Divergent Adaptation Strategies of Vascular Facultative Epiphytes to Bark and Soil Habitats: Insights from Stoichiometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Species
2.2. Field Sampling and Greenhouse Culture
2.3. Laboratory Analysis
2.4. Data Analysis and Statistics
3. Results
3.1. Stoichiometric Patterns of C, N, P Concentrations and Their Ratios in Different Organs in Two Habitats in the Field
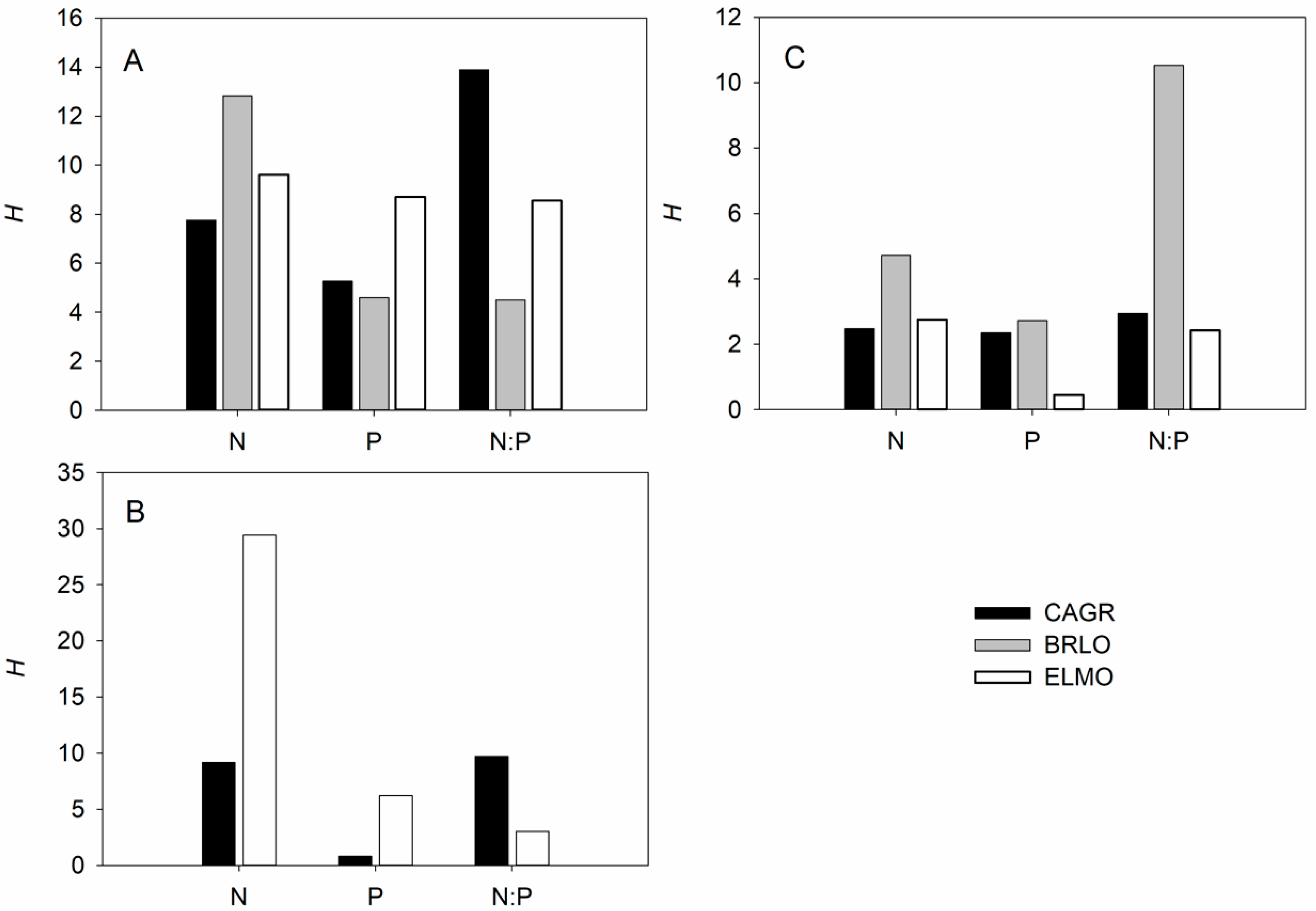
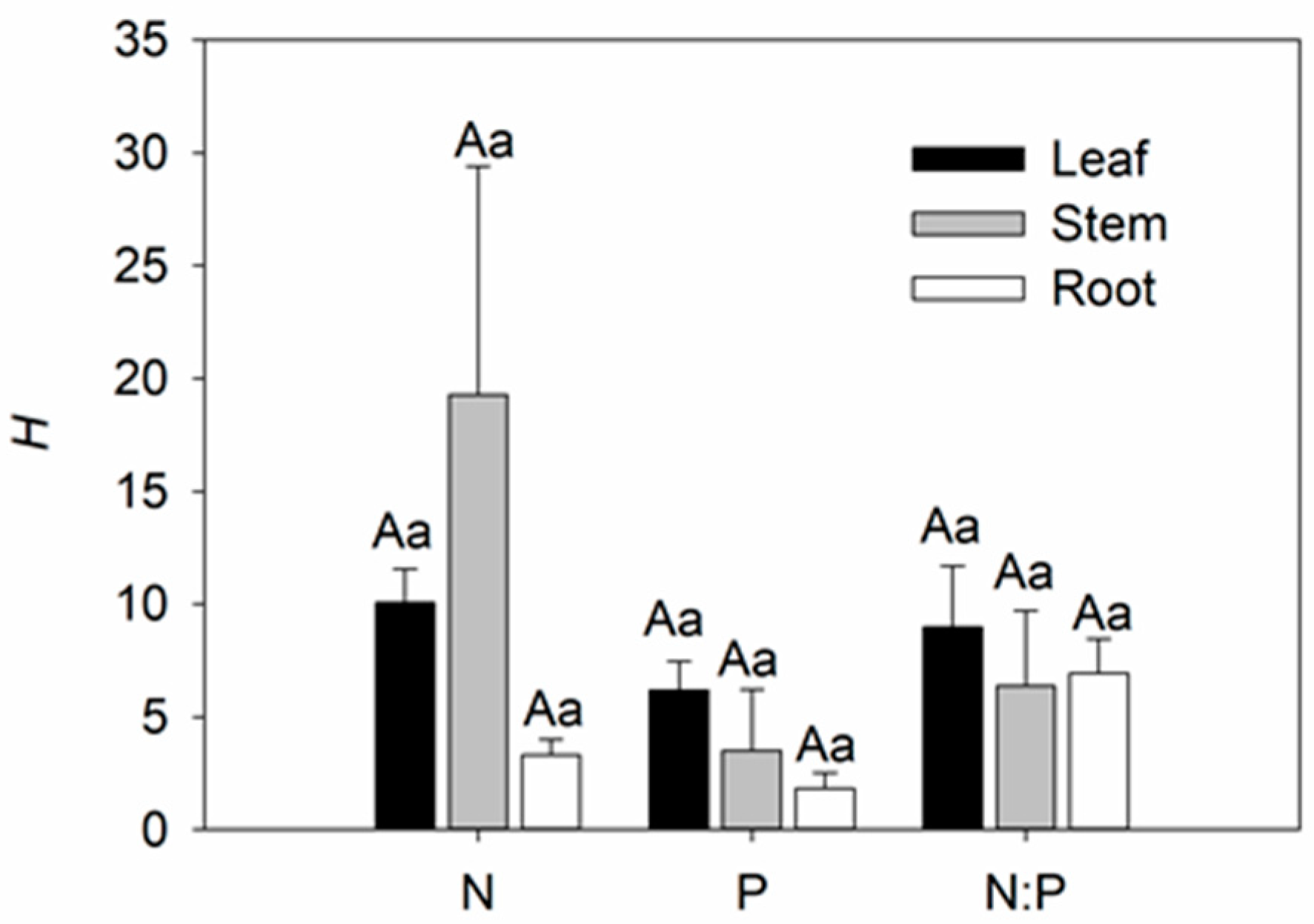
3.2. Variation of Stoichiometric Homeostasis among Different Species, Organs, and Elements in the Field
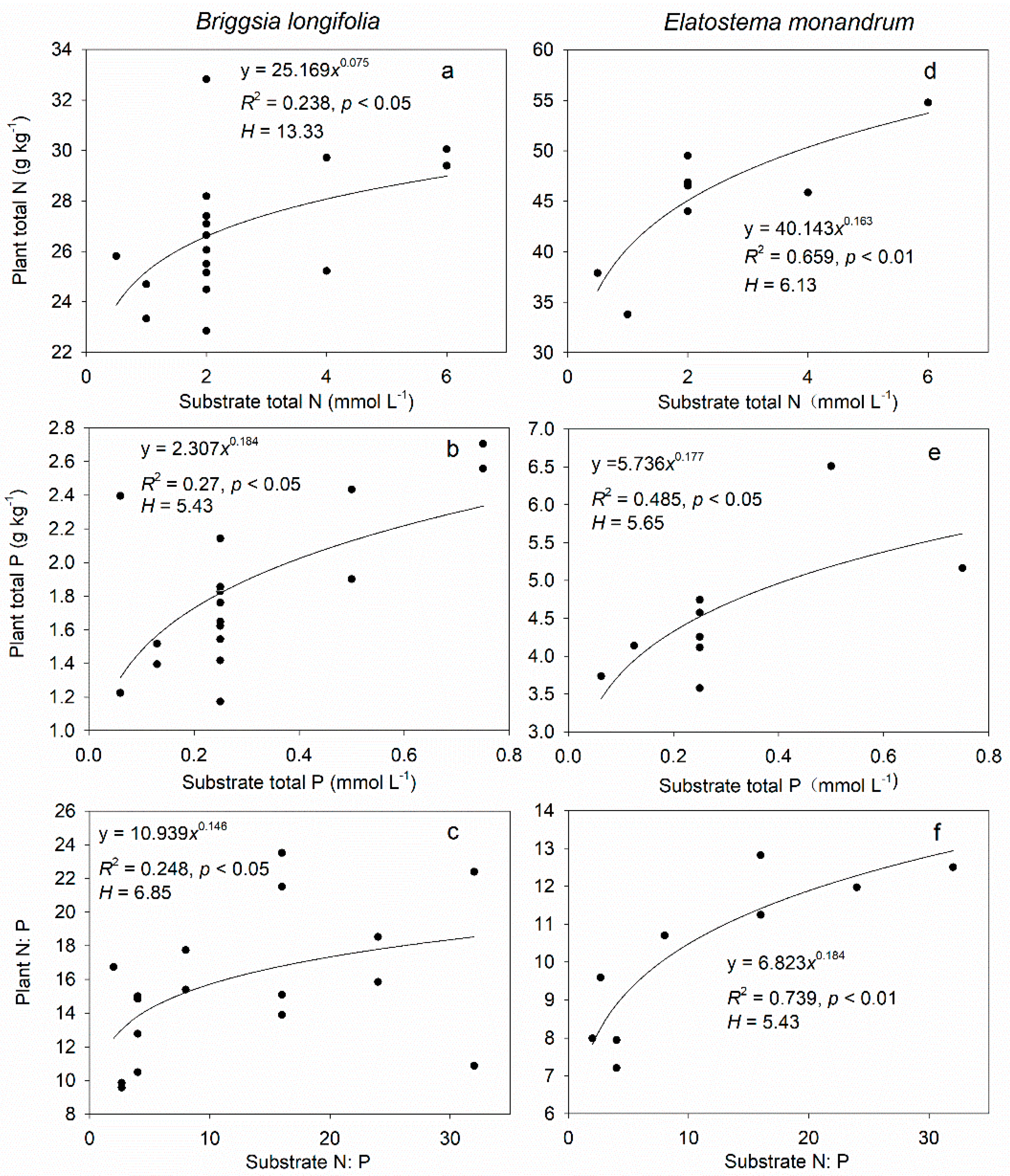
3.3. Stoichiometric Homeostasis of Leaf N, P, and N:P in Greenhouse Cultivation Experiment
4. Discussion
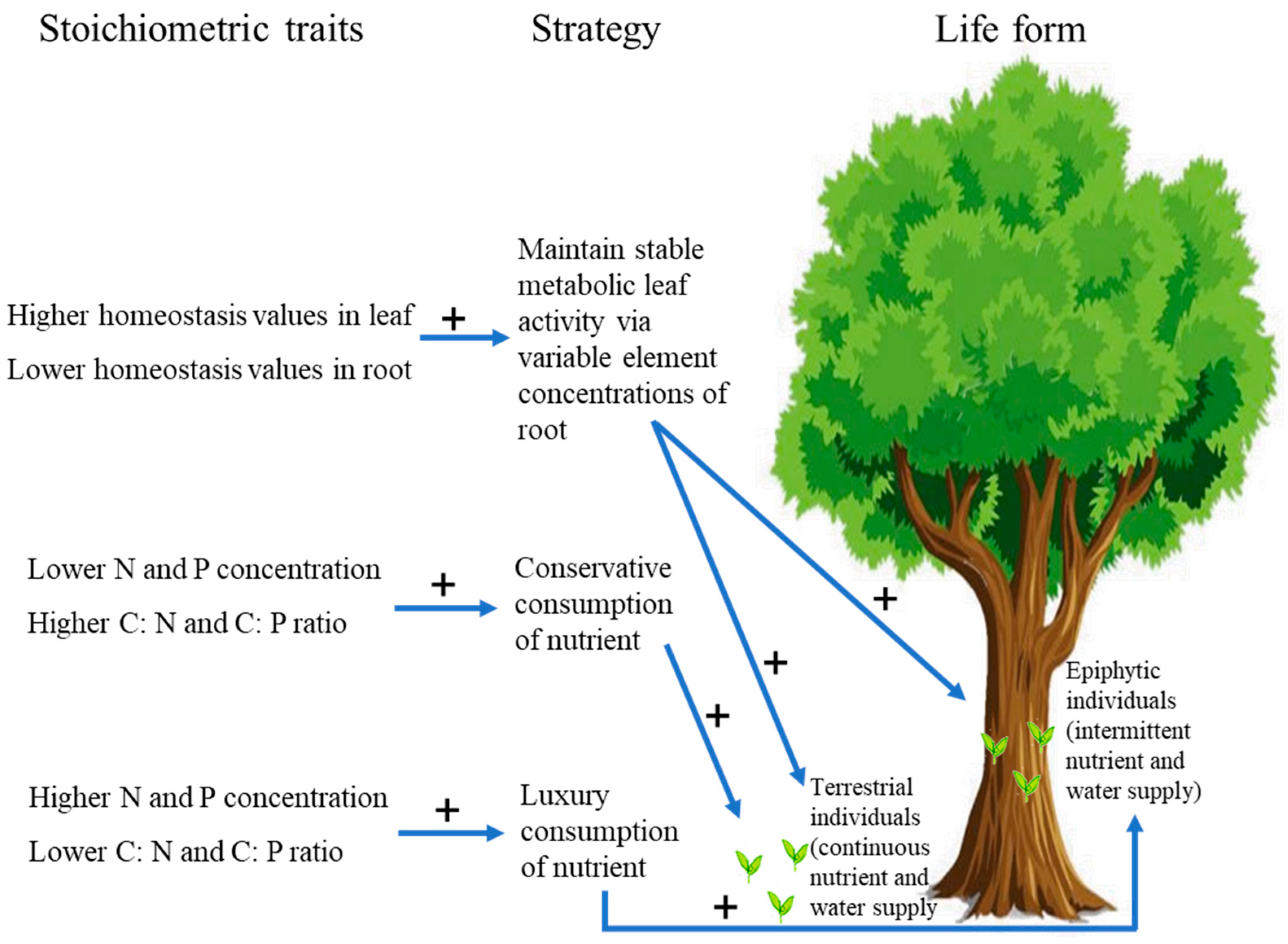
4.1. Differences in Nutrient Use Strategy between Epiphytic and Terrestrial Individuals of Facultative Epiphytes
4.2. Effects of Nutrient Limitation on the Distribution of Three Facultative Epiphytes
4.3. Differences in Stoichiometric Homeostasis among Species, Organs, and Elements of Facultative Epiphytes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lowman, M.D. Plants in the forest canopy: Some reflections on current research and future direction. Plant Ecol. 2001, 153, 39–50. [Google Scholar] [CrossRef]
- Lowman, M.D.; Schowalter, T.; Franklin, J.F.; Lowman, M.D.; Schowalter, T.; Franklin, J. Methods in Forest Canopy Research; University of California Press: Oakland, CA, USA, 2012. [Google Scholar]
- Zotz, G.; Winkler, U. Aerial roots of epiphytic orchids: The velamen radicum and its role in water and nutrient uptake. Oecologia 2013, 171, 733–741. [Google Scholar] [CrossRef]
- Benzing, D.H. Vascular Epiphytes: General Biology and Related Biota; Cambridge University Press: New York, NY, USA, 1990. [Google Scholar]
- Lu, H.Z.; Liu, W.Y.; Yu, F.H.; Song, L.; Xu, X.L.; Wu, C.S.; Zheng, Y.L.; Li, Y.P.; Gong, H.D.; Chen, K. Higher clonal integration in the facultative epiphytic fern Selliguea griffithiana growing in the forest canopy compared with the forest understory. Ann. Bot. 2015, 116, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Zotz, G. ‘Hemiepiphyte’: A confusing term and its history. Ann. Bot. 2013, 111, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Z.; Brooker, R.; Song, L.; Liu, W.Y.; Sack, L.; Zhang, J.L.; Yu, F.H. When facilitation meets clonal integration in forest canopies. New Phytol. 2020, 225, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, W.Y.; Li, D.W.; Song, L.; Chen, K.; Fu, Y. Slower rates of litter decomposition of dominant epiphytes in the canopy than on the forest floor in a subtropical montane forest, southwest China. Soil Biol. Biochem. 2014, 70, 211–220. [Google Scholar] [CrossRef]
- Théry, M. Forest light and its influence on habitat selection. Plant Ecol. 2001, 153, 251–261. [Google Scholar] [CrossRef]
- Zotz, G.; Hietz, P. The physiological ecology of vascular epiphytes: Current knowledge, open questions. J. Exp. Bot. 2001, 52, 2067. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, J.W.; Li, B.G.; Cao, K.F. The effect of drought on photosynthesis in two epiphytic and two terrestrial tropical fern species. Photosynthetica 2009, 47, 128–132. [Google Scholar] [CrossRef]
- Zotz, G. Plants on Plants—The Biology of Vascular Epiphytes; Springer Nature Press: Cham, Switzerland, 2016. [Google Scholar]
- Chen, Q.; Lu, H.Z.; Liu, W.Y.; Wu, Y.; Song, L.; Li, S. Obligate to facultative shift of two epiphytic Lepisorus species during subtropical forest degradation: Insights from functional traits. Forest Ecol. Manag. 2019, 435, 66–76. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Prieto, I.; Torres, P.; Campoy, M.; Alguacil, M.M.; Roldan, A. Water-spender strategy is linked to higher leaf nutrient concentrations across plant species colonizing a dry and nutrient-poor epiphytic habitat. Environ. Exp. Bot. 2018, 153, 302–310. [Google Scholar] [CrossRef]
- Testo, W.; Sundue, M. Primary hemiepiphytism in Colysis ampla (Polypodiaceae) provides new insight into the evolution of growth habit in ferns. Int. J. Plant Sci. 2014, 175, 526–536. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Elser, J.J.; Hamilton, A. Stoichiometry and the new biology: The future is now. PLoS Biol. 2007, 5, e181. [Google Scholar] [CrossRef] [PubMed]
- Sistla, S.A.; Appling, A.P.; Lewandowska, A.M.; Taylor, B.N.; Wolf, A.A. Stoichiometric flexibility in response to fertilization along gradients of environmental and organismal nutrient richness. Oikos 2015, 124, 949–959. [Google Scholar] [CrossRef]
- Yan, Z.B.; Guan, H.Y.; Han, W.X.; Han, T.S.; Guo, Y.L.; Fang, J.Y. Reproductive organ and young tissues show constrained elemental composition in Arabidopsis thaliana. Ann. Bot. 2016, 117, 431. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Q.S.; Elser, J.J.; He, N.P.; Wu, H.; Zhang, G.M.; Wu, J.G.; Bai, Y.F.; Han, X.G. Linking stoichiometric homoeostasis with ecosystem structure, functioning and stability. Ecol. Lett. 2010, 13, 1390–1399. [Google Scholar] [CrossRef]
- Yu, Q.; Elser, J.J.; He, N.P.; Wu, H.H.; Chen, Q.S.; Zhang, G.M.; Han, X.G. Stoichiometric homeostasis of vascular plants in the Inner Mongolia grassland. Oecologia 2011, 166, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.; Chapin, F.S., III. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar]
- Güsewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Güsewell, S. Responses of wetland graminoids to the relative supply of nitrogen and phosphorus. Plant Ecol. 2005, 176, 35–55. [Google Scholar] [CrossRef]
- Peæuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 3934. [Google Scholar]
- Ma, W.; Li, J.; Jimoh, S.O.; Zhang, Y.; Guo, F.; Ding, Y.; Li, X.; Hou, X. Stoichiometric ratios support plant adaption to grazing moderated by soil nutrients and root enzymes. PeerJ 2019, 7, e7047. [Google Scholar] [CrossRef] [PubMed]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Wanek, W.; Zotz, G. Are vascular epiphytes nitrogen or phosphorus limited? A study of plant 15N fractionation and foliar N: P stoichiometry with the tank bromeliad Vriesea sanguinolenta. New Phytol. 2011, 192, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Koojiman, S.A.L.M. The Stoichiometry of Animal Energetics. J. Theor. Biol. 1995, 177, 139–149. [Google Scholar] [CrossRef]
- Yu, Q.; Wilcox, K.; La, P.K.; Knapp, A.K.; Han, X.; Smith, M.D. Stoichiometric homeostasis predicts plant species dominance, temporal stability, and responses to global change. Ecology 2015, 96, 2328–2335. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Pendall, E.; Morgan, J.A.; Blumenthal, D.M.; Carrillo, Y.; Lecain, D.R.; Follett, R.F.; Williams, D.G. Climate change alters stoichiometry of phosphorus and nitrogen in a semiarid grassland. New Phytol. 2012, 196, 807–815. [Google Scholar] [CrossRef]
- Persson, J.; Fink, P.; Goto, A.; Hood, J.M.; Jonas, J.; Kato, S. To be or not to be what you eat: Regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 2010, 119, 741–751. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Reich, P.B.; Ian, W.F.; Wang, Z.H. Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol. Lett. 2011, 14, 788–796. [Google Scholar] [CrossRef]
- Karimi, R.; Folt, C.L. Beyond macronutrients: Element variability and multielement stoichiometry in freshwater invertebrates. Ecol. Lett. 2006, 9, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.Y.; Xu, W.T.; Zhou, G.Y.; Bai, Y.F.; Li, J.X.; Tang, X.L.; Chen, D.M.; Liu, Q.; Ma, W.H.; Xiong, G.M.; et al. Patterns of plant carbon, nitrogen, and phosphorus concentration in relation to productivity in China’s terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2018, 115, 4033–4038. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; Zeng, C.S.; Shao, J.J.; Zhou, X.H. Plant nutrient dynamics and stoichiometric homeostasis of invasive species Spartina alterniflora and native Cyperus malaccensis var. brevifolius in the Minjiang River estuarine wetlands. Chin. J. Plant Ecol. 2017, 41, 405–460. [Google Scholar]
- Schreeg, L.A.; Santiago, L.S.; Wright, S.J.; Turner, B.L. Stem, root, and older leaf N:P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 2014, 95, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Li, D.-D.; Jiao, F.; Yao, J.; Du, H.-T. The Latitudinal Patterns of Leaf and Soil C: N: P Stoichiometry in the Loess Plateau of China. Front. Plant Sci. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fang, X.; Tang, X.; Wang, W.; Zhou, G.; Xu, S.; Huang, W.; Wang, G.; Yan, J.; Ma, K.; et al. Patterns and controlling factors of plant nitrogen and phosphorus stoichiometry across China’s forests. Biogeochemistry 2019, 143, 191–205. [Google Scholar] [CrossRef]
- Tian, D.; Yan, Z.; Ma, S.; Ding, Y.; Luo, Y.; Chen, Y.; Du, E.; Han, W.; Kovacs, E.D.; Shen, H.; et al. Family-level leaf nitrogen and phosphorus stoichiometry of global terrestrial plants. Sci. China Life Sci. 2019, 62, 1047–1057. [Google Scholar] [CrossRef]
- Rinker, H.B.; Lowman, M. Forest Canopies; Elsevier Academic Press: London, UK, 2004. [Google Scholar]
- Huang, J.-B.; Liu, W.-Y.; Li, S.; Song, L.; Lu, H.-Z.; Shi, X.-M.; Chen, X.; Hu, T.; Liu, S.; Liu, T. Ecological stoichiometry of the epiphyte community in a subtropical forest canopy. Ecol. Evol. 2019, 9, 14394–14406. [Google Scholar] [CrossRef] [PubMed]
- Young, S.S.; Herwitz, S.R. Floristic Diversity and Co-Occurrences in a Subtropical Broad-Leaved Forest and Two Contrasting Regrowth Stands in Central-West Yunnan Province, China. Vegetatio 1995, 119, 1–13. [Google Scholar]
- Li, S.; Liu, W.; Wang, L.; Ma, W.; Song, L. Biomass, diversity and composition of epiphytic macrolichens in primary and secondary forests in the subtropical Ailao Mountains, SW China. Forest Ecol. Manag. 2011, 261, 1760–1770. [Google Scholar] [CrossRef]
- Annaselvam, J.; Parthasarathy, N. Diversity and distribution of herbaceous vascular epiphytes in a tropical evergreen forest at Varagalaiar, Western Ghats, India. Biodivers. Conserv. 2001, 10, 317–329. [Google Scholar] [CrossRef]
- Vance, E.D.; Nadkarni, N.M. Microbial biomass and activity in canopy organic matter and the forest floor of a tropical cloud forest. Soil Biol. Biochem. 1990, 22, 677–684. [Google Scholar] [CrossRef]
- Wania, R.; Hietz, P.; Wanek, W. Natural N-15 abundance of epiphytes depends on the position within the forest canopy: Source signals and isotope fractionation. Plant Cell Environ. 2002, 25, 581–589. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Penuelas, J. The C: N: P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavenderbares, J.; Chapin, F.S.; Cornelissen, J.H.C.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Winkler, U.; Zotz, G. ‘And then there were three’: Highly efficient uptake of potassium by foliar trichomes of epiphytic bromeliads. Ann. Bot. 2010, 106, 421–427. [Google Scholar] [CrossRef]
- de Freitas, C.A.; Scarano, F.R.; Biesboer, D.D. Morphological variation in two facultative epiphytic bromeliads growing on the floor of a swamp forest. Biotropica 2003, 35, 546–550. [Google Scholar] [CrossRef]
- Yan, Z.B.; Tian, D.; Han, W.X.; Tang, Z.Y.; Fang, J.Y. An assessment on the uncertainty of the nitrogen to phosphorus ratio as a threshold for nutrient limitation in plants. Ann. Bot. 2017, 120, 937–942. [Google Scholar] [CrossRef]
- Lasso, E.; Ackerman, J.D. Nutrient limitation restricts growth and reproductive output in a tropical montane cloud forest bromeliad: Findings from a long-term forest fertilization experiment. Oecologia 2013, 171, 165–174. [Google Scholar] [CrossRef]
- Mondragón, D.; Valverde, T.; Hernandez-Apolinar, M. Population ecology of epiphytic angiosperms: A review. Trop. Ecol. 2015, 51, 1–39. [Google Scholar]
- Demott, W.R.; Pape, B.J. Stoichiometry in an ecological context: Testing for links between Daphnia P-content, growth rate and habitat preference. Oecologia 2005, 142, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Townsend, A.R.; Cleveland, C.C.; Asner, G.P.; Bustamante, M.M.C. Controls over foliar N: P ratios in tropical rain forests. Ecology 2007, 88, 107–118. [Google Scholar] [CrossRef]
- Yang, D.X.; Song, L.; Jin, G.Z. The soil C: N: P stoichiometry is more sensitive than the leaf C: N: P stoichiometry to nitrogen addition: A four-year nitrogen addition experiment in a Pinus koraiensis plantation. Plant Soil 2019, 442, 183–198. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wu, H.H.; Yu, Q.; Wang, Z.W.; Wei, C.Z.; Long, M.; Kattge, J.; Smith, M.; Han, X.G. Sampling Date, Leaf Age and Root Size: Implications for the Study of Plant C: N: P Stoichiometry. PLoS ONE 2013, 8, e60360. [Google Scholar] [CrossRef]
- He, J.S.; Fang, J.Y.; Wang, Z.H.; Guo, D.L.; Dan, F.B.F.; Geng, Z. Stoichiometry and large-scale patterns of leaf carbon and nitrogen in the grassland biomes of China. Oecologia 2006, 149, 115. [Google Scholar] [CrossRef]
- Zhao, N.; Yu, G.R.; He, N.P.; Wang, Q.F.; Jia, Y.L. Coordinated pattern of multi-element variability in the leaves and roots across Chinese forest biomes. Glob. Ecol. Biogeogr. 2016, 25, 359–367. [Google Scholar] [CrossRef]
- Xing, W.; Shi, Q.; Liu, H.; Liu, G.H. Growth rate, protein: RNA ratio and stoichiometric homeostasis of submerged macrophytes under eutrophication stress. Knowl. Manag. Aquat. Ecosys. 2016, 417, 25. [Google Scholar] [CrossRef][Green Version]
- Avolio, M.L.; Koerner, S.E.; Pierre, K.J.L.; Wilcox, K.R.; Wilson, G.W.T.; Smith, M.D.; Collins, S.L. Changes in plant community composition, not diversity, during a decade of nitrogen and phosphorus additions drive above-ground productivity in a tallgrass prairie. J. Ecol. 2014, 102, 1649–1660. [Google Scholar] [CrossRef]

| Treatment | N (mmol L−1) | P (mmol L−1) | N: P |
|---|---|---|---|
| N1P3 | 0.5 | 0.25 | 2 |
| N2P3 | 1 | 0.25 | 4 |
| N3P3 | 2 | 0.25 | 8 |
| N4P3 | 4 | 0.25 | 16 |
| N5P3 | 6 | 0.25 | 24 |
| P1N3 | 2 | 0.0625 | 3.2 |
| P2N3 | 2 | 0.125 | 16 |
| P4N3 | 2 | 0.5 | 4 |
| P5N3 | 2 | 0.75 | 2.7 |
| Organ | Variable | Habitat | Species | Habitat × Species | |||
|---|---|---|---|---|---|---|---|
| F | df | F | df | F | df | ||
| Leaf | C | 3.67 | 1, 93 | 634.20 ** | 2, 93 | 1.54 | 2, 93 |
| N | 4.56 * | 1, 93 | 91.22 ** | 2, 93 | 1.29 | 2, 93 | |
| P | 10.83 ** | 1, 89 | 43.99 ** | 2, 89 | 3.01 | 2, 89 | |
| C:N | 0.56 | 1, 93 | 170.12 ** | 2, 93 | 1.10 | 2, 93 | |
| C:P | 7.73 ** | 1, 89 | 94.48 ** | 2, 89 | 3.70 * | 2, 89 | |
| N:P | 3.99 * | 1, 89 | 4.53 * | 2, 89 | 0.89 | 2, 89 | |
| Stem | C | 7.06 * | 1, 57 | 206.77 ** | 1, 57 | 2.03 | 1, 57 |
| N | 1.43 | 1, 57 | 93.92 ** | 1, 57 | 0.70 | 1, 57 | |
| P | 11.38 ** | 1, 44 | 2.84 | 1, 44 | 1.07 | 1, 44 | |
| C:N | 4.40 * | 1, 57 | 84.56 ** | 1, 57 | 0.82 | 1, 57 | |
| C:P | 3.74 | 1, 44 | 8.92 ** | 1, 44 | 0.02 | 1, 44 | |
| N:P | 3.24 | 1, 44 | 10.39 ** | 1, 44 | 2.25 | 1, 44 | |
| Root | C | 23.39 ** | 1, 90 | 21.88 ** | 2, 90 | 1.93 | 2, 90 |
| N | 4.69 * | 1, 90 | 34.30 ** | 2, 90 | 0.75 | 2, 90 | |
| P | 3.38 | 1, 65 | 13.60 ** | 2, 65 | 0.81 | 2, 65 | |
| C:N | 0.09 | 1, 90 | 62.97 ** | 2, 90 | 3.73 * | 2, 90 | |
| C:P | 0.10 | 1, 65 | 9.03 ** | 2, 65 | 0.72 | 2, 65 | |
| N:P | 0.00 | 1, 65 | 4.34 * | 2, 65 | 3.20 * | 2, 65 | |
| Variable | Species | Treatment | Species × Level | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | df | p | F | df | p | F | df | p | |
| N | 226.11 | 1 | 0.00 | 4.17 | 8 | 0.03 | 1.32 | 8 | 0.22 |
| P | 241.34 | 1 | 0.00 | 4.94 | 8 | 0.02 | 1.97 | 8 | 0.18 |
| N:P | 8.95 | 1 | 0.02 | 0.73 | 8 | 0.67 | 0.20 | 8 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Liu, W.; Hu, T.; Tang, D.; Mo, Y.; Wu, Y. Divergent Adaptation Strategies of Vascular Facultative Epiphytes to Bark and Soil Habitats: Insights from Stoichiometry. Forests 2021, 12, 16. https://doi.org/10.3390/f12010016
Zhang T, Liu W, Hu T, Tang D, Mo Y, Wu Y. Divergent Adaptation Strategies of Vascular Facultative Epiphytes to Bark and Soil Habitats: Insights from Stoichiometry. Forests. 2021; 12(1):16. https://doi.org/10.3390/f12010016
Chicago/Turabian StyleZhang, Tingting, Wenyao Liu, Tao Hu, Dandan Tang, Yuxuan Mo, and Yi Wu. 2021. "Divergent Adaptation Strategies of Vascular Facultative Epiphytes to Bark and Soil Habitats: Insights from Stoichiometry" Forests 12, no. 1: 16. https://doi.org/10.3390/f12010016
APA StyleZhang, T., Liu, W., Hu, T., Tang, D., Mo, Y., & Wu, Y. (2021). Divergent Adaptation Strategies of Vascular Facultative Epiphytes to Bark and Soil Habitats: Insights from Stoichiometry. Forests, 12(1), 16. https://doi.org/10.3390/f12010016






