The Root Mycobiota of Betula aetnensis Raf., an Endemic Tree Species Colonizing the Lavas of Mt. Etna (Italy)
Abstract
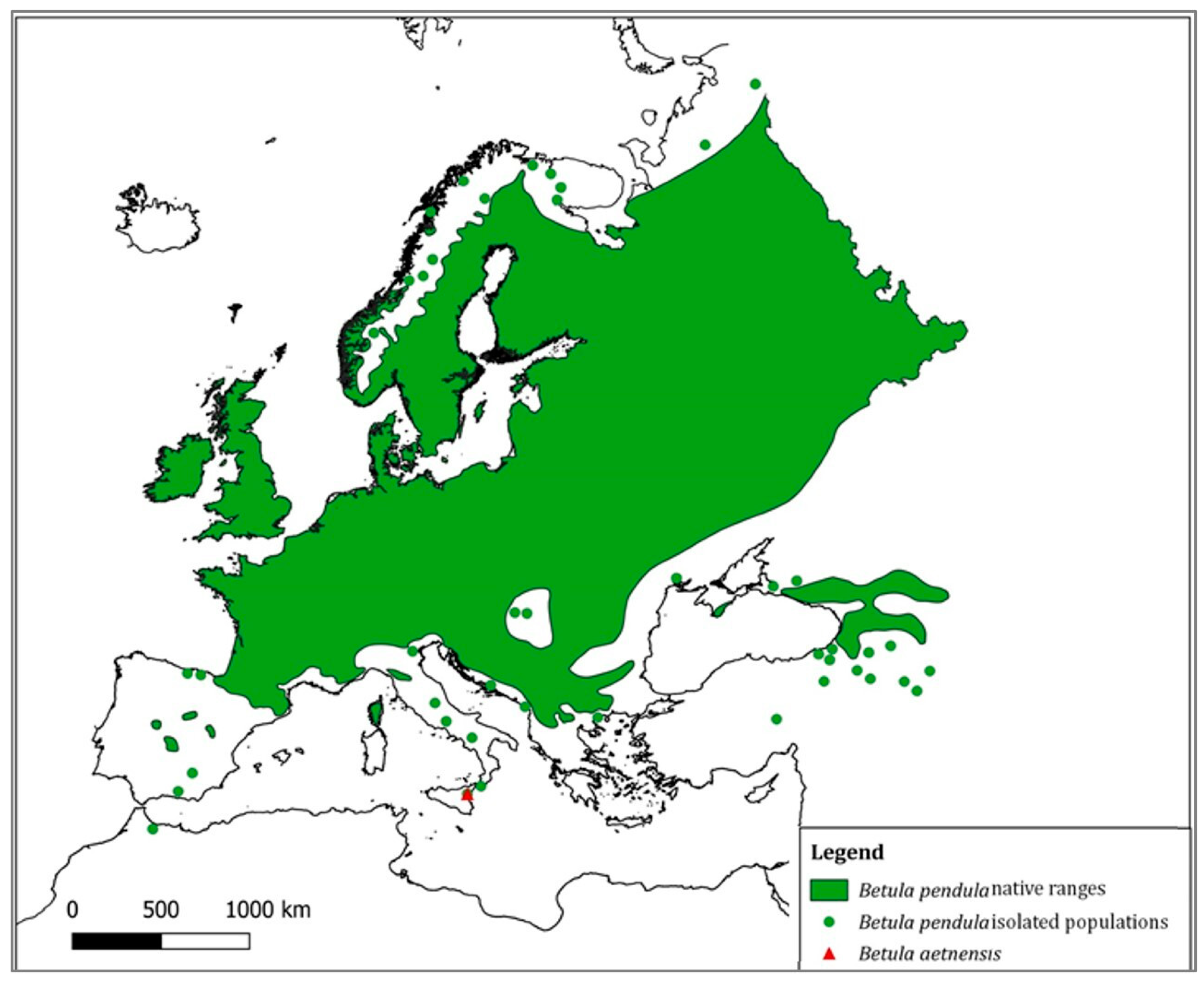
:1. Introduction
2. Materials and Methods
2.1. Collection Sites and Plant Traits
2.2. Soil Analysis
2.3. Ectomycorrhizal Colonization
2.4. Arbuscular Mycorrhizal and Endophytic Colonization
2.5. DNA Extraction
2.6. Automated Ribosomal Intergenic Spacer Analysis (ARISA)
2.7. PCR-DGGE of Fungal Endophytes
2.8. Illumina MiSeq Sequencing and Data Analysis
3. Results
3.1. Plant Traits and Substrate Characteristics
3.2. Ectomycorrhizal Structures
3.3. Arbuscular Mycorrhizal and Endophytic Structures
3.4. Bacterial and Fungal Diversity
3.5. Diversity of Fungal Root Endophytes
3.6. Identification of B. aetnensis Root Mycobiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- La Mantia, T.; Pasta, S. The Sicilian phanerophytes: Still a noteworthy patrimony, soon a lost resource? IUFRO Conference 15 November 2003, Firenze “Monitoring and indicators of forest biodiversity in Europe—From ideas to operationality”. EFI Proc. 2005, 51, 515–526. [Google Scholar]
- Brullo, C.; Brullo, S.; Del Galdo, G.G.; Guarino, R.; Siracusa, G.; Sciandrello, S. The class Querco-Fagetea sylvaticae in Sicily: An example of boreo-temperate vegetation in the central Mediterranean region. Ann. Bot. 2012, 2, 19–38. [Google Scholar] [CrossRef]
- Beck, P.; Tinner, W.; Caudullo, G.; De Rigo, D. Betula pendula, Betula pubescens and other birches in Europe: Distribution, habitat, usage and threats. Eur. Atlas For. Tree Species 2016, 70–73. [Google Scholar] [CrossRef]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia; Edagricole: Bologna, Italy, 2017; Volume 1, ISBN 8850652429. [Google Scholar]
- Leonardi, S.; Rapp, M.; Failla, M.; Komaromy, E. Organic matter and nutrient cycling within an endemic birch stand in the Etna massif (Sicily): Betula aetnensis Rafin. Vegetatio 1994, 111, 45–57. [Google Scholar]
- Řehounková, K.; Lencová, K.; Prach, K. Spontaneous establishment of woodland during succession in a variety of central European disturbed sites. Ecol. Eng. 2018, 111, 94–99. [Google Scholar] [CrossRef]
- Kompała-Bąba, A.; Bierza, W.; Błońska, A.; Sierka, E.; Magurno, F.; Chmura, D.; Besenyei, L.; Radosz, Ł.; Woźniak, G. Vegetation diversity on coal mine spoil heaps–how important is the texture of the soil substrate? Biologia (Bratisl) 2019, 74, 419–436. [Google Scholar] [CrossRef] [Green Version]
- Bierza, W.; Bierza, K.; Trzebny, A.; Greń, I.; Dabert, M.; Ciepał, R.; Trocha, L.K. The communities of ectomycorrhizal fungal species associated with Betula pendula R oth and Pinus sylvestris L. growing in heavy-metal contaminated soils. Plant Soil 2020, 457, 321–338. [Google Scholar] [CrossRef]
- De Luca, D.; Paino, L.; Del Guacchio, E. The genetic structure of silver birch (Betula pendula Roth) in Campania (southern Italy). Delpinoa 2016–2017, 58–59, 41–53. Available online: http://www.biologiavegetale.unina.it/delpinoa_files/58-59_41-53.pdf. (accessed on 17 November 2021).
- Sidoti, A.; Lione, G.; Gugliemo, F.; Giordano, L.; Pasotti, L.G.P. Indagini preliminari su struttura e distribuzione delle popolazioni di Armillaria mellea e Heterobasidion annosum associate a piante deperienti di Betula aetnensis in Sicilia. Micol. Ital. 2013, 42, 68–72. [Google Scholar]
- Napoli, M. Ricerche micocenologiche in betuleti dell’Etna. Micol. Veg. Mediterr. 1993, 8, 113–124. [Google Scholar]
- Smith, S.; Read, D. Mycorrhizal Symbiosis; Academic Press: London, UK, 2008. [Google Scholar]
- Terhonen, E.; Blumenstein, K.; Kovalchuk, A.; Asiegbu, F.O. Forest Tree Microbiomes and Associated Fungal Endophytes: Functional Roles and Impact on Forest Health. Forests 2019, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Badalamenti, E.; La Mantia, T.; Quatrini, P. Arbuscular mycorrhizal fungi positively affect growth of Ailanthus altissima (Mill.) Swingle seedlings and show a strong association with this invasive species in Mediterranean woodlands. J. Torrey Bot. Soc. 2015, 142, 127–139. [Google Scholar] [CrossRef]
- Wang, Z.; Johnston, P.R.; Takamatsu, S.; Spatafora, J.W.; Hibbett, D.S. Toward a phylogenetic classificationof the Leotiomycetes based on rDNA data. Mycologia 2006, 98, 1066–1076. [Google Scholar] [CrossRef]
- Burke, D.J.; Dunham, S.M.; Kretzer, A.M. Molecular analysis of bacterial communities associated with the roots of Douglas fir (Pseudotsuga menziesii) colonized by different ectomycorrhizal fungi. FEMS Microbiol. Ecol. 2008, 65, 299–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mucha, J.; Peay, K.G.; Smith, D.P.; Reich, P.; Stefański, A.; Hobbie, S.E. Effect of Simulated Climate Warming on the Ectomycorrhizal Fungal Community of Boreal and Temperate Host Species Growing Near Their Shared Ecotonal Range Limits. Microb. Ecol. 2017, 75, 348–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pautasso, M.; Schlegel, M.; Holdenrieder, O. Forest Health in a Changing World. Microb. Ecol. 2014, 69, 826–842. [Google Scholar] [CrossRef] [PubMed]
- Klironomos, J.N. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 2002, 417, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Bever, J.D.; Platt, T.G.; Morton, E.R. Microbial Population and Community Dynamics on Plant Roots and Their Feedbacks on Plant Communities. Annu. Rev. Microbiol. 2012, 66, 265–283. [Google Scholar] [CrossRef] [Green Version]
- Bever, J.D. Soil community feedback and the coexistence of competitors: Conceptual frameworks and empirical tests. New Phytol. 2003, 157, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Callaway, R.M.; Thelen, G.C.; Rodriguez, A.; Holben, W.E. Soil biota and exotic plant invasion. Nature 2004, 427, 731–733. [Google Scholar] [CrossRef]
- Kolaříková, Z.; Kohout, P.; Krüger, C.; Janoušková, M.; Mrnka, L.; Rydlová, J. Root-associated fungal communities along a primary succession on a mine spoil: Distinct ecological guilds assemble differently. Soil Biol. Biochem. 2017, 113, 143–152. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kouch, J.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un système radiculaire: Recherche de méthodes d’estimation ayant une signification fonctionnelle. In Les Mycorhizes: Physiologie et Génétique, 1er Séminaire Européen sur les Mycorhizes; Gianinazzi, S., Ed.; Dijon INRA: Dijon, France, 1986; pp. 217–221. [Google Scholar]
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; University Arizona Press: Tucson, AZ, USA, 1996. [Google Scholar]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Badalamenti, E.; Gristina, L.; Laudicina, V.A.; Novara, A.; Pasta, S.; La Mantia, T. The impact of Carpobrotus cfr. acinaciformis (L.) L. Bolus on soil nutrients, microbial communities structure and native plant communities in Mediterranean ecosystems. Plant Soil 2016, 409, 19–34. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture: Washington, DC, USA, 1954.
- Mehlich, A. Use of Triethanolamine Acetate-Barium Hydroxide Buffer for the Determination of Some Base Exchange Properties and Lime Requirement of Soil. Soil Sci. Soc. Am. J. 1939, 3, 162–166. [Google Scholar] [CrossRef]
- Agerer, R. Fungal relationships and structural identity of their ectomycorrhizae. Mycol. Prog. 2006, 5, 67–107. [Google Scholar] [CrossRef]
- Brundrett, M.; Bougher, N.; Dell, B.; Grove, T.; Malajczuk, N. Working with Mycorrhizas in Forestry and Agriculture. Ann. Bot. 1996, 102, 374. [Google Scholar] [CrossRef]
- Brundrett, M.C. Mycorrhizal associations and other means of nutrition of vascular plants: Understnding global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Barillot, C.D.C.; Sarde, C.-O.; Bert, V.; Tarnaud, E.; Cochet, N. A standardized method for the sampling of rhizosphere and rhizoplan soil bacteria associated to a herbaceous root system. Ann. Microbiol. 2012, 63, 471–476. [Google Scholar] [CrossRef]
- Cardinale, M.; Brusetti, L.; Quatrini, P.; Borin, S.; Puglia, A.M.; Rizzi, A.; Sorlini, C.; Corselli, C.; Zanardini, E.; Daffonchio, D. Desenvolvimento de Matrizes Tridimensionais Poliméricas para Aplicação em Engenharia de Tecido Ósseo. Appl. Environ. Microbiol. 2004, 70, 6147–6156. [Google Scholar] [CrossRef] [Green Version]
- Ranjard, L.; Poly, F.; Lata, J.-C.; Mougel, C.; Thioulouse, J.; Nazaret, S. Characterization of Bacterial and Fungal Soil Communities by Automated Ribosomal Intergenic Spacer Analysis Fingerprints: Biological and Methodological Variability. Appl. Environ. Microbiol. 2001, 67, 4479–4487. [Google Scholar] [CrossRef] [Green Version]
- La Marca, E.C.; Catania, V.; Tagliavia, M.; Mannino, A.M.; Chemello, R.; Quatrini, P. Temporal dynamic of biofilms enhances the settlement of the central-Mediterranean reef-builder Dendropoma cristatum (Biondi, 1859). Mar. Environ. Res. 2021, 172, 105484. [Google Scholar] [CrossRef] [PubMed]
- Schuelke, M. An economic method for the fluorescent labeling of PCR fragments. Nat. Biotechnol. 2000, 18, 233–234. [Google Scholar] [CrossRef]
- Novara, A.; Catania, V.; Tolone, M.; Gristina, L.; Laudicina, V.A.; Quatrini, P. Cover Crop Impact on Soil Organic Carbon, Nitrogen Dynamics and Microbial Diversity in a Mediterranean Semiarid Vineyard. Sustainability 2020, 12, 3256. [Google Scholar] [CrossRef] [Green Version]
- Anderson, I.C.; Campbell, C.D.; Prosser, J.I. Diversity of fungi in organic soils under a moorland—Scots pine (Pinus sylvestris L.) gradient. Environ. Microbiol. 2003, 5, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.D.; Lee, S.; Taylor, J. Analysis of phylogenetic relationships by amplification and direct sequencing of ribosomal RNA genes. PCR Protoc. A Guid. Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [Green Version]
- Simon, L.; Lalonde, M.; Bruns, T.D. Specific amplification of 18S fungal ribosomal genes from vesicular-arbuscular mycorrhizal fungal communities. Appl. Environ. Microbiol. 1992, 58, 291–295. [Google Scholar] [CrossRef] [Green Version]
- Yergeau, E.; Vujanovic, V.; St-Arnaud, M. Changes in Communities of Fusarium and Arbuscular Mycorrhizal Fungi as Related to Different Asparagus Cultural Factors. Microb. Ecol. 2006, 52, 104–113. [Google Scholar] [CrossRef]
- Helgason, T.; Daniell, T.J.; Husband, R.; Fitter, A.H.; Young, J.P.W. Ploughing up the wood-wide web? Nature 1998, 394, 431. [Google Scholar] [CrossRef]
- Kowalchuk, G.A.; De Souza, F.A.; Van Veen, J.A. Community analysis of arbuscular mycorrhizal fungi associated with Ammophila arenaria in Dutch coastal sand dunes. Mol. Ecol. 2002, 11, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.; Finlay, R.D.; Tehler, A. Molecular analysis of arbuscular mycorrhizal fungi colonising a semi-natural grassland along a fertilisation gradient. New Phytol. 2006, 172, 159–168. [Google Scholar] [CrossRef]
- Catania, V.; Cappello, S.; Di Giorgi, V.; Santisi, S.; Di Maria, R.; Mazzola, A.; Vizzini, S.; Quatrini, P. Microbial communities of polluted sub-surface marine sediments. Mar. Pollut. Bull. 2018, 131, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, L.; Dahlberg, A.; Nilsson, M.; Zackrisson, O.; Kårén, O. Ectomycorrhizal fungal communities in late-successional Swedish boreal forests, and their composition following wildfire. Mol. Ecol. 2003, 8, 205–215. [Google Scholar] [CrossRef]
- Ingleby, K.; Mason, P.A.; Last, F.T. Identification of Ectomycorrhizas; HMSO: London, UK, 1990.
- James, P.; Chester, D.; Duncan, A.M. Development and spatial distribution of soils on an active volcano: Mt Etna, Sicily. CATENA 2016, 137, 277–297. [Google Scholar] [CrossRef]
- Jones, T.E. Evolving approaches to volcanic tourism crisis management: An investigation of long-term recovery models at Toya-Usu Geopark. J. Hosp. Tour. Manag. 2016, 28, 31–40. [Google Scholar] [CrossRef]
- Hopkins, D.W.; Badalucco, L.; English, L.C.; Meli, S.M.; Chudek, J.A.; Ioppolo, A. Plant litter decomposition and microbial characteristics in volcanic soils (Mt Etna, Sicily) at different stages of development. Biol. Fertil. Soils 2006, 43, 461–469. [Google Scholar] [CrossRef]
- D’Antone, C.; Punturo, R.; Vaccaro, C. Rare earth elements distribution in grapevine varieties grown on volcanic soils: An example from Mount Etna (Sicily, Italy). Environ. Monit. Assess. 2017, 189, 1914. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, Y.-L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef]
- Dhillion, S.S. Ectomycorrhizae, arbuscular mycorrhizae, and Rhizoctonia sp. of alpine and boreal Salix spp. in Norway. Arct. Alp. Res. 1994, 26, 304–307. [Google Scholar] [CrossRef]
- Van der Heijden, E.W. Differential benefits of arbuscular mycorrhizal and ectomycorrhizal infection of Salix repens. Mycorrhiza 2001, 10, 185–193. [Google Scholar] [CrossRef]
- Chilvers, G.A.; Lapeyrie, F.F.; Horan, D.P. Ectomycorrhizal vs Endomycorrhizal Fungi within the Same Root System. New Phytol. 1987, 107, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, L.M.; Nilsson, M.-C.; Wardle, D.A.; Zackrisson, O. Context dependent effects of ectomycorrhizal species richness on tree seedling productivity. Oikos 2001, 93, 353–364. [Google Scholar] [CrossRef]
- Neilson, J.W.; Jordan, F.L.; Maier, R.M. Analysis of artifacts suggests DGGE should not be used for quantitative diversity analysis. J. Microbiol. Methods 2013, 92, 256–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amend, A. From Dandruff to Deep-Sea Vents: Malassezia-like Fungi Are Ecologically Hyper-diverse. PLoS Pathog. 2014, 10, e1004277. [Google Scholar] [CrossRef] [Green Version]
- Jumpponen, A. Dark septate endophytes—Are they mycorrhizal? Mycorrhiza 2001, 11, 207–211. [Google Scholar] [CrossRef]
- Hassan, S.E.D.; Boon, E.; St-Arnaud, M.; Hijri, M. Molecular biodiversity of arbuscular mycorrhizal fungi in trace metal-polluted soils. Mol. Ecol. 2011, 20, 3469–3483. [Google Scholar] [CrossRef]
- Bellemain, E.; Carlsen, T.; Brochmann, C.; Coissac, E.; Taberlet, P.; Kauserud, H. ITS as an environmental DNA barcode for fungi: An in silico approach reveals potential PCR biases. BMC Microbiol. 2010, 10, 189. [Google Scholar] [CrossRef] [Green Version]
- Dasila, K.; Pandey, A.; Samant, S.S.; Pande, V. Endophytes associated with Himalayan silver birch (Betula utilis D. Don) roots in relation to season and soil parameters. Appl. Soil Ecol. 2020, 149, 103513. [Google Scholar] [CrossRef]
- Kříbek, B.; Míková, J.; Knésl, I.; Mihaljevič, M.; Sýkorová, I. Uptake of trace elements and isotope fractionation of Cu and Zn by birch (Betula pendula) growing on mineralized coal waste pile. Appl. Geochem. 2020, 122, 104741. [Google Scholar] [CrossRef]
- Vrålstad, T. Are ericoid and ectomycorrhizal fungi part of a common guild? New Phytol. 2004, 164, 7–10. [Google Scholar] [CrossRef]
- Perotto, S.; Daghino, S.; Martino, E. Ericoid mycorrhizal fungi and their genomes: Another side to the mycorrhizal symbiosis? New Phytol. 2018, 220, 1141–1147. [Google Scholar] [CrossRef] [Green Version]
- Abuzinadah, R.A.; Read, D.J. The role of proteins in the nitrogen nutrition of ectomycorrhizal plants: V. Nitrogen transfer in birch (Betula pendula) grown in association with mycorrhizal and non-mycorrhizal fungi. New Phytol. 1989, 112, 61–68. [Google Scholar] [CrossRef]
- Qian, X.; El-Ashker, A.; Kottke, I.; Oberwinkler, F. Studies of pathogenic and antagonistic microfungal populations and their potential interactions in the mycorrhizoplane of Norway spruce (Picea abies (L.) Karst.) and beech (Fagus sylvatica L.) on acidified and limed plots. Plant Soil 1998, 199, 111–116. [Google Scholar] [CrossRef]
- Grelet, G.; Johnson, D.; Paterson, E.; Anderson, I.C.; Alexander, I.J. Reciprocal carbon and nitrogen transfer between an ericaceous dwarf shrub and fungi isolated from Piceirhiza bicolorata ectomycorrhizas. New Phytol. 2009, 182, 359–366. [Google Scholar] [CrossRef]
- Selosse, M.; Vohník, M.; Chauvet, E. Out of the rivers: Are some aquatic hyphomycetes plant endophytes? New Phytol. 2008, 178, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Lukešová, T.; Kohout, P.; Větrovský, T.; Vohník, M. The Potential of Dark Septate Endophytes to Form Root Symbioses with Ectomycorrhizal and Ericoid Mycorrhizal Middle European Forest Plants. PLoS ONE 2015, 10, e0124752. [Google Scholar] [CrossRef] [Green Version]
- Galitskaya, P.; Biktasheva, L.; Blagodatsky, S.; Selivanovskaya, S. Response of bacterial and fungal communities to high petroleum pollution in different soils. Sci. Rep. 2021, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Rho, H.; Hsieh, M.; Kandel, S.L.; Cantillo, J.; Doty, S.L.; Kim, S.-H. Do Endophytes Promote Growth of Host Plants Under Stress? A Meta-Analysis on Plant Stress Mitigation by Endophytes. Microb. Ecol. 2017, 75, 407–418. [Google Scholar] [CrossRef]
- Caldwell, B.A.; Jumpponen, A.; Trappe, J.M. Utilization of Major Detrital Substrates by Dark-Septate, Root Endophytes. Mycologia 2000, 92, 230. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Sato, H.; Tanabe, A.; Hidaka, A.; Kadowaki, K.; Toju, H. Spatial Segregation and Aggregation of Ectomycorrhizal and Root-Endophytic Fungi in the Seedlings of Two Quercus Species. PLoS ONE 2014, 9, e96363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toju, H.; Sato, H. Root-Associated Fungi Shared Between Arbuscular Mycorrhizal and Ectomycorrhizal Conifers in a Temperate Forest. Front. Microbiol. 2018, 9, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterflinger, K.; Tesei, D.; Zakharova, K. Fungi in hot and cold deserts with particular reference to microcolonial fungi. Fungal Ecol. 2012, 5, 453–462. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological Control of Plant-Parasitic Nematodes by Filamentous Fungi Inducers of Resistance: Trichoderma, Mycorrhizal and Endophytic Fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]





| Sample Code | Height (cm) | Basal Diameter (cm) | Age (Years) | Ectomycorrhizal Colonization | Arbuscular Mycorrhizal Colonization | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed Root Tips (N) | Observed Root Length (cm) | Colonization (%) | F% | M% | m% | A% | a% | ||||
| NAT1 | 39 | 0.40 | 8 | 2.730 | 217.0 | 68.4 | 94.4 | 54.0 | 55.9 | 15.0 | 27.8 |
| NAT2 | 50 | 0.55 | 12 | 1.077 | 115.0 | 81.0 | 95.7 | 46.3 | 48.5 | 7.35 | 15.8 |
| NURS1 | 37 | 0.47 | 1 | 3.534 | 275.3 | 93.1 | 97.2 | 51.8 | 53.7 | 11.2 | 21.6 |
| NURS2 | 37 | 0.63 | 1 | 4.081 | 404.3 | 95.3 | 95.8 | 55.6 | 57.4 | 12.2 | 21.9 |
| NURS3 | 36 | 0.63 | 1 | 5.496 | 650.0 | 94.3 | 98.7 | 57.0 | 58.8 | 11.4 | 20.0 |
| Sample | Band n° | Sequence Length (bp) | Division | Class | Order | Putative Ecology | Closest Sequence Match | Similarity (%) | Accession Number |
|---|---|---|---|---|---|---|---|---|---|
| NAT1 | 1 | 142 | Ascomycota | Leotiomycetes | Helotiales | DSE, ERM, SAP | Uncultured Phialocephala clone otu19_mt2015 | 98% | AB354287.1 |
| 3 | 259 | Uncultured Phialocephala | 99% | HF947843.1 | |||||
| 4 | 242 | Phialocephala helvetica isolate RSF_Q104 | 95% | EU103612.1 | |||||
| 6 | 169 | Pezizomycetes | Pezizales | DSE | Uncultured Tricharina isolate DGGE gel band ZA4 | 94% | KM200057.1 | ||
| 5 | 240 | Basidiomycota | Exobasidiomycetes | Malasseziales | DSE | Uncultured Malassezia clone TS1-9013 18S | 95% | KC525787.1 | |
| NAT2 | 9 | 177 | Ascomycota | Leotiomycetes | Helotiales | DSE, ERM, SAP | Phialocephala fortinii isolate FFP810 18S | 97% | JQ711957.1 |
| 10 | 239 | Uncultured Phialocephala clone WD_S2_8_55a_1 1 | 95% | JX630399.1 | |||||
| 11 | 243 | Uncultured Helotiales clone AhedenL36 | 98% | FJ475791.1 | |||||
| 13 | 148 | Phialocephala fortinii isolate m14 | 98% | MH931279.1 | |||||
| NURS1+2+3 | 15 | 177 | Ascomycota | Leotiomycetes | Helotiales | DSE, ERM, SAP | Phialocephala fortinii isolate m14 | 98% | MH931279.1 |
| 16 | 258 | Phialocephala fortinii isolate m14 | 97% | MH931279.1 | |||||
| 20 | 257 | Pezizomycetes | Pezizales | DSE | Uncultured Tricharina isolate DGGE gel band ZA4 | 96% | KM200057.1 |
| Index | Sample | |||
|---|---|---|---|---|
| NAT1R | NAT2R | NAT1RS | NAT2RS | |
| Taxa_S | 7 | 15 | 9 | 12 |
| Simpson_1-D | 0.686 | 0.8706 | 0.6042 | 0.8407 |
| Shannon_H | 1.295 | 2.342 | 1.269 | 2.043 |
| Evenness_e^H/S | 0.5217 | 0.6933 | 0.3953 | 0.6428 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badalamenti, E.; Catania, V.; Sofia, S.; Sardina, M.T.; Sala, G.; La Mantia, T.; Quatrini, P. The Root Mycobiota of Betula aetnensis Raf., an Endemic Tree Species Colonizing the Lavas of Mt. Etna (Italy). Forests 2021, 12, 1624. https://doi.org/10.3390/f12121624
Badalamenti E, Catania V, Sofia S, Sardina MT, Sala G, La Mantia T, Quatrini P. The Root Mycobiota of Betula aetnensis Raf., an Endemic Tree Species Colonizing the Lavas of Mt. Etna (Italy). Forests. 2021; 12(12):1624. https://doi.org/10.3390/f12121624
Chicago/Turabian StyleBadalamenti, Emilio, Valentina Catania, Serena Sofia, Maria Teresa Sardina, Giovanna Sala, Tommaso La Mantia, and Paola Quatrini. 2021. "The Root Mycobiota of Betula aetnensis Raf., an Endemic Tree Species Colonizing the Lavas of Mt. Etna (Italy)" Forests 12, no. 12: 1624. https://doi.org/10.3390/f12121624
APA StyleBadalamenti, E., Catania, V., Sofia, S., Sardina, M. T., Sala, G., La Mantia, T., & Quatrini, P. (2021). The Root Mycobiota of Betula aetnensis Raf., an Endemic Tree Species Colonizing the Lavas of Mt. Etna (Italy). Forests, 12(12), 1624. https://doi.org/10.3390/f12121624








