Detecting Heterobasidion irregulare in Minnesota and Assessment of Indigenous Fungi on Pines
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Surveys
2.2. qPCR Analysis
2.3. Culture Methods
2.4. Spore Surveys
3. Results
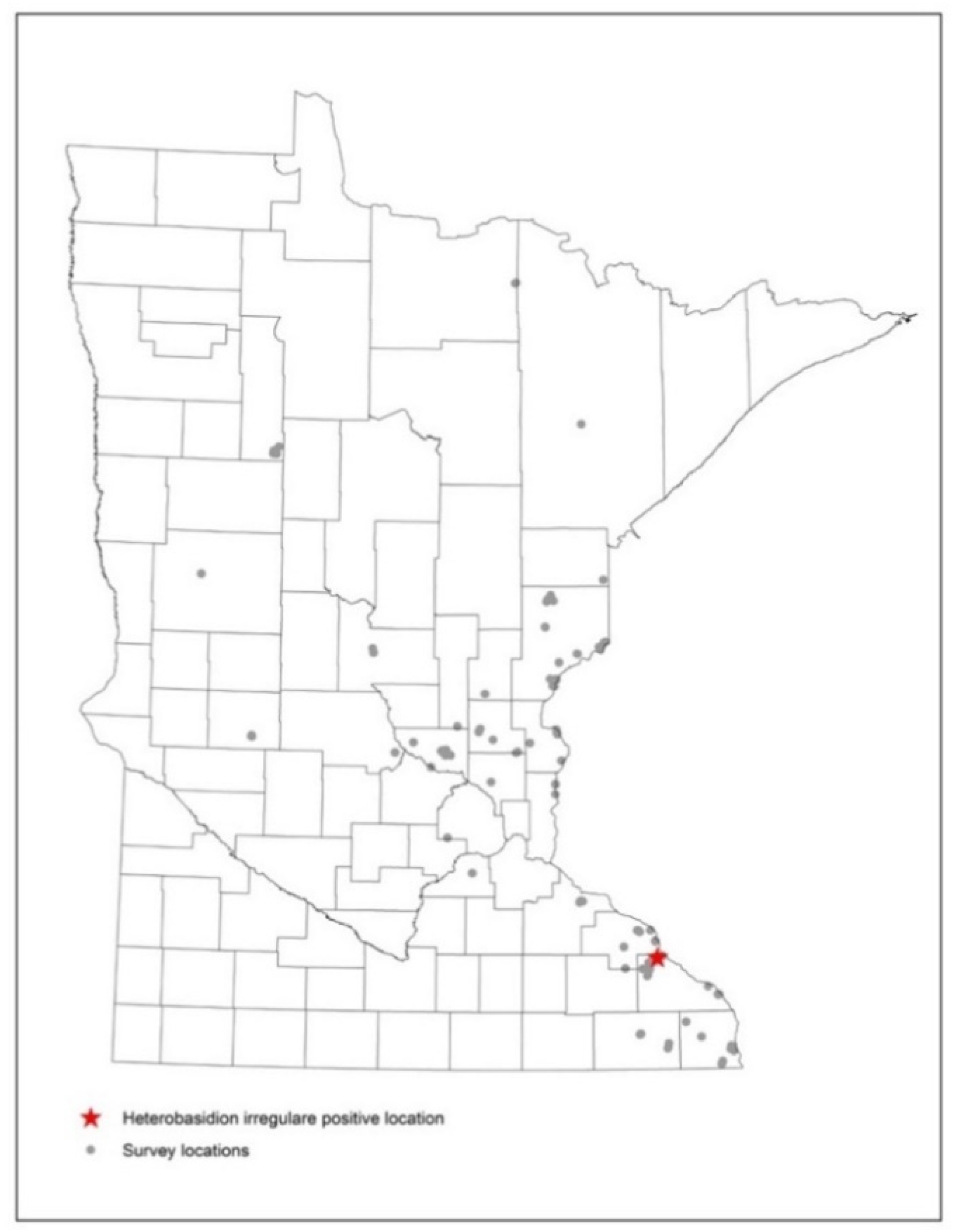
3.1. Field Surveys
3.2. Spore Surveys
4. Discussion
4.1. Field Surveys
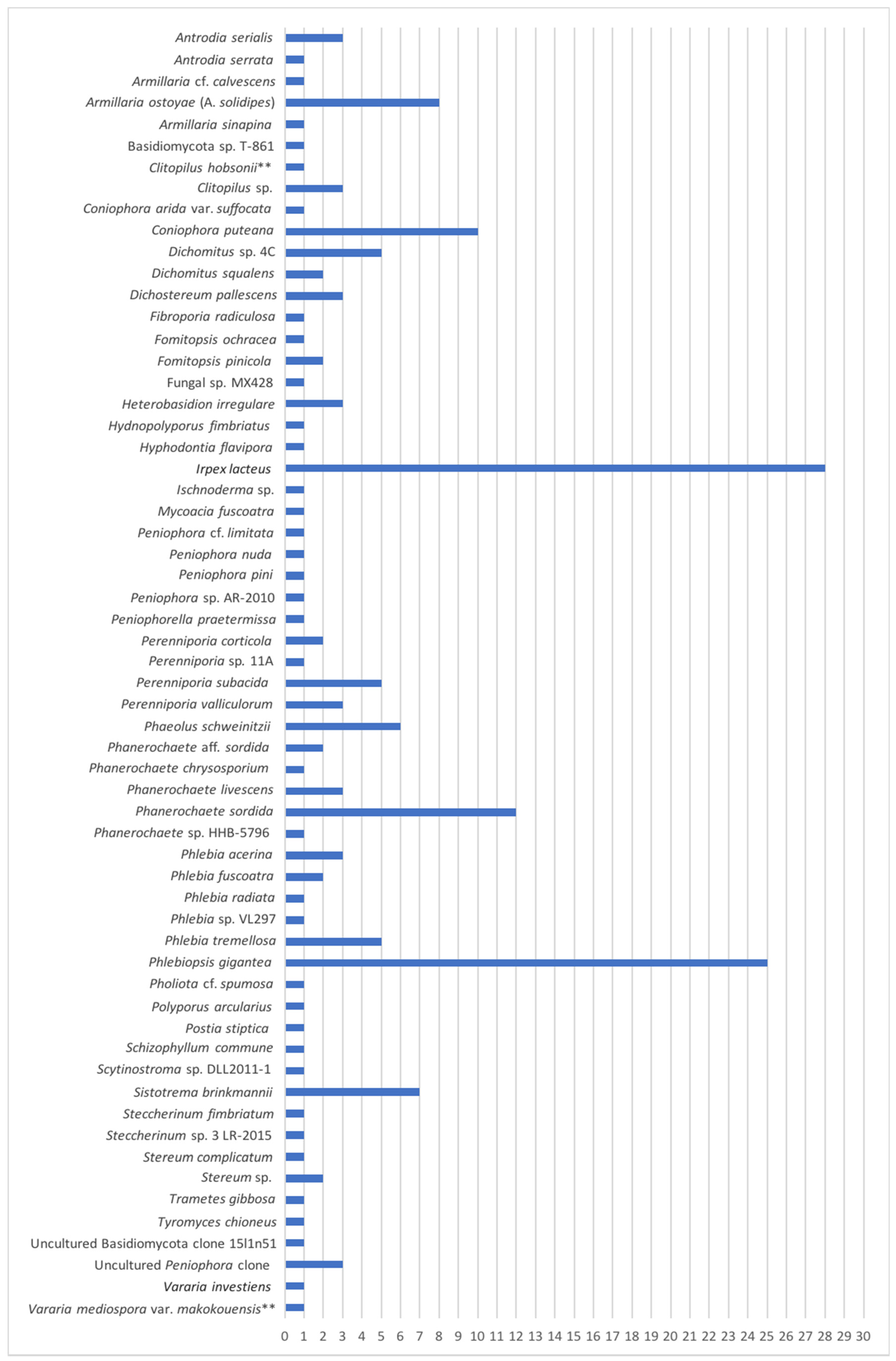
4.2. Basidiomycota Isolated
4.3. Spore Surveys
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filip, G.M.; Morrison, D.J. North America. In Heterobasidion annosum: Biology, Ecology, Impact and Control; CABI: Wallingford, UK, 1998; pp. 405–427. [Google Scholar]
- Rishbeth, J. Observations on the biology of Fomes annosus, with particular reference to East Anglian pine plantations. Ann. Bot. 1951, 15, 1–22. [Google Scholar] [CrossRef]
- Korhonen, K.; Stenlid, J. Biology of Heterobasidion annosum. In Heterobasidion annosum: Biology, Ecology, Impact and Control; CABI: Wallingford, UK, 1998; pp. 43–70. [Google Scholar]
- Stenlid, J.; Redfern, D.B. Spread within the tree and stand. In Heterobasidion annosum: Biology, Ecology, Impact and Control; CABI: Wallingford, UK, 1998; pp. 125–141. [Google Scholar]
- Stanosz, G.R.; Smith, D.R.; Juzwik, J. Seasonal availability of the Heterobasidion root disease pathogen in central Wisconsin. Can. J. For. Res. 2016, 46, 1076–1080. [Google Scholar] [CrossRef]
- Myers, L.J.; Smith, D.R.; Stanosz, G.R. Survival of Heterobasidion irregulare on red pine discs in cold temperatures. For. Pathol. 2018, 49, e12480. [Google Scholar] [CrossRef]
- Capretti, P.; Korhonen, K.; Mugnai, L.; Romagnoli, C. An intersterility group of Heterobasidion annousm specialized to Abies alba. Eur. J. For. Pathol. 1990, 20, 231–240. [Google Scholar] [CrossRef]
- Chase, T.E.; Urlich, R.C. Heterobasidion annosum root and butt rot of trees. Adv. Plant. Pathol. 1988, 6, 501–510. [Google Scholar]
- Korhonen, K. Intersterility groups of Heterobasidion annosum. In Proceedings of the Communicationes Instituti Forestalis Fenniae, Helsinki, Finland, 1978; Volume 94, pp. 1–25. [Google Scholar]
- Niemela, T.; Korhonen, K. Taxonomy of the genus Heterobasidion. In Heterobasidion annosum: Biology, Ecology, Impact and Control; CABI: Wallingford, UK, 1998; pp. 27–33. [Google Scholar]
- Otrosina, W.J.; Garbelotto, M. Heterobasidion occidentale sp. nov. and Heterobasidion irregulare nom. nov.: A disposition of North American Heterobasidion biological species. Fungal Biol. 2010, 114, 16–25. [Google Scholar] [CrossRef]
- Stanosz, G.R. Root rot of red pine caused by Heterobasidion annosum in Wisconsin. Plant Dis. 1995, 79, 859. [Google Scholar] [CrossRef]
- Blanchette, R.A.; Held, B.W.; Mollov, D.; Blake, J.; D’Amato, A.W. First report of Heterobasidion irregulare causing root rot and mortality of red pines in Minnesota. Plant Dis. 2015, 99, 1038. [Google Scholar] [CrossRef]
- Minnesota DNR. Past Climate Data. Available online: https://www.dnr.state.mn.us/climate/historical/acis_stn_meta.html (accessed on 8 December 2020).
- Wisconsin DNR. Heterobasidion Root Disease (HRD) (Formerly Annosus Root Rot). Available online: https://dnr.wi.gov/topic/foresthealth/annosumrootrot.html (accessed on 8 December 2020).
- Dumas, M.T.; Laflamme, G. Efficacy of two Phlebiopsis gigantea formulations in preventing Heterobasidion irregulare colonization of red pine stumps in Eastern Canada. Phytoprotection 2013, 93, 25–31. [Google Scholar] [CrossRef]
- Nicolotti, G.; Gonthier, P. Stump treatment against Heterobasidion with Phlebiopsis gigantea and some chemicals in Picea abies stands in the Western Alps. For. Pathol. 2005, 35, 365–374. [Google Scholar] [CrossRef]
- Pratt, J.E.; Niemi, M.; Sierota, Z.H. Comparison of three products based on Phlebiopsis gigantea for the control of Heterobasidion annosum in Europe. Biocontrol Sci. Technol. 2000, 10, 467–477. [Google Scholar] [CrossRef]
- Terhonen, E.; Sun, H.; Buee, M.; Kasanen, R.; Paulin, L.; Asiegbu, F.O. Effects of the use of biocontrol agent (Phlebiopsis gigantea) on fungal communities on the surface of Picea abies stumps. For. Ecol. Manag. 2013, 310, 428–433. [Google Scholar] [CrossRef]
- Blanchette, R.A.; Held, B.W.; Hellmann, L.; Millman, L.; Büntgen, U. Arctic driftwood reveals unexpectedly rich fungal diversity. Fungal Ecol. 2016, 23, 28–65. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Worrall, J.J. Media for selective isolation of hymenomycetes. Mycologia 1991, 83, 296–302. [Google Scholar] [CrossRef]
- Bérubé, J.A.; Potvin, A.; Stewart, D. Importance of local and long-distance Heterobasidion irreugulare aerial basidiospore dispersal for future infection centres in thinned red pine plantation in Quebec. For. Chron. 2017, 93, 241–245. [Google Scholar] [CrossRef]
- Bérubé, J.A.; Dubé, J.; Potvin, A. Incidence of Heterobasidion irregulare aerial basidiospores at different locations in southern Quebec. Can. J. Plant. Pathol. 2017, 40, 34–38. [Google Scholar] [CrossRef]
- Lamarche, J.; Potvin, A.; Stewart, D.; Blais, M.; Pelletier, G.; Shamoun, S.F.; Hamelin, R.C.; Tanguay, P. Real-time PCR assays for the detection of Heterobasidion irregulare, H. occidentale, H. annosum sensu stricto and the Heterobasidion annosum complex. For. Pathol. 2016, 47, e12321. [Google Scholar] [CrossRef]
- Pfender, W.; Graw, R.; Bradley, W.; Carney, M.; Maxwell, L. Emission rates, survival, and modeled dispersal of viable pollen of creeping bentgrass. Crop. Sci. 2007, 47, 2529–2539. [Google Scholar] [CrossRef]
- Sinclair, W.A.; Lyon, H.H. Diseases of Trees and Shrubs, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 2006; p. 616. [Google Scholar]
- Gonthier, P.; Warner, R.; Nicolotti, G.; Mazzagila, A. Pathogen introduction as a collateral effect of military activity. Mycol. Res. 2004, 108, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Stenlid, J. Regional Differentiation in Heterobasidion annosum. In Proceedings of the Eighth International Conference on Root and Butt Roots, Wik, Sweeden/Haikko, Finland, 9–16 August 1993; Johansson, M., Stenlid, J., Eds.; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1994; pp. 243–248. [Google Scholar]
- Garbelotto, M.; Gonthier, P. Biology, Epidemiology, and Control of Heterobasidion Species Worldwide. Annu. Rev. Phytopathol. 2013, 51, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Kallio, T. Aerial distribution of the root-rot fungus Fomes annosus (Fr.) Cooke in Finland. Acta For. Fenn. 1970, 107, 1–55. [Google Scholar] [CrossRef]
- Ryvarden, L.; Gilbertson, R.L. European polypores: Part. 1: Abortiporus—Lindtneria; Fungiflora A/S: Oslo, Norway, 1993; p. 387. [Google Scholar]
- Burdsall, H.H. A contribution to the taxonomy of the genus Phanerochaete (Corticiaceae, Aphyllophorales). Mycol. Mem. 1985, 10, 123–128. [Google Scholar]
- Whitney, R.D. Root-rotting fungi in white spruce, black spruce, and balsam fir in Northern Ontario. Can. J. For. Res. 1995, 25, 1209–1230. [Google Scholar] [CrossRef]
- Kromroy, K.W.; Blanchette, R.A.; Grigal, D.F. Armillaria species on small woody plants, small woody debris, and root fragments in red pine stands. Can. J. For. Res. 2005, 35, 1487–1495. [Google Scholar] [CrossRef]
- Edmunds, R.L.; Driver, C.H. Dispersion and deposition of spores of Fomes annosus and fluorescent particles. Phytopathology 1974, 64, 1313–1321. [Google Scholar] [CrossRef]
- Gonthier, P.; Garbelotto, M.M.; Nicolotti, G. Seasonal patterns of spore deposition of Heterobasidion species in four forests of the western Alps. Phytopathology 2005, 95, 759–767. [Google Scholar] [CrossRef]
- James, R.L.; Cobb, F.W. Spore deposition by Heterobasidion annosum in forests of California. Plant. Dis. 1984, 68, 246–248. [Google Scholar] [CrossRef]
- Rishbeth, J. Dispersal of Fomes annosus and Peniophora gigantea. Trans. Br. Mycol. Soc. 1959, 42, 243–260. [Google Scholar] [CrossRef]
- Punter, D. Fomes annosus in Eastern Canada. In Root Diseases of Soil-Borne Pathogens; Toussoun, T.A., Bega, R.V., Nelson, P.E., Eds.; University of California Press: Berkeley, CA, USA, 1970; pp. 156–170. [Google Scholar]
- Moykkynen, T.; Kontiokari, J. Spore deposition of Heterobasidion annosum coll. in Picea abies stands on North Karelia, eastern Finland. For. Pathol. 2001, 31, 107–114. [Google Scholar] [CrossRef]
- Keriö, S.; Niemi, S.M.; Haapanen, M.; Daniel, G.; Asiegbu, F.O. Infection of Picea abies clones with a homokaryotic isolate of Heterobasidion parviporum under field conditions. Can. J. For. Res. 2014, 45, 227–235. [Google Scholar] [CrossRef]
- Korhonen, K.; Piri, T. The main hosts and distribution of the S and P groups of Heterobasidion annosum in Finland. In Proceedings of the 8th International Conference on Root and Butt Rots, Wik, Sweden/Haikko, Finland, 9–16 August 1993; Johansson, M., Stenlid, J., Eds.; Swedish University of Agricultural Sciences: Uppsala, Sweden; pp. 260–267. [Google Scholar]
- Lamarche, J.; Potvin, A.; Pelletier, G.; Stewart, D.; Feau, N.; Alayon, D.I.O.; Dale, A.L.; Coelho, A.; Uzunovic, A.; Bilodeau, G.J.; et al. Molecular detection of 10 of the most unwanted alien forest pathogens in Canada using real-time PCR. PLoS ONE 2015, 10, e0134265. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, E.D.; Duceppe, M.; Bérubé, J.A.; Kimoto, T.; Lemieux, C.; Bilodeau, G.J. Screening for exotic forest pathogens to increase survey capacity using metagenomics. Phytopathology 2018, 108, 1509–1521. [Google Scholar] [CrossRef]

| Location | Sampling Week | Total Spore Count | Spores Per m3 | Spores Per m−2 h−1 | Cumulative Spores Deposited on a 30 cm Stump |
|---|---|---|---|---|---|
| Burr Oak, WI | 18 September 2018 | 63,776.07 | 1832.25 | 149.33 | 129.51 |
| Minneiska | 18 September 2018 | 412.58 | 13.55 | 1.26 | 0.96 |
| Great River Bluffs State Park | 26 September 2018 | 100.75 | 3.31 | 0.31 | 0.23 |
| Hay Creek | 26 September 2018 | 33.53 | 1.10 | 0.10 | 0.08 |
| Elba | 03 October 2018 | 33.04 | 1.09 | 0.10 | 0.08 |
| Vinegar Ridge Recreation Area | 03 October 2018 | 75.10 | 2.47 | 0.23 | 0.17 |
| William O’Brien State Park | 10 October 2018 | 163.10 | 5.36 | 0.50 | 0.38 |
| Wild River State Park | 10 October 2018 | 85.23 | 2.80 | 0.26 | 0.10 |
| Itasca State Park (Location 1) | 23 October 2018 | 0 | 0 | 0 | 0 |
| Itasca State Park (Location 2) | 23 October 2018 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otto, E.; Held, B.; Redford, S.; Blanchette, R.A. Detecting Heterobasidion irregulare in Minnesota and Assessment of Indigenous Fungi on Pines. Forests 2021, 12, 57. https://doi.org/10.3390/f12010057
Otto E, Held B, Redford S, Blanchette RA. Detecting Heterobasidion irregulare in Minnesota and Assessment of Indigenous Fungi on Pines. Forests. 2021; 12(1):57. https://doi.org/10.3390/f12010057
Chicago/Turabian StyleOtto, Eric, Benjamin Held, Samuel Redford, and Robert A. Blanchette. 2021. "Detecting Heterobasidion irregulare in Minnesota and Assessment of Indigenous Fungi on Pines" Forests 12, no. 1: 57. https://doi.org/10.3390/f12010057
APA StyleOtto, E., Held, B., Redford, S., & Blanchette, R. A. (2021). Detecting Heterobasidion irregulare in Minnesota and Assessment of Indigenous Fungi on Pines. Forests, 12(1), 57. https://doi.org/10.3390/f12010057






