Seasonal Variations in Carbon, Nitrogen, and Phosphorus Stoichiometry of a Robinia pseudoacacia Plantation on the Loess Hilly Region, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Data Collection
2.4. Statistical Analysis
3. Results
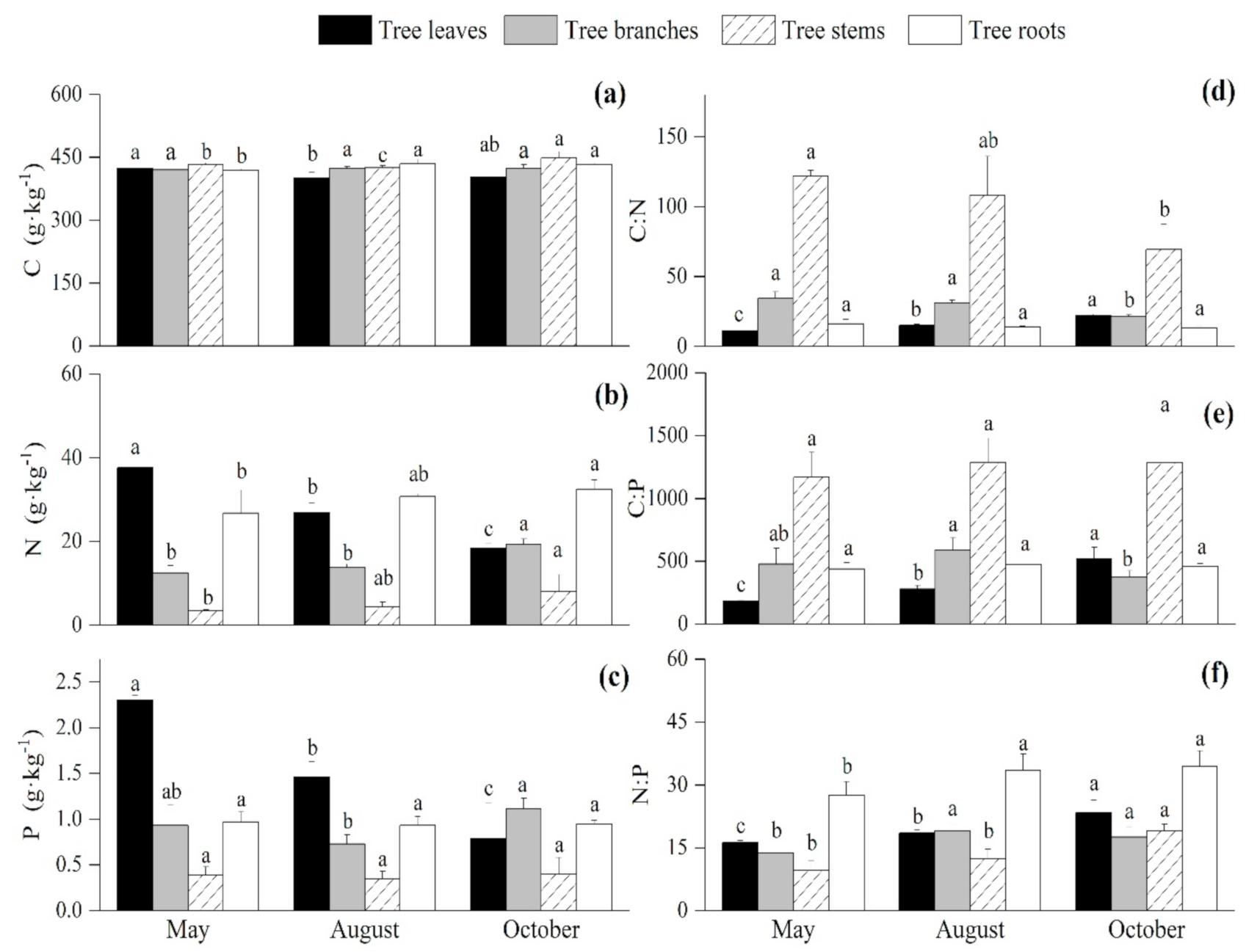
3.1. Seasonal Variations of the C:N:P Stoichiometry in the Arbor Layer
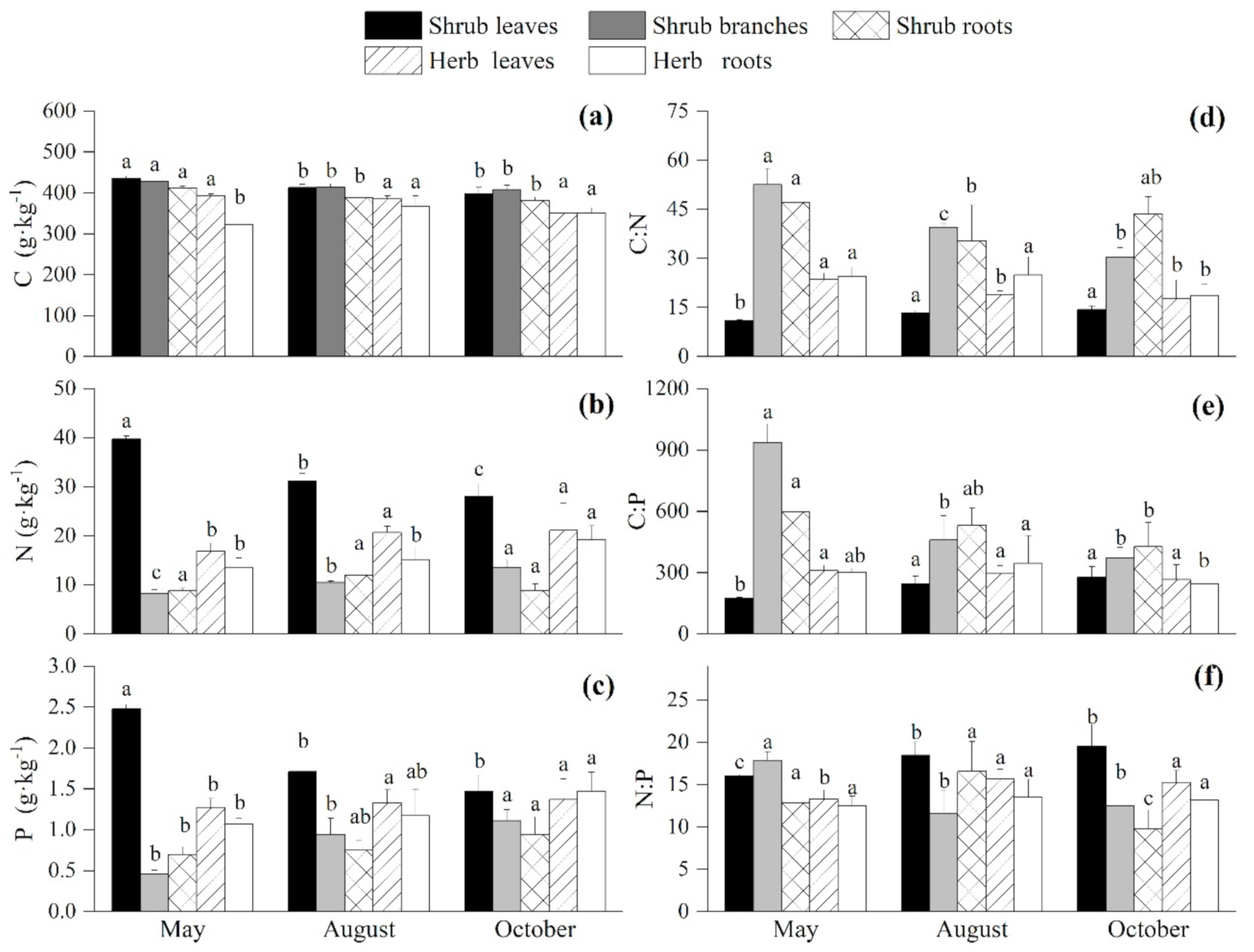
3.2. Seasonal Variations of the C:N:P Stoichiometry in the Undergrowth Layer
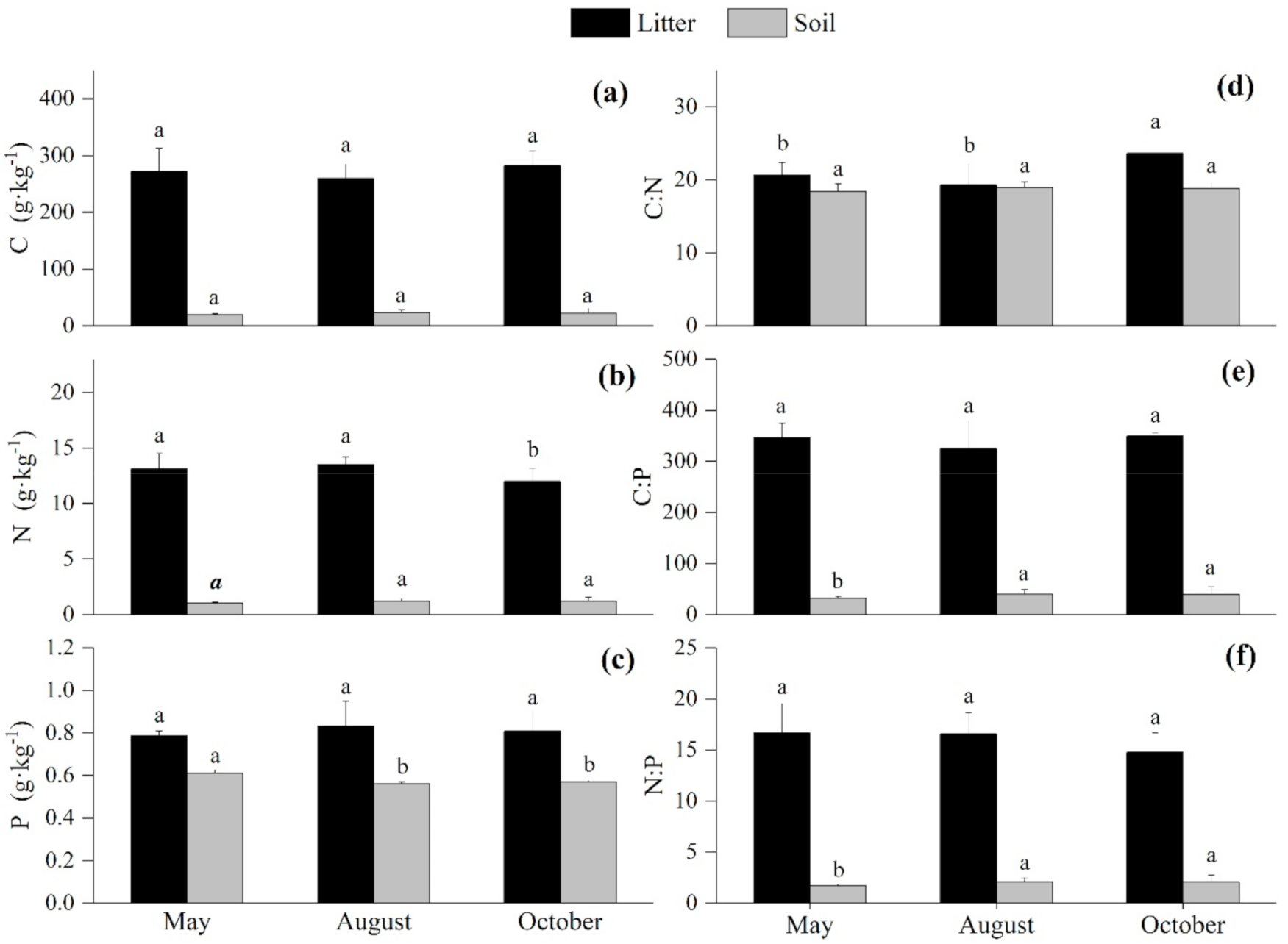
3.3. Seasonal Variations of the C:N:P Stoichiometry in the Litter and Soil
3.4. Correlations of the C:N:P Stoichiometry Among the Plants, Litter, and Soil
4. Discussion
4.1. Seasonal Variations of the C, N, and P Contents and Ratios of the Plants
4.2. Seasonal Variation of the C:N:P Stoichiometry in the Litter and Soil
4.3. Correlation Analysis of Plants, Litter and Soil C, N, P Stoichiometry
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elser, J.J.; Hamilton, A. Stoichiometry and the new biology: The future is now. PLoS Biol. 2007, e181. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 358–377. [Google Scholar] [CrossRef]
- Klausmeier, C.A.; Litchman, E.; Daufresne, T.; Levin, S.A. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 2004, 429, 171–174. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. The role of plants in the effects of global change on nutrient availability and stoichiometry in the Plant-Soil System. Plant Physiol. 2012, 160, 1741–1761. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, B.; Gao, X.P.; Li, X.Y.; Li, C.D. Nitrogen and phosphorus addition differentially affect plant ecological stoichiometry in desert grassland. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Maas-Hebner, K.G.; Emmingham, W.H.; Larson, D.J.; Chan, S.S. Establishment and growth of native hardwood and conifer seedlings underplanted in thinned Douglas-fir stands. For. Ecol. Manag. 2005, 208, 331–345. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.R.; An, S.S. Ecological stoichiometry in leaves, roots, litters and soil among different plant communities in a desertified region of Northern China. Catena 2018, 166, 328–338. [Google Scholar] [CrossRef]
- Berg, B. Litter decomposition and organic matter turnover in northern forest soils. For. Ecol. Manag. 2000, 133, 13–22. [Google Scholar] [CrossRef]
- Bai, X.J.; Wang, B.R.; An, S.S.; Zeng, Q.C.; Zhang, H.X. Response of forest species to C:N:P in the plant-litter-soil system and stoichiometric homeostasis of plant tissues during afforestation on the Loess Plateau, China. Catena 2019, 183, 104186. [Google Scholar] [CrossRef]
- Manzoni, S.; Jackson, R.B.; Trofymow, J.A.; Porporato, A. The global stoichiometry of litters nitrogen mineralization. Science 2008, 321, 684–686. [Google Scholar] [CrossRef]
- Gao, D.X.; Zhang, W.; Ren, C.J.; Dai, Y.Y.; Qiao, W.J.; Chen, Z.X.; Yang, G.H.; Han, X.H. Ecological stoichiometry characteristics of soil and leaves during the recovery process of typical vegetation on the Loess Plateau. Acta Ecol. Sin. 2019, 39, 3622–3630. (In Chinese) [Google Scholar]
- Fan, H.B.; Wu, J.P.; Liu, W.F.; Yuan, Y.H.; Hu, L.; Cai, Q.K. Linkages of plant and soil C:N:P stoichiometry and their relationships to forest growth in subtropical plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]
- Führer, E. Forest functions, ecosystem stability and management. For. Ecol. Manag. 2000, 132, 29–38. [Google Scholar] [CrossRef]
- Loreau, M.; De Mazancourt, C.; Duffy, E. Biodiversity and ecosystem stability: A synthesis of underlying mechanisms. Ecol. Lett. 2013, 16, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.; Reed, S.; Hou, S.L.; Hu, Y.Y.; Wei, H.W.; Lü, F.M.; Cui, Q.; Han, X.G. Temporal variability of foliar nutrients: Responses to nitrogen deposition and prescribed fire in a temperate steppe. Biogeochemistry 2017, 133, 295–305. [Google Scholar] [CrossRef]
- Zhao, Q.Q.; Bai, J.H.; Liu, Q.; Lu, Q.Q.; Gao, Z.Q.; Wang, J.J. Spatial and seasonal variations of soil carbon and nitrogen content and stock in a Tidal Salt Marsh with Tamarix chinensis, China. Wetlands 2016, 36, 145–152. [Google Scholar] [CrossRef]
- Ågren, G.I. Stoichiometry and nutrition of plant growth in natural communities. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 153–170. [Google Scholar] [CrossRef]
- Yang, Y.H.; Luo, Y.Q. Carbon:nitrogen stoichiometry in forest ecosystems during stand development. Glob. Ecol. Biogeogr. 2011, 20, 354–361. [Google Scholar] [CrossRef]
- Wright, I.J.; Westoby, M. Nutrient concentration, resorption and lifespan: Leaf traits of Australian sclerophyll species. Funct. Ecol. 2003, 17, 11–19. [Google Scholar] [CrossRef]
- Grigal, D.F.; Ohmann, L.F.; Brander, R.B. Seasonal dynamics of tall shrubs in northeastern Minnesota: Biomass and nutrient element changes. For. Sci. 1976, 195–208. [Google Scholar] [CrossRef]
- Kerkhoff, A.J.; Enquist, B.J.; Elser, J.J.; Fagan, W.F. Plant allometry, stoichiometry and the temperature-dependence of primary productivity. Glob. Ecol. Biogeogr. 2005, 14, 585–598. [Google Scholar] [CrossRef]
- Liu, F.D.; Liu, Y.H.; Wang, G.M.; Song, Y.; Liu, Q.; Li, D.S.; Mao, P.L.; Zhang, H. Seasonal Variations of C:N:P stoichiometry and their trade-offs in different organs of Suaeda salsa in Coastal Wetland of Yellow River Delta, China. PLoS ONE 2015, 10, e138169. [Google Scholar] [CrossRef]
- Jing, L.; Li, J.P.; Zhang, Y.; Ma, H.Y.; Li, J. Study on dry soil layers under different land-use systerms in the Loess Plateau. Pratacult. Sci. 2018, 35, 1829–1835. (In Chinese) [Google Scholar]
- Wu, D.Y.; Jiao, J.Y.; Yu, W.J.; Xu, H.Y.; Chen, Y.X. Respone characteristics of Locust growth, understory vegetation and soil moisture on planting density in northern Shaanxi. Acta Bot. Boreali-Occident. Sin. 2017, 37, 346–355. (In Chinese) [Google Scholar]
- Wu, Z.B.; Yang, X.M. Artificial Robinia peseudoacacia growth and soil moisture variation in Loessial Hilly-Gully region. Res. Soil Water Conserv. 2004, 11, 117–120. (In Chinese) [Google Scholar]
- Cao, Y.; Chen, Y.M. Coupling of plant and soil C:N:P stoichiometry in black locust (Robinia pseudoacacia) plantations on the Loess Plateau, China. Trees 2017, 31, 1559–1570. [Google Scholar] [CrossRef]
- Du, M.; Feng, H.; Zhang, L.; Pei, S.; Tang, X. Variations in carbon, nitrogen and phosphorus stoichiometry during a growth season within a Platycladus orientalis plantation. Pol. J. Environ. Stud. 2020, 29. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zhang, P.; Peng, S.Z.; Chen, Y.M.; Cao, Y. The coupling of leaf, litter, and soil nutrients in warm temperate forests in northwestern China. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, J.A.; Allen, S.E. A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun. Soil Sci. Plant Anal. 2009, 6, 1–11. [Google Scholar] [CrossRef]
- Thomas, S.C.; Martin, A.R. Carbon content of tree tissues: A synthesis. Forests 2012, 3, 332–352. [Google Scholar] [CrossRef]
- Abbas, M.; Ebeling, A.; Oelmann, Y.; Ptacnik, R.; Roscher, C.; Weigelt, A.; Weisse, W.W.; Wilcke, W.; Hillebrand, H. Biodiversity effects on plant stoichiometry. PLoS ONE 2013, 8, e58179. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Crabbe, M.J.C.; Xu, F.L.; Wang, W.L.; Niu, R.L.; Gao, X.; Zhang, P.; Chen, H.K. Seasonal Variations in carbon, nitrogen and phosphorus concentrations and C:N:P stoichiometry in the leaves of differently aged Larix principis-rupprechtii Mayr. Plantations. Forests 2017, 8, 373. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, J.G.; ClarK, C.M.; Pan, Q.M.; Zhang, L.X.; Chen, S.P.; Wang, Q.B.; Han, X.Q. Grazing alters ecosystem functioning and C:N:P stoichiometry of grasslands along a regional precipitation gradient. J. Appl. Ecol. 2012, 49, 1204–1215. [Google Scholar] [CrossRef]
- Yang, J.J.; Zhang, X.R.; MA, L.S.; Chen, Y.A.; Dang, T.H.; An, S.S. Ecological stoichiometric relationships between components of Robinia pseudoacacia forest in loess plateau. Acta Pedol. Sin. 2014, 51, 133–142. (In Chinese) [Google Scholar]
- Ye, Y.S.; Liang, X.Q.; Chen, Y.X.; Li, L.; Ji, Y.J.; Zhu, C.Y. Carbon, Nitrogen and phosphorus accumulation and partitioning, and C:N:P stoichiometry in late-season rice under different water and nitrogen managements. PLoS ONE 2014, 9, e101776. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.N.; Lu, J.Y.; Yang, H.M.; Zhang, X.; Luo, C.L.; Zhao, Y.X. Resorption of nitrogen, phosphorus and potassium from leaves of lucerne stands of different ages. Plant Soil. 2014, 383, 301–312. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peuelas, J. Factors affecting nutrient concentration and stoichiometry of forest trees in Catalonia (NE Spain). For. Ecol. Manag. 2011, 262, 2024–2034. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, H.H.; He, N.P.; Lu, X.T.; Wang, Z.P.; Elser, J.J. Testing the Growth Rate Hypothesis in vascular plants with above- and below-ground biomass. PLoS ONE 2012, 7, e32162. [Google Scholar] [CrossRef]
- Guo, H.; Zhuang, W.W.; Li, J. Dynamic analysis of stoichiometric ratios of C, N, and P during different growth periods in four herbaceous plants from Gurbantünggüt Desert. Plant Sci. J. 2019, 37, 356–366. (In Chinese) [Google Scholar]
- Santa Regina, I.; Rico, M.; Gallego, M.H.A. Seasonal variation in nutrient concentration in leaves and branches of Quercus pyrenaica. J. Veg. Sci. 1997, 8, 651–654. [Google Scholar] [CrossRef]
- Townsend, A.R.; Cleveland, C.C.; Asner, G.P.; Bustamante, M.M. Controls over foliar N:P ratios in tropical rain forests. Ecology 2007, 88, 107–118. [Google Scholar] [CrossRef]
- Aerts, R. Nutrient resorption from senescing leaves of perennials: Are there general patterns. J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, J.; Biswas, A.; Cao, J.; Qi, X. Seasonal dynamics of leaf stoichiometry of phragmites australis: A case study from Yangguan Wetland, Dunhuang, China. Plants 2020, 9, 1323. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Sterner, R.W.; Gorokhova, E.; Fagan, W.F.; Markow, T.A.; Cotner, J.B.; Harrison, J.F.; Hobbie, S.E.; Odell, G.M.; Weider, L.W. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000, 3, 540–550. [Google Scholar] [CrossRef]
- Sun, J.N.; Gao, P.; Li, C.; Wang, R.J.; Niu, X.; Wang, B. Ecological stoichiometry characteristics of the leaf-litter-soil continuum of Quercus acutissima Carr. and Pinus densiflora Sieb. in Northern China. Environ. Earth Sci. 2019, 78. [Google Scholar] [CrossRef]
- Chen, C.; Wang, G.J.; Zhao, Y.; Zhou, G.X.; Li, L.; Gao, J.Q. Seasonal dynamics and allometric growth relationships of C, N and P stoichiometry in the organs of Cunninghamia lanceolata from Huitong. Acta Ecol. Sin. 2016, 36, 7614–7623. [Google Scholar] [CrossRef][Green Version]
- Forja, J.M.; Blasco, J.; Gómez-Parra, A. Spatial and seasonal variation of in situ benthic fluxes in the bay of Cadiz (South-west Spain). Estuar. Coast. Shelf Sci. 1994, 39, 127–141. [Google Scholar] [CrossRef]
- Waive, J.; Larsson, U. Seasonal changes in Baltic Sea seston stoichiometry: The influence of diazotrophic cyanobacteria. Mar. Ecol. Prog. 2010, 13–25. [Google Scholar] [CrossRef]
- Li, H.L.; Crabbe, M.J.C.; Xu, F.L.; Wang, W.L.; Ma, L.H.; Niu, R.L.; Gao, X.; Li, X.X.; Zhang, P.; Ma, X.; et al. Seasonal variations in carbon, nitrogen and phosphorus concentrations and C:N:P stoichiometry in different organs of a Larix principis-rupprechtii Mayr. plantation in the Qinling Mountains, China. PLoS ONE 2017, 12, e185163. [Google Scholar] [CrossRef] [PubMed]
- Grace, J. Toward models of resource allocation by plants. Plant Resour. Alloc. 1997, 279–291. [Google Scholar] [CrossRef]
- Vitousek, P. Nutrient cycling and nutrient use efficiency. Am. Nat. 1982, 119, 553–572. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Tian, H.Q.; Chen, G.S.; Zhang, C.; Melillo, J.M.; Hall, C.A.S. Pattern and variation of C:N:P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Hatton, P.J.; Castanha, C.; Torn, M.S.; Bird, J.A. Litter type control on soil C and N stabilization dynamics in a temperate forest. Glob. Chang. Biol. 2015, 1358–1367. [Google Scholar] [CrossRef]
- Kang, H.; Freeman, C. Soil enzyme analysis for leaf litter decomposition in Global Wetlands. Commun. Soil Sci. Plant Anal. 2009, 40, 3323–3334. [Google Scholar] [CrossRef]
- Ren, S.J.; Cao, M.K.; Tao, B.; Li, K.R. The effects of nitrogen limitation on terrestrial ecosystem carbon cycle: A review. Prog. Geogr. 2006, 25, 58–67. (In Chinese) [Google Scholar]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio”for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Yang, Y.H.; Fang, J.Y.; Guo, D.L.; Ji, C.J.; Ma, W.H. Vertical patterns of soil carbon, nitrogen and carbon: Nitrogen stoichiometry in Tibetan grasslands. Biogeosci. Discuss. 2010, 7, 1–24. [Google Scholar] [CrossRef]
- Neufeldt, H.; Da Silva, J.E.D.; Ayarza, M.A.; Zech, W. Land-use effects on phosphorus fractions in Cerrado oxisols. Biol. Fertil. Soils 2000, 31, 30–37. [Google Scholar] [CrossRef]
- Post, W.M.; Pastor, J.; Zinke, P.J.; Stangenberger, A.G. Global patterns of soil nitrogen storage. Nature 1985, 317, 613–616. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Vitousek, P.M. Nutrient limitation of decomposition in Hawaiian forests. Ecology 2000, 81, 1867–1877. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, F. Impact of vegetation succession on leaf-litter-soil C:N:P stoichiometry and their intrinsic relationship in the Ziwuling Area of China’s Loess Plateau. J. For. Res. 2020. [Google Scholar] [CrossRef]
- Walker, T.W.; Adams, A.F.R. Studies on soil organic matter. Soil Sci. 1959, 85, 307–318. [Google Scholar] [CrossRef]
| Component | C | N | P | C:N | C:P | N:P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AV/g·kg−1 | CV/% | AV/g·kg−1 | CV/% | AV/g·kg−1 | CV/% | AV | CV/% | AV | CV/% | AV | CV/% | |
| TL | 409.02 ± 19.82 | 4.85 | 27.61 ± 8.15 | 29.53 | 1.52 ± 0.64 | 42.15 | 16.08 ± 4.64 | 28.87 | 326.97 ± 153.01 | 46.8 | 19.44 ± 3.45 | 17.74 |
| TB | 419.47 ± 8.37 | 1.99 | 15.18 ± 3.28 | 21.64 | 0.93 ± 0.22 | 23.86 | 28.9 ± 6.22 | 21.54 | 481.16 ± 127.44 | 26.49 | 16.75 ± 2.97 | 17.72 |
| TS | 435.13 ± 10.05 | 3.13 | 5.28 ± 3.10 | 58.8 | 0.38 ± 0.12 | 31.78 | 99.79 ± 34.82 | 34.89 | 1248.53 ± 350.31 | 28.06 | 13.71 ± 4.54 | 33.14 |
| TR | 428.32 ± 13.64 | 2.35 | 30.01 ± 4.11 | 13.71 | 0.95 ± 0.09 | 9.13 | 14.55 ± 2.14 | 14.73 | 455.62 ± 47.28 | 10.38 | 31.79 ± 4.72 | 14.83 |
| SL | 415.7 ± 18.57 | 4.47 | 32.97 ± 5.31 | 16.1 | 1.88 ± 0.47 | 24.97 | 12.85 ± 1.61 | 12.53 | 233.2 ± 55.74 | 23.9 | 18.01 ± 2.95 | 16.39 |
| SB | 416.68 ± 12.07 | 2.9 | 10.76 ± 2.46 | 22.83 | 0.84 ± 0.31 | 37.11 | 40.77 ± 9.80 | 24.05 | 589.99 ± 267.43 | 45.33 | 14 ± 3.62 | 25.84 |
| SR | 393.69 ± 15.82 | 4.02 | 9.87 ± 2.53 | 25.67 | 0.8 ± 0.18 | 22.9 | 41.99 ± 8.66 | 20.64 | 519.23 ± 116.37 | 22.41 | 13.07 ± 4.91 | 37.53 |
| HL | 376.67 ± 31.24 | 8.29 | 19.57 ± 3.83 | 19.55 | 1.32 ± 0.18 | 13.68 | 19.96 ± 4.24 | 21.25 | 290.74 ± 51.68 | 17.77 | 14.75 ± 1.56 | 10.59 |
| HR | 347.16 ± 26.82 | 7.73 | 15.97 ± 3.36 | 21.05 | 1.24 ± 0.28 | 22.76 | 22.64 ± 4.91 | 21.68 | 297.98 ± 91.36 | 30.66 | 13.04 ± 1.47 | 11.27 |
| LI | 270.96 ± 50.37 | 18.59 | 12.86 ± 2.15 | 16.76 | 0.81 ± 0.09 | 10.63 | 21.19 ± 2.72 | 12.84 | 340 ± 79.50 | 23.38 | 15.99 ± 2.74 | 17.14 |
| SO | 21.31 ± 5.95 | 27.9 | 1.13 ± 0.27 | 23.57 | 0.58 ± 0.02 | 3.89 | 18.72 ± 0.87 | 4.63 | 36.64 ± 10.59 | 28.91 | 1.94 ± 0.48 | 24.72 |
| Plants | Components | C | N | P |
|---|---|---|---|---|
| Tree | Leaves–Branches | 0.404 * | −0.847 ** | −0.587 ** |
| Leaves-Stems | 0.532 ** | −0.613 ** | −0.183 | |
| Leaves-Roots | −0.352 | −0.597 ** | −0.189 | |
| Branches-Stems | −0.298 | 0.724 ** | 0.602 ** | |
| Branches-Roots | −0.142 | 0.557 ** | 0.458 * | |
| Stems-Roots | 0.030 | 0.085 | 0.421 * | |
| Shrub | Leaves-Branch | 0.893 ** | −0.728 ** | −0.835 ** |
| Leaves-Root | 0.761 ** | −0.118 | −0.294 | |
| Branches-Roots | 0.680 ** | 0.142 | 0.439 * | |
| Herb | Leaves-Roots | −0.103 | 0.684 ** | 0.717 ** |
| Component | C | N | P | C:N | C:P | N:P |
|---|---|---|---|---|---|---|
| TL-SL | 0.613 ** | 0.919 ** | 0.928 ** | 0.759 ** | 0.806 ** | 0.794 ** |
| TL-AH | −0.299 | −0.494 ** | −0.24 | −0.582 ** | −0.539 ** | 0.279 |
| TL-LI | 0.44 * | 0.492 ** | −0.092 | 0.542 ** | 0.066 | −0.173 |
| TL-SO | −0.523 ** | −0.312 | 0.785 ** | 0.221 | 0.182 | 0.082 |
| SL-AH | 0.143 | −0.345 | −0.014 | −0.213 | −0.241 | 0.202 |
| SL-LI | 0.241 | 0.21 | −0.044 | 0.547 ** | 0.367 | 0.208 |
| SL-SO | −0.586 ** | −0.232 | 0.858 ** | 0.05 | −0.126 | −0.263 |
| AH-LI | −0.334 | −0.116 | 0.629 ** | 0.074 | 0.377 | −0.031 |
| AH-SO | 0.193 | 0.771 ** | −0.15 | −0.318 | −0.368 | 0.572 ** |
| LI-SO | −0.547 ** | −0.554 ** | −0.073 | 0.09 | −0.404 * | −0.512 ** |
| Item | Area | C/(g·kg−1) | N/(g·kg−1) | P/(g·kg−1) | C:N | C:P | N:P | Date Source |
|---|---|---|---|---|---|---|---|---|
| Tree leaves | The Loess Plateau | 409.02 ± 19.8 | 27.61 ± 8.1 | 1.52 ± 0.6 | 16.08 ± 4.4 | 326.97 ± 86 | 19.44 ± 3.4 | This study |
| China | - | 20.2 ± 8.4 | 1.46 ± 1.0 | - | - | 13.83 ± 9.3 | Han | |
| Global | 464.0 ± 3.2 | 20.6 ± 1.2 | 1.99 ± 0.1 | 22.5 ± 1.9 | 232.0 ± 72.4 | 12.7 ± 10.1 | Elser | |
| Global | 461.3 ± 72.2 | 20.1 ± 8.7 | 1.77 ± 1.1 | 23.8 ± 17.3 | 300.9 ± 236.8 | 13.8 ± 9.5 | Reich and Oleksyn | |
| Litter | The Loess Plateau | 270.96 ± 50.3 | 12.86 ± 2.1 | 0.81 ± 0.1 | 21.19 ± 2.7 | 340.0 ± 79.5 | 15.99 ± 2.7 | This study |
| China | 508.16 ± 28.2 | 10.85 ± 1.9 | 0.46 ± 0.2 | 54.1 ± 16.4 | 1355.74 ± 80.1 | 25.47 ± 2.4 | Wang | |
| Global | 467.7 ± 2.7 | 10.0 ± 0.1 | 0.7 ± 0.0 | 52.9 ± 1.3 | 1183.0 ± 70 | 19.2 ± 0.5 | Yuan | |
| Global | - | 10.93 ± 6.5 | 0.85 ± 0.7 | - | - | 18.32 ± 11.2 | Kang | |
| Soil | The Loess Plateau | 21.31 ± 5.9 | 1.13 ± 0.3 | 0.58 ± 0.0 | 18.72 ± 0.8 | 36.64 ± 10.5 | 1.94 ± 0.4 | This study |
| China | 11.12 ± 1.6 | 1.07 ± 0.1 | 2.05 ± 0.7 | 11.9 ± 0.2 | 60.0 ± 1.1 | 5.1 ± 0.1 | Tian | |
| Global | - | - | - | 14.3 ± 0.5 | 186.0 ± 12.9 | 13.1 ± 0.8 | Cleveland |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, C.; Qiao, Y.; Cao, Y.; Chen, Y.; Wu, X.; Xue, W. Seasonal Variations in Carbon, Nitrogen, and Phosphorus Stoichiometry of a Robinia pseudoacacia Plantation on the Loess Hilly Region, China. Forests 2021, 12, 214. https://doi.org/10.3390/f12020214
Dong C, Qiao Y, Cao Y, Chen Y, Wu X, Xue W. Seasonal Variations in Carbon, Nitrogen, and Phosphorus Stoichiometry of a Robinia pseudoacacia Plantation on the Loess Hilly Region, China. Forests. 2021; 12(2):214. https://doi.org/10.3390/f12020214
Chicago/Turabian StyleDong, Congguo, Yuning Qiao, Yang Cao, Yunming Chen, Xu Wu, and Wenyan Xue. 2021. "Seasonal Variations in Carbon, Nitrogen, and Phosphorus Stoichiometry of a Robinia pseudoacacia Plantation on the Loess Hilly Region, China" Forests 12, no. 2: 214. https://doi.org/10.3390/f12020214
APA StyleDong, C., Qiao, Y., Cao, Y., Chen, Y., Wu, X., & Xue, W. (2021). Seasonal Variations in Carbon, Nitrogen, and Phosphorus Stoichiometry of a Robinia pseudoacacia Plantation on the Loess Hilly Region, China. Forests, 12(2), 214. https://doi.org/10.3390/f12020214






