Abstract
Several eucalypt species are known for their capacity to massively regenerate through seeds in recently burned areas, becoming an ecological problem in regions where the species is not native. Here we study the demography and the development of highly dense Eucalyptus globulus wildling populations established one year after a fire and test two methods to control these populations. We monitored five mixed E. globulus stands across one year, in Central Portugal. We established a set of plots in each stand, with three treatments: mechanical cutting, herbicide spraying and no disturbance (control plots). Herbicide was applied in four concentrations. We tagged randomly selected plants in the control plots to monitor their growth. The initial mean wildling density was 322,000 plants ha−1, the highest ever recorded in the introduced range. Wildling density was significantly dependent on the density of surrounding adult E. globulus trees. Wildling density in control plots decreased 30% in one year, although showing positive variations over time because of new recruitment. Despite seasonal growth differences, wildlings showed a high growth rate throughout the year, reaching 15.6 cm month−1 in the summer. The growth rate of tagged wildings was positively affected by solar radiation and negatively affected by evapotranspiration and maximum temperature. Mechanical cutting reduced wildling density by 97% while herbicide treatment reduced density between 80% (for the lowest concentration) and 99% (for the highest concentration). Herbicide-treated plants were more likely to resprout than cut plants. Regardless of the control method adopted (cutting or herbicide), management strategies should include the follow-up of the treated areas, to detect the establishment of new recruits and resprouting.
1. Introduction
Eucalyptus globulus Labill, commonly known as Tasmanian blue gum, is native to Tasmania, the Bass Strait Islands and southeast Australia [1]. The species has been introduced to several world regions outside the native range, being used mostly for paper pulp production. It is currently one of the most popular and widespread fast-growth eucalypt species, estimated to cover around 2.3 million ha worldwide [2,3]. Eucalyptus globulus is also the eucalypt species which has been the focus of more research regarding its invasiveness, being reported as invasive in several countries where it was introduced [4]. Portugal currently has a large area of E. globulus plantations, estimated as 845,000 ha, corresponding to 26% of the Portuguese forest [5]. Most research on naturalization and invasiveness of E. globulus has been developed in Portugal, a region where the species seems to find particularly favourable conditions to successfully reproduce, both within [6,7] and around [8,9] planted areas. Previous studies developed in Portugal and Australia, suggest that the average density of naturally-established E. globulus seedlings (wildlings) in Portugal is several times higher than in the native range [10,11]. The factors that contribute to the naturalization of the species in Portugal include a long introduction history, a large planted area [6,10,12], the abandonment of forest plantations and recurrent wildfires [7,13]. Abandonment and poor management are key factors that may explain the existence of high densities of wildlings in many areas across Portugal and Spain [14] but studies at a local scale have been mostly carried out in commercial well-managed plantations [8,9].
Like many other eucalypts, E. globulus evolved in a fire-prone environment and developed a set of fire adaptations [15]. The ability to regenerate the burned canopy and the fire-triggered seed dispersal are important fire-adaptive traits [16,17]. The fruits of E. globulus are woody pseudo-capsules with four narrow cracks (valves) that open and release the seeds following capsule desiccation [18]. Seed shedding occurs throughout the year, but dehiscence peaks are more likely after wildfires and hot, dry periods [19,20]. Seeds are relatively small (1–2 mm) and are released in high quantities, sufficient to satiate seed predators [21]. They are mainly dispersed through gravity and wind [20], readily germinating when exposed to moisture [18], namely in burned areas [22]. As a result, burned E. globulus stands may present high densities of successful regeneration. Águas, et al. [6] found an average of 4800 E. globulus wildlings ha−1 reaching a mean height of 270 cm, five to seven years after fire, in a large survey carried out in Central and Northern Portugal. However, despite the evidence of these high densities, there are no field-based data on the demography and growth rate that resulted in these wildling populations. Previous studies [18,23] suggest that fire should deplete the canopy seed bank, resulting in one single post-fire recruitment event. Nevertheless, no one has ever, to the best of our knowledge, monitored wildling populations of E. globulus to validate this hypothesis. The only studies that assessed the early developmental stages of E. globulus seedlings were sowing experiments [22,24,25,26,27]. None of these studies has analysed the demography of well-established seedlings, i.e., the survivors after the first dry summer season, when an important mortality is expected to occur because of water stress [25]. Only one study was developed in burned areas [22], and none of these studies has monitored seedling growth.
The naturalization of E. globulus may result in negative impacts on local ecosystems [2] and increased fire hazard [28]. The species is also known for having a strong allelopathic impact on other organisms [29,30,31]. These characteristics call for the adoption of proper management practices in industrial plantations [32]. The spread of wildlings within and around plantations may also have a negative impact on the local forest economy because of increased competition for resources and higher management costs [32]. Therefore, the control of post-fire wildlings is required either from an economic or an environmental perspective. The management of E. globulus has been reported to involve the highest expenditure among 22 invasive species in Spain [33]. The control of eucalypts, particularly E. globulus, should be relatively easy when compared to species that form long-living soil seed banks or regenerate through root suckers, as with several Acacia spp. As with most eucalypts, seeds from E. globulus lack dormancy, being very sensitive and vulnerable to burying, fungal attack or predation [34]). Herbicides, sometimes associated with cutting, have proven to be effective at controlling some common invasive woody species such as Acacia dealbata and Ailanthus altissima [35,36]. Cutting young E. globulus wildlings near the ground using a brush cutter can be an effective alternative, provided that no significant resprouting occurs after cutting. As for herbicide application, the only studies on E. globulus are related to the control of weeds in plantations e.g., [37] or the killing of stumps [38,39]. One of the reasons for this knowledge gap is the fact that E. globulus is not legally recognized, at least in Portugal and Spain (Decree-Law 92/2019 and Royal Decree 630/2013, respectively), as an invasive species. On the other hand, the control of E. globulus wildlings does not present the same challenge as it does the control of species that develop a long-lasting seed bank, such as many Fabaceae. However, it is not known if and how well young E. globulus wildlings can survive or regenerate after herbicide application or cutting because of the species remarkable ability to resprout [17,40].
In this study, we present the results of a one-year experiment established in unmanaged mixed stands of E. globulus and other species, burned by a major fire event occurred on the 15 October 2017 in Central Portugal. The stands were extensively colonized by highly dense wildling populations of E. globulus, providing an opportunity to study the natural dynamics of these populations and the methods to control them. The main goals of this study are: (a) to assess the post-fire demography of E. globulus wildlings one year after fire; (b) to measure the vegetative growth of those populations and determine the drivers of that growth; (c) to test the efficiency of two control methods in the elimination of young E. globulus wildlings.
2. Materials and Methods
2.1. Experimental Design
We conducted an experiment in the municipality of Santa Comba Dão in Central Portugal aimed at studying the early-stage dynamics (growth and demography) of naturally-established E. globulus wildlings and the efficiency of different control treatments. According to the Köppen classification, the region has a Warm-summer Mediterranean climate (Csb) [41] with an average precipitation of 998 mm per year. Soils are derived from granite, the local bedrock. The Santa Comba Dão territory is heavily occupied by E. globulus, present in 43% of forest stands [42]. Many of these forests are mixed, due to poor management and land abandonment, partly promoted by the recurrent wildfires [13]. The land plots are mostly private and very fragmented with an average surface below one hectare [43].
The study was established at five forest stands (Colmeosa, Barba, Póvoa, Soito and Torroal; coordinates in Table 1), representative of these mixed forests, composed by E. globulus and other tree species, mostly pines (Pinus pinaster), oaks (Quercus spp.) and the exotic Acacia dealbata. The understory was strongly dominated by E. globulus wildlings, with some native shrub (Cistus psilosepalus, Rubus sp., Ulex sp.) and herbaceous (mostly Pteridium aquilinum and Rubia peregrina) species. The five forest stands were separated by less than 3 km (distances between 338 and 2657 m) at similar altitude (between 269 and 299 m; Table 1) and around 57 km from the coast. The five forest stands were burnt by a large wildfire which severely affected the study region on 15 October 2017, resulting in massive recruitment of eucalypt wildlings, reaching hundreds of plants per square meter in some areas. There are no records of other wildfires in these forest stands since 1975. Stands were very diverse among them, both in terms of tree density and species composition, forming a good representation of the chaotic mixtures of exotic and native trees from different origins (natural regeneration and human agency) typical of this region.

Table 1.
Characterisation of forest stands. DBH: Diameter at breast height (mean ± SE) of Eucalyptus globulus (Eg) and other tree species (other spp.); Proportion Eg: the proportion of E. globulus trees in the stand; Closest Eg: distance from transect to the closest E. globulus tree. Average density/average height: initial characteristics of the E. globulus wildling population. Uppercase letters show the result of multiple comparisons among forest stands using a post-hoc Nemenyi test. Different letters correspond to statistically significant differences (p < 0.05).
We adopted a hierarchical sampling design, with five transects as the basic sampling units, one for each forest stand. Initially, we established a series of five experimental blocks of treatments across each transect aimed at testing alternatives to eliminate the eucalypt wildlings. Each block was composed of three 1 × 1 m plots corresponding to the following treatments: a Control plot (no disturbance); a Cutting plot, where all wildlings were cut at ground level with pruning scissors; and a Herbicide plot, where all plants were sprayed with glyphosate. The plots were arranged sequentially and oriented diagonally across the transect, i.e., the transect was coincident with the diagonal (1.4 m long) of the squared plot. Therefore, all transects were 21 m long (1.4 m × 15 plots). To assess demography and growth at the individual level, five randomly selected plants were tagged for monitoring, within each plot. Blocks had an increasing glyphosate concentration (from 0.5% to 2.5%, with a 0.5% step) across each transect so that concentrations were not replicated within each transect but only among transects. The 2.0% concentration was later discarded because some of these plots had been established in patches with very low wildling densities. However, although only four herbicide concentrations were retained for analysis (0.5%, 1.0%, 1.5% and 2.5%), the Cutting and the Control treatments associated with each 2% block were still included in the final dataset. The commercial product used was glyphosate in the form of isopropyl ammonium salt with a concentration of 360 g L−1 of active ingredient. The product was then diluted in water, according to the different concentrations adopted in the experiment. The water pH was lowered to pH = 4, using wine vinegar (6%), a common technique to increase herbicide efficiency. The herbicide was sprayed uniformly over all plants in each plot, using a backpack sprayer equipped with an anti-drip nozzle TeeJet AI8004 (TeeJet Technologies, Glendale Heights, IL, USA). A plastic cover was installed around the plot to prevent the drift of glyphosate into adjacent plots. Glyphosate is a non-selective, systemic herbicide, meaning that the active ingredient is absorbed by the plant organs [44]. Glyphosate was chosen because of its wide usage, including the control of woody invasive plants with a strong resprouting ability such as Ailanthus altissima [36].
2.2. Data Collection
The monitoring of the experimental plots started on 19 November 2018, approximately 13 months after the major wildfire which affected this region. Treatments were applied on 27 November in all forest stands. Monitoring was performed approximately every two months until 4 December 2019 (380 days), so there were six surveys: 19 November 2018 (survey 1); 14 February 2019 (survey 2); 23 May 2019 (survey 3); 18 July 2019 (survey 4); 9 October 2019 (survey 5); and 4 December 2019 (survey 6). In each survey, we counted the number of plants that were alive in each plot and registered the presence of resprouting and recruitment. The five tagged individuals of each plot were measured (plant height) to assess plant growth across time.
Overall, we should have 450 (6 surveys × 5 transects × 5 blocks × treatment plots) plot observations (including wildling counts and height measurements) per treatment but some plot observations were lost (24, mostly in Control plots) due to missing data and destruction by unknown agents. Considering that the 2% Herbicide treatment was also dropped, we ended up with 401 plot observations over the whole experiment: 130 in Control plots, 146 in Cutting plots and 125 in Herbicide plots.
The forest stands were characterised as to tree density, structure and composition. Tree density was estimated using the point-centered quarter method [45]. With that purpose, we divided the transect into three sections measuring 7 m each. At the middle of each section, we established four orthogonal quadrants and in each quadrant, we registered the distance from the closest tree to the intersection. Therefore, we registered information for 12 trees in each transect (3 quadrants × 4 trees). For each tree, we measured the diameter at breast height (DBH) and registered the tree species. Only individuals taller than 4 m were recorded for stand characterisation.
We collected meteorological data that could potentially help explain plant growth [25]. Meteorological data from the nearest station (ca. 29 km from the study area) was supplied by the Portuguese Institute for Sea and Atmosphere (IPMA). This information included: maximum, minimum and average daily temperature (℃); maximum, minimum and average daily relative humidity (%); daily precipitation (mm); average daily wind speed (km h−1); average daily solar radiation (MJ m−1); and daily potential evapotranspiration (mm). Based on the potential evapotranspiration, we computed crop evapotranspiration (ETc) using the Penman–Monteith equation [46]. Averages were computed for each period between consecutive surveys, so there were five sets of meteorological data, for the whole period and all forest stands.
2.3. Data Analysis
To relate the initial wildling density and wildling height with the local conditions of each forest stand, we built univariate generalized linear models (GLM) with a normal error distribution and an identity link (n = 5 forest stands). The explanatory variables were the average DBH of E. globulus trees, E. globulus density, the distance (of the transect) to the closest E. globulus tree, and the basal area (m2 ha−1) of E. globulus trees. We checked whether there were significant differences in wildling density and wildling height among the five forest stands at the beginning of the study, through a Kruskal–Wallis test, followed by a post-hoc Nemenyi test, using the R package PMCMR [47].
Plant growth was analysed using the plants tagged in Control plots, by pooling height measurements into one single mean value per plot and survey (wildling height). Changes in wildling height between consecutive surveys were used to estimate the average growth rate (cm month−1). We used a generalized linear mixed model (GLMM) to assess the influence of two population variables (wildling density and wildling height, measured at the beginning of each growth period) and weather variables (see Section 2.2) on the average growth rate (n = 97) of surviving plants. Wildling height was introduced as a covariate, to control its influence on the model output. The model was built with a Gaussian error distribution. The transect and the block within each transect were used as nested random effects, following the structure described by Bates et al. [48]. To avoid multi-collinearity between explanatory variables, we computed variance inflation factors (VIF) to select the final set of predictors, using the R package car [49]. We adopted VIF > 4 [50] as the threshold for discarding highly correlated variables. The GLMM was built using the R package lme4 [48]. The variance explained by the model was estimated using the R package MuMIn, according to the method proposed by Nakagawa and Schielzeth [51]. This method delivers a marginal pseudo-R2, representing the variance explained by the fixed effects and a conditional pseudo-R2, representing the variance explained by the whole model, including the random effects.
We assessed the effect of treatments (Cutting and Herbicide in different concentrations) on the net demography of the wildling populations, also using a GLMM with a negative binomial error distribution. We chose a GLMM because mixed-effects modelling can cope with unbalanced data such as in our case (different sample sizes for the tested treatments) [50]. Net demography corresponds to the difference between the initial wildling density (plants m−2) in each plot and the current wildling density, observed in the following surveys. This response variable was chosen as an alternative to wildling density, to avoid a zero-inflated model [50] resulting from the strong mortality in most of the cut and herbicide-treated plots. The only dependent variable was Treatment with six levels (Control, Cutting, Herbicide 0.5%, Herbicide 1.0%, Herbicide 1.5% and Herbicide 2.5%). Model structure and the variance estimation were similar to those previously described for the growth model. Model overdispersion was assessed using the function overdisp_fun from the same package [52].
The presence of recruitment and resprouting in all plots were analysed to test the independence between each of these binary variables and the three plot types (Control, Cutting, Herbicide), using a Fisher’s exact test for count data with a simulated p-value. The different herbicide concentrations were pooled into one single treatment, so the tests were performed on 2 × 3 tables (presence/absence, three treatments). Pooling was necessary to get a balanced sample but also because the main goal was to contrast the three treatments, regardless of the herbicide concentration. For the same reason incomplete blocks were discarded, so we ended up with 60 plots, 20 for each treatment. The presence of resprouting and recruitment was registered for all plots that had at least one observation of resprouting or recruitment, respectively, over the six surveys. Individual counts were also performed but these were not analysed because there was a risk of repeated counts of the same individual. We have used R software to produce all the analyses and graphs presented in this study [53].
3. Results
3.1. Characterisation of Forest Stands
The characteristics of the five burned forest stands listed in Table 1 correspond to the information collected in survey 1. Colmeosa was the site with the highest percentage of E. globulus, being also the site with the highest density of eucalypts. Torroal had the largest E. globulus trees (higher average DBH). Soito was the site with the lowest: tree density, percentage of E. globulus and average DBH, and with the largest distance to the nearest E. globulus tree. The initial average density of E. globulus wildlings found in all plots (n = 75) was 32.2 ± 1.4 plants m−2, ranging between 80.4 ± 2.4 in Colmeosa and 15.8 ± 0.9 in Soito. The Kruskal–Wallis test showed significant differences between sites (Chi-squared = 37.311, df = 4, p-value < 0.001), with Colmeosa presenting a significantly different density, compared to the other forest stands (post-hoc Nemenyi test). The density of E. globulus trees (obtained from the proportion of eucalypts within the estimated tree density) was the only dependent variable that significantly explained the variability of wildling densities among forest stands. The GLM showed a positive relationship between the two variables (coeff. = 0.03; p = 0.010; n = 5, R2 = 0.92). Most plots (52%) did not show any presence of other plant species, being completely covered with eucalypt wildlings. At the beginning of the experiment, the maximum height of tagged wildlings was 186 cm, the minimum was 12 cm and the pooled average height was 73.1 ± 3.0 cm (n = 75). The Kruskal–Wallis test showed significant differences between sites (Chi-squared = 46.665, df = 4, p < 0.001). The average wildling height at Torroal was significantly higher than at Soito and Póvoa and the average wildling height at Soito was significantly lower than at Colmeosa, Barba and Torroal. The GLM developed for the average wildling height did not reveal any significant relationships between this variable and the stand variables.
3.2. Demography
Wildling density decreased throughout the experiment in Control plots. This reduction was 30%, from 33.4 ± 2.7 plants m−2 (n = 25), to 23.4 ± 2.6 plants m−2 (n = 20) observed in the last survey. Two surveys (4 and 6) showed an increase of wildling density, compared to the previous survey (see Section 3.4) because of the recruitment of new plants. At the end of the experiment, the overall mortality of tagged plants in Control plots was 40%. We did not consider the 25 plants of discarded plots, so this rate corresponds to 100 plants in total. The mortality these 100 plants was low in surveys 2, 3 and 4 (7%, 3% and 2%, respectively), but it sharply increased in the last two surveys (11% and 17%) corresponding to the summer and autumn months.
3.3. Growth
At the beginning of the experiment, the average height of tagged plants in Control plots was 71.1 ± 5.1 cm (n = 25) and the average height in the last survey was 173.1 ± 20.2 cm (n = 14) (Figure 1). The initial average height in the 14 remaining plots (with alive individuals in the last survey), was 66.5 ± 7.9 cm. The average growth in all plots over the experiment was 8.4 ± 0.7cm month−1 (Figure 2). The highest growth rates were observed in Spring and Summer, with 10.5 ± 1.4 cm month−1 and 15.6 ± 1.5 cm month−1, respectively (Figure 2). Winter was the season with the lowest growth rate, with 3.4 ± 0.4 cm month−1. The growth rate was positively influenced by solar radiation and negatively influenced by maximum temperature and evapotranspiration (Table 2). The wildling height was the only population variable retained by the model, showing a positive influence on growth rate. The marginal R2 of the GLMM was 62.6% and the conditional R2 was 62.8%.
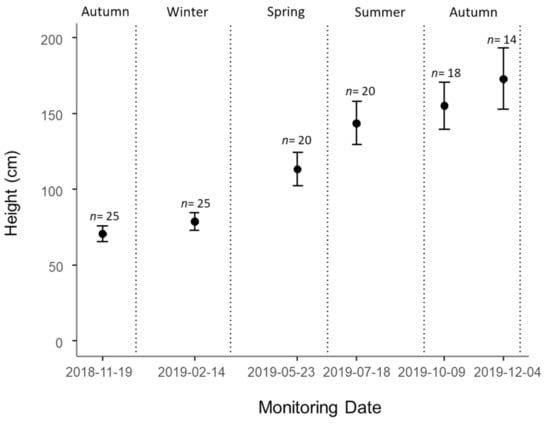
Figure 1.
Average height of E. globulus wildlings (mean ± SE) registered in each survey, using the plants tagged in Control plots. Dashed lines represent the limits of each season.
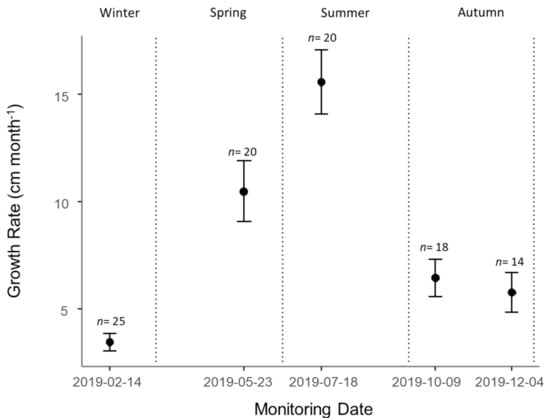
Figure 2.
Average growth rate of E. globulus wildings tagged in Control plots (mean ± SE) between consecutive surveys. Dashed lines represent the limits of each season.

Table 2.
Generalized linear mixed model presenting the effect of wildling height, maximum daily temperature, evapotranspiration and solar radiation on wildling growth (cm month−1). The table shows the coefficients of the model, the respective standard errors, the z statistic and the associated probabilities: ≤0.001 = ***; >0.05 = ns.
3.4. Treatments
The effect of treatments on wildling density in comparison with the Control plots is illustrated in Figure 3. Considering the start and the end of the experiment, wildling density was reduced by 97% in the Cutting treatment, by 80% in the Herbicide 0.5%, by 88% in the Herbicide 1.0%, by 99% in the Herbicide 1.5% and by 99% in the Herbicide 2.5%. The reduction was abrupt in the Cutting treatment (97% between the first and the second surveys) and Herbicide 2.5% (94% for the same period) and more gradual for lower herbicide concentrations, in these latter cases taking two survey intervals to reach a stable density.
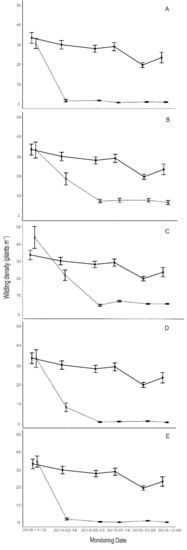
Figure 3.
Graphs showing the effect of each treatment (dashed lines) on wildling density (mean ± SE), compared with the Control plots (solid line): (A) Cutting; (B) Herbicide 0.5%; (C) Herbicide 1.0%; (D) Herbicide 1.5%; (E) Herbicide 2.5%.
The GLMM developed to assess the influence of treatments, showed that all treatments had a positive influence on the net demography of E. globulus wildlings, with Herbicide 1.5% showing the highest coefficient (1.16; Table 3). Cutting and Herbicide 0.5% presented the lowest coefficients (0.79 and 0.76, respectively). The marginal R2 was 10% and the conditional R2 was 90%, showing a very high influence of plot location.

Table 3.
Effect of treatments on the net demography of E. globulus wildlings as shown by a Generalised Linear Mixed Model. The table shows the coefficients of the model, the respective standard errors, the z statistic and the associated probabilities: ≤0.001 = ***; ≤0.05 = *.
The Fisher’s exact test applied to the distribution of resprouting plants by the three treatments (Control, Cutting and Herbicide) showed the existence of a dependent relationship between the two variables (p < 0.001) (Figure 4). Pairwise comparisons showed that plants sprayed with herbicide were more likely to resprout than plants in the control plots (p < 0.001). The lowest recruitment was registered in plots sprayed with herbicide (four out of 20) even though this difference was not statistically significant. Both resprouting and recruitment were first registered in survey 3 and continued in further surveys until the end of the experiment. These results were in line with the distribution of recruits and resprouts per treatment. The counts of recruits were 50, 61 and 8 plants for the Control, the Cutting and the Herbicide treatments, respectively. Resprouting was observed in 19 and 37 plants, in the Cutting and Herbicide treatments, respectively.
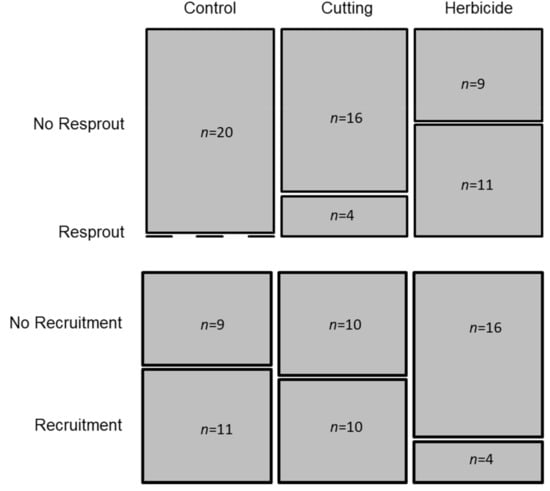
Figure 4.
Frequency of plots with presence and absence of resprouting (upper graph) and recruitment (lower graph), over the experiment (n = 60), distributed by treatment.
4. Discussion
4.1. Wildling Establishment
We should consider the local nature of our study and the particular establishment conditions because the experiment was conducted in a small territory in a particular year. The sampled forest stands were chosen for being a representative example of the massive post-fire establishment that occurred in large areas of Central and Northern Portugal, after the 2017 wildfires. Therefore, our study can be considered an extreme example of the potential post-fire establishment and early-stage development of E. globulus wildlings, in the introduced range, under very favourable circumstances. We found much higher wildling densities (up to 804,000 plants ha−1) compared to other studies on E. globulus and even on other eucalypt species. The highest eucalypt wildling densities registered in the available literature were in burned stands. Ruthrof, et al. [54] and Ruthrof [55] have reported densities between 2610 (E. cladocalyx) and 5200 plants ha−1 (E. megacornuta) in an urban park in Western Australia (non-native range) eight and 12 years after fire, respectively. Águas, et al. [6] have found 4800 plants ha−1 in a large survey of burned eucalypt and pine-dominated stands, across Central and North Portugal. Calviño-Cancela and Rubido-Bará [27], estimated maximum recruitment as 20,000 plants ha−1, for E. globulus seeds fallen within the first 5 m from the plantation edge. Therefore, to the best of our knowledge, the present work reports the highest eucalypt wildling densities ever recorded, exceeding by far the values reported by other studies. The reasons for this disparity are multiple and should be addressed.
The age of the wildlings is an important aspect to consider because the density of tree populations tends to decrease over time. Considering that seed dehiscence occurs within the first five weeks after fire and that most germination occurs within the first two weeks after dehiscence, [23,25] it is reasonable to assume that most wildlings were about one-year-old at the beginning of the experiment. Therefore we can assume that most of the 234,000 plants ha−1 found at the end of the experiment were two-years-old. If we apply an average mortality rate of 30% (according to our results) we would expect to have 80,262 plants ha−1 after five years and 39,328 plants ha−1 after seven years, still much above the density reported by Águas, et al. [6] five to seven years after fire. Therefore, the young age of the wildling populations surveyed in this study does not seem to explain alone, the high densities observed.
The abandonment of E. globulus stands has been related to higher wildling establishment, compared to managed plantations [6,8]. Stand characteristics (mixed, uneven-aged stands) suggest that the sampled stands have resulted from the abandonment of former plantations. On the other hand, wildling density was directly related to the density of adult eucalypt trees, some of them presenting a DBH, greatly beyond what is commonly found in managed plantations. Bigger trees correspond to increased seed production and dispersal, which may contribute to the high wildling densities recorded [56]. Wildling establishment was also positively influenced by environmental factors. According to the models developed by previous authors [11,14,57], the study region has good conditions for E. globulus establishment, in terms of annual precipitation, altitude, and distance from the sea.
The circumstances related to the fire season have probably played a major role in the very high wildling densities recorded in our study. Seeds have higher germination success if adequate moisture conditions are met shortly after dehiscence. The shorter the time-lag between dehiscence and germination cues, the higher will be the probability that seeds will escape predation and natural decay [58,59]. The fire occurred unusually late in early autumn (15 October), two days before significant rainfall (13 mm). The resulting soil moisture has probably created optimal conditions for rapid seed germination and seedling establishment. These conditions have probably allowed many established plants to develop and escape the water stress, typical of summer months. Previous studies have also found higher seedling survival in autumn sowings compared to spring sowings, thereby supporting this hypothesis [24,27].
4.2. Wildling Demography
There was a considerable reduction of wildling densities in Control plots, over one year. High mortality at this stage is expected due to the high density of plants and consequent intraspecific competition. Previous studies on E. globulus demography report very low survival rates during the first year, but provide no information for further developmental stages. Nereu, et al. [25] registered 0% survival one year after sowing, Fernandes, et al. [24] registered a maximum survival of 1.3%, 300 days after sowing, and Calviño-Cancela and Rubido-Bará [27] estimated (based on a sowing experiment) E. globulus survival to be between 3.7% and 19.9%, 180 days after dehiscence. The survival rate of tagged plants in Control plots was 60% and, therefore, much higher than the values reported by these studies. Besides the reasons previously outlined, related to the age of the plants, the stand characteristics, the season of the fire and the environmental conditions, differences in demography were also probably related with the characteristics of the experiments presented in the cited studies. Sowing experiments use a limited number of seeds, and can hardly mimic natural conditions, such as those reported in our study. Seed availability is a key factor that strongly affects plant abundance [60] since it influences the probability that some seeds will reach microsites with good conditions for seedling survival [61].
The presence of new emerging wildlings during our study contradicts the expectation created by previous studies, of a single fire-triggered dehiscence event leading to the depletion of the canopy seed bank [18,23]. The uneven-sized populations of E. globulus wildlings found by Águas, et al. [6] in burned areas could be the result of continuous recruitment after fire. Eucalyptus globulus does not form a seed bank [34] so recruitment should have originated mostly from capsules in the canopy of neighbouring eucalypt trees. It is also possible that encapsulated seeds on the ground may have been preserved, and released later due to disturbances caused by animals, people or wind [23]. Regardless of the involved mechanisms, recruitment was very likely higher than the small increase of wildling density registered in the Control plots in the fourth and sixth surveys, because this increase represented the difference between mortality and recruitment. Considering that the average number of tagged plants in Control plots has decreased 10% more than the overall density of these plots (40% vs. 30%), we can reasonably assume that this value should be close to the overall new recruitment that was alive at the end of the experiment. This percentage corresponds to 3.3 plants m−2 year−1 (11% of the initial average wildling density in Control plots), a high number that does not include the plants that established and died during the experiment.
4.3. Wildling Growth
One year after fire the tagged plants that survived were on average 71 cm height, reaching 173 cm at the end of the second year. Comparisons with other studies are difficult because there is no published information about the early growth of E. globulus wildlings. The few previous studies (sowing experiments) report growth rates that are much lower (less than 10 cm year−1) than the values registered in our study [22,25]. The wildlings surveyed by Águas, et al. [6] five to seven years after fires in Central and Northern Portugal were 270 cm height on average, although with a wide variation among samples. Site productivity was found to be an important driver of plant height. Similarly, we found significant height differences among forest stands, revealing that local site conditions can be determinant for early wildling development.
Growth data was associated with strong seasonality, showing a peak in spring and early summer. The difference between spring-early summer growth and winter growth was about five-fold. In Mediterranean climates, most plant species, including E. globulus, should find the most favourable growth conditions in spring-early summer, as opposed to late summer (hydric stress) and winter (low temperature and low solar radiation) [62]. Despite seasonal differences, we should highlight the continuous growth over the year, as opposed to many native tree species which become dormant in the cold season. The GLMM developed to explain the growth rate has confirmed the strong relationships between weather variables and growth, showing a positive effect of solar radiation and a negative effect of evapotranspiration and maximum temperature. The first variable is the fundamental driver of photosynthesis, while the latter two are limiting factors directly related to water stress. Although these effects were expected [62] we should stress the fact that these variables were more important than the structural characteristics of the wildling population (wilding density and height) in explaining growth variability among populations and across time.
4.4. Effectiveness of Control Methods
Our research shows that the application of chemical or physical control methods can significantly reduce the densities of young E. globulus wildlings. After 380 days, cutting and the highest concentrations of glyphosate (1.5% and 2.5%) caused a 97% to 99% reduction in wildling density. It is important to note the very high value of the conditional R2, compared to the marginal R2, showing that the effect of treatments was strongly site-dependent. We also observed that resprouting was significantly more frequent in Herbicide plots, compared to Control plots, than in Cutting plots. This result was surprising since glyphosate is rapidly absorbed and translocated to the tissues of higher metabolic activity [44] acting through enzyme blockage [63], which should strongly affect resprouting. Although several studies report the development of resistance mechanisms to glyphosate [64], it is very unlikely that E. globulus wildling populations would have developed such mechanisms. However, we should stress the strong resprouting capacity of E. globulus associated with the presence of a lignotuber [17,65]. Although the long-lasting effects of lignotubers on the survival of decapitated seedlings have been demonstrated [65], our results suggest that resprouting can also occur when a chemical disturbance is applied.
Our experiment shows the need to apply high concentrations of herbicide for it to be effective. Considering the concentration of the solution, and the volume of the product applied per square meter, the four herbicide treatments ranged from 1.9 kg ha−1 (weight of active ingredient) in the lowest concentration up to 14.0 kg ha−1 in the highest concentration. The technical recommendations of the manufacturer point to a maximum of 2.5 kg ha−1, which means that only the least concentrated solution (0.5%) met that recommendation. In current forestry practices, the application rate should not exceed 4 kg ha−1 [66] although there are references of application rates even higher than the 2.5% concentration e.g., [67]. We observed that one year after treatment there were still 6.6 plants m2 in the 0.5% herbicide plots, which suggests that E. globulus wildlings may present considerable resistance to standard applications of herbicide.
Considering that the 1.5% concentration has delivered similar results to the 2.5% concentration, it seems reasonable to use the lower herbicide concentration both from an economical as from an environmental point of view. The cost of the herbicide (current prices in Portugal) amounts to 219 € ha−1, for a 1.5% herbicide application, to which we should add around 80€ of labour costs. The application of cutting operations using a brush cutter ranges from 383 € ha−1 to 1150 € ha−1 (according to the official tables from the Portuguese authority for forests), including manpower. Therefore, although similarly effective, cutting seems to be a more expensive solution compared to herbicide application.
5. Conclusions
Although our study did not aim to assess ecosystem impacts, there are ecological consequences that should be drawn from our results. The high plant density, the limited mortality and the fast growth of E. globulus wildlings portrayed by our dataset suggest that the naturalization of E. globulus under favourable post-fire conditions can have a strong impact on local ecosystems. The complete absence of other plant species in more than half of the surveyed plots supports this hypothesis. Moreover, our results show that recruitment is a continuous process not limited to a single post-fire episode, which calls for additional attention to the problem of post-fire management in recently burned eucalypt stands. Nonetheless, the fire event that triggered E. globulus dehiscence and the massive plant recruitment had special characteristics since it occurred in mid-autumn, followed by significant rain, providing optimal soil moisture that allowed high seed germination rates. Therefore, special attention should be paid to fire events that occur late or after the fire season, because of the increased risk of high wildling establishment.
Both herbicide and cutting treatments proved to be effective alternatives to control young wildling populations of E. globulus. Although herbicide may be a more economical alternative compared to cutting, we should consider the strict regulations for herbicide application and the potential impacts on the environment, especially the risk to watercourses [68]. On the other hand, herbicide was able to match the performance of the cutting treatment only for high product concentrations, beyond what is technically advised, and was more prone to resprouting. Therefore, under the conditions of our study, cutting should be a better alternative for managing young E. globulus wildlings. Regardless of the control method adopted, the monitoring of the treated areas is of paramount importance, to detect resprouting plants and new recruitment, as observed in our study.
Until recently, most literature on eucalypts and particularly on E. globulus has been focused on wood production from plantations and the respective environmental impacts. Therefore, the ecology of E. globulus as a naturalized species in the introduced range was and still is, poorly known. The data presented in this study show for the first time the early behaviour of recently established wildling populations in a post-fire environment and the ways to control it. This information can be useful for knowledge-based management of these novel ecosystems (in the sense of Hobbs, et al. 2006) [69] in their areas of expansion in western Iberia (Portugal and Spain) and elsewhere in regions prone to E. globulus naturalization [2]. However, more focused research is needed to assess the real impact of the establishment of E. globulus wildlings on local plant communities.
Author Contributions
Conceptualization, J.S.S. and C.J.; Data curation, M.N. and S.P.; Formal analysis, M.N.; Funding acquisition, J.S.S.; Investigation, J.S.S.; Methodology, J.S.S.; Project administration, J.S.S.; Resources, J.S.S.; Writing—original draft, J.S.S., L.Q. and E.D.; Writing—review and editing, J.S.S., M.N., S.P., L.Q., C.J. and E.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Portuguese Foundation for Science and Technology (FCT), through project Wildgum II, grant number PTDC/ASP-SIL/30435/2017.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the Santa Comba Dão municipality for the logistic support to this study and to the Portuguese Institute for Sea and Atmosphere (IPMA) for providing the meteorological data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jacobs, M.R. Eucalypts for Planting; Food and Agriculture Organization of the United Nations: Rome, Italy, 1981. [Google Scholar]
- Rejmánek, M.; Richardson, D. Eucalypts. In Encyclopedia of Biological Invasions; Simberloff, D., Rejmánek, M., Eds.; University of California Press: Los Angeles, CA, USA, 2011; pp. 203–209. [Google Scholar]
- Potts, B.M.; Vaillancourt, R.E.; Jordan, G.J.; Dutkowski, G.W.; Costa e Silva, J.; McKinnon, G.E.; Steane, D.A.; Volker, P.W.; Lopez, G.A.; Apiolaza, L.A.; et al. Exploration of the Eucalyptus globulus gene pool. In Proceedings of the Eucalyptus in a Changing World, Aveiro, Portugal, 11–15 October; pp. 46–61.
- Rejmánek, M.; Richardson, D.M. Trees and shrubs as invasive alien species–2013 update of the global database. Divers. Distrib. 2013, 19, 1093–1094. [Google Scholar] [CrossRef]
- ICNF. 6º Inventário Florestal Nacional-Relatório Final; Instituto da Conservação da Natureza e das Florestas: Lisboa, Portugal, 2019; p. 58. [Google Scholar]
- Águas, A.; Ferreira, A.; Maia, P.; Fernandes, P.M.; Roxo, L.; Keizer, J.; Silva, J.S.; Rego, F.C.; Moreira, F. Natural establishment of Eucalyptus globulus Labill. in burnt stands in Portugal. For. Ecol. Manag. 2014, 323, 47–56. [Google Scholar]
- Vaz, A.S.; Honrado, J.P.; Lomba, A. Replacement of pine by eucalypt plantations: Effects on the diversity and structure of tree assemblages under land abandonment and implications for landscape management. Landsc. Urban Plan. 2019, 185, 61–67. [Google Scholar]
- Fernandes, P.; Antunes, C.; Pinho, P.; Máguas, C.; Correia, O. Natural regeneration of Pinus pinaster and Eucalyptus globulus from plantation into adjacent natural habitats. For. Ecol. Manag. 2016, 378, 91–102. [Google Scholar] [CrossRef]
- Deus, E.; Silva, J.S.; Larcombe, M.J.; Catry, F.X.; Queirós, L.; Santos, P.D.; Matias, H.; Águas, A.; Rego, F.C. Investigating the invasiveness of Eucalyptus globulus in Portugal: Site-scale drivers, reproductive capacity and dispersal potential. Biol. Invasions 2019, 21, 2027–2044. [Google Scholar]
- Águas, A.; Larcombe, M.J.; Matias, H.; Deus, E.; Potts, B.M.; Rego, F.C.; Silva, J.S. Understanding the naturalization of Eucalyptus globulus in Portugal: A comparison with Australian plantations. Eur. J. For. Res. 2017, 3, 433–446. [Google Scholar] [CrossRef]
- Larcombe, M.J.; Silva, J.S.; Vaillancourt, R.E.; Potts, B.M. Assessing the invasive potential of Eucalyptus globulus in Australia: Quantification of wildling establishment from plantations. Biol. Invasions 2013, 15, 2763–2781. [Google Scholar] [CrossRef]
- Deus, E.d.; Silva, J.S.; Castro-Díez, P.; Lomba, A.; Ortiz, M.L.; Vicente, J. Current and future conflicts between eucalypt plantations and high biodiversity areas in the Iberian Peninsula. J. Nat. Conserv. 2018, 45, 107–117. [Google Scholar] [CrossRef]
- Silva, J.S.; Vaz, P.; Moreira, F.; Catry, F.; Rego, F.C. Wildfires as a major driver of landscape dynamics in three fire-prone areas of Portugal. Landscape Urban Plann. 2011, 101, 349–358. [Google Scholar] [CrossRef]
- Queirós, L.; Deus, E.; Silva, J.S.; Vicente, J.; Ortiz, L.; Fernandes, P.M.; Castro-Díez, P. Assessing the drivers and the recruitment potential of Eucalyptus globulus in the Iberian Peninsula. For. Ecol. Manage. 2020, 466, 118147. [Google Scholar] [CrossRef]
- Gill, A.M. Eucalypts and fires: Interdependent or independent? In Eucalypt Ecology: Individuals to Ecosystems; Williams, J.E., Woinarsk, J.C.Z., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 151–167. [Google Scholar]
- Nicolle, D. A classification and census of regenerative strategies in the eucalypts (Angophora, Corymbia and Eucalyptus-Myrtaceae), with special reference to the obligate seeders. Aust. J. Bot. 2006, 54, 391–407. [Google Scholar] [CrossRef]
- Catry, F.X.; Moreira, F.; Tujeira, R.; Silva, J.S. Post-fire survival and regeneration of Eucalyptus globulus in forest plantations in Portugal. For. Ecol. Manag. 2013, 310, 194–203. [Google Scholar] [CrossRef]
- Silva, J.S.; dos Santos, P.; Sério, A.; Gomes, F. Effects of heat on dehiscence and germination in Eucalyptus globulus Labill. Int. J. Wildland Fire 2016, 25, 478–483. [Google Scholar] [CrossRef]
- Stoneman, G.L. Ecology and physiology of establishment of eucalypt seedlings from seed: A review. Aust. For. 1994, 57, 11–29. [Google Scholar]
- Cremer, K. How eucalypt fruits release their seed. Aust. J. Bot. 1965, 13, 11–16. [Google Scholar]
- Vivian, L.M.; Cary, G.J.; Bradstock, R.A.; Gill, A.M. Influence of fire severity on the regeneration, recruitment and distribution of eucalypts in the Cotter River Catchment, Australian Capital Territory. Austral Ecol. 2008, 33, 55–67. [Google Scholar] [CrossRef]
- Calviño-Cancela, M.; Lorenzo, P.; González, L. Fire increases Eucalyptus globulus seedling recruitment in forested habitats: Effects of litter, shade and burnt soil on seedling emergence and survival. For. Ecol. Manag. 2018, 409, 826–834. [Google Scholar] [CrossRef]
- Santos, P.; Matias, H.; Deus, E.; Águas, A.; Silva, J.S. Fire effects on capsules and encapsulated seeds from Eucalyptus globulus in Portugal. Plant Ecol. 2015, 216, 1611–1621. [Google Scholar] [CrossRef]
- Fernandes, P.; Maguas, C.; Correia, O. Combined effects of climate, habitat, and disturbance on seedling establishment of Pinus pinaster and Eucalyptus globulus. Plant Ecol. 2017, 218, 501–515. [Google Scholar] [CrossRef]
- Nereu, M.; Silva, J.S.; Deus, E.; Nunes, M.; Potts, B. The effect of management operations on the demography of Eucalyptus globulus seedlings. For. Ecol. Manag. 2019, 453, 117630. [Google Scholar] [CrossRef]
- Becerra, P.I.; Bustamante, R.O. The effect of herbivory on seedling survival of the invasive exotic species Pinus radiata and Eucalyptus globulus in a Mediterranean ecosystem of Central Chile. For. Ecol. Manag. 2008, 256, 1573–1578. [Google Scholar]
- Calviño-Cancela, M.; Rubido-Bará, M. Invasive potential of Eucalyptus globulus: Seed dispersal, seedling recruitment and survival in habitats surrounding plantations. For. Ecol. Manag. 2013, 305, 129–137. [Google Scholar]
- Silva, J.S.; Moreira, F.; Vaz, P.; Catry, F.; Godinho-Ferreira, P. Assessing the relative fire proneness of different fores types in Portugal. Plant Biosyst. 2009, 143, 597–608. [Google Scholar]
- Souto, X.C.; Bolaño, J.C.; González, L.; Reigosa, M.J. Allelopathic effects of tree species on some soil microbial populations and herbaceous plants. Biol. Plant. 2001, 44, 269–275. [Google Scholar] [CrossRef]
- Babu, R.C.; Kandasamy, O. Allelopathic effect of Eucalyptus globulus Labill. on Cyperus rotundus L. and Cynodon dactylon L. Pers. J. Agron. Crop Sci. 1997, 179, 123–126. [Google Scholar]
- Jayakumar, M.; Eyini, M. Allelopathic effect of Eucalyptus globulus Labil in groundnut and corn. Comp. Physiol. Ecol. 1990, 15, 109–113. [Google Scholar]
- Brundu, G.; Pauchard, A.; Pyšek, P.; Pergl, J.; Bindewald, A.M.; Brunori, A.; Canavan, S.; Campagnaro, T.; Celesti-Grapow, L.; Dechoum, M.D.S.; et al. Global guidelines for the sustainable use of non-native trees to prevent tree invasions and mitigate their negative impacts. NeoBiota 2020, 61, 65–116. [Google Scholar] [CrossRef]
- Andreu, J.; Vila, M.; Hulme, P.E. An assessment of stakeholder perceptions and management of noxious alien plants in Spain. Environ. Manag. 2009, 43, 1244–1255. [Google Scholar]
- Boland, D.J.; Brooker, M.I.H.; Turnbull, J.W. Eucalyptus Seed; CSIRO publishing: Canberra, Australia, 1980; p. 204. [Google Scholar]
- Souza-Alonso, P.; Lorenzo, P.; Rubido-Bará, M.; González, L. Effectiveness of management strategies in Acacia dealbata Link invasion, native vegetation and soil microbial community responses. For. Ecol. Manag. 2013, 304, 464–472. [Google Scholar]
- Constán-Nava, S.; Bonet, A.; Pastor, E.; Lledó, M.J. Long-term control of the invasive tree Ailanthus altissima: Insights from Mediterranean protected forests. For. Ecol. Manag. 2010, 260, 1058–1064. [Google Scholar]
- Vargas, F.; Rubilar, R.; Gonzalez-Benecke, C.A.; Sanchez-Olate, M.; Aracena, P. Long-term response to area of competition control in Eucalyptus globulus plantations. New For. 2018, 49, 383–398. [Google Scholar] [CrossRef]
- Bean, C.; Russo, M.J. Elemental Stewardship Abstract for Eucalyptus globulus; The Nature Conservancy: Richmond, VA, USA, 1989; Available online: http://www.invasive.org (accessed on 10 August 2020).
- Boyd, D. Eucalyptus removal on Angel Island. In Proceedings of the California Exotic Pest Plant Council 1997 Symposium Proceedings, Concord, CA, USA, 2–4 October; pp. 1–3.
- Burrows, G.E. Buds, bushfires and resprouting in the eucalypts. Aust. J. Bot. 2013, 61, 331–349. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z 2006, 15, 259–263. [Google Scholar]
- CMDFCI. Plano Municipal de Defesa da Floresta Contra Incêndios-Caderno I Diagnóstico; Câmara Municipal de Santa Comba Dão: Santa Comba Dão, Portugal, 2015. [Google Scholar]
- DGRF. Estratégia Nacional Para as Florestas; DGRF: Lisboa, Portugal, 2006; p. 189. [Google Scholar]
- Sprankle, P.; Meggitt, W.F.; Penner, D. Absorption, action, and translocation of glyphosate. Weed Sci. 1975, 23, 235–240. [Google Scholar]
- Cottam, G.; Curtis, J.T.; Hale, B.W. Some sampling characteristics of a population of randomly dispersed individuals. Ecology 1953, 34, 741–757. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration-Guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56. FAO Rome 1998, 300, D05109. [Google Scholar]
- Pohlert, T. The Pairwise Multiple Comparison of Mean Ranks (PMCMR)R Package. 2014. Available online: https://cran.r-project.org/web/packages/PMCMR/vignettes/PMCMR.pdf (accessed on 15 May 2020).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage publications: Los Angeles, CA, USA, 2018; p. 608. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed effects models and extensions in ecology with R. Springer: New York, NY, USA, 2009. [Google Scholar]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Thomas, R.; Vaughan, I.; Lello, J.; Medeiros, R.; Pollard, A.; Seward, A.; Smith, J.; Vafidis, J. Data Analysis with R Statistical Software: A Guidebook for Scientists; Eco-explore: Cardiff, South Wales, UK, 2015; p. 285. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Ruthrof, K.X.; Loneragan, W.A.; Yates, C.J. Comparative population dynamics of Eucalyptus cladocalyx in its native habitat and as an invasive species in an urban bushland in south-western Australia. Divers. Distrib. 2003, 9, 469–483. [Google Scholar]
- Ruthrof, K.X. Invasion by Eucalyptus megacornuta of an Urban Bushland in Southwestern Australia. Weed Technol. 2004, 18, 1376–1380. [Google Scholar]
- Simberloff, D. The role of propagule pressure in biological invasions. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 81–102. [Google Scholar]
- Catry, F.X.; Moreira, F.; Deus, E.; Silva, J.S.; Águas, A. Assessing the extent and the environmental drivers of Eucalyptus globulus wildling establishment in Portugal: Results from a countrywide survey. Biol. Invasions 2015, 17, 3163–3181. [Google Scholar] [CrossRef]
- Bond, W.J. Fire survival of Cape Proteaceae-influence of fire season and seed predators. Vegetatio 1984, 56, 65–74. [Google Scholar] [CrossRef]
- Deus, E.; Silva, J.S.; Marchante, H.; Marchante, E.; Félix, C. Are post-dispersed seeds of Eucalyptus globulus predated in the introduced range? Evidence from an experiment in Portugal. Web Ecol. 2018, 18, 67–79. [Google Scholar] [CrossRef]
- Ehrlén, J.; Münzbergova, Z.; Diekmann, M.; Eriksson, O. Long-term assessment of seed limitation in plants: Results from an 11-year experiment. J. Ecol. 2006, 94, 1224–1232. [Google Scholar] [CrossRef]
- Bailey, T.G.; Davidson, N.J.; Close, D.C. Understanding the regeneration niche: Microsite attributes and recruitment of eucalypts in dry forests. For. Ecol. Manag. 2012, 269, 229–238. [Google Scholar]
- Sanz-Perez, V.; Castro-Diez, P.; Joffre, R. Seasonal carbon storage and growth in Mediterranean tree seedlings under different water conditions. Tree Physiol. 2009, 29, 1105–1116. [Google Scholar] [CrossRef]
- Rubin, J.L.; Gaines, C.G.; Jensen, R.A. Enzymological basis for herbicidal action of glyphosate. Plant Physiol. 1982, 70, 833–839. [Google Scholar] [CrossRef]
- Sammons, R.D.; Gaines, T.A. Glyphosate resistance: State of knowledge. Pest Manag. Sci. 2014, 70, 1367–1377. [Google Scholar] [CrossRef]
- Borzak, C.L.; Potts, B.M.; O’Reilly-Wapstra, J.M. Survival and recovery of Eucalyptus globulus seedlings from severe defoliation. For. Ecol. Manag. 2016, 379, 243–251. [Google Scholar] [CrossRef]
- Rolando, C.; Baillie, B.; Thompson, D.; Little, K. The risks associated with glyphosate-based herbicide use in planted forests. Forests 2017, 8, 208. [Google Scholar] [CrossRef]
- Patten, K. Comparison of chemical and mechanical control efforts for invasive Spartina in Willapa Bay, Washington. In Proceedings of the Conference on Invasive Spartina, San Francisco, CA, USA, 8–9 November 2004. [Google Scholar]
- Reoyo-Prats, B.; Aubert, D.; Menniti, C.; Ludwig, W.; Sola, J.; Pujo-Pay, M.; Conan, P.; Verneau, O.; Palacios, C. Multicontamination phenomena occur more often than expected in Mediterranean coastal watercourses: Study case of the Têt River (France). Sci. Total Environ. 2017, 579, 10–21. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Arico, S.; Aronson, J.; Baron, J.S.; Bridgewater, P.; Cramer, V.A.; Epstein, P.R.; Ewel, J.J.; Klink, C.A.; Lugo, A.E.; et al. Novel ecosystems: Theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr. 2006, 15, 1–7. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).