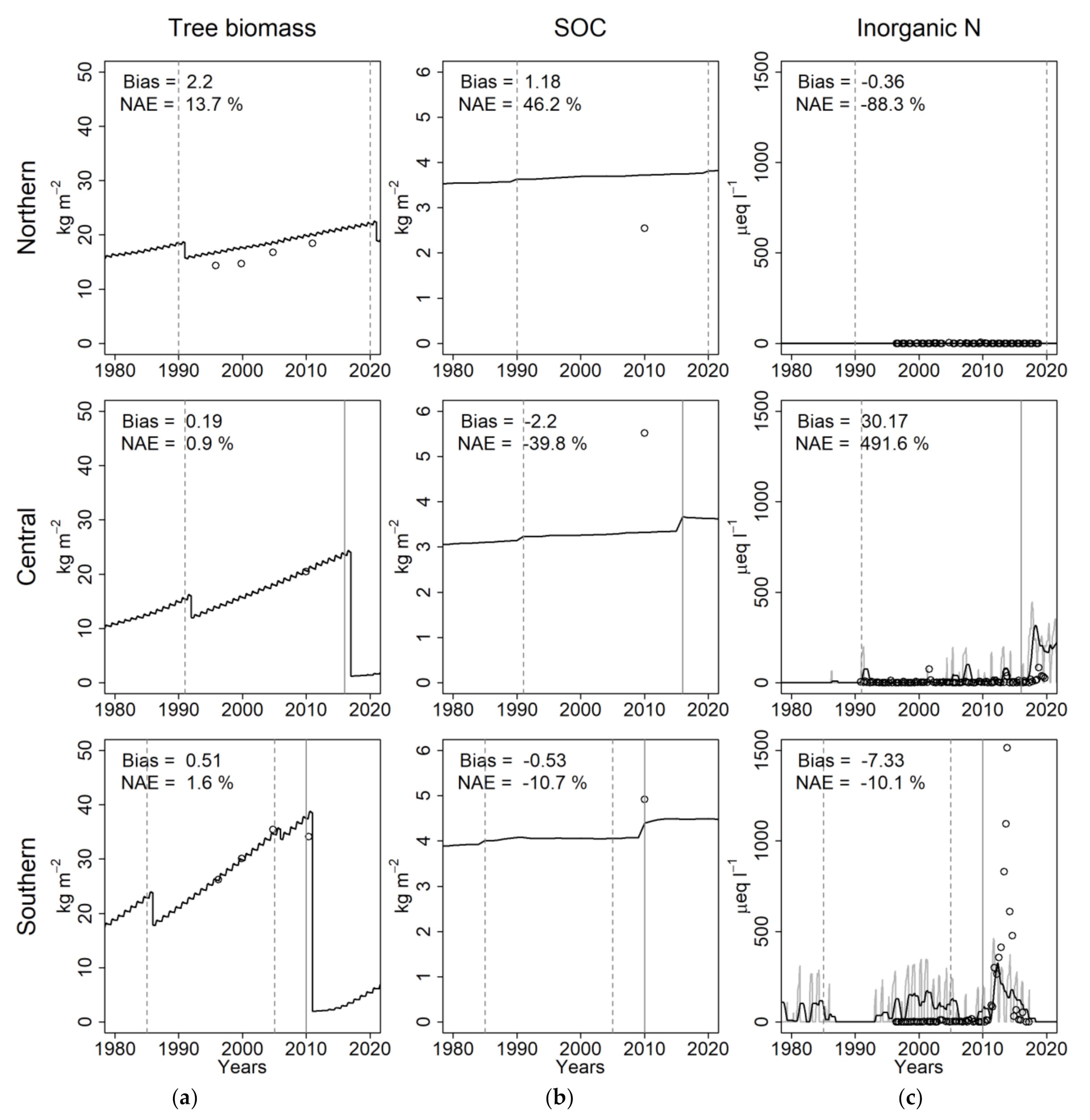
3.1. Model Evaluation
3.1.1. Tree Growth
3.1.2. SOC
3.1.3. N Leaching
3.2. Fertilization Effects
3.2.1. Tree Growth
3.2.2. SOC
3.2.3. N Leaching
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Database | Number of Sites | Site Names | Average (%) | St Dev |
|---|---|---|---|---|
| NORDSOIL 1 | 16 | See [89] | 12 | 8 |
| SWETHRO 2 | 9 | L05, LO7, M10, BD06, O35B, M16B, P95B, P95C, P95D | 25 | 15 |
| Whole-tree harvesting experiments 3 | 4 | Tönnersjöheden, Kosta, Lövliden, Lund | 9 | 7 |
| Total | 29 | 15 | 13 |
Appendix B
| Layer | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Horizon | O | A | AB | B | C |
| Thickness (m) | 0.03 | 0.08 | 0.09 | 0.2 | 0.13 |
| Clay (kg/kg) | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 |
| Silt (kg/kg) | 0.01 | 0.04 | 0.05 | 0.06 | 0.02 |
| Sand (kg/kg) | 0.12 | 0.71 | 0.68 | 0.70 | 0.59 |
| Gravel (kg/kg) | 0.01 | 0.21 | 0.21 | 0.20 | 0.36 |
| Stoniness (m3/m3) | 0.00 | 0.20 | 0.20 | 0.20 | 0.20 |
| Org mtrl (kg/kg) | 0.86 | 0.03 | 0.05 | 0.03 | 0.02 |
| Bulk density (kg/dm3) | 174 | 1140 | 1154 | 1356 | 1471 |
| Kgibb | 6.5 | 6.5 | 7.6 | 8.6 | 9.2 |
| CEC (keq/kg) | 0.25 | 0.06 | 0.04 | 0.02 | 0.02 |
| Base saturation (%) | 77.40 | 18.80 | 21.20 | 31.00 | 38.80 |
| Albite (%) | 2.89 | 19.26 | 19.15 | 23.84 | 22.62 |
| Anorthite (%) | 0.20 | 1.34 | 1.88 | 2.09 | 1.91 |
| Apatite (%) | 0.01 | 0.10 | 0.34 | 0.33 | 0.30 |
| Biotite (%) | 0.04 | 0.28 | 1.03 | 1.47 | 1.36 |
| Chlorite1 (%) | |||||
| Chlorite2 (%) | 0.02 | 0.15 | 0.55 | 0.79 | 0.73 |
| Clinopyroxene (%) | 0.03 | 0.17 | 0.59 | 0.81 | 0.74 |
| Epidote (%) | 0.18 | 1.17 | 1.63 | 1.80 | 1.65 |
| Hornblende (%) | 0.04 | 0.27 | 0.97 | 1.35 | 1.25 |
| Illite1 (%) | |||||
| Illite2 (%) | 0.81 | 5.41 | 10.57 | 5.18 | 4.63 |
| K-Feldspar90 (%) | 3.00 | 20.00 | 13.48 | 19.67 | 18.29 |
| K-Feldspar100 (%) | |||||
| Muscovite (%) | |||||
| Vermiculite1 (%) | 0.05 | 0.36 | 1.35 | 1.96 | 1.81 |
| Vermiculite2 (%) |
| Layer | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Horizon | O | A | AB | B | C |
| Thickness (m) | 0.06 | 0.12 | 0.2 | 0.15 | 0.13 |
| Clay (kg/kg) | 0.00 | 0.02 | 0.02 | 0.02 | 0.02 |
| Silt (kg/kg) | 0.01 | 0.09 | 0.09 | 0.09 | 0.11 |
| Sand (kg/kg) | 0.06 | 0.80 | 0.61 | 0.71 | 0.70 |
| Gravel (kg/kg) | 0.00 | 0.07 | 0.27 | 0.16 | 0.15 |
| Stoniness (m3/m3) | 0.00 | 0.05 | 0.05 | 0.05 | 0.05 |
| Org mtrl (kg/kg) | 0.93 | 0.02 | 0.01 | 0.02 | 0.02 |
| Bulk density (kg/dm3) | 232 | 1309 | 1501 | 1390 | 1515 |
| Kgibb | 6.5 | 6.5 | 7.6 | 8.6 | 9.2 |
| CEC (keq/kg) | 0.32 | 0.02 | 0.02 | 0.02 | 0.02 |
| Base saturation (%) | 35.00 | 17.20 | 10.70 | 12.70 | 16.10 |
| Albite (%) | 1.17 | 23.35 | 19.71 | 20.44 | 22.78 |
| Anorthite (%) | 0.17 | 3.50 | 2.29 | 2.67 | 2.68 |
| Apatite (%) | 0.00 | 0.04 | 0.04 | 0.12 | 0.20 |
| Biotite (%) | |||||
| Chlorite1 (%) | 0.02 | 0.31 | 0.48 | 0.53 | 0.44 |
| Chlorite2 (%) | 0.01 | 0.21 | 0.30 | 0.36 | 0.28 |
| Clinopyroxene (%) | |||||
| Epidote (%) | 0.16 | 3.11 | 3.39 | 2.38 | 3.36 |
| Hornblende (%) | 0.02 | 0.38 | 0.54 | 0.65 | 0.53 |
| Illite1 (%) | 0.06 | 1.22 | 2.50 | 1.50 | 4.12 |
| Illite2 (%) | |||||
| K-Feldspar90 (%) | |||||
| K-Feldspar100 (%) | 0.88 | 17.57 | 11.56 | 14.00 | 13.43 |
| Muscovite (%) | 0.12 | 2.34 | 4.99 | 2.85 | 5.15 |
| Vermiculite1 (%) | 0.03 | 0.51 | 1.50 | 0.89 | 1.65 |
| Vermiculite2 (%) | 0.01 | 0.20 | 0.28 | 0.35 | 0.28 |
| Layer | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Horizon | O | A | AB | B | C |
| Thickness (m) | 0.05 | 0.06 | 0.2 | 0.2 | 0.14 |
| Clay (kg/kg) | 0.01 | 0.04 | 0.05 | 0.03 | 0.02 |
| Silt (kg/kg) | 0.04 | 0.24 | 0.25 | 0.18 | 0.13 |
| Sand (kg/kg) | 0.08 | 0.57 | 0.51 | 0.64 | 0.68 |
| Gravel (kg/kg) | 0.00 | 0.09 | 0.14 | 0.11 | 0.15 |
| Stoniness (m3/m3) | 0.00 | 0.20 | 0.20 | 0.20 | 0.20 |
| Org mtrl (kg/kg) | 0.87 | 0.06 | 0.05 | 0.04 | 0.02 |
| Bulk density (kg/dm3) | 181 | 959 | 1062 | 1279 | 1446 |
| Kgibb | 6.5 | 6.5 | 7.6 | 8.6 | 9.2 |
| CEC (keq/kg) | 0.21 | 0.05 | 0.04 | 0.02 | 0.02 |
| Base saturation (%) | 0.43 | 0.05 | 0.06 | 0.07 | 0.05 |
| Albite (%) | 1.58 | 17.93 | 18.31 | 19.23 | 20.00 |
| Anorthite (%) | 0.01 | 2.10 | 2.31 | 2.42 | 2.60 |
| Apatite (%) | 0.42 | 0.14 | 0.21 | 0.28 | 0.33 |
| Biotite (%) | |||||
| Chlorite1 (%) | 0.20 | 0.30 | 0.49 | 0.59 | 0.70 |
| Chlorite2 (%) | 0.13 | 0.21 | 0.33 | 0.40 | 0.48 |
| Clinopyroxene (%) | |||||
| Epidote (%) | 0.01 | 1.88 | 2.07 | 2.16 | 2.32 |
| Hornblende (%) | 0.02 | 0.36 | 0.58 | 0.70 | 0.82 |
| Illite1 (%) | 0.32 | 1.80 | 3.24 | 3.12 | 2.59 |
| Illite2 (%) | |||||
| K-Feldspar90 (%) | |||||
| K-Feldspar100 (%) | 1.15 | 15.37 | 12.88 | 13.93 | 15.65 |
| Muscovite (%) | 0.73 | 3.44 | 6.19 | 5.95 | 4.89 |
| Vermiculite1 (%) | 0.11 | 0.51 | 0.81 | 0.99 | 1.18 |
| Vermiculite2 (%) | 0.12 | 0.20 | 0.32 | 0.39 | 0.46 |
Appendix C
| Mineral | Si | Ti | Al | Fe | Mg | Ca | Na | K | P | H |
|---|---|---|---|---|---|---|---|---|---|---|
| Albite | 3 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Anorthite | 2 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Apatite | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 3 | 1 |
| Biotite | 2.18 | 0.09 | 1.36 | 0.9 | 0.9 | 0 | 0.04 | 0.68 | 0 | 1.62 |
| Chlorite1 | 0.69 | 0.005 | 0.62 | 0.62 | 0.535 | 0.015 | 0.01 | 0 | 0 | 2.21 |
| Chlorite2 | 0.435 | 0.005 | 0.5 | 0.29 | 0.515 | 0 | 0 | 0 | 0 | 1.51 |
| Clinopyroxene | 0.943 | 0.007 | 0.053 | 0.107 | 0.433 | 0.390 | 0.020 | 0 | 0 | 0 |
| Epidote | 0.62 | 0 | 0.48 | 0.15 | 0 | 0.4 | 0 | 0 | 0 | 0.22 |
| Hornblende | 1.01 | 0.0183 | 0.36 | 0.3 | 0.35 | 0.2767 | 0.09 | 0.03 | 0 | 0.3133 |
| Illite1 | 0.68 | 0 | 0.52 | 0 | 0 | 0 | 0 | 0.12 | 0 | 0.4 |
| Illite2 | 0.66 | 0 | 0.42 | 0.08 | 0.04 | 0.02 | 0 | 0.13 | 0 | 0.4 |
| K-Feldspar90 | 3 | 0 | 1 | 0 | 0 | 0 | 0.1 | 0.9 | 0 | 0 |
| K-Feldspar100 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Muscovite | 1.2 | 0.02 | 0.96 | 0.12 | 0.08 | 0 | 0.02 | 0.44 | 0 | 0.94 |
| Vermiculite1 | 1.465 | 0 | 0.81 | 0.91 | 0.515 | 0.1 | 0 | 0 | 0 | 4.02 |
| Vermiculite2 | 0.615 | 0 | 0.34 | 0.11 | 0.515 | 0.05 | 0 | 0 | 0 | 2.45 |
| Quartz | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
References
- Swedish Government. Regeringens Proposition 2008/09: 163. En Sammanhållen Klimat-Och Energipolitik–Energi. Available online: https://www.regeringen.se/rattsliga-dokument/proposition/2009/03/prop.-200809163/ (accessed on 15 January 2021).
- Börjesson, P.; Hansson, J.; Berndes, G. Future Demand For Forest-Based Biomass For Energy Purposes In Sweden. For. Ecol. Manag. 2017, 383, 17–26. [Google Scholar] [CrossRef]
- Lindkvist, A.; Kardell, Ö.; Nordlund, C. Intensive Forestry As Progress Or Decay? An Analysis Of The Debate About Forest Fertilization In Sweden, 1960–2010. Forests 2011, 2, 112–146. [Google Scholar] [CrossRef]
- Johnson, D.W.; Curtis, P.S. Effects Of Forest Management On Soil C And N Storage: Meta Analysis. For. Ecol. Manag. 2001, 140, 227–238. [Google Scholar] [CrossRef]
- Sathre, R.; Gustavsson, L.; Bergh, J. Primary Energy And Greenhouse Gas Implications Of Increasing Biomass Production Through Forest Fertilization. Biomass Bioenergy 2010, 34, 572–581. [Google Scholar] [CrossRef]
- Aber, J.D.; Nadelhoffer, K.J.; Steudler, P.; Melillo, J.M. Nitrogen Saturation In Northern Forest Ecosystems. Bioscience 1989, 39, 378–386. [Google Scholar] [CrossRef]
- Aber, J. Nitrogen Saturation In Temperate Forest Ecosystems: Current Theory, Remaining Questions And Recent Advances. In Progress in Plant Nutrition: Plenary Lectures of the XIV International Plant Nutrition Colloquium; Springer: Dordrecht, The Netherlands, 2002; pp. 179–188. [Google Scholar] [CrossRef]
- Emmett, B. Nitrogen Saturation Of Terrestrial Ecosystems: Some Recent Findings And Their Implications For Our Conceptual Framework. Water Air Soil Pollut. Focus 2007, 7, 99. [Google Scholar] [CrossRef]
- Cowling, E.; Galloway, J.; Furiness, C.; Barber, M.; Bresser, T.; Cassman, K.; Erisman, J.W.; Haeuber, R.; Howarth, R.; Melillo, J.; et al. Optimizing Nitrogen Management In Food And Energy Production And Environmental Protection: Summary Statement From The Second International Nitrogen Conference. Sci. World J. 2001, 1 (Suppl. 2), 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tamm, C.O. Nitrogen In Terrestrial Ecosystems: Questions of Productivity, Vegetational Changes, and Ecosystem Stability; Springer: Dordrecht, The Netherlands, 1991. [Google Scholar]
- Karlsson, P.E.; Pihl Karlsson, G.; Hellsten, S.; Akselsson, C.; Ferm, M.; Hultberg, H. Total Deposition Of Inorganic Nitrogen To Norway Spruce Forests–Applying A Surrogate Surface Method Across A Deposition Gradient In Sweden. Atmos. Environ. 2019, 217. [Google Scholar] [CrossRef]
- Akselsson, C.; Belyazid, S.; Hellsten, S.; Klarqvist, M.; Pihl-Karlsson, G.; Karlsson, P.-E.; Lundin, L. Assessing The Risk of N Leaching From Forest Soils Across A Steep N Deposition Gradient In Sweden. Environ. Pollut. 2010, 158, 3588–3595. [Google Scholar] [CrossRef]
- Akselsson, C.; Westling, O.; Orlander, G. Regional Mapping Of Nitrogen Leaching From Clearcuts In Southern Sweden. For. Ecol. Manag. 2004, 202, 235–243. [Google Scholar] [CrossRef]
- Swedish Forest Agency. Kvävegödsling av Skogsmark; Meddelande 2: 2007; Skogsstyrelsen: Jönköping, Sweden, 2007.
- Swedish Forest Agency. Föreskrifter om Ändring i Skogsstyrelsens föreskrifter och allmänna råd (SKSFS 2011:7) till Skogsvårdslagen; SKSFS 2013:2; Skogsstyrelsen: Jönköping, Sweden, 2014.
- Högberg, P.; Larsson, S.; Lundmark, T.; Moen, J.; Nilsson, U.; Nordin, A. Effekter Av Kvävegödsling På Skogsmark Kunskapssammanställning Utförd Av Slu På Begäran Av Skogsstyrelsen; Swedish Forest Agency: Linköping, Sweden, 2014. [Google Scholar]
- Jacobson, S.; Pettersson, F. Growth Responses Following Nitrogen and NPK Mg Additions to Previously N-Fertilized Scots Pine and Norway Spruce Stands on Mineral Soils in Sweden. Can. J. For. Res. 2001, 31, 899–908. [Google Scholar] [CrossRef]
- Nohrstedt, H.-Ö. Response Of Coniferous Forest Ecosystems On Mineral Soils To Nutrient Additions: A Review of Swedish Experiences. Scand. J. For. Res. 2001, 16, 555–573. [Google Scholar] [CrossRef]
- Andersson, F.; Brække, F.H.; Hallbäcken, L. Nutrition and Growth of Norway Spruce Forests in a Nordic Climate and Deposition Gradient; TemaNord 1998:566; Nordic Council of Ministers: Copenhagen, Denmark, 1998. [Google Scholar]
- Tamm, C.O.; Aronsson, A.; Popovic, B.; Flower-Ellis, J. Optimum Nutrition and Nitrogen Saturation in Scots Pine Stands; Studia Forestalia Suecica No 206; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1999. [Google Scholar]
- Mälkönen, E. Estimation of Nitrogen Saturation on the Basis of Long-Term Fertilization Experiments. Plant Soil 1990, 128, 75–82. [Google Scholar] [CrossRef]
- Sikstrom, U. Effects of Low-Dose Liming and Nitrogen Fertilization on Stemwood Growth and Needle Properties of Picea Abies and Pinus Sylvestris. For. Ecol. Manag. 1997, 95, 261. [Google Scholar] [CrossRef]
- Bergh, J.; Nilsson, U.; Allen, H.L.; Johansson, U.; Fahlvik, N. Long-Term Responses of Scots Pine and Norway Spruce Stands in Sweden to Repeated Fertilization and Thinning. For. Ecol. Manag. 2014, 320, 118–128. [Google Scholar] [CrossRef]
- Ring, E.; Jacobson, S.; Nohrstedt, H.-Ö. Soil-Solution Chemistry in a Coniferous Stand after Adding Wood Ash and Nitrogen. Can. J. For. Res. 2006, 36, 153–163. [Google Scholar] [CrossRef]
- Högbom, L.; Nohrstedt, H.-Ö.; Nordlund, S. Nitrogen Fertilization Effects on Stream Water Cadmium Concentration. J. Environ. Qual. 2001, 30, 189–193. [Google Scholar] [CrossRef]
- Binkley, D.; Burnham, H.; Lee Allen, H. Water Quality Impacts Of Forest Fertilization With Nitrogen And Phosphorus. For. Ecol. Manag. 1999, 121, 191–213. [Google Scholar] [CrossRef]
- Gundersen, P.; Schmidt, I.K.; Raulund-Rasmussen, K. Leaching of Nitrate From Temperate Forests–Effects of Air Pollution and Forest Management. Environ. Rev. 2006, 14, 1–57. [Google Scholar] [CrossRef]
- Ring, E. Effects of Previous N Fertilizations on Soil—Water Ph and N Concentrations after Clear—Felling and Soil Scarification at a Pinus Sylvestris Site. Scand. J. For. Rese. 1996, 11, 7–16. [Google Scholar] [CrossRef]
- Berdén, M.; Nilsson, S.I.; Nyman, P. Ion Leaching before and after Clear-Cutting in a Norway Spruce Stand—Effects of Long-Term Application of Ammonium Nitrate and Superphosphate. Water Air Soil Pollut. 1997, 93, 1–26. [Google Scholar] [CrossRef]
- Ring, E.; Bergholm, J.; Olsson, B.A.; Jansson, G. Urea Fertilizations of a Norway Spruce Stand: Effects on Nitrogen in Soil Water and Field-Layer Vegetation after Final Felling. Can. J. For. Res. 2003, 33, 375–384. [Google Scholar] [CrossRef]
- Hedwall, P.-O.; Grip, H.; Linder, S.; Lövdahl, L.; Nilsson, U.; Bergh, J. Effects of Clear-Cutting and Slash Removal on Soil Water Chemistry and Forest-Floor Vegetation in a Nutrient Optimised Norway Spruce Stand. Silva Fenn. 2013, 47, 933. [Google Scholar] [CrossRef]
- Ring, E.; Högbom, L.; Nohrstedt, H.O. Effects of Brash Removal after Clear Felling on Soil and Soil-Solution Chemistry and Field-Layer Biomass in an Experimental Nitrogen Gradient. Sci. World J. 2001, 1 (Suppl. 2), 457–466. [Google Scholar] [CrossRef]
- Ring, E.; Högbom, L.; Jacobson, S.; Jansson, G.; Nohrstedt, H.-Ö. Long-Term Effects on Soil-Water Nitrogen and Ph of Clearcutting and Simulated Disc Trenching of Previously Nitrogen-Fertilised Pine Plots. Can. J. For. Res. 2018, 48, 1115–1123. [Google Scholar] [CrossRef]
- Wallman, P.; Svensson, M.; Sverdrup, H.; Belyazid, S. Forsafe—An Integrated Process-Oriented Forest Model for Long-Term Sustainability Assessments. For. Ecol. Manag. 2005, 207, 19–36. [Google Scholar] [CrossRef]
- Belyazid, S.; Westling, O.; Sverdrup, H. Modelling Changes in Forest Soil Chemistry at 16 Swedish Coniferous Forest Sites Following Deposition Reduction. Environ. Pollut. 2006, 144, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Pihl Karlsson, G.; Akselsson, C.; Hellsten, S.; Karlsson, P.E. Reduced European Emissions of S and N—Effects on Air Concentrations, Deposition and Soil Water Chemistry in Swedish Forests. Environ. Pollut. 2011, 159, 3571–3582. [Google Scholar] [CrossRef] [PubMed]
- Langner, J. Air Pollution Assessment Study Using the Match Modelling System: Application to Sulphur and Nitrogen Compounds over Sweden 1994; SMHI: Norrköping, Sweden, 1996.
- Alveteg, M. Dynamics of Forest Soil Chemistry; Lund University: Lund, Sweden, 1998. [Google Scholar]
- Aber, J.D.; Federer, C.A. A Generalized, Lumped-Parameter Model of Photosynthesis, Evapotranspiration and Net Primary Production in Temperate and Boreal Forest Ecosystems. Oecologia 1992, 92, 463–474. [Google Scholar] [CrossRef]
- Walse, C.; Berg, B.; Sverdrup, H. Review and Synthesis of Experimental Data on Organic Matter Decomposition with Respect to the Effect of Temperature, Moisture, and Acidity. Environ. Rev. 1998, 6, 25–40. [Google Scholar] [CrossRef]
- Wallman, P.; Belyazid, S.; Svensson, M.G.E.; Sverdrup, H. Decomp—A Semi-Mechanistic Model of Litter Decomposition. Environ. Model. Softw. 2006, 21, 33–44. [Google Scholar] [CrossRef]
- Lindström, G.; Gardelin, M. Model structure. In Modelling Groundwater Response to Acidification; Sandén, P., Warfvinge, P., Eds.; SMHI: Norrköping, Sweden, 1992. [Google Scholar]
- Yu, L.; Zanchi, G.; Akselsson, C.; Wallander, H.; Belyazid, S. Modeling the forest phosphorus nutrition in a southwestern Swedish forest site. Ecol. Model. 2018, 369, 88–100. [Google Scholar] [CrossRef]
- Zanchi, G.; Belyazid, S.; Akselsson, C.; Yu, L.; Bishop, K.; Köhler, S.J.; Grip, H. A Hydrological Concept Including Lateral Water Flow Compatible with the Biogeochemical Model Forsafe. Hydrology 2016, 3, 11. [Google Scholar] [CrossRef]
- Sverdrup, H.; Stjernquist, I. (Eds.) Developing Principles and Models for Sustainable Forestry in Sweden; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Kjellström, E.; Sveriges Meteorologiska Och Hydrologiska Institut. A 140-Year Simulation of European Climate with the New Version of the Rossby Centre Regional Atmospheric Climate Model (RCA3); SMHI: Norrköping, Sweden, 2005.
- Roeckner, E.; Brokopf, R.; Esch, M.; Giorgetta, M.; Hagemann, S.; Kornblueh, L.; Manzini, E.; Schlese, U.; Schulzweida, U. Sensitivity of Simulated Climate to Horizontal and Vertical Resolution in the Echam5 Atmosphere Model. J. Clim. 2006, 19, 3771. [Google Scholar] [CrossRef]
- Nakicenovic, N.; Swart, R. Special Report on Emissions Scenarios (SRES)—A Special Report of Working Group III of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambrige, UK, 2000; Volume 559. [Google Scholar]
- Yang, W.; Andréasson, J.; Graham, L.P.; Olsson, J.; Rosberg, J.; Wetterhall, F. Distribution-Based Scaling to Improve Usability of Regional Climate Model Projections for Hydrological Climate Change Impacts Studies. Hydrol. Res. 2010, 41, 211–229. [Google Scholar] [CrossRef]
- Johansson, B. Areal Precipitation and Temperature in the Swedish Mountains: An Evaluation from a Hydrological Perspective. Hydrol. Res. 2000, 31, 207–228. [Google Scholar] [CrossRef]
- Hempel, S.; Frieler, K.; Warszawski, L.; Schewe, J.; Piontek, F. A Trend-Preserving Bias Correction—The Isi-Mip Approach. Earth Syst. Dyn. 2013, 4, 219–236. [Google Scholar] [CrossRef]
- David, R.; (University of Gothenburg, Gothenburg, Sweden). Personal communication, 2010.
- Robertson, L.; Langner, J.; Engardt, M. An Eulerian Limited-Area Atmospheric Transport Model. J. Appl. Meteorol. 1999, 38, 190–210. [Google Scholar] [CrossRef]
- Engardt, M.; Langner, J. Simulations of Future Sulphur and Nitrogen Deposition over Europe Using Meteorological Data from Three Regional Climate Projections. Tellus B Chem. Phys. Meteorol. 2013, 65, 20348. [Google Scholar] [CrossRef]
- Munthe, J.; Arnell, J. (Eds.) Klimatförändringen och Miljömål; Report 6705; Swedish Environmental Protection Agency: Stockholm, Sweden, 2016.
- Lamarque, J.F.; Bond, T.C.; Eyring, V.; Granier, C.; Heil, A.; Klimont, Z.; Lee, D.; Liousse, C.; Mieville, A.; Owen, B.; et al. Historical (1850–2000) Gridded Anthropogenic and Biomass Burning Emissions of Reactive Gases and Aerosols: Methodology and Application. Atmos. Chem. Phys. 2010, 10, 7017–7039. [Google Scholar] [CrossRef]
- Lampa, M.E.; Belyazid, S.; Zanchi, G.; Akselsson, C. Effects of Whole-Tree Harvesting on Soil, Soil Water and Tree Growth—A Dynamic Modelling Exercise in Four Long-Term Experiments. Ecol. Model. 2019, 414. [Google Scholar] [CrossRef]
- Lindo, Z.; Nilsson, M.-C.; Gundale, M. Bryophyte-Cyanobacteria Associations as Regulators of the Northern Latitude Carbon Balance in Response to Global Change. Global Chang. Biol. 2013, 19, 2022–2035. [Google Scholar] [CrossRef]
- Zanchi, G.; Belyazid, S.; Akselsson, C.; Yu, L. Modelling the Effects of Management Intensification on Multiple Forest Services: A Swedish Case Study. Ecol. Model. 2014, 284, 48–59. [Google Scholar] [CrossRef]
- Yu, L.; Belyazid, S.; Akselsson, C.; Van Der Heijden, G.; Zanchi, G. Storm Disturbances in a Swedish Forest-A Case Study Comparing Monitoring and Modelling. Ecol. Model. 2016, 320, 102–113. [Google Scholar] [CrossRef]
- Posch, M.; Kurz, D. A2m—A Program to Compute All Possible Mineral Modes from Geochemical Analyses. Comput. Geosci. 2007, 33, 563–572. [Google Scholar] [CrossRef]
- Belyazid, S. Dynamic Modelling of Biogeochemical Processes in Forest Ecosystems. Ph.D. Thesis, Lund University, Lund, Sweden, 2006. [Google Scholar]
- Aber, J.D.; Ollinger, S.V.; Federer, C.A.; Reich, P.B.; Goulden, M.L.; Kicklighter, D.W.; Melillo, J.M.; Lathrop, R.G. Predicting the Effects of Climate Change on Water Yield and Forest Production in the Northeastern United States. Clim. Res. 1995, 5, 207–222. [Google Scholar] [CrossRef]
- Ågren Göran, I.; Hyvönen, R.; Nilsson, T. Are Swedish Forest Soils Sinks or Sources for CO2: Model Analyses Based on Forest Inventory Data. Biogeochemistry 2007, 82, 217–227. [Google Scholar] [CrossRef]
- Aber, J.D.; Ollinger, S.V.; Driscoll, C.T. Modeling Nitrogen Saturation in Forest Ecosystems in Response to Land Use and Atmospheric Deposition. Ecol. Model. 1997, 101, 61–78. [Google Scholar] [CrossRef]
- Gustafson, E.J.; Miranda, B.R. Pnet-Succession V3.1 Extension User Guide. Available online: https://sites.google.com/site/landismodel/extensions/pnet-succession (accessed on 15 January 2021).
- Swedish Forest Agency. Gallringsmallar: Norra Sverige; Skogsstyrelsen: Jönköping, Sweden, 1985.
- Swedish Forest Agency. Gallringsmallar: Södra Sverige; Skogsstyrelsen: Jönköping, Sweden, 1985.
- Janssen, P.H.M.; Heuberger, P.S.C. Calibration of Process-Oriented Models. Ecol. Model. 1995, 83, 55–66. [Google Scholar] [CrossRef]
- Nash, J.E.; Sutcliffe, J.V. River Flow Forecasting through Conceptual Models Part I—A Discussion of Principles. J. Hydrol. 1970, 10, 282–290. [Google Scholar] [CrossRef]
- Belyazid, S.; Giuliana, Z. Water Limitation Can Negate the Effect of Higher Temperatures on Forest Carbon Sequestration. Eur. J. For. Res. 2019, 138, 287–297. [Google Scholar] [CrossRef]
- Schrumpf, M.; Schulze, E.D.; Kaiser, K.; Schumacher, J. How Accurately Can Soil Organic Carbon Stocks and Stock Changes Be Quantified by Soil Inventories? Biogeosciences 2011, 8, 1193–1212. [Google Scholar] [CrossRef]
- Yurova, A.Y.; Lankreijer, H. Carbon Storage in the Organic Layers of Boreal Forest Soils under Various Moisture Conditions: A Model Study for Northern Sweden Sites. Ecol. Model. 2007, 204, 475–484. [Google Scholar] [CrossRef]
- Stendahl, J. Skogsdata 2017: [Aktuella Uppgifter om de Svenska Skogarna Från Riksskogstaxeringen]; Institutionen För Skoglig Resurshushållning, SLU: Umeå, Sweden, 2017. [Google Scholar]
- Fahey, T.J.; Siccama, T.G.; Driscoll, C.T.; Likens, G.E.; Campbell, J.; Johnson, C.E.; Battles, J.J.; Aber, J.D.; Cole, J.J.; Fisk, M.C.; et al. The Biogeochemistry of Carbon at Hubbard Brook. Biogeochemistry 2005, 75, 109–176. [Google Scholar] [CrossRef]
- Johnson, D.W.; Todd, J.D.E.; Trettin, C.F.; Sedinger, J.S. Soil Carbon and Nitrogen Changes in Forests of Walker Branch Watershed, 1972 to 2004. Soil Sci. Soc. Am. J. 2007, 71, 1639–1646. [Google Scholar] [CrossRef]
- Kiser, L.C.; Kelly, J.M.; Mays, P.A. Changes in Forest Soil Carbon and Nitrogen after a Thirty-Year Interval. Soil Sci. Soc. Am. J. 2009, 73, 647–653. [Google Scholar] [CrossRef]
- Pihl Karlsson, G.; Hellsten, S.; Akselsson, C.; Karlsson, P.E. Försurning Och Övergödning I Skåne Län Resultat Från Krondroppsnätet Till Och Med 2018/19; Report C535; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2020. [Google Scholar]
- Futter, M.N.; Ring, E.; Högbom, L.; Entenmann, S.; Bishop, K.H. Consequences of Nitrate Leaching Following Stem-Only Harvesting of Swedish Forests Are Dependent on Spatial Scale. Environ. Pollut. 2010, 158, 3552–3559. [Google Scholar] [CrossRef]
- Emmett, B.A.; Anderson, J.M.; Hornung, M. The Controls on Dissolved Nitrogen Losses Following Two Intensities of Harvesting in a Sitka Spruce Forest (N. Wales). For. Ecol. Manag. 1991, 41, 65–80. [Google Scholar] [CrossRef]
- Blanes, M.C.; Emmett, B.A.; Viñegla, B.; Carreira, J.A. Alleviation of P Limitation Makes Tree Roots Competitive for N against Microbes in a N-Saturated Conifer Forest: A Test through P Fertilization and 15n Labelling. Soil Biol. Biochem. 2012, 48, 51–59. [Google Scholar] [CrossRef]
- Vitousek Peter, M.; Porder, S.; Houlton Benjamin, Z.; Chadwick Oliver, A. Terrestrial Phosphorus Limitation: Mechanisms, Implications, And Nitrogen—Phosphorus Interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Likens, G.E.; Driscoll, C.T.; Buso, D.C. Long-Term Effects of Acid Rain: Response and Recovery of a Forest Ecosystem. Science 1996, 272, 244–246. [Google Scholar] [CrossRef]
- From, F.; Strengbom, J.; Nordin, A. Residual Long-Term Effects of Forest Fertilization on Tree Growth and Nitrogen Turnover in Boreal Forest. Forests 2015, 6, 1145–1156. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, X.; Geng, Q.; Shi, Z.; Luo, Y.; Xu, X.; Morellato, P. Leaf Litter Traits Predominantly Control Litter Decomposition in Streams Worldwide. Glob. Ecol. Biogeogr. 2019, 28, 1469–1486. [Google Scholar] [CrossRef]
- Peltoniemi, M.; Mäkipää, R.; Liski, J.; Tamminen, P. Changes in Soil Carbon With Stand Age—An Evaluation of a Modelling Method with Empirical Data. Glob. Chang. Biol. 2004, 10, 2078–2091. [Google Scholar] [CrossRef]
- Frey, S.D.; Knorr, M.; Parrent, J.L.; Simpson, R.T. Chronic Nitrogen Enrichment Affects the Structure and Function of the Soil Microbial Community in Temperate Hardwood and Pine Forests. For. Ecol. Manag. 2004, 196, 159–171. [Google Scholar] [CrossRef]
- Tipping, E.; Davies, J.A.C.; Henrys, P.A.; Kirk, G.J.D.; Lilly, A.; Dragosits, U.; Carnell, E.J.; Dore, A.J.; Sutton, M.A.; Tomlinson, S.J. Long-Term Increases In Soil Carbon Due to Ecosystem Fertilization by Atmospheric Nitrogen Deposition Demonstrated by Regional-Scale Modelling and Observations. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stendahl, J.; Akselsson, C.; Melkerud, P.-A.; Belyazid, S. Pedon-Scale Silicate Weathering: Comparison of the Profile Model and the Depletion Method at 16 Forest Sites in Sweden. Geoderma 2013, 211–212, 65–74. [Google Scholar] [CrossRef]
- Brandtberg, P.-O.; Olsson, B.A. Changes in the Effects of Whole-Tree Harvesting on Soil Chemistry during 10 years of Stand Development. For. Ecol. Manag. 2012, 277, 150–162. [Google Scholar] [CrossRef]

| Pool | kpot (year−1) | f(T) | g(θ) | φ(pH) | ||
|---|---|---|---|---|---|---|
| Ea (kJ mol−1) | KW | nW | KpH (kmol−3) | m | ||
| EDCa | 24 | 18 | 9.4 | 3.4 | 65,600 | 1 |
| Holocellulose | 1.1 | 33 | 9.4 | 3.4 | 20,500 | 1 |
| Lignin | 0.17 | 50 | 9.4 | 3.4 | 1050 | 1 |
| Resistant | 0.003 | 53 | 9.4 | 3.4 | 1050 | 1 |
| DOC | 15 | 451 | 110 | 2.454 | 20,500 | 1 |
| Parameters | Northern Site (Högbränna) 1 | Central Site (Södra Averstad) 2 | Southern Site (Västra Torup) 3 |
|---|---|---|---|
| Coordinates (N, E) | 65.41, 18.10 | 59.01, 13.11 | 56.14, 13.51 |
| Year of planting | 1916 | 1932 | 1941 |
| Year of final felling | - | 2016 | 2010 |
| Standing stem biomass (kg m−2) | 9443 (year 2011) | 11,680 (year 2010) | 18,841 (year 2010) |
| Average annual temp. (°C) 4 | 1.0 | 7.2 | 8.0 |
| Average annual prec. (mm) 4 | 720 | 744 | 859 |
| Measured throughfall deposition 5 | |||
| N deposition (kg ha−1 year−1) | 0.5 ± 0.2 | 4.6 ± 1.2 | 9.4 ± 1.8 |
| S deposition (kg ha−1 year−1) | 0.8 ± 0.2 | 5.8 ± 3.6 | 11.7 ± 6.7 |
| Cl deposition (kg ha−1 year−1) | 1.9 ± 0.4 | 29.2 ± 6.8 | 37.0 ± 8.0 |
| Ca + Mg + Na + K dep. (kg ha−1 year−1) | 7.9 ± 2.1 | 37.6 ± 5.4 | 46.8 ± 7.7 |
| Measured open field deposition 5 | |||
| N deposition (kg ha−1 year−1) | 1.2 ± 0.2 | 6.7 ± 1.5 | 11.4 ± 2.9 |
| S deposition (kg ha−1 yar−1) | 1.0 ± 0.2 | 4.4 ± 1.7 | 6.9 ± 2.0 |
| Cl deposition (kg ha−1 year−1) | 1.4 ± 0.4 | 8.5 ± 3.7 | 24.8 ± 13.6 |
| Ca + Mg + Na + K dep. (kg ha−1 year−1) | 3.0 ± 0.8 | 8.3 ± 4.0 | 20.8 ± 10.9 |
| Parameter | Definition (Units) | Value |
|---|---|---|
| FolReten | Foliage retention time (year) | (1/FolRet = 0.489 − 0.0063 * Latitude) |
| WoodTurnover | Fractional mortality of live wood (year−1) | 0.01 (Southern site) 0.025 (Central and Northern sites) |
| WUECnst | Constant for calculating water use efficiency | 15.9 |
| HalfSat | Half saturation constant for light (μmol/m2/s) | 170 |
| Site | Thinning | Final Felling | Fertilization (150 kg ha−1) |
|---|---|---|---|
| Northern | 1990, 2020 | 1915, 2055 | 2025, 2035, 2045 |
| Central | 1961, 1991, 2045, 2065 | 1931, 2016, 2095 | 2067, 2076, 2085 |
| Southern | 1965, 1985, 2005, 2035, 2055 | 1940, 2010, 2080 | 2057, 2064, 2070 |
| Before Final Felling | 20 Years after Final Felling | |||
|---|---|---|---|---|
| Biomass | SOC | Biomass | SOC | |
| Northern | 21% | 2% | 14% | 4% |
| Central | 6% | 1% | 4% | 2% |
| Southern | 5% | 1% | 7% | 1% |
| Site | Before Final Felling (kg ha−1) | 20 Years after Final Felling (kg ha−1) | Fraction Leached (%) | ||
|---|---|---|---|---|---|
| BAS | FERT | BAS | FERT | ||
| Northern | 4 | 96 | 49 | 48 | 20% |
| Central | 2 | 152 | 87 | 88 | 34% |
| Southern | 30 | 210 | 60 | 56 | 39% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucander, K.; Zanchi, G.; Akselsson, C.; Belyazid, S. The Effect of Nitrogen Fertilization on Tree Growth, Soil Organic Carbon and Nitrogen Leaching—A Modeling Study in a Steep Nitrogen Deposition Gradient in Sweden. Forests 2021, 12, 298. https://doi.org/10.3390/f12030298
Lucander K, Zanchi G, Akselsson C, Belyazid S. The Effect of Nitrogen Fertilization on Tree Growth, Soil Organic Carbon and Nitrogen Leaching—A Modeling Study in a Steep Nitrogen Deposition Gradient in Sweden. Forests. 2021; 12(3):298. https://doi.org/10.3390/f12030298
Chicago/Turabian StyleLucander, Klas, Giuliana Zanchi, Cecilia Akselsson, and Salim Belyazid. 2021. "The Effect of Nitrogen Fertilization on Tree Growth, Soil Organic Carbon and Nitrogen Leaching—A Modeling Study in a Steep Nitrogen Deposition Gradient in Sweden" Forests 12, no. 3: 298. https://doi.org/10.3390/f12030298
APA StyleLucander, K., Zanchi, G., Akselsson, C., & Belyazid, S. (2021). The Effect of Nitrogen Fertilization on Tree Growth, Soil Organic Carbon and Nitrogen Leaching—A Modeling Study in a Steep Nitrogen Deposition Gradient in Sweden. Forests, 12(3), 298. https://doi.org/10.3390/f12030298






