Weather–Growth Responses Show Differing Adaptability of Scots Pine Provenances in the South-Eastern Parts of Baltic Sea Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trials, Provenances, and Measurements
2.2. Data Analysis
3. Results
3.1. Local Linear Weather–Growth Relationships
3.2. Regional Nonlinear Responses
4. Discussion
4.1. Plasticity of Growth Responses
4.2. Regional Weather Drivers of Radial Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buras, A.; Menzel, A. Projecting Tree Species Composition Changes of European Forests for 2061–2090 Under RCP 4.5 and RCP 8.5 Scenarios. Front. Plant Sci. 2019, 9, 1986. [Google Scholar] [CrossRef] [Green Version]
- Morin, X.; Fahse, L.; Jactel, H.; Scherer-Lorenzen, M.; García-Valdés, R.; Bugmann, H. Long-term response of forest productivity to climate change is mostly driven by change in tree species composition. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Isaac-Renton, M.; Montwé, D.; Hamann, A.; Spiecker, H.; Cherubini, P.; Treydte, K. Northern forest tree populations are physiologically maladapted to drought. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkerk, P.J.; Costanza, R.; Hetemäki, L.; Kubiszewski, I.; Leskinen, P.; Nabuurs, G.J.; Potočnik, J.; Palahí, M. Climate-Smart Forestry: The missing link. For. Policy Econ. 2020, 115, 102164. [Google Scholar] [CrossRef]
- Breed, M.F.; Harrison, P.A.; Bischoff, A.; Durruty, P.; Gellie, N.J.C.; Gonzales, E.K.; Havens, K.; Karmann, M.; Kilkenny, F.F.; Krauss, S.L.; et al. Priority actions to improve provenance decision-making. Bioscience 2018, 68, 510–516. [Google Scholar] [CrossRef]
- Nabuurs, G.-J.; Verkerk, P.J.; Schelhaas, M.-J.; Ramón González Olabarria, J.; Trasobares, A.; Cienciala, E. Climate-Smart Forestry: Mitigation impacts in three European regions. In From Science to Policy 6; European Forest Institute: Joensuu, Finland, 2018; p. 32. [Google Scholar]
- Breed, M.F.; Stead, M.G.; Ottewell, K.M.; Gardner, M.G.; Lowe, A.J. Which provenance and where? Seed sourcing strategies for revegetation in a changing environment. Conserv. Genet. 2013, 14, 1–10. [Google Scholar] [CrossRef]
- Aitken, S.N.; Bemmels, J.B. Time to get moving: Assisted gene flow of forest trees. Evol. Appl. 2016, 9, 271–290. [Google Scholar] [CrossRef]
- Berlin, M.; Persson, T.; Jansson, G.; Haapanen, M.; Ruotsalainen, S.; Bärring, L.; Gull, B.A. Scots pine transfer effect models for growth and survival in Sweden and Finland. Silva Fenn. 2016, 50, 1562. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, S.G.; Ding, C.; Hamann, A.; Hacke, U.G.; Thomas, B.R.; Brouard, J.S. Frost hardiness vs. growth performance in trembling aspen: An experimental test of assisted migration. J. Appl. Ecol. 2013, 50, 939–949. [Google Scholar] [CrossRef] [Green Version]
- MacLachlan, I.R.; Wang, T.; Hamann, A.; Smets, P.; Aitken, S.N. Selective breeding of lodgepole pine increases growth and maintains climatic adaptation. For. Ecol. Manag. 2017, 391, 404–416. [Google Scholar] [CrossRef]
- Harvey, J.E.; Smiljanić, M.; Scharnweber, T.; Buras, A.; Cedro, A.; Cruz-García, R.; Drobyshev, I.; Janecka, K.; Jansons, Ā.; Kaczka, R.; et al. Tree growth influenced by warming winter climate and summer moisture availability in northern temperate forests. Glob. Chang. Biol. 2020, 26, 2505–2518. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, T.; Cochard, H.; Segura, V.; Rozenberg, P. Native-source climate determines the Douglas-fir potential of adaptation to drought. For. Ecol. Manag. 2019, 444, 9–20. [Google Scholar] [CrossRef]
- Alakärppä, E.; Salo, H.M.; Valledor, L.; Cañal, M.J.; Häggman, H.; Vuosku, J. Natural variation of DNA methylation and gene expression may determine local adaptations of Scots pine populations. J. Exp. Bot. 2018, 69, 5293–5305. [Google Scholar] [CrossRef]
- Nabais, C.; Hansen, J.K.; David-Schwartz, R.; Klisz, M.; López, R.; Rozenberg, P. The effect of climate on wood density: What provenance trials tell us? For. Ecol. Manag. 2018, 408, 148–156. [Google Scholar] [CrossRef]
- Cavin, L.; Jump, A.S. Highest drought sensitivity and lowest resistance to growth suppression are found in the range core of the tree Fagus sylvatica L. not the equatorial range edge. Glob. Chang. Biol. 2017, 23, 362–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, K.; Wang, X.; Liang, P.; An, H.; Sun, H.; Han, W.; Li, Q. Tree-ring widths are good proxies of annual variation in forest productivity in temperate forests. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Moran, E.; Lauder, J.; Musser, C.; Stathos, A.; Shu, M. The genetics of drought tolerance in conifers. New Phytol. 2017, 216, 1034–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valladares, F.; Matesanz, S.; Guilhaumon, F.; Araújo, M.B.; Balaguer, L.; Benito-Garzón, M.; Cornwell, W.; Gianoli, E.; Kleunen, M.; Naya, D.E.; et al. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 2014, 17, 1351–1364. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Blackman, C.J.; Choat, B.; Duursma, R.A.; Rymer, P.D.; Medlyn, B.E.; Tissue, D.T. Tree hydraulic traits are coordinated and strongly linked to climate-of-origin across a rainfall gradient. Plant. Cell Environ. 2018, 41, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Restaino, C.M.; Peterson, D.L.; Littell, J. Increased water deficit decreases Douglas fir growth throughout western US forests. Proc. Natl. Acad. Sci. USA 2016, 113, 9557–9562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, J.A.; Esteban, L.G.; de Palacios, P.; Fernández, F.G. Variation in wood anatomical traits of Pinus sylvestris L. between Spanish regions of provenance. Trees-Struct. Funct. 2010, 24, 1017–1028. [Google Scholar] [CrossRef]
- Li, Y.; Suontama, M.; Burdon, R.D.; Dungey, H.S. Genotype by environment interactions in forest tree breeding: Review of methodology and perspectives on research and application. Tree Genet. Genomes 2017, 13, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Matisons, R.; Elferts, D.; Krišāns, O.; Schneck, V.; Gärtner, H.; Bast, A.; Wojda, T.; Kowalczyk, J.; Jansons, Ā. Non-linear regional weather-growth relationships indicate limited adaptability of the eastern Baltic Scots pine. For. Ecol. Manag. 2021, 479, 118600. [Google Scholar] [CrossRef]
- Matisons, R.; Jansone, D.; Elferts, D.; Adamovičs, A.; Schneck, V.; Jansons, Ā. Plasticity of response of tree-ring width of Scots pine provenances to weather extremes in Latvia. Dendrochronologia 2019, 54, 1–10. [Google Scholar] [CrossRef]
- Leites, L.P.; Rehfeldt, G.E.; Robinson, A.P.; Crookston, N.L.; Jaquish, B. Possibilities and limitations of using historic provenance tests to infer forest species growth responses to climate change. Nat. Resour. Model. 2012, 25, 409–433. [Google Scholar] [CrossRef]
- Chakraborty, D.; Wang, T.; Andre, K.; Konnert, M.; Lexer, M.J.; Matulla, C.; Schueler, S. Selecting populations for non-analogous climate conditions using universal response functions: The case of Douglas-fir in Central Europe. PLoS ONE 2015, 10, e0136357. [Google Scholar] [CrossRef]
- de Villemereuil, P.; Gaggiotti, O.E.; Mouterde, M.; Till-Bottraud, I. Common garden experiments in the genomic era: New perspectives and opportunities. Heredity 2016, 116, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Housset, J.M.; Nadeau, S.; Isabel, N.; Depardieu, C.; Duchesne, I.; Lenz, P.; Girardin, M.P. Tree rings provide a new class of phenotypes for genetic associations that foster insights into adaptation of conifers to climate change. New Phytol. 2018, 218, 630–645. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Babst, F.; Bellassen, V.; Frank, D.; Launois, T.; Tan, K.; Ciais, P.; Poulter, B. Converging Climate Sensitivities of European Forests Between Observed Radial Tree Growth and Vegetation Models. Ecosystems 2018, 21, 410–425. [Google Scholar] [CrossRef] [Green Version]
- Rosner, S.; Světlík, J.; Andreassen, K.; Børja, I.; Dalsgaard, L.; Evans, R.; Karlsson, B.; Tollefsrud, M.M.; Solberg, S. Wood density as a screening trait for drought sensitivity in Norway spruce. Can. J. For. Res. 2013, 44, 154–161. [Google Scholar] [CrossRef]
- Henttonen, H.M.; Mäkinen, H.; Heiskanen, J.; Peltoniemi, M.; Laurén, A.; Hordo, M. Response of radial increment variation of Scots pine to temperature, precipitation and soil water content along a latitudinal gradient across Finland and Estonia. Agric. For. Meteorol. 2014, 198–199, 294–308. [Google Scholar] [CrossRef]
- Matisons, R.; Krišāns, O.; Kārkliņa, A.; Adamovičs, A.; Jansons, Ā.; Gärtner, H. Plasticity and climatic sensitivity of wood anatomy contribute to performance of eastern Baltic provenances of Scots pine. For. Ecol. Manag. 2019, 452, 117568. [Google Scholar] [CrossRef]
- Heer, K.; Behringer, D.; Piermattei, A.; Bässler, C.; Brandl, R.; Fady, B.; Jehl, H.; Liepelt, S.; Lorch, S.; Piotti, A.; et al. Linking dendroecology and association genetics in natural populations: Stress responses archived in tree rings associate with SNP genotypes in silver fir (Abies alba Mill.). Mol. Ecol. 2018, 27, 1428–1438. [Google Scholar] [CrossRef]
- Cuny, H.E.; Fonti, P.; Rathgeber, C.B.K.; Arx, G.; Peters, R.L.; Frank, D.C. Couplings in cell differentiation kinetics mitigate air temperature influence on conifer wood anatomy. Plant. Cell Environ. 2019, 42, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Jyske, T.; Mäkinen, H.; Kalliokoski, T.; Nöjd, P. Intra-annual tracheid production of Norway spruce and Scots pine across a latitudinal gradient in Finland. Agric. For. Meteorol. 2014, 194, 241–254. [Google Scholar] [CrossRef]
- Wilmking, M.; Maaten-Theunissen, M.; Maaten, E.; Scharnweber, T.; Buras, A.; Biermann, C.; Gurskaya, M.; Hallinger, M.; Lange, J.; Shetti, R.; et al. Global assessment of relationships between climate and tree growth. Glob. Chang. Biol. 2020, 26, 3212–3220. [Google Scholar] [CrossRef] [Green Version]
- Billings, S.A.; Glaser, S.M.; Boone, A.S.; Stephen, F.M. Nonlinear tree growth dynamics predict resilience to disturbance. Ecosphere 2015, 6, art242. [Google Scholar] [CrossRef] [Green Version]
- Hofgaard, A.; Ols, C.; Drobyshev, I.; Kirchhefer, A.J.; Sandberg, S.; Söderström, L. Non-stationary response of tree growth to climate trends along the arctic margin. Ecosystems 2019, 22, 434–451. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, A.H.; Duffy, P.A.; Mann, D.H. Nonlinear responses of white spruce growth to climate variability in interior Alaska. Can. J. For. Res. 2013, 43, 331–343. [Google Scholar] [CrossRef]
- Way, D.A.; Oren, R. Differential responses to changes in growth temperature between trees from different functional groups and biomes: A review and synthesis of data. Tree Physiol. 2010, 30, 669–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matías, L.; Linares, J.C.; Sánchez-Miranda, Á.; Jump, A.S. Contrasting growth forecasts across the geographical range of Scots pine due to altitudinal and latitudinal differences in climatic sensitivity. Glob. Chang. Biol. 2017, 23, 4106–4116. [Google Scholar] [CrossRef]
- Brzeziecki, B.; Kienast, F. Classifying the life-history strategies of trees on the basis of the Grimian model. For. Ecol. Manag. 1994, 69, 167–187. [Google Scholar] [CrossRef]
- Hytteborn, H.; Maslov, A.; Nazimova, D.; Rysin, L.P. Boreal forests of Eurasia. In Coniferous Forests, Ecosystems of the World; Elsevier: Amsterdam, The Netherlands, 2005; pp. 23–99. [Google Scholar]
- Jansson, G.; Hansen, J.K.; Haapanen, M.; Kvaalen, H.; Steffenrem, A. The genetic and economic gains from forest tree breeding programmes in Scandinavia and Finland. Scand. J. For. Res. 2017, 32, 273–286. [Google Scholar] [CrossRef]
- Dering, M.; Kosiński, P.; Wyka, T.P.; Pers-Kamczyc, E.; Boratyński, A.; Boratyńska, K.; Reich, P.B.; Romo, A.; Zadworny, M.; Żytkowiak, R.; et al. Tertiary remnants and Holocene colonizers: Genetic structure and phylogeography of Scots pine reveal higher genetic diversity in young boreal than in relict Mediterranean populations and a dual colonization of Fennoscandia. Divers. Distrib. 2017, 23, 540–555. [Google Scholar] [CrossRef] [Green Version]
- Giertych, M.; Mátyás, C. Genetics of Scots Pine; Elsevier: Amsterdam, The Netherlands, 1991; Volume 3, ISBN 9781483291635. [Google Scholar]
- Martínez-Vilalta, J.; Sala, A.; Piñol, J. The hydraulic architecture of Pinaceae—A review. Plant Ecol. 2004, 171, 3–13. [Google Scholar] [CrossRef]
- Loehle, C. Height growth rate tradeoffs determine northern and southern range limits for trees. J. Biogeogr. 1998, 25, 735–742. [Google Scholar] [CrossRef]
- Taeger, S.; Zang, C.; Liesebach, M.; Schneck, V.; Menzel, A. Impact of climate and drought events on the growth of Scots pine (Pinus sylvestris L.) provenances. For. Ecol. Manag. 2013, 307, 30–42. [Google Scholar] [CrossRef]
- Grattapaglia, D.; Silva-Junior, O.B.; Resende, R.T.; Cappa, E.P.; Müller, B.S.; Tan, B.; Isik, F.; Ratcliffe, B.; El-Kassaby, Y.A. Quantitative genetics and genomics converge to accelerate forest tree breeding. Front. Plant Sci. 2018, 9, 1693. [Google Scholar] [CrossRef] [PubMed]
- Kohlstock, N.; Schneck, H. Scots pine breeding (Pinus sylvestris L.) at Waldsieversdorf and its impact on pine management in the north eastern German lowland. Silvae Genet. 1992, 41, 174–180. [Google Scholar]
- Jansons, Ā.; Baumanis, I. Growth dynamics of scots pine geographical provenances in Latvia. Balt. For. 2005, 11, 29–37. [Google Scholar]
- Hartmann, D.L.; Klein Tank, A.M.G.; Rusticucci, M.; Alexander, L.V.; Brönnimann, S.; Charabi, Y.A.R.; Dentener, F.J.; Dlugokencky, E.J.; Easterling, D.R.; Kaplan, A.; et al. Observations: Atmosphere and surface. In Climate Change 2013 the Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; Volume 9781107057999, pp. 159–254. ISBN 9781107415324. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- Avotniece, Z.; Klavins, M.; Rodinovs, V. Changes of extreme climate events in Latvia. Environ. Clim. Technol. 2012, 9, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Taeger, S.; Sparks, T.H.; Menzel, A. Effects of temperature and drought manipulations on seedlings of Scots pine provenances. Plant Biol. 2015, 17, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, H.; Nievergelt, D. The core-microtome: A new tool for surface preparation on cores and time series analysis of varying cell parameters. Dendrochronologia 2010, 28, 85–92. [Google Scholar] [CrossRef]
- Holmes, R. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Cook, E.R. The decomposition of tree-ring series for environmental studies. Tree Ring Bull. 1987, 47, 37–59. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. Dendroclimatic calibration in R: The bootRes package for response and correlation function analysis. Dendrochronologia 2013, 31, 68–74. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef] [Green Version]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 2020, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2011, 73, 3–36. [Google Scholar] [CrossRef] [Green Version]
- von Arx, G.; Arzac, A.; Fonti, P.; Frank, D.; Zweifel, R.; Rigling, A.; Galiano, L.; Gessler, A.; Olano, J.M. Responses of sapwood ray parenchyma and non-structural carbohydrates of Pinus sylvestris to drought and long-term irrigation. Funct. Ecol. 2017, 31, 1371–1382. [Google Scholar] [CrossRef] [Green Version]
- Olsson, T.; Ericsson, T. Genetic parameter estimates of growth and survival of Pinus sylvestris with mixed model multiple-trait restricted maximum likelihood analysis. Scand. J. For. Res. 2002, 17, 103–110. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: http://www.r-project.org/ (accessed on 5 December 2019).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, Second ed. Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 12 March 2021).
- Ohse, B.; Ohse, B.; Jansen, F.; Wilmking, M. Do limiting factors at Alaskan treelines shift with climatic regimes? Environ. Res. Lett. 2012, 7, 015505. [Google Scholar] [CrossRef]
- Carrer, M.; Urbinati, C. Long-term change in the sensitivity of tree-ring growth to climate forcing in Larix decidua. New Phytol. 2006, 170, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Carrer, M.; Nola, P.; Motta, R.; Urbinati, C. Contrasting tree-ring growth to climate responses of Abies alba toward the southern limit of its distribution area. Oikos 2010, 119, 1515–1525. [Google Scholar] [CrossRef]
- Merlin, M.; Perot, T.; Perret, S.; Korboulewsky, N.; Vallet, P. Effects of stand composition and tree size on resistance and resilience to drought in sessile oak and Scots pine. For. Ecol. Manag. 2015, 339, 22–33. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-González, C.; Sampedro, L.; Rozas, V.; Zas, R. Climate drives intraspecific differentiation in the expression of growth-defence trade-offs in a long-lived pine species. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Glasner, E.; Weiss, B. Sensitive dependence on initial conditions. Nonlinearity 1993, 6, 1067. [Google Scholar] [CrossRef] [Green Version]
- Cuny, H.E.; Rathgeber, C.B.K.; Lebourgeois, F.; Fortin, M.; Fournier, M. Life strategies in intra-annual dynamics of wood formation: Example of three conifer species in a temperate forest in north-east France. Tree Physiol. 2012, 32, 612–625. [Google Scholar] [CrossRef] [Green Version]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef] [Green Version]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Castagneri, D.; Fonti, P.; von Arx, G.; Carrer, M. How does climate influence xylem morphogenesis over the growing season? Insights from long-term intra-ring anatomy in Picea abies. Ann. Bot. 2017, 119, mcw274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Tang, J.; Mustard, J.F.; Lee, J.-E.; Rossini, M.; Joiner, J.; Munger, J.W.; Kornfeld, A.; Richardson, A.D. Solar-induced chlorophyll fluorescence that correlates with canopy photosynthesis on diurnal and seasonal scales in a temperate deciduous forest. Geophys. Res. Lett. 2015, 42, 2977–2987. [Google Scholar] [CrossRef]
- Rathgeber, C.B.K.; Cuny, H.E.; Fonti, P. Biological basis of tree-ring formation: A crash course. Front. Plant Sci. 2016, 7, 734. [Google Scholar] [CrossRef] [Green Version]
- Hansen, J.; Beck, E. Seasonal changes in the utilization and turnover of assimilation products in 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees 1994, 8, 172–182. [Google Scholar] [CrossRef]
- Popkova, M.I.; Vaganov, E.A.; Shishov, V.V.; Babushkina, E.A.; Rossi, S.; Fonti, M.V.; Fonti, P. Modeled tracheidograms disclose drought influence on Pinus sylvestris tree-rings structure from siberian forest-steppe. Front. Plant Sci. 2018, 9, 1144. [Google Scholar] [CrossRef]
- Strand, M.; Löfvenius, M.O.; Bergsten, U.; Lundmark, T.; Rosvall, O. Height growth of planted conifer seedlings in relation to solar radiation and position in Scots pine shelterwood. Forest Ecol. Manag. 2006, 224, 258–265. [Google Scholar] [CrossRef]
- Fonti, M.V.; Vaganov, E.A.; Wirth, C.; Shashkin, A.V.; Astrakhantseva, N.V.; Schulze, E.-D. Age-Effect on Intra-Annual δ13C-Variability within Scots Pine Tree-Rings from Central Siberia. Forests 2018, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Lanner, R.M. Patterns of shoot development in Pinus and their relationship to growth potential. In Tree Physiology and Yield Improvement; Cannell, M.G.R., Last, F.T., Eds.; Academic Press: London, UK, 1976; pp. 223–243. [Google Scholar]
- Hacket-Pain, A.J.; Ascoli, D.; Vacchiano, G.; Biondi, F.; Cavin, L.; Conedera, M.; Drobyshev, I.; Liñán, I.D.; Friend, A.D.; Grabner, M.; et al. Climatically controlled reproduction drives interannual growth variability in a temperate tree species. Ecol. Lett. 2018, 21, 1833–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tierney, G.L.; Fahey, T.J.; Groffman, P.M.; Hardy, J.P.; Fitzhugh, R.D.; Driscoll, C.T. Soil freezing alters fine root dynamics in a northern hardwood forest. Biogeochemistry 2001, 56, 175–190. [Google Scholar] [CrossRef]
- Martin-Benito, D.; Beeckman, H.; Cañellas, I. Influence of drought on tree rings and tracheid features of Pinus nigra and Pinus sylvestris in a mesic Mediterranean forest. Eur. J. For. Res. 2012, 132, 33–45. [Google Scholar] [CrossRef]
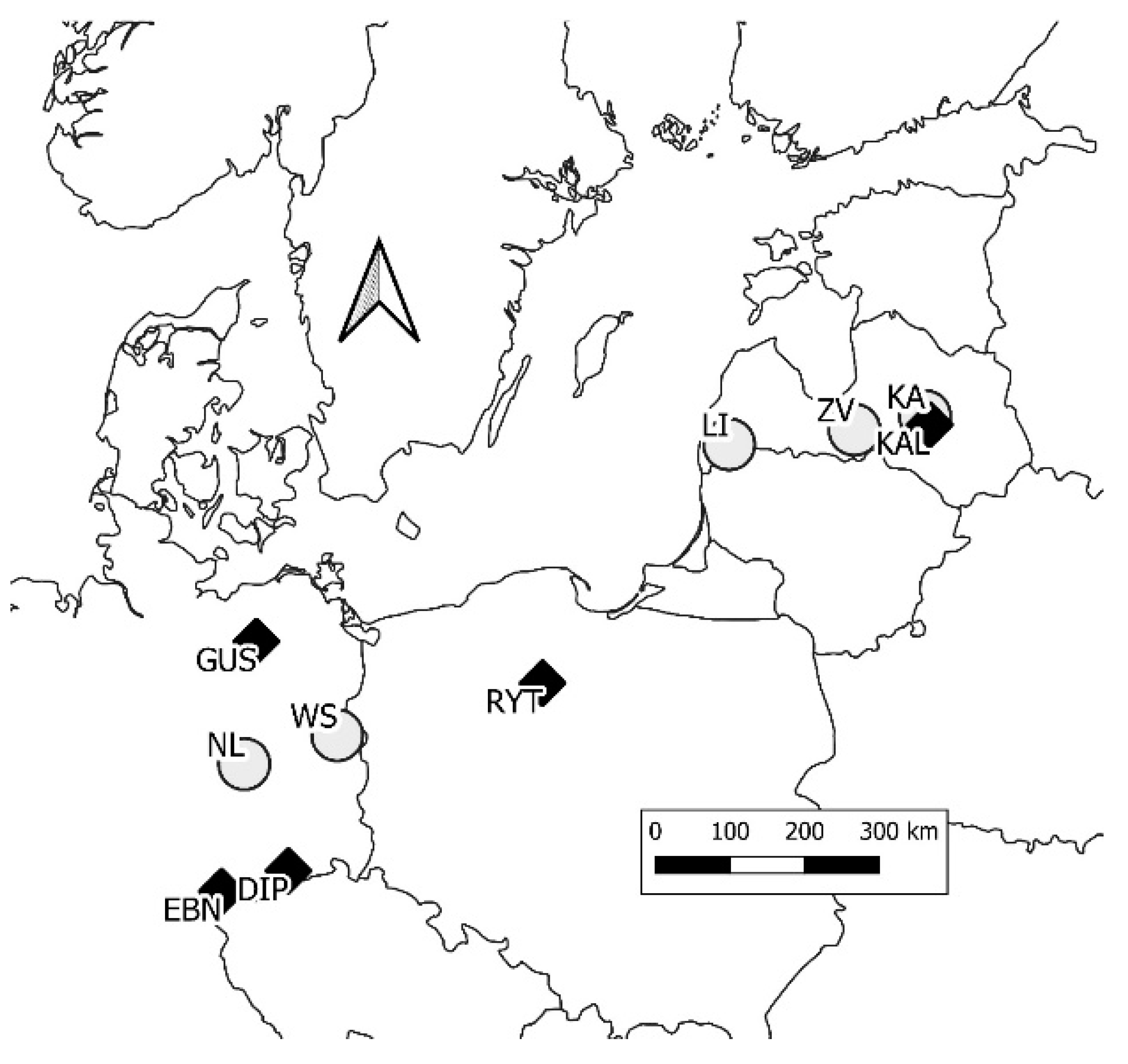



| LI | ZV | KA | WS | NL | |
|---|---|---|---|---|---|
| Vicinity | Liepaja (Latvia) | Zvirgzde (Latvia) | Kalsnava (Latvia) | Waldsieversdorf (Germany) | Nedlitz (Germany) |
| Latitude, °N | 56.45 | 56.65 | 56.8 | 52.53 | 52.02 |
| Longitude, °E | 21.63 | 24.37 | 25.93 | 14.05 | 12.33 |
| Elevation, m a.s.l. | 15 | 50 | 220 | 60 | 115 |
| Annual temperature | 7.5 ± 0.6 | 7.2 ± 0.7 | 6.4 ± 0.7 | 9.2 ± 0.7 | 9.6 ± 0.6 |
| May–September temperature | 15.0 ± 0.7 | 15.2 ± 0.8 | 14.8 ± 0.8 | 16.2 ± 0.7 | 16.6 ± 0.07 |
| January temperature | −1.9 ± 2.4 | −3 ± 2.6 | −4.2 ± 2.7 | 0.1 ± 2.5 | 0.4 ± 2.4 |
| July temperature | 17.8 ± 1.6 | 18.2 ± 1.6 | 17.9 ± 1.6 | 18.7 ± 1.6 | 19.3 ± 1.7 |
| Annual precipitation sum | 789 ± 91 | 659 ± 75 | 689 ± 81 | 568 ± 80 | 662 ± 73 |
| May–September precipitation sum | 353 ± 71 | 333 ± 63 | 349 ± 66 | 290 ± 66 | 294 ± 59 |
| Dippoldiswalde (DIP) | Eibenstock (EBN) | Kalsnava (KAL) | Güstrow (GUS) | Rytel (RYT) | |
|---|---|---|---|---|---|
| Latitude, °N | 50.82 | 50.45 | 56.7 | 53.75 | 53.67 |
| Longitude, °E | 13.87 | 12.43 | 25.9 | 12.25 | 18.02 |
| Elevation, m a.s.l. | 590 | 710 | 190 | 25 | 130 |
| Annual temperature, °C | 6.6 | 6.0 | 6.3 | 8 | 7.9 |
| Temperature May–September, °C | 13.1 | 12.5 | 14.6 | 14.8 | 15 |
| Temperature Jan, °C | −2.5 | −3.1 | −4.3 | −0.9 | −1.4 |
| Temperature Jul, °C | 15.3 | 14.8 | 18.0 | 16.8 | 17.2 |
| Annual precipitation, mm | 809 | 993 | 650 | 586 | 597 |
| Precipitation May–September, mm | 405 | 501 | 340 | 303 | 301 |
| Trial | Provenance | N | TRW | r-bar | GINI | AC1 | SENS | SNR | EPS |
|---|---|---|---|---|---|---|---|---|---|
| LI | DIP | 12 | 2.58 ± 1.57 | 0.44 | 0.34 | 0.79 | 0.24 | 11.36 | 0.92 |
| LI | EBN | 11 | 2.87 ± 1.32 | 0.36 | 0.25 | 0.80 | 0.20 | 7.69 | 0.88 |
| LI | GUS | 11 | 3.30 ± 1.62 | 0.36 | 0.27 | 0.78 | 0.19 | 7.65 | 0.88 |
| LI | KAL | 12 | 3.07 ± 1.45 | 0.33 | 0.25 | 0.78 | 0.19 | 7.28 | 0.88 |
| LI | RYT | 12 | 3.38 ± 1.74 | 0.37 | 0.27 | 0.82 | 0.19 | 8.78 | 0.90 |
| ZV | DIP | 9 | 2.00 ± 1.06 | 0.43 | 0.30 | 0.77 | 0.23 | 9.04 | 0.90 |
| ZV | EBN | 9 | 2.11 ± 1.19 | 0.41 | 0.31 | 0.79 | 0.23 | 8.39 | 0.89 |
| ZV | GUS | 10 | 2.45 ± 1.11 | 0.41 | 0.24 | 0.80 | 0.19 | 8.58 | 0.90 |
| ZV | KAL | 10 | 2.25 ± 1.01 | 0.41 | 0.25 | 0.77 | 0.21 | 9.14 | 0.90 |
| ZV | RYT | 10 | 2.86 ± 1.25 | 0.38 | 0.23 | 0.68 | 0.22 | 7.84 | 0.89 |
| KA | DIP | 10 | 2.02 ± 0.94 | 0.34 | 0.27 | 0.77 | 0.23 | 6.76 | 0.87 |
| KA | EBN | 13 | 2.02 ± 1.02 | 0.39 | 0.28 | 0.78 | 0.24 | 10.26 | 0.91 |
| KA | GUS | 12 | 2.37 ± 0.97 | 0.42 | 0.23 | 0.74 | 0.22 | 10.88 | 0.92 |
| KA | KAL | 14 | 2.36 ± 0.90 | 0.37 | 0.22 | 0.70 | 0.21 | 9.87 | 0.91 |
| KA | RYT | 12 | 2.77 ± 1.00 | 0.44 | 0.20 | 0.75 | 0.19 | 11.50 | 0.92 |
| NL | DIP | 12 | 2.21 ± 0.98 | 0.30 | 0.23 | 0.58 | 0.26 | 6.19 | 0.86 |
| NL | EBN | 13 | 2.09 ± 0.84 | 0.35 | 0.21 | 0.60 | 0.24 | 8.48 | 0.90 |
| NL | GUS | 13 | 2.33 ± 0.89 | 0.37 | 0.20 | 0.59 | 0.25 | 9.24 | 0.90 |
| NL | KAL | 13 | 2.07 ± 0.74 | 0.37 | 0.19 | 0.46 | 0.27 | 9.55 | 0.90 |
| NL | RYT | 15 | 2.55 ± 0.75 | 0.37 | 0.16 | 0.45 | 0.23 | 10.60 | 0.91 |
| WS | DIP | 13 | 2.18 ± 1.22 | 0.46 | 0.27 | 0.52 | 0.30 | 13.43 | 0.93 |
| WS | EBN | 12 | 2.22 ± 1.10 | 0.44 | 0.25 | 0.48 | 0.31 | 11.53 | 0.92 |
| WS | GUS | 15 | 2.35 ± 1.04 | 0.55 | 0.22 | 0.52 | 0.27 | 21.88 | 0.96 |
| WS | KAL | 12 | 2.27 ± 1.04 | 0.53 | 0.24 | 0.49 | 0.30 | 16.76 | 0.94 |
| WS | RYT | 16 | 2.36 ± 0.91 | 0.48 | 0.20 | 0.45 | 0.26 | 17.45 | 0.95 |
| DIP | EBN | KAL | RYT | GUS | |
|---|---|---|---|---|---|
| Fixed effects, effective degree of freedom and F-value | |||||
| Temp. prev. June | 1.00 (30.6) *** | 1.00 (31.2) *** | 1.76 (10.46) *** | 1.78 (7.5) *** | 1.00 (10.45) ** |
| Temp. March | 1.57 (3.1) | 1.00 (5.06) * | 1.00 (3.57) | 1.47 (4.78) * | 1.00 (7.28) ** |
| Temp. May | 1.89 (9.29) ** | 1.94 (14.28) *** | 1.94 (17.44) *** | 1.93 (11.23) *** | 1.96 (20.94) *** |
| Temp. July | 1.35 (10.02) *** | 1.91 (8.34) *** | 1.62 (5.30) ** | 1.89 (10.03) ** | 1.96 (16.08) *** |
| Prec. prev. August | 1.27 (20.56) *** | 1.63 (8.45) ** | 1.00 (10.41) ** | 1.80 (13.59) *** | 1.00 (18.89) *** |
| Prec. prev. December | 1.92 (7.34) ** | 1.11 (1.32) | 1.85 (9.49) ** | 1.62 (6.37) * | 1.89 (8.02) ** |
| SPEI. May | 1.90 (12.64) *** | 1.28 (9.36) ** | 1.00 (15.16) *** | 1.66 (38.78) *** | 1.00 (29.02) *** |
| SPEI. June | 1.69 (1.69) | 1.87 (3.38) | 1.92 (6.99) ** | 1.93 (12.99) *** | 1.94 (19.08) *** |
| SPEI. July | 1.51 (8.09) * | 1.00 (13.5) *** | 1.00 (10.39) ** | 1.00 (31.23) *** | 1.66 (21.33) *** |
| Random effects, variance | |||||
| Year | 2.03 × 10−3 | 1.00 × 10−6 | 3.24 × 10−4 | 1.21 × 10−4 | 6.25 × 10−4 |
| Trial | 7.94 × 10−3 | 1.02 × 10−2 | 8.10 × 10−3 | 7.06 × 10−3 | 7.92 × 10−3 |
| Replication | 1.00 × 10−6 | 1.00 × 10−6 | 1.00 × 10−6 | 1.00 × 10−6 | 1.00 × 10−6 |
| Tree | 3.39 × 10−2 | 2.92 × 10−2 | 3.88 × 10−2 | 2.13 × 10−2 | 2.31 × 10−2 |
| Residual | 3.60 × 10−5 | 1.21 × 10−4 | 2.50 × 10−5 | 4.90 × 10−5 | 4.00 × 10−6 |
| Model performance | |||||
| Adjusted R2 | 0.28 | 0.24 | 0.21 | 0.28 | 0.30 |
| RMSE | 0.21 | 0.19 | 0.21 | 0.17 | 0.18 |
| RMSE (verification) | 0.19 | 0.18 | 0.18 | 0.17 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matisons, R.; Jansone, D.; Bāders, E.; Dubra, S.; Zeltiņš, P.; Schneck, V.; Jansons, Ā. Weather–Growth Responses Show Differing Adaptability of Scots Pine Provenances in the South-Eastern Parts of Baltic Sea Region. Forests 2021, 12, 1641. https://doi.org/10.3390/f12121641
Matisons R, Jansone D, Bāders E, Dubra S, Zeltiņš P, Schneck V, Jansons Ā. Weather–Growth Responses Show Differing Adaptability of Scots Pine Provenances in the South-Eastern Parts of Baltic Sea Region. Forests. 2021; 12(12):1641. https://doi.org/10.3390/f12121641
Chicago/Turabian StyleMatisons, Roberts, Diāna Jansone, Endijs Bāders, Stefānija Dubra, Pauls Zeltiņš, Volker Schneck, and Āris Jansons. 2021. "Weather–Growth Responses Show Differing Adaptability of Scots Pine Provenances in the South-Eastern Parts of Baltic Sea Region" Forests 12, no. 12: 1641. https://doi.org/10.3390/f12121641
APA StyleMatisons, R., Jansone, D., Bāders, E., Dubra, S., Zeltiņš, P., Schneck, V., & Jansons, Ā. (2021). Weather–Growth Responses Show Differing Adaptability of Scots Pine Provenances in the South-Eastern Parts of Baltic Sea Region. Forests, 12(12), 1641. https://doi.org/10.3390/f12121641








