Potential Differences and Methods of Determining Gypsy Moth Female Flight Capabilities: Implications for the Establishment and Spread in Novel Habitats
Abstract
1. Introduction
2. Gypsy Moth Population Dynamics (Establishment and Spread) and Potential Impacts of Female Flight
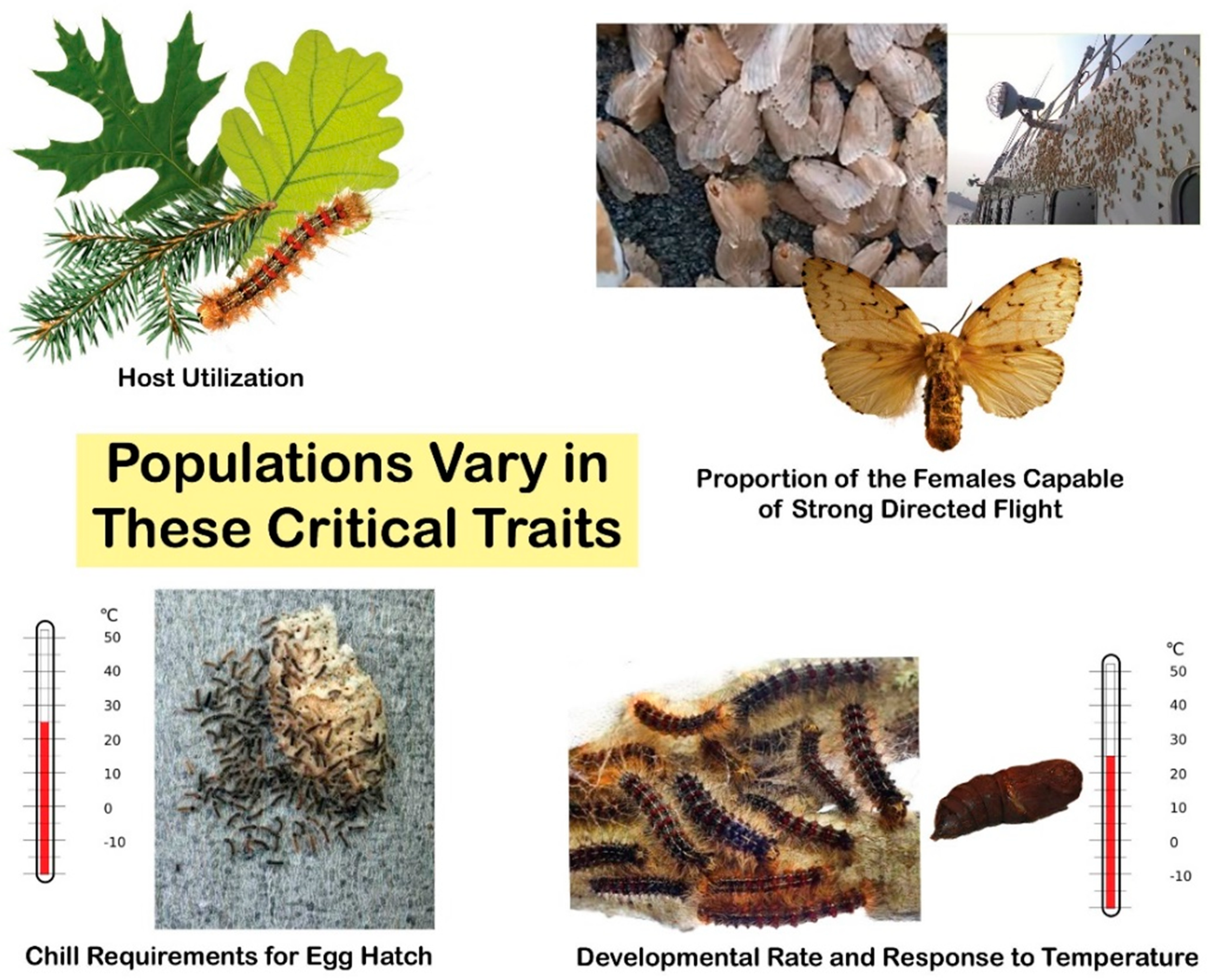
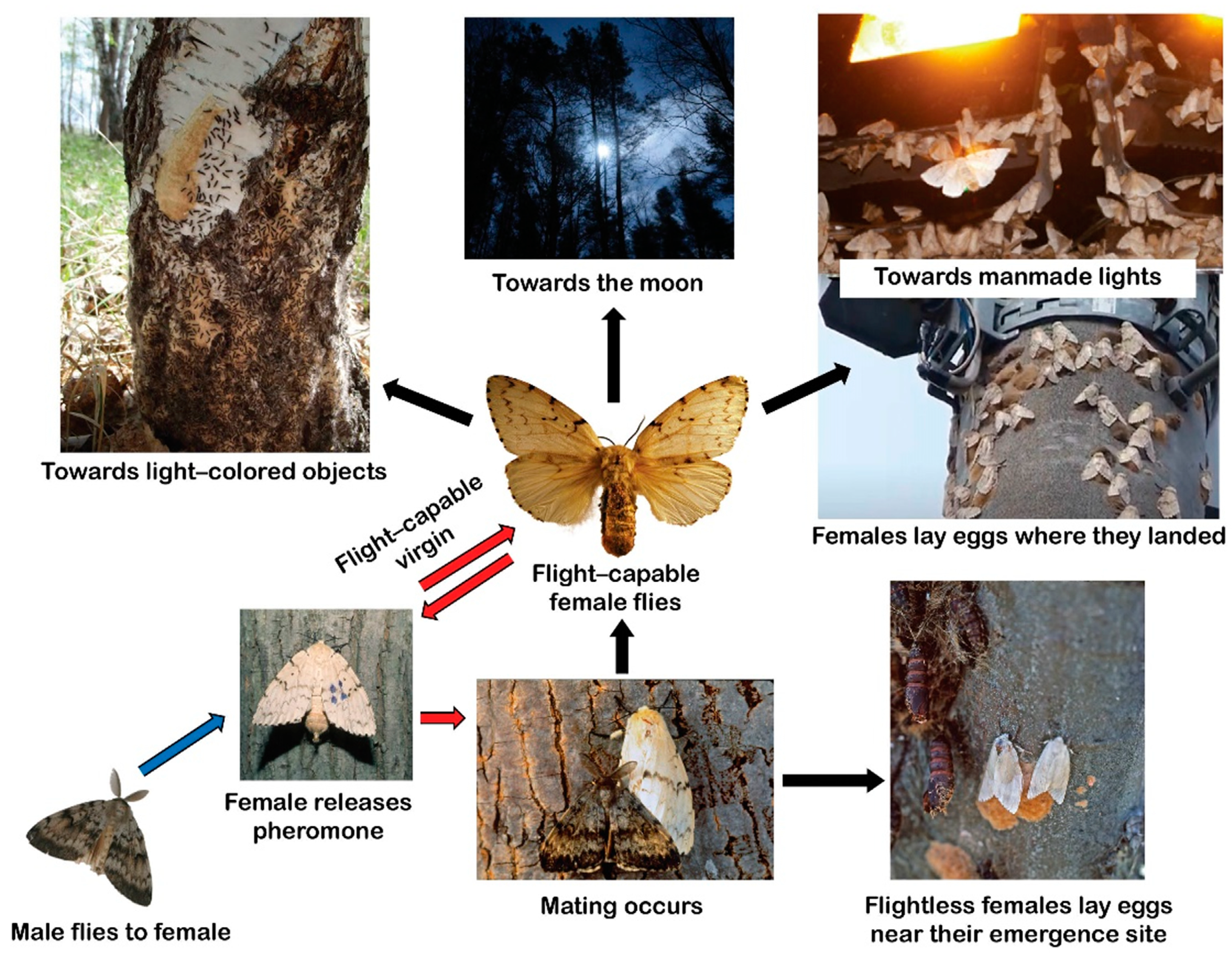
3. Variation in Female Flight Capability and Flight Distances
3.1. Female Flight Capability
“In a freely hybridizing population, the amount of flight capability maintained would depend on several factors: initial ratio of flight capable to flightless females, costs versus fitness of flight in the particular environment, propensity of different hybrids to mate, etc. Should females with full flight capability be introduced into North America in an area where the flightless females are already established, the populations would hybridize, and the ability of L. dispar to spread could be increased”.
3.2. Female Flight Distance
4. Identification of Gypsy Moth Subspecies and Specific Traits within Populations
4.1. Genetic and Genomic Analyses of Subspecies and Populations
4.2. Physical Differences among Subspecies and Populations
4.3. Identification of Flight-Capable Populations
5. Conclusions: Current Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gninenko, Y.I.; Orlinskii, A.D. Outbreaks of Lymantria dispar in Russian forests during the 1990s. EPPO Bull. 2003, 33, 325–329. [Google Scholar] [CrossRef]
- Bigsby, K.M.; Ambrose, M.J.; Tobin, P.C.; Sills, E.O. The cost of gypsy moth sex in the city. Urban For. Urban Green. 2014, 13, 459–468. [Google Scholar] [CrossRef]
- Liebhold, A.; Gottschalk, K.; Muzika, R.M.; Montgomery, M.E.; Young, R.; O’Day, K.; Kelley, B. Suitability of North American Tree Species to the Gypsy Moth: A Summary of Field and Laboratory Tests; USDA For. Serv; Northeastern Experiment Station: Radnor, PA, USA, 1995; Volume GTR-NE-211, pp. 1–34. [Google Scholar]
- Keena, M.A.; Richards, J.Y. Comparison of survival and development of gypsy moth Lymantria dispar L. (Lepidoptera: Erebidae) populations from different geographic areas on North American conifers. Insects 2020, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Pogue, M.G.; Schaefer, P.W. A Review of Selected Species of Lymantria (Hübner 1819) Including Three New Species (Lepidoptera: Noctuidae: Lymantriinae) from Subtropical and Temperate Regions of Asia; North AmericaForest Health Technology Enterprise Team: Morgantown, WV, USA, 2007; Volume FHTET-2006-07, pp. 201–217. [Google Scholar]
- Djoumad, A.; Nisole, A.; Zahiri, R.; Freschi, L.; Picq, S.; Gundersen-Rindal, D.E.; Sparks, M.E.; Dewar, K.; Stewart, D.; Maaroufi, H.; et al. Comparative analysis of mitochondrial genomes of geographic variants of the gypsy moth, Lymantria dispar, reveals a previously undescribed genotypic entity. Sci. Rep. UK 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Keena, M.A.; Cote, M.J.; Grinberg, P.S.; Wallner, W.E. World distribution of female flight and genetic variation in Lymantria dispar (Lepidoptera: Lymantriidae). Environ. Entomol. 2008, 37, 636–649. [Google Scholar] [CrossRef]
- Gray, D.R. Hitchhikers on trade routes: A phenology model estimates the probabilities of gypsy moth introduction and establishment. Ecol. Appl. 2010, 20, 2300–2309. [Google Scholar] [CrossRef]
- Tobin, P.C. Space-time patterns during the establishment of a nonindigenous species. Popul. Ecol. 2007, 49, 257–263. [Google Scholar] [CrossRef]
- McManus, M.; Csoka, G. History and impact of gypsy moth in North America and comparison of recent outbreaks in Europe. Acta Silv. Lign. Hung. 2007, 3, 47–64. [Google Scholar]
- Liebhold, A.M.; Halverson, J.A.; Elmes, G.A. Gypsy moth invasion in North America: A quantitative analysis. J. Biogeogr. 1992, 19, 513–520. [Google Scholar] [CrossRef]
- Roberts, E.A.; Tobin, P.C.; Wu, J. Decision Support System for the Gypsy Moth Slow-the-Spread Program. Available online: http://yt.ento.vt.edu/old-da/ (accessed on 17 January 2021).
- Hajek, A.E. Pathology and epizootiology of Entomophaga maimaiga infections in forest Lepidoptera. Microbiol. Mol. Biol. Rev. 1999, 63, 814–835. [Google Scholar] [CrossRef]
- Dwyer, G.; Dushoff, J.; Yee, S.H. The combined effects of pathogens and predators on insect outbreaks. Nature 2004, 430, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Mannu, R.; Cocco, A.; Luciano, P.; Lentini, A. Influence of Bacillus thuringiensis application timing on population dynamics of gypsy moth in Mediterranean cork oak forests. Pest Manag. Sci. 2020, 76, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Haynes, K.J.; Liebhold, A.M.; Johnson, D.M. Spatial analysis of harmonic oscillation of gypsy moth outbreak intensity. Oecologia 2009, 159, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.M.; Liebhold, A.M.; Tobin, P.C.; Bjørnstad, O.N. Allee effects and pulsed invasion by the gypsy moth. Nature 2006, 444, 361–363. [Google Scholar] [CrossRef]
- Liebhold, A.; Elkinton, J.; Williams, D.; Muzika, R.M. What causes outbreaks of the gypsy moth in North America? Popul. Ecol. 2000, 42, 257–266. [Google Scholar] [CrossRef]
- Johnson, D.M.; Liebhold, A.M.; Bjornstad, O.N.; McManus, M.L. Circumpolar variation in periodicity and synchrony among gypsy moth populations. J. Anim. Ecol. 2005, 74, 882–892. [Google Scholar] [CrossRef]
- Weiser, J. Patterns over place and time. In Epizootiology of Insect Diseases; Fuxa, J.R., Tanda, Y., Eds.; Wiley: New York, NK, USA, 1987; pp. 215–244. [Google Scholar]
- Hlásny, T.; Trombik, J.; Holuša, J.; Lukášová, K.; Grendár, M.; Turčáni, M.; Zúbrik, M.; Tabaković-Tošić, M.; Hirka, A.; Buksha, I.; et al. Multi-decade patterns of gypsy moth fluctuations in the Carpathian mountains and options for outbreak forecasting. J. Pest Sci. 2016, 89, 413–425. [Google Scholar] [CrossRef]
- Davidson, C.B.; Gottschalk, K.W.; Johnson, J.E. Tree mortality following defoliation by the European gypsy moth (Lymantria dispar L.) in the United States: A review. For. Sci. 1999, 45, 74–84. [Google Scholar]
- Morin, R.S.; Liebhold, A.M. Invasive forest defoliator contributes to the impending downward trend of oak dominance in eastern North America. Forestry 2016, 89, 284–289. [Google Scholar] [CrossRef]
- Aukema, J.E.; Leung, B.; Kovacs, K.; Chivers, C.; Britton, K.O.; Englin, J.; Frankel, S.J.; Haight, R.G.; Holmes, T.P.; Liebhold, A.M.; et al. Economic impacts of non-native forest insects in the continental United States. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Animal Palnt Health Insepction Service [APHIS]—United States Department of Agriculture. Asian Gypsy Moth Survey and Response Guidelines. Available online: https://www.aphis.usda.gov/plant_health/plant_pest_info/gypsy_moth/downloads/AGMSurveyResponseGuidelines.pdf (accessed on 17 January 2021).
- Washington State Department of Agriculture-Plant Protection Division Pest Program. Cooperative Gypsy Moth Eradication Program Snohomish County. Washington State Environmental Assessment; Washington State Department of Agriculture: Olympia, WA, USA, 2020.
- Wallner, W.E.; Humble, L.M.; Levin, R.E.; Baranchikov, Y.N.; Carde, R.T. Response of adult lymantriid moths to illumination devices in the Russian Far-East. J. Econ. Entomol. 1995, 88, 337–342. [Google Scholar] [CrossRef]
- Schaefer, P.W.; Strothkamp, K.G. Mass flights of Lymantria dispar japonica and Lymantria mathura (Erebidae: Lymantriinae) to commercial lighting, with notes on female viability and fecundity. J. Lepid. Soc. 2014, 68, 124–129. [Google Scholar]
- Keena, M.A. Inheritance and world variation in thermal requirements for egg hatch in Lymantria dispar (Lepidoptera: Erebidae). Environ. Entomol. 2016, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Limbu, S.; Keena, M.; Chen, F.; Cook, G.; Nadel, H.; Hoover, K. Effects of temperature on development of Lymantria dispar asiatica and Lymantria dispar japonica (Lepidoptera: Erebidae). Environ. Entomol. 2017, 46, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Griess, V.C.; Keena, M.A. Assessing the potential distribution of Asian gypsy moth in Canada: A comparison of two methodological approaches. Sci. Rep. UK 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Keena, M.A.; Grinberg, P.S.; Wallner, W.E. Inheritance of female flight in Lymantria dispar (Lepidoptera: Lymantriidae). Environ. Entomol. 2007, 36, 484–494. [Google Scholar] [CrossRef]
- Tobin, P.C.; Blackburn, L.M. Long-distance dispersal of the gypsy moth (Lepidoptera: Lymantriidae) facilitated its initial invasion of Wisconsin. Environ. Entomol. 2008, 37, 87–93. [Google Scholar] [CrossRef]
- Gray, R.H.; Lorimer, C.G.; Tobin, P.C.; Raffa, K.F. Preoutbreak dynamics of a recently established invasive herbivore: Roles of natural enemies and habitat structure in stage-specific performance of gypsy moth (Lepidoptera: Lymantriidae) populations in northeastern Wisconsin. Environ. Entomol. 2008, 37, 1174–1184. [Google Scholar] [CrossRef]
- Elkinton, J.S.; Liebhold, A.M. Population-dynamics of gypsy moth in North America. Annu. Rev. Entomol. 1990, 35, 571–596. [Google Scholar] [CrossRef]
- Herrick, O.W.; Gansner, D.A. Gypsy moth on a new frontier: Forest tree defoliation and mortality. North. J. Appl. For. 1987, 4, 128–133. [Google Scholar] [CrossRef]
- Lentini, A.; Mannu, R.; Cocco, A.; Ruiu, P.A.; Cerboneschi, A.; Luciano, P. Long-term monitoring and microbiological control programs against lepidopteran defoliators in Sardinian cork oak forests (Italy). Ann. Silvic. Res. 2020, 45, 21–30. [Google Scholar]
- Allee, W.C. Animal Aggregations: A Study in General Sociology; University of Chicago Press: Chicago, IL, USA, 1932. [Google Scholar]
- Whitmire, S.L.; Tobin, P.C. Persistence of invading gypsy moth populations in the United States. Oecologia 2006, 147, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.M.; Hastings, A. Allee effects in biological invasions. Ecol. Lett. 2005, 8, 895–908. [Google Scholar] [CrossRef]
- Contarini, M.; Onufrieva, K.S.; Thorpe, K.W.; Raffa, K.F.; Tobin, P.C. Mate-finding failure as an important cause of allee effects along the leading edge of an invading insect population. Entomol. Exp. Appl. 2009, 133, 307–314. [Google Scholar] [CrossRef]
- Tobin, P.C.; Robinet, C.; Johnson, D.M.; Whitmire, S.L.; Bjornstad, O.N.; Liebhold, A.M. The role of allee effects in gypsy moth, Lymantria dispar (L.), invasions. Popul. Ecol. 2009, 51, 373–384. [Google Scholar] [CrossRef]
- Jankovic, M.; Petrovskii, S. Gypsy moth invasion in North America: A simulation study of the spatial pattern and the rate of spread. Ecol. Complex. 2013, 14, 132–144. [Google Scholar] [CrossRef]
- Sharov, A.A.; Roberts, E.A.; Liebhold, A.M.; Ravlin, F.W. Gypsy moth (Lepidoptera: Lymantriidae) spread in the central Appalachians: Three methods for species boundary estimation. Environ. Entomol. 1995, 24, 1529–1538. [Google Scholar] [CrossRef]
- Tobin, P.C.; Bai, B.B.; Eggen, D.A.; Leonard, D.S. The ecology, geopolitics, and economics of managing Lymantria dispar in the United States. Int. J. Pest Manag. 2012, 58, 195–210. [Google Scholar] [CrossRef]
- Keena, M.A.; Wallner, W.E.; Grinberg, P.S.; Carde, R.T. Female flight propensity and capability in Lymantria dispar (Lepidoptera: Lymantriidae) from Russia, North America, and their reciprocal f-1 hybrids. Environ. Entomol. 2001, 30, 380–387. [Google Scholar] [CrossRef]
- Gray, D.R. Unwanted spatial bias in predicting establishment of an invasive insect based on simulated demographics. Int. J. Biometeorol. 2014, 58, 949–961. [Google Scholar] [CrossRef]
- Gray, D.R. The gypsy moth life stage model: Landscape-wide estimates of gypsy moth establishment using a multi-generational phenology model. Ecol. Model. 2004, 176, 155–171. [Google Scholar] [CrossRef]
- Soukhovolsky, V.G.; Ponomarev, V.I.; Sokolov, G.I.; Tarasova, O.V.; Krasnoperova, P.A. Gypsy moth Lymantria dispar L. in the southern Urals: Patterns in population dynamics and modeling. Biol. Bull. Rev. 2016, 6, 57–69. [Google Scholar] [CrossRef]
- May, C.; Hillerbrand, N.; Thompson, L.M.; Faske, T.M.; Martinez, E.; Parry, D.; Agosta, S.J.; Grayson, K.L. Geographic variation in larval metabolic rate between northern and southern populations of the invasive gypsy moth. J. Insect Sci. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Tobin, P.C.; Gray, D.R.; Liebhold, A.M. Supraoptimal temperatures influence the range dynamics of a non-native insect. Divers. Distrib. 2014, 20, 813–823. [Google Scholar] [CrossRef]
- Faske, T.M.; Thompson, L.M.; Banahene, N.; Levorse, A.; Herrera, M.Q.; Sherman, K.; Timko, S.E.; Yang, B.R.; Gray, D.R.; Parry, D.; et al. Can gypsy moth stand the heat? A reciprocal transplant experiment with an invasive forest pest across its southern range margin. Biol. Invasions 2019, 21, 1365–1378. [Google Scholar] [CrossRef]
- Banahene, N.; Salem, S.K.; Faske, T.M.; Byrne, H.M.; Glackin, M.; Agosta, S.J.; Eckert, A.J.; Grayson, K.L.; Thompson, L.M. Thermal sensitivity of gypsy moth (Lepidoptera: Erebidae) during larval and pupal development. Environ. Entomol. 2018, 47, 1623–1631. [Google Scholar] [CrossRef]
- Peterson, A.T.; Williams, R.; Chen, G. Modeled global invasive potential of Asian gypsy moths, Lymantria dispar. Entomol. Exp. Appl. 2007, 125, 39–44. [Google Scholar] [CrossRef]
- Charlton, R.E.; Carde, R.T.; Wallner, W.E. Synchronous crepuscular flight of female Asian gypsy moths: Relationships of light intensity and ambient and body temperatures. J. Insect Behav. 1999, 12, 517–531. [Google Scholar] [CrossRef]
- Iwaizumi, R.; Arakawa, K.; Koshio, C. Nocturnal flight activities of the female Asian gypsy moth, Lymantria dispar (Linnaeus) (Lepidoptera: Lymantriidae). Appl. Entomol. Zool. 2010, 45, 121–128. [Google Scholar] [CrossRef]
- Chen, F.; Shi, J.; Keena, M. Evaluation of the effects of light intensity and time interval after the start of scotophase on the female flight propensity of Asian gypsy moth (Lepidoptera: Erebidae). Environ. Entomol. 2016, 45, 404–409. [Google Scholar] [CrossRef]
- Keena, M.A.; Grinberg, P.S.; Wallner, W.E. Comparison of female flight capability of Lymantria dispar L. reared on artificial diet versus foliage. In US Department of Agriculture Interagency Gypsy Moth Research Forum 1997; Fosbroke, S.L.C., Gottschalk, K., Eds.; Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1997; p. 58. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Turcani, M.; Kamata, N. Inference of adult female dispersal from the distribution of gypsy moth egg masses in a Japanese city. Agric. For. Entomol. 2008, 10, 69–73. [Google Scholar] [CrossRef]
- Yang, F.; Luo, Y.; Shi, J. The influence of geographic population, age, and mating status on the flight activity of the Asian gypsy moth Lymantria dispar (Lepidoptera: Erebidae) in China. Appl. Entomol. Zool. 2017, 52, 265–270. [Google Scholar] [CrossRef]
- Robinet, C.; Lance, D.R.; Thorpe, K.W.; Onufrieva, K.S.; Tobin, P.C.; Liebhold, A.M. Dispersion in time and space affect mating success and Allee effects in invading gypsy moth populations. J. Anim. Ecol. 2008, 77, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Munson, A.S.; Leonard, D.; Mastro, T.; McGovern, T.; Levy, J.; Kucera, D. Russian Far East Lymantrid Monitoring Project: Project Summary 1993–1994; United Sates Department of Agriculture Forest Service Intermountain Region: Ogden, UT, USA, 1995.
- Trotter, R.T., III; Limbu, S.; Hoover, K.; Nadel, H.; Keena, M.A. Comparing Asian gypsy moth [Lymantria dispar asiatica (Lepidoptera: Erebidae) and L. dispar japonica] trap data from East Asian ports with lab parameterized phenology models: New tools and questions. Ann. Entomol. Soc. Am. 2020, 113, 125–138. [Google Scholar] [CrossRef]
- Garner, K.J.; Slavicek, J.M. Identification and characterization of a RAPD-PCR marker for distinguishing Asian and North American gypsy moths. Insect Mol. Biol. 1996, 5, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.; Zahiri, R.; Djoumad, A.; Freschi, L.; Lamarche, J.; Holden, D.; Cervantes, S.; Ojeda, D.I.; Potvin, A.; Nisole, A.; et al. A multi-species TaqMan PCR assay for the identification of Asian gypsy moths (Lymantria spp.) and other invasive lymantriines of biosecurity concern to North America. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Stewart, D.; Nisole, A.; Djoumad, A.; Zahiri, R.; Lamarche, J.; Levesque, R.C.; Hamelin, R.C.; Cusson, M. A needle in a haystack: A multigene TaqMan assay for the detection of Asian gypsy moths in bulk pheromone trap samples. Biol. Invasions 2019, 21, 1843–1856. [Google Scholar] [CrossRef]
- Djoumad, A.; Nisole, A.; Stewart, D.; Holden, D.; Zahiri, R.; Inoue, M.N.; Martemyanov, V.V.; Levesque, R.C.; Hamelin, R.C.; Cusson, M. Reassessment of the status of Lymantria albescens and Lymantria postalba (Lepidoptera: Erebidae: Lymantriinae) as distinct ‘Asian gypsy moth’ species, using both mitochondrial and nuclear sequence data. Syst. Entomol. 2020, 45, 493–504. [Google Scholar] [CrossRef]
- Wu, Y.; Molongoski, J.J.; Winograd, D.F.; Bogdanowicz, S.M.; Louyakis, A.S.; Lance, D.R.; Mastro, V.C.; Harrison, R.G. Genetic structure, admixture and invasion success in a holarctic defoliator, the gypsy moth (Lymantria dispar, Lepidoptera: Erebidae). Mol. Ecol. 2015, 24, 1275–1291. [Google Scholar] [CrossRef]
- Picq, S.; Keena, M.; Havill, N.; Stewart, D.; Pouliot, E.; Boyle, B.; Levesque, R.C.; Hamelin, R.C.; Cusson, M. Assessing the potential of genotyping-by-sequencing-derived single nucleotide polymorphisms to identify the geographic origins of intercepted gypsy moth (Lymantria dispar) specimens: A proof-of-concept study. Evol. Appl. 2017. [Google Scholar] [CrossRef]
- Hamelin, R.C.; Roe, A.D. Genomic biosurveillance of forest invasive alien enemies: A story written in code. Evol. Appl. 2020, 13, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Getahun, M.N.; Thoma, M.; Lavista-Llanos, S.; Keesey, I.; Fandino, R.A.; Knaden, M.; Wicher, D.; Olsson, S.B.; Hansson, B.S. Intracellular regulation of the insect chemoreceptor complex impacts odour localization in flying insects. J. Exp. Biol. 2016, 219, 3428–3438. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.C.; Grosse-Wilde, E.; Wheeler, D.; Mescher, M.C.; Hansson, B.S.; De Moraes, C.M. Comparing the expression of olfaction-related genes in gypsy moth (Lymantria dispar) adult females and larvae from one flightless and two flight-capable populations. Front. Ecol. Evol. 2017, 5. [Google Scholar] [CrossRef]
- Hebert, F.O.; Freschi, L.; Blackburn, G.; Beliveau, C.; Dewar, K.; Boyle, B.; Gundersen-Rindal, D.E.; Sparks, M.E.; Cusson, M.; Hamelin, R.C.; et al. Expansion of lines and species-specific DNA repeats drives genome expansion in Asian gypsy moths. Sci. Rep. UK 2019, 9. [Google Scholar] [CrossRef]
- Zhang, J.; Cong, Q.; Rex, E.A.; Hallwachs, W.; Janzen, D.H.; Grishin, N.V.; Gammon, D.B. Gypsy moth genome provides insights into flight capability and virus-host interactions. Proc. Natl. Acad. Sci. USA 2019, 116, 1669–1678. [Google Scholar] [CrossRef]
- Marden, J.H. Maximum lift production during takeoff in flying animals. J. Exp. Biol. 1987, 130, 235–258. [Google Scholar]
- Shi, J.; Chen, F.; Keena, M.A. Differences in wing morphometrics of Lymantria dispar (Lepidoptera: Erebidae) between populations that vary in female flight capability. Ann. Entomol. Soc. Am. 2015, 108, 528–535. [Google Scholar] [CrossRef]
- Bierl, B.A.; Beroza, M.; Collier, C.W. Potent sex attractant of gypsy moth—Its isolation, identification, and synthesis. Science 1970, 170. [Google Scholar] [CrossRef]
- Roe, A.D.; Torson, A.S.; Bilodeau, G.; Bilodeau, P.; Blackburn, G.S.; Cui, M.M.; Cusson, M.; Doucet, D.; Griess, V.C.; Lafond, V.; et al. Biosurveillance of forest insects: Part integration and application of genomic tools to the surveillance of non-native forest insects. J. Pest Sci. 2019, 92, 51–70. [Google Scholar] [CrossRef]
| Origin | Sustained Flight | Gliding Flight | |
|---|---|---|---|
| Female | Male | ||
| Russia | Russia | 88% | 11% |
| Russia | North America | 0% | 51% |
| North America | Russia | 2% | 65% |
| North America | North America | 0% | 0% |
| Authors | Year | Population | Distance | Notes |
|---|---|---|---|---|
| Rozkhov and Vasilyeva | 1982 | Asia (unspecified) | 100 km | |
| Baranchikov | 1986 | Russia Far East | 3–5 km | |
| Savotikov et al. | 1995 | Asia (unspecified) | 20–40 km | |
| Liebhold et al. | 2008 | Japan (Kanazawa) | ≤1 km | max distance, one night |
| Iwaizumi et al. | 2010 | Japan (Yokahoma City, Chiba City) | 746 m | max distance, one night, virgin female |
| 511 m | max distance, one night, mated female | |||
| 226 m | mean distance, one night, virgin female | |||
| 269 m | mean distance, one night, mated female | |||
| Iwaizumi and Arakawa | 2010 | Japan (Tomakomai) | 659 ± 335 m | mean distance, one night |
| Japan (Hachinohe) | 188 ± 202 m | mean distance, one night | ||
| Japan (Kobe) | 356 ± 351 m | mean distance, one night | ||
| Japan (Chiba) | 255 ± 116 m | mean distance, one night | ||
| Yang et al. | 2017 | China (Jining, Inner Mongolia) | 3.95 ± 0.29 km | mean distance, one night; one-day-old females; measured with flight mill |
| China (Sandeli, Liaoning) | 6.63 ± 1.40 km | mean distance, one night; one-day-old females; measured with flight mill | ||
| China (Yanzikou, Beijing) | 5.56 ± 1.16 km | mean distance, one night; one-day-old females; measured with flight mill | ||
| China (Longhua, Hebei) | 4.03 ± 0.99 km | mean distance, one night; one-day-old females; measured with flight mill | ||
| China (Lianyungang, Jiangsu) | 5.79 ± 1.44 km | mean distance, one night; one-day-old females; measured with flight mill | ||
| China (Liuan, Anhui) | 6.54 ± 1.12 km | mean distance, one night; one-day-old females; measured with flight mill | ||
| China (Xifeng, Guizhou) | 7.50 ± 2.28 km | mean distance, one night; one-day-old females; measured with flight mill |
| Country | City Port | Distance from Port to Forest Edge (km) | Notes |
|---|---|---|---|
| Republic of Korea | Donghae | 2 | |
| Republic of Korea | Okgye | 1 | |
| Republic of Korea | Incheon | 2 | |
| Republic of Korea | Pyongtaek | 2–5 | Depends on size of forest needed |
| Republic of Korea | Busan | 2 | |
| Republic of Korea | Pohang | 2 | |
| Republic of Korea | Ulsan | 3 | |
| Republic of Korea | Gunsan | 1–3 | Depends on size of forest needed |
| Republic of Korea | Mokpo | 1–2.5 | Depends on size of forest needed |
| Republic of Korea | Gwangyang | 1.5 | |
| Republic of Korea | Yeongilman | 1.5 | |
| Republic of Korea | Onsan | 2–4.5 | Depends on size of forest needed |
| Republic of Korea | Daesan | 1 | |
| Japan | Kokura | 3–5 | Depends on size of forest needed |
| Japan | Ube | 4 | |
| Japan | Oita | 3–8 | Depends on size of forest needed |
| Japan | Hirosihima | 3–4 | Depends on size of forest needed |
| Japan | Matsunaga | 2 | |
| Japan | Tsuruga | 1.5 | |
| Japan | Kanazawa | 1–7 | Depends on size of forest needed |
| Japan | Chiba | 5–8 | Depends on size of forest needed |
| Japan | Fushiki | 7 | |
| Japan | Toyama-shinko | 6–14 | Depends on size of forest needed |
| Japan | Sakata | 0 | Port directly next to forest |
| Japan | Hachinohe | 5 | |
| Japan | Nagahama | 4.5 | |
| Japan | Aomori | 4 | |
| Japan | Hakodate | 0.5–4 | Depends on port location used |
| Japan | Tomakomai | 3 | |
| Japan | Otaru | 1 | |
| Russia | Vladivostok | 3.5–9 | 9 k to closest trap; 3.5 k to closest forest edge |
| Russia | Nakhodka | 0–3 | 3 k to closest trap; 0 k to closest forest edge |
| Russia | Vostochny | 0.5 | |
| Russia | Olga | 0 | Port directly next to forest |
| Russia | Slavyanka | 1 | |
| Russia | Zarubino | 0 | Port directly next to forest |
| Russia | Posyet | 0 | Port directly next to forest |
| Russia | Plastun | 0 | Port directly next to forest |
| Russia | Vanino | 0.2 | |
| Russia | Kozmino | 0 | Port directly next to forest |
| Russia | Korsakov | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, V.; Keena, M.A.; Maennicke, G.E.; Hamelin, R.C.; Griess, V.C. Potential Differences and Methods of Determining Gypsy Moth Female Flight Capabilities: Implications for the Establishment and Spread in Novel Habitats. Forests 2021, 12, 103. https://doi.org/10.3390/f12010103
Srivastava V, Keena MA, Maennicke GE, Hamelin RC, Griess VC. Potential Differences and Methods of Determining Gypsy Moth Female Flight Capabilities: Implications for the Establishment and Spread in Novel Habitats. Forests. 2021; 12(1):103. https://doi.org/10.3390/f12010103
Chicago/Turabian StyleSrivastava, Vivek, Melody A. Keena, Galen E. Maennicke, Richard C. Hamelin, and Verena C. Griess. 2021. "Potential Differences and Methods of Determining Gypsy Moth Female Flight Capabilities: Implications for the Establishment and Spread in Novel Habitats" Forests 12, no. 1: 103. https://doi.org/10.3390/f12010103
APA StyleSrivastava, V., Keena, M. A., Maennicke, G. E., Hamelin, R. C., & Griess, V. C. (2021). Potential Differences and Methods of Determining Gypsy Moth Female Flight Capabilities: Implications for the Establishment and Spread in Novel Habitats. Forests, 12(1), 103. https://doi.org/10.3390/f12010103






