Intake of Radionuclides in the Trees of Fukushima Forests 5. Earthquake Could Have Caused an Increase in Xyloglucan in Trees †
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Trees
2.2. Immuno-Staining of Stem Sections
3. Results and Discussion
3.1. Megathrust Earthquake and Aftershocks in Fukushima
3.2. Xyloglucan Signals in the Annual Rings of Oak Trees
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yasukawa, C.; Aoki, S.; Nonaka, M.; Itakura, M.; Tsubokura, M.; Baba, K.; Ohbayashi, H.; Sugawara, I.; Seyama, T.; Uehara, I.; et al. Intake of radionuclides in the trees of Fukushima forests. 1. Field study. Forests 2019, 10, 652. [Google Scholar] [CrossRef]
- Aoki, S.; Nonaka, M.; Yasukawa, C.; Itakura, M.; Tsubokura, M.; Baba, K.; Ohbayashi, H.; Seyama, T.; Uehara, I.; Kaida, R.; et al. Intake of radionuclides in the trees of Fukushima forests. 2. Study of radiocesium flow to poplar seedlings as a model tree. Forests 2019, 10, 736. [Google Scholar] [CrossRef]
- Seyama, T.; Arakawa, R.; Machida, S.; Yoshida, S.; Maru, A.; Baba, K.; Kobayashi, Y.; Kaida, R.; Taji, T.; Sakata, Y.; et al. Intake of radionuclides in the trees of Fukushima forests. 3. Removal of radiocesium from stem wood. Forests 2020, 11, 589. [Google Scholar] [CrossRef]
- Nonaka, M.; Yasukawa, C.; Aoki, S.; Itakura, M.; Willför, S.; Capek, P.; Shoseyov, O.; Tsubokura, M.; Baba, K.; Kaida, R.; et al. Intake of radionuclides in the trees of Fukushima forests. 4. Binding of radioiodine to xyloglucan. Forests 2020, 11, 957. [Google Scholar] [CrossRef]
- Labavitch, J.M.; Ray, P.M. Turnover of cell wall polysaccharides in elongating pea stem segments. Plant Physiol. 1974, 53, 669–673. [Google Scholar] [CrossRef][Green Version]
- Hayashi, T.; Wong, Y.-S.; Maclachlan, G. Pea xyloglucan and cellulose. II. Hydrolysis by pea endo-1,4-β-glucanases. Plant Physiol. 1984, 75, 605–610. [Google Scholar] [CrossRef]
- Talmadge, K.W.; Keegstra, K.; Bauer, W.D.; Albersheim, P. The structure of plant cell walls. I. The macromolecular components of the walls of suspension-cultured sycamore cells with a detailed analysis of the pectic polysaccharides. Plant Physiol. 1973, 51, 15–73. [Google Scholar] [CrossRef]
- Bauer, W.D.; Talmadge, K.W.; Keegstra, K.; Albersheim, P. The structure of plant cell walls. II. The hemicellulose of the walls of suspension-cultured sycamore cells. Plant Physiol. 1973, 51, 174–187. [Google Scholar] [CrossRef]
- Keegstra, K.; Talmadge, K.W.; Bauer, W.D.; Albersheim, P. The structure of plant cell walls. III. A model of the walls of suspension-cultured sycamore cells based on the interconnections of the macromolecular components. Plant Physiol. 1973, 51, 188–197. [Google Scholar] [CrossRef]
- Valent, B.S.; Albersheim, P. The structure of plant cell walls. V. On the binding of xyloglucan to cellulose fibers. Plant Physiol. 1974, 54, 105–108. [Google Scholar] [CrossRef]
- Hayashi, T.; Marsden, M.P.F.; Delmer, D.P. Pea xyloglucan and cellulose. V. Xyloglucan-cellulose interaction in vitro and in vivo. Plant Physiol. 1987, 83, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Ogawa, K.; Mitsuishi, Y. Characterization of the adsorption of xyloglucan to cellulose. Plant Cell Physiol. 1994, 35, 1199–1205. [Google Scholar] [CrossRef]
- Zhao, Z.; Crespi, V.H.; Kubicki, J.D.; Cosgrove, D.J.; Zhonget, L. Molecular dynamics simulation study of xyloglucan adsorption on cellulose surfaces: Effects of surface hydrophobicity and side-chain variation. Cellulose 2014, 21, 1025–1039. [Google Scholar] [CrossRef]
- Thompson, J.E.; Fry, S.C. Evidence for covalent linkage between xyloglucan and acidic pectins in suspension-cultured rose cells. Planta 2000, 211, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Popper, Z.A.; Fry, S.C. Wide spread occurrence of a covalent linkage between xyloglucan and acidic polysaccharides in suspension-cultured angiosperm cells. Ann. Bot. 2005, 96, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Shiono, K. Focal mechanisms of major earthquakes in southwest Japan and their tectonic significance. J. Phys. Earth 1977, 25, 1–26. [Google Scholar] [CrossRef][Green Version]
- Tanioka, Y.; Ruff, L.; Satake, K. What controls the lateral variation of large earthquake occurrence along the Japan Trench? Island Arc 1997, 6, 261–266. [Google Scholar] [CrossRef]
- Zhao, D.; Ochi, F.; Hasegawa, A.; Yamamoto, A. Evidence for the location and cause of large crustal earthquakes in Japan. J. Geophys. Res. 2000, 105, 13579–13594. [Google Scholar] [CrossRef]
- Uchida, N.; Matsuzawa, T. Coupling coefficient, hierarchical structure, and earthquake cycle for the source area of the 2011 off the Pacific coast of Tohoku earthquake inferred from small repeating earthquake data. Earth Planets Space 2011, 63, 675–679. [Google Scholar] [CrossRef]
- Bassett, D.; Sandwell, D.T.; Fialko, Y.; Watts, A.B. Upper-plate controls on co-seismic slip in the 2011 magnitude 9.0 Tohoku-oki earthquake. Nature 2016, 531, 92–96. [Google Scholar] [CrossRef]
- Kunugi, T. Relationship between Japan Meteorological Agency instrumental intensity and instrumental modified Mercalli intensity obtained from K-NET strong-motion data. Zisin 2000, 53, 89–93. (In Japanese) [Google Scholar] [CrossRef]
- Wald, D.J.; Quitoriano, V.; Heaton, T.H.; Kanamori, H.; Scrivner, C.W.; Worden, C.B. TriNet “Shake Maps”: Rapid generation of instrumental ground motion and intensity maps for earthquakes in southern California. Earthq. Spectra 1999, 15, 537–555. [Google Scholar] [CrossRef]
- Marcus, S.E.; Verhertbruggen, Y.; Herve, C.; Ordaz-Ortiz, J.J.; Farkas, V.; Pedersen, H.L.; Willats, W.G.T.; Knox, J.P. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 2008, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.F.; Pattison, R.R.; Brownlee, A.H.; Cahoon, S.M.P.; Hollingsworth, T.N. Effect of tree-ring detrending method on apparent growth trends of black and white spruce in interior Alaska. Environ. Res. Lett. 2016, 11, 114007. [Google Scholar] [CrossRef]
- Barros, J.; Serk, H.; Granlund, I.; Pesquet, E. The cell biology of lignification in higher plants. Ann. Bot. 2015, 115, 1053–1074. [Google Scholar] [CrossRef]
- Bloknina, O.; Laitinen, T.; Hatakeyama, Y.; Delhomme, N.; Paasela, T.; Zhao, L.; Street, N.R.; Wada, H.; Karkonen, A.; Fagerstedt, K. Ray parenchymal cells contribute to lignification of tracheids in developing xylem of Norway spruce. Plant Physiol. 2019, 181, 1552–1572. [Google Scholar] [CrossRef]
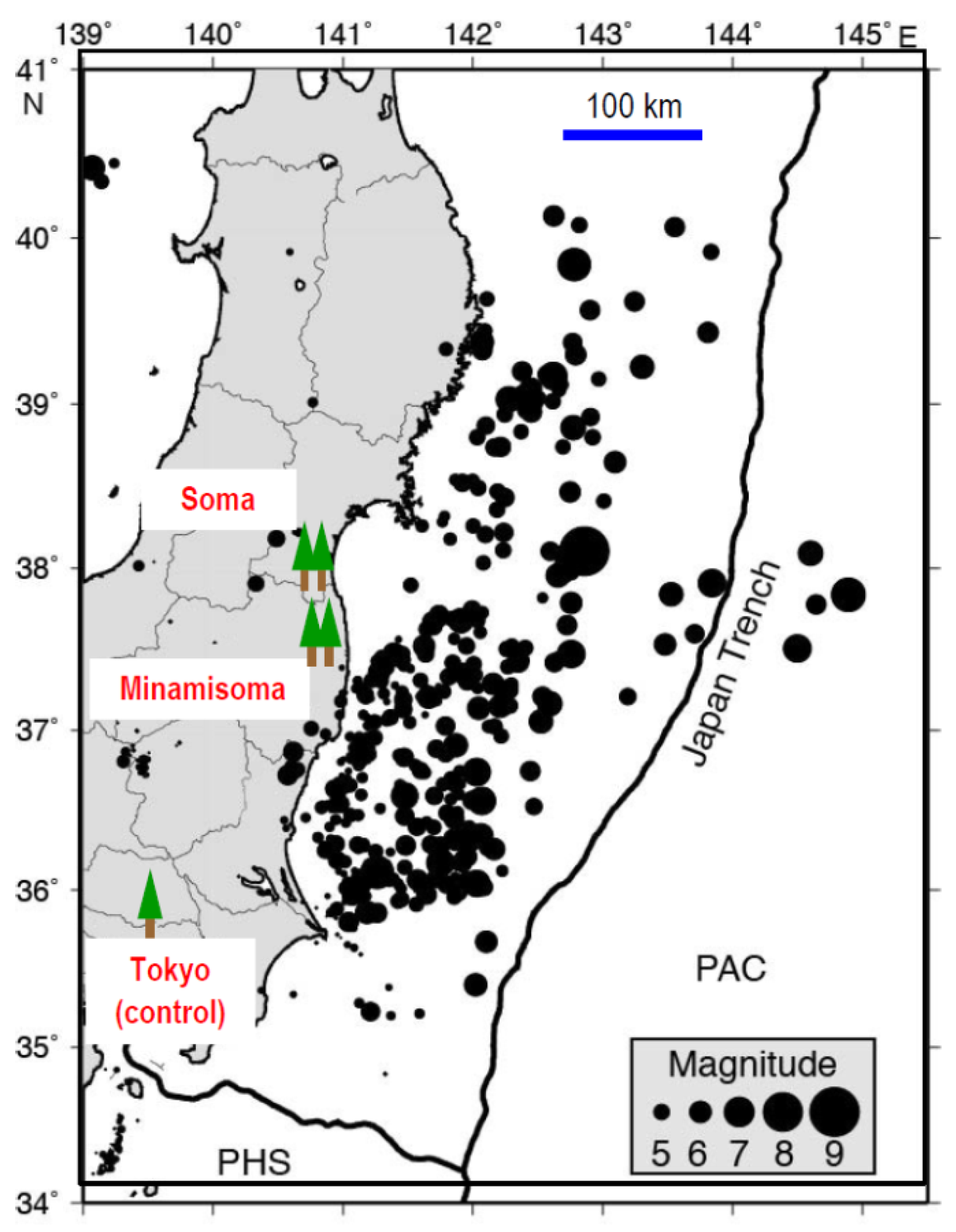
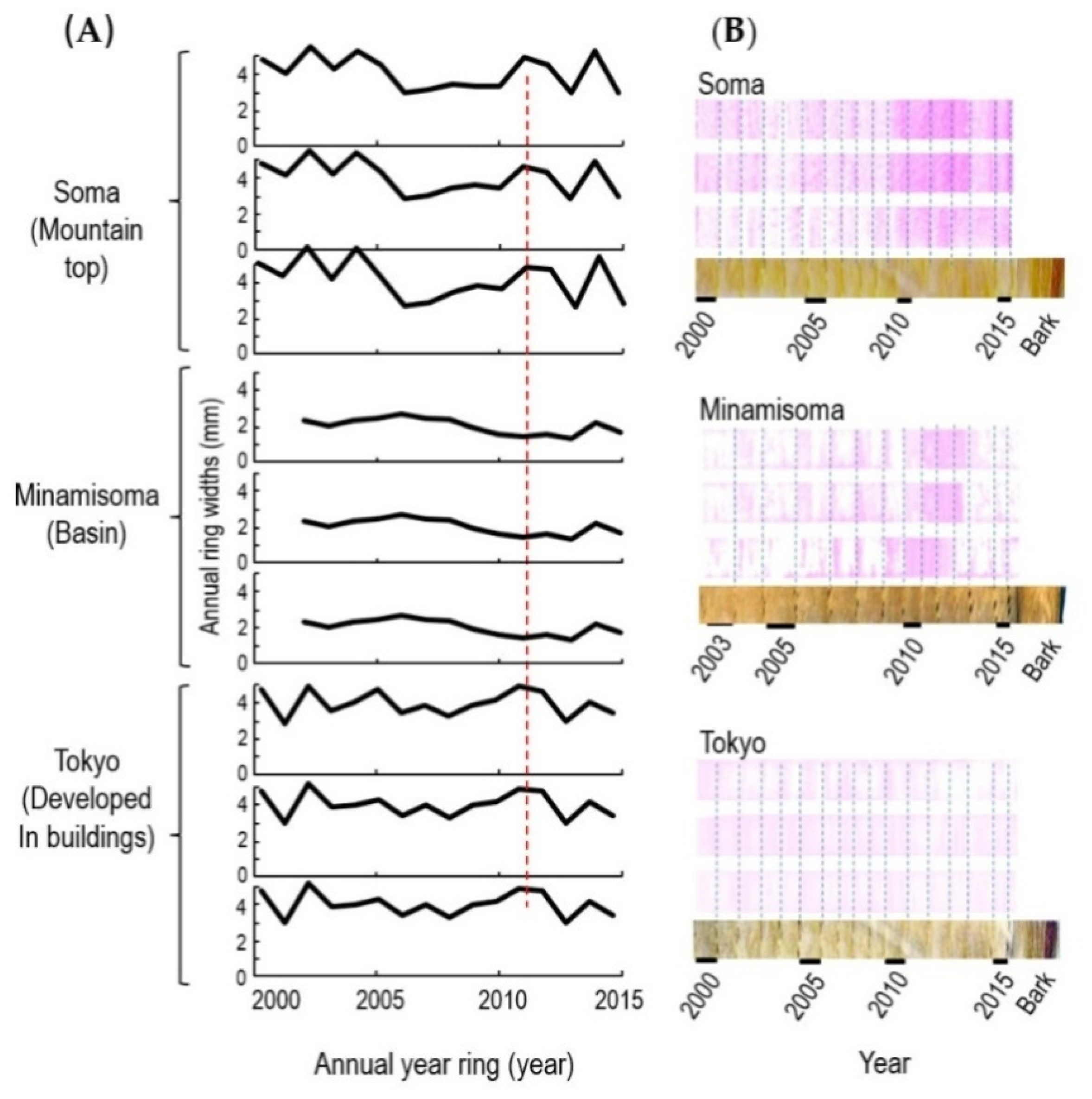
| Year | Annual Earthquake § (Number) | Mean Wind Force ‡ (m/s) | Annual Rainfall ‡ (mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Soma | Minamisoma | Tokyo | Soma | Minamisoma | Tokyo | Soma | Minamisoma | Tokyo | |
| 2007 | 1 | 3 | 0 | 2.3 | 0.9 | 3.4 | 1394 | 1706 | 1332 |
| 2008 | 8 | 9 | 0 | 2.2 | 0.9 | 2.8 | 1656 | 1653 | 1858 |
| 2009 | 5 | 5 | 1 | 2.3 | 0.9 | 2.9 | 1516 | 1468 | 1802 |
| 2010 | 5 | 4 | 0 | 2.1 | 1.2 | 2.9 | 1437 | 1720 | 1680 |
| 2011 | 102 | 98 | 8 | 2.3 | 1.3 | 2.9 | 1316 | 1274 | 1480 |
| 2012 | 21 | 21 | 7 | 2.3 | 1.4 | 3.0 | 1406 | 1482 | 1570 |
| 2013 | 14 | 16 | 5 | 2.3 | 1.5 | 3.1 | 1135 | 1260 | 1614 |
| 2014 | 10 | 13 | 6 | 2.4 | 1.4 | 2.9 | 1561 | 1780 | 1808 |
| 2015 | 9 | 9 | 2 | 2.3 | 1.3 | 2.8 | 1671 | 1775 | 1782 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaida, R.; Sasaki, Y.; Ozaki, K.; Baba, K.; Momoi, T.; Ohbayashi, H.; Taji, T.; Sakata, Y.; Hayashi, T. Intake of Radionuclides in the Trees of Fukushima Forests 5. Earthquake Could Have Caused an Increase in Xyloglucan in Trees. Forests 2020, 11, 966. https://doi.org/10.3390/f11090966
Kaida R, Sasaki Y, Ozaki K, Baba K, Momoi T, Ohbayashi H, Taji T, Sakata Y, Hayashi T. Intake of Radionuclides in the Trees of Fukushima Forests 5. Earthquake Could Have Caused an Increase in Xyloglucan in Trees. Forests. 2020; 11(9):966. https://doi.org/10.3390/f11090966
Chicago/Turabian StyleKaida, Rumi, Yuya Sasaki, Kaho Ozaki, Kei’ichi Baba, Takao Momoi, Hiroya Ohbayashi, Teruaki Taji, Yoichi Sakata, and Takahisa Hayashi. 2020. "Intake of Radionuclides in the Trees of Fukushima Forests 5. Earthquake Could Have Caused an Increase in Xyloglucan in Trees" Forests 11, no. 9: 966. https://doi.org/10.3390/f11090966
APA StyleKaida, R., Sasaki, Y., Ozaki, K., Baba, K., Momoi, T., Ohbayashi, H., Taji, T., Sakata, Y., & Hayashi, T. (2020). Intake of Radionuclides in the Trees of Fukushima Forests 5. Earthquake Could Have Caused an Increase in Xyloglucan in Trees. Forests, 11(9), 966. https://doi.org/10.3390/f11090966




