The Need to Improve Riparian Forests Management in Uranium Mining Areas Based on Assessment of Heavy Metal and Uranium Contamination
Abstract
1. Introduction
2. Materials and Methods
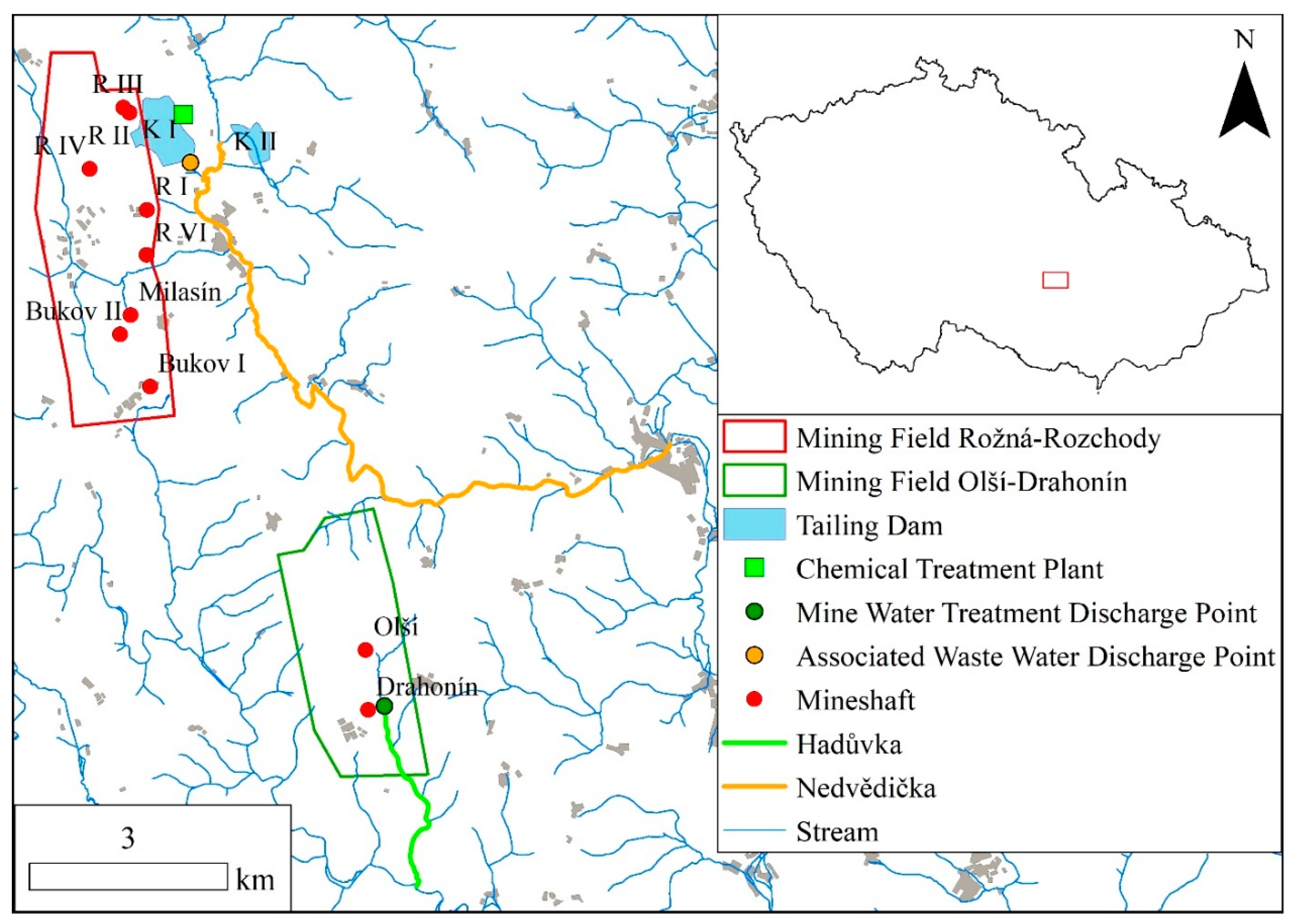
2.1. Study Area
2.2. Sampling Design
2.3. Sample Collection and Preparation
2.4. Sample Analysis
2.5. Data Analysis
3. Results and Discussion
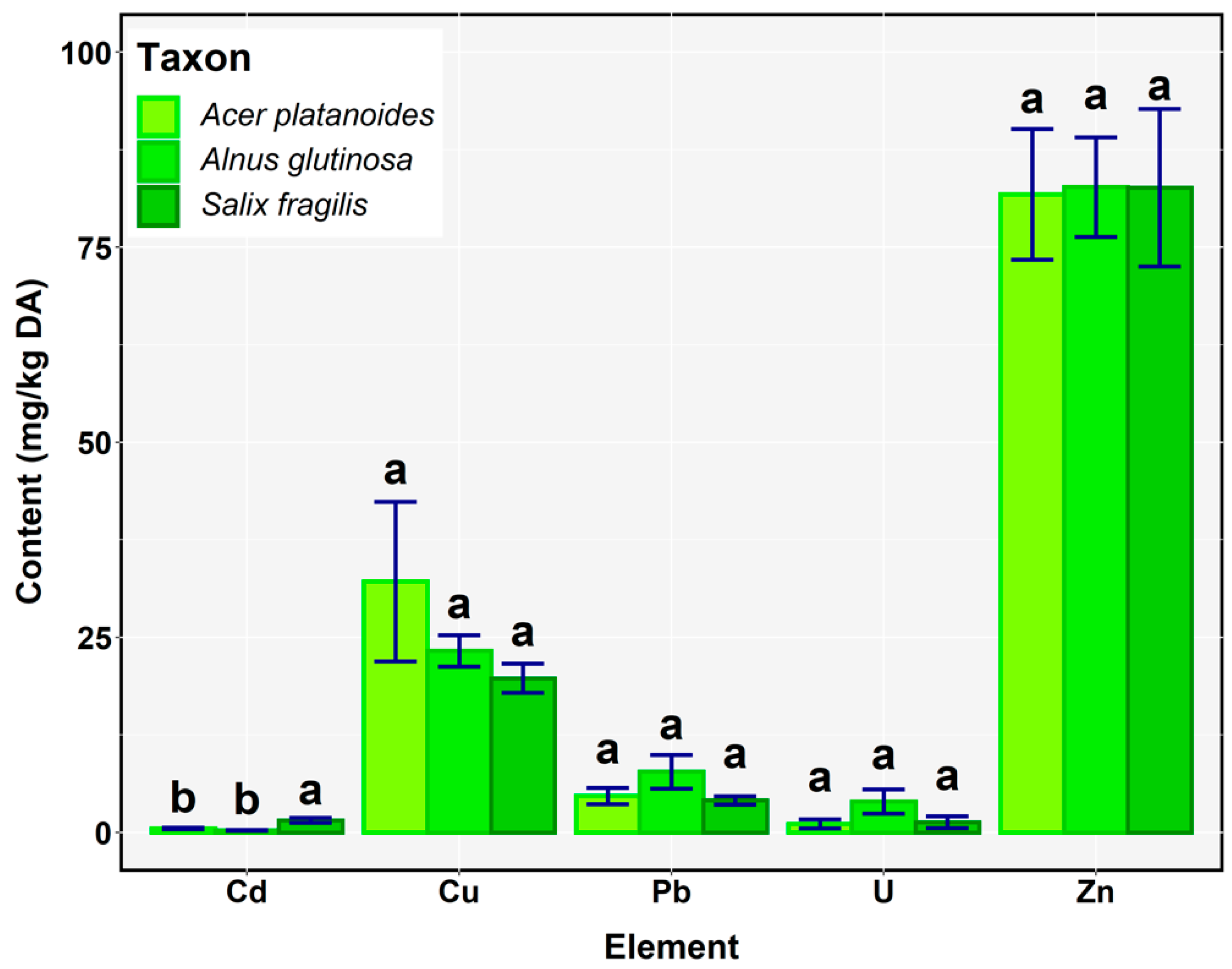
3.1. Evaluation of Heavy Metals and Uranium in Vegetation
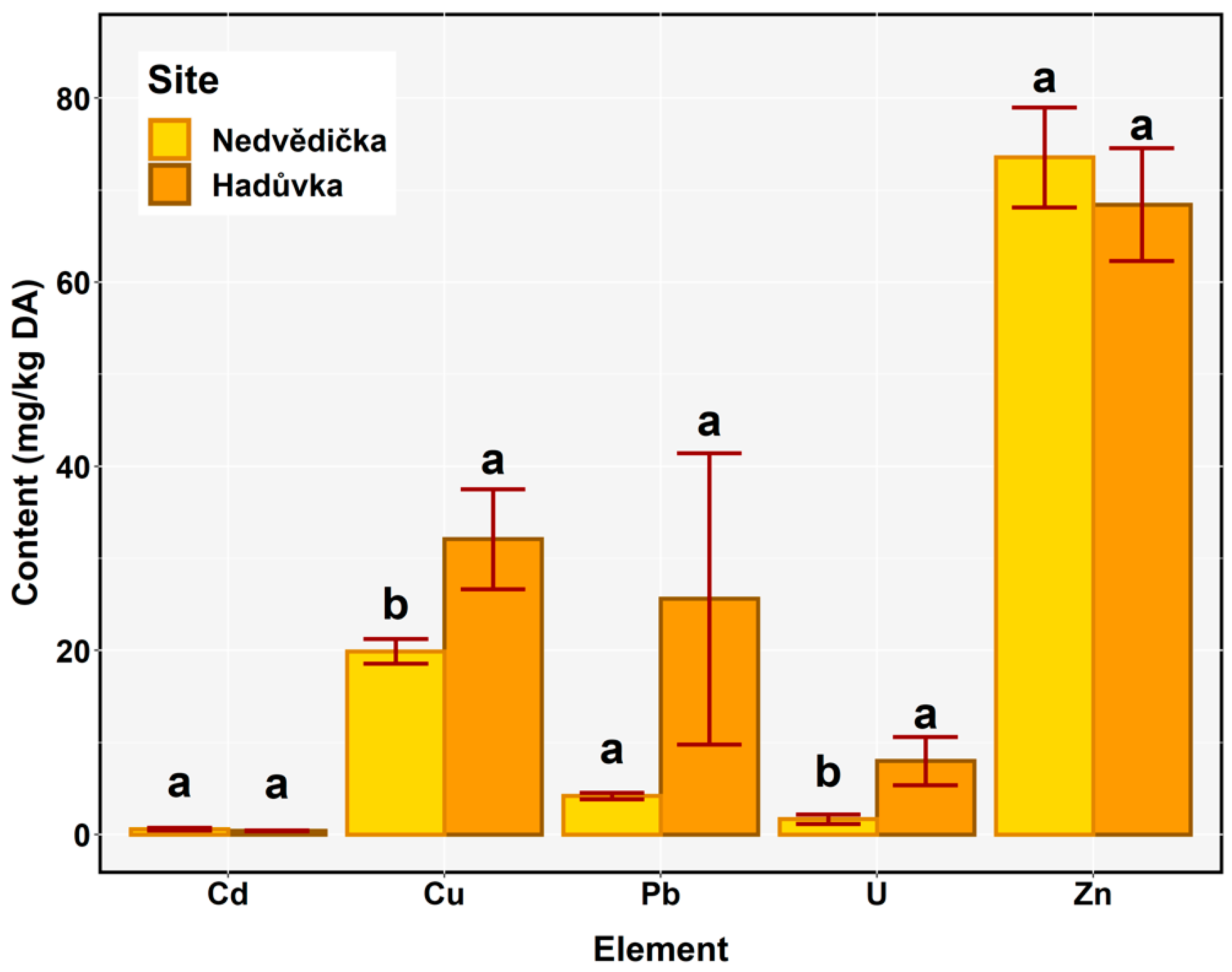
3.2. Comparison of Pollution at the Sites of the Short-Term and Long-Term Closure of Mining
3.3. Distribution of Contamination Depending on the Distance from the Source of Pollution
3.4. Correlation Analysis
3.5. Measures against the Spread of Contamination in the Environment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hudcová, H.; Badurová, J.; Rozkošný, M.; Sova, J.; Funková, R.; Svobodová, J. Quality and mutagenicity of water and sediment of the streams impacted by the former uranium mine area OlŠí-Drahonín (Czech Republic). J. Environ. Radioact. 2013, 116, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Baumann, N.; Arnold, T.; Haferburg, G. Uranium contents in plants and mushrooms grown on a uranium-contaminated site near Ronneburg in Eastern Thuringia/Germany. Environ. Sci. Pollut. Res. 2014, 21, 6921–6929. [Google Scholar] [CrossRef] [PubMed]
- Haribala; Hu, B.; Wang, C.; Gerilemandahu; Xu, X.; Zhang, S.; Bao, S.; Li, Y. Assessment of radioactive materials and heavy metals in the surface soil around uranium mining area of Tongliao, China. Ecotoxicol. Environ. Saf. 2016, 130, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Neiva, A.M.R.; Antunes, I.M.H.R.; Carvalho, P.C.S.; Santos, A.C.T. Uranium and arsenic contamination in the former Mondego Sul uranium mine area, Central Portugal. J. Geochem. Explor. 2016, 162, 1–15. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, H.; Liu, X. Health risk assessment of heavy metals in the soil-water-rice system around the Xiazhuang uranium mine, China. Environ. Sci. Pollut. Res. 2019, 26, 5904–5912. [Google Scholar] [CrossRef]
- Bai, H.; Hu, B.; Wang, C.; Bao, S.; Sai, G.; Xu, X.; Zhang, S.; Li, Y. Assessment of radioactive materials and heavy metals in the surface soil around the Bayanwula prospective uranium mining area in China. Int. J. Environ. Res. Public Health 2017, 14, 300. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9780429192036. [Google Scholar]
- Favas, P.J.C.; Pratas, J.; Mitra, S.; Sarkar, S.K.; Venkatachalam, P. Biogeochemistry of uranium in the soil-plant and water-plant systems in an old uranium mine. Sci. Total Environ. 2016, 568, 350–368. [Google Scholar] [CrossRef]
- Gao, N.; Huang, Z.; Liu, H.; Hou, J.; Liu, X. Advances on the toxicity of uranium to different organisms. Chemosphere 2019, 237, 124548. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.H.; Hu, N.; Wang, Y.D.; Chen, S.Y.; Zou, C.; Dai, Z.R.; Zhang, H.; Ding, D.X. Enhanced phytoremediation of uranium contaminated soil by artificially constructed plant community plots. J. Environ. Radioact. 2019, 208, 106036. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, P.; Singh, A. Phytoremediation strategies for remediation of uranium-contaminated environments: A review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2575–2647. [Google Scholar] [CrossRef]
- Ouyang, J.; Liu, Z.; Ye, T.; Zhang, L. Uranium pollution status and speciation analysis in the farmland-rice system around a uranium tailings mine in southeastern China. J. Radioanal. Nucl. Chem. 2019, 322, 1011–1022. [Google Scholar] [CrossRef]
- Razo, I.; Carrizales, L.; Castro, J.; Díaz-Barriga, F.; Monroy, M. Arsenic and Heavy Metal Pollution of Soil, Water and Sediments in a Semi-Arid Climate Mining Area in Mexico. Water Air Soil Pollut. 2004, 152, 129–152. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Kafka, J. Rudné a Uranové Hornictví České Republiky; DIAMO: Dolní Rožínka, Czech Republic, 2003. [Google Scholar]
- Cimala, Z. Po Stopách Průzkumu a Těžby Uranových Ložisek na Moravě a Východních Čechách; DIAMO: Dolní Rožínka, Czech Republic, 1997. [Google Scholar]
- Kříbek, B.; Hájek, A. Uranové Ložisko Rožná: Model. Pozdně Variských a Povariských Mineralizací; Česká Geologická Služba: Prague, Czech Republic, 2005. [Google Scholar]
- Sedláček, B. Uplynulých 50 let dobývání na ložisku; DIAMO: Stráž pod Ralskem, Czech Republic, 2007. [Google Scholar]
- Lusk, K.; Hájek, A. Analýza Zaplavování Uranových Dolů v České Republice. Available online: https://slon.diamo.cz/hpvt/2003/sekce_z/PZ15P.htm (accessed on 17 May 2020).
- Veselý, M. Provozně Manipulační Rád: Odkaliště KI; DIAMO: Stráž pod Ralskem, Czech Republic, 2006. [Google Scholar]
- Šenk, B. Likvidace U-rud Olší okres Žďár nad Sázavou. Available online: https://slon.diamo.cz/hpvt/2001/sekce/sanace/11/S11.htm (accessed on 17 May 2020).
- Czech Hydrometeorological Institute (CHMI). Hydrological Yearbook of the Czech Republic 2017; Czech Hydrometeorological Institute: Prague, Czech Republic, 2018. [Google Scholar]
- PMO. Radiologické Ukazatele ve Dvouletí 2017–2018. Available online: http://www.pmo.cz/vhc/kvalita2017_2018/Radiochemicky_monitoring_2017_18.pdf (accessed on 5 May 2020).
- United States Environmental Protection Agency. Edition of the Drinking Water Standards and Health Advisories Tables; Office of Water U.S.: Washington, DC, USA, 2018.
- Juřička, D.; Mikl, L.; Muchová, M.; Cihlářová, H.; Hladký, J.; Rosická, Z.; Novotná, J.; Frýzová, R.; Brtnický, M.; Kynický, J. Metal pollution of forest phytomass from uranium industry in Czech Republic and its ecological management perspectives. Acta Univ. Agric. Silvic. Mendel. Brun. 2017, 65, 51–59. [Google Scholar] [CrossRef]
- Luyssaert, S.; Raitio, H.; Mertens, J.; Vervaeke, P.; Lust, N. Should foliar cadmium concentrations be expressed on a dry weight or dry ash weight basis? J. Environ. Monit. 2002, 4, 408–412. [Google Scholar] [CrossRef]
- Vandecasteele, B.; De Vos, B.; Tack, F.M.G. Cadmium and Zinc uptake by volunteer willow species and elder rooting in polluted dredged sediment disposal sites. Sci. Total Environ. 2002, 299, 191–205. [Google Scholar] [CrossRef]
- Claussen, T. Dry ash, a better reference base than dry matter for heavy metals and other persistent pollutants. Plant. Soil 1990, 127, 91–95. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils. Revision 2; United States Environmental Protection Agency: Washington, DC, USA, 1996.
- United States Environmental Protection Agency. Method 200.8: Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma-Mass Spectrometry. Revision 5; United States Environmental Protection Agency: Washington, DC, USA, 1994.
- R Core Team. A Language and Environment for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 15 May 2020).
- RStudio Team. RStudio: Integrated Development for R. Available online: http://www.rstudio.com/ (accessed on 15 May 2020).
- Wickham, H. ggplot2; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Wickham, H.; Francois, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. R package Version 0.8.5. Available online: https://cran.r-project.org/package=dplyr (accessed on 15 May 2020).
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall International: London, UK, 1984; ISBN 978-013-077-595-5. [Google Scholar]
- Beaujean, A.A. BaylorEdPsych: R Package for Baylor University Educational Psychology Quantitative Courses. R Package Version 0.5. Available online: http://cran.r-project.org/package=BaylorEdPsych (accessed on 18 May 2020).
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-2. Available online: https://cran.r-project.org/package=agricolae (accessed on 15 April 2020).
- Faraway, R.J. Linear Models with R.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 1-58488-425-8. [Google Scholar]
- Gross, J.; Ligges, U. Nortest: Tests for Normality. R Package Version 1.0-4. Available online: http://cran.r-project.org/package=nortest (accessed on 17 May 2020).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 15 May 2020).
- Pekár, S.; Brabec, M. Modern Analysis of Biological Data. Generalized Linear Models in R; Masaryk University Press: Brno, Czech Republic, 2016; ISBN 978-80-210-8019-5. [Google Scholar]
- Li, G.Y.; Hu, N.; Ding, D.X.; Zheng, J.F.; Liu, Y.L.; Wang, Y.D.; Nie, X.Q. Screening of plant species for phytoremediation of uranium, thorium, barium, nickel, strontium and lead contaminated soils from a uranium mill tailings repository in South China. Bull. Environ. Contam. Toxicol. 2011, 86, 646–652. [Google Scholar] [CrossRef]
- Divrikli, U.; Horzum, N.; Soylak, M.; Elci, L. Trace heavy metal contents of some spices and herbal plants from western Anatolia, Turkey. Int. J. Food Sci. Technol. 2006, 41, 712–716. [Google Scholar] [CrossRef]
- Michaud, A.M.; Chappellaz, C.; Hinsinger, P. Copper phytotoxicity affects root elongation and iron nutrition in durum wheat (Triticum turgidum durum L.). Plant. Soil 2008, 310, 151–165. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Henriques, F.S. Biochemical, physiological, and structural effects of excess copper in plants. Bot. Rev. 1991, 57, 246–273. [Google Scholar] [CrossRef]
- Radojevic, A.A.; Serbula, S.M.; Kalinovic, T.S.; Kalinovic, J.V.; Steharnik, M.M.; Petrovic, J.V.; Milosavljevic, J.S. Metal/metalloid content in plant parts and soils of Corylus spp. influenced by mining–metallurgical production of copper. Environ. Sci. Pollut. Res. 2017, 24, 10326–10340. [Google Scholar] [CrossRef] [PubMed]
- Huseyinova, R.; Kutbay, H.G.; Bilgin, A.; Kiliç, D.; Horuz, A.; Kirmanoǧlu, C. Sulphur and some heavy metal contents in foliage of Corylus avellana and some roadside native plants in Ordu province, Turkey. Ekoloji 2009, 19, 10–16. [Google Scholar] [CrossRef]
- Stojanović, M.D.; Stevanović, D.R.; Milojković, J.V.; Grubišić, M.S.; Ileš, D.A. Phytotoxic effect of the uranium on the growing up and development the plant of corn. Water Air Soil Pollut. 2010, 209, 401–410. [Google Scholar] [CrossRef]
- Hou, J.; Wang, C.; Zhou, Y.; Li, S.; Hayat, T.; Alsaedi, A.; Wang, X. Effects of uranium stress on physiological and biochemical characteristics in seedlings of six common edible vegetables. J. Radioanal. Nucl. Chem. 2018, 316, 1001–1010. [Google Scholar] [CrossRef]
- Srivastava, S.; Bhainsa, K.C.; D’Souza, S.F. Investigation of uranium accumulation potential and biochemical responses of an aquatic weed Hydrilla verticillata (L.f.) Royle. Bioresour. Technol. 2010, 101, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- Horemans, N.; Van Hees, M.; Saenen, E.; Van Hoeck, A.; Smolders, V.; Blust, R.; Vandenhove, H. Influence of nutrient medium composition on uranium toxicity and choice of the most sensitive growth related endpoint in Lemna minor. J. Environ. Radioact. 2016, 151, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.G.; Knox, A.S.; Kaplan, D.I.; Sharitz, R. Phytoextraction of uranium and thorium by native trees in a contaminated wetland. J. Radioanal. Nucl. Chem. 2005, 264, 417–422. [Google Scholar] [CrossRef]
- Říčka, A. Proudění a geochemie podzemních vod v ložiskové oblasti Rožná. Ph.D. Thesis, Masaryk University, Brno, Czech Republic, 2010. [Google Scholar]
- DIAMO. Oddělení Ekologie—Bilance Množství Důlních a Oplachových vod Ložiska Rožná; DIAMO: Dolní Rožínka, Czech Republic, 2017. [Google Scholar]
- Faměra, M.; Kotková, K.; Tůmová; Elznicová, J.; Matys Grygar, T. Pollution distribution in floodplain structure visualised by electrical resistivity imaging in the floodplain of the Litavka River, the Czech Republic. Catena 2018, 165, 157–172. [Google Scholar] [CrossRef]
- Duquène, L.; Vandenhove, H.; Tack, F.; Meers, E.; Baeten, J.; Wannijn, J. Enhanced phytoextraction of uranium and selected heavy metals by Indian mustard and ryegrass using biodegradable soil amendments. Sci. Total Environ. 2009, 407, 1496–1505. [Google Scholar] [CrossRef]
| Nedvědička (Site 1) | Hadůvka (Site 2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plot | Tree Species | Cd | Cu | Pb | U | Zn | Tree Species | Cd | Cu | Pb | U | Zn |
| 1 | Alnus glutinosa L. | 0.17 | 21.9 | 7.36 | 0.30 | 108 | Aesculus hippocastanum L. | 0.73 | 24.7 | 326 | 51.8 | 38.6 |
| Salix fragilis L. | 0.59 | 23.3 | 5.90 | 3.60 | 63.4 | Corylus avellana L. | 0.78 | 118 | 11.6 | 10.5 | 86.6 | |
| 2 | Alnus glutinosa | 0.31 | 22.5 | 6.41 | <0.1 | 59.6 | Fagus sylvatica L. | 0.38 | 48.3 | 15.4 | 10.1 | 57.0 |
| Alnus glutinosa | 0.30 | 12.8 | 4.28 | <0.1 | 96.2 | Acer platanoides L. | 0.23 | 35.6 | 8.81 | 1.81 | 78.8 | |
| 3 | Cornus sanguinea L. | 0.15 | 10.9 | 2.59 | 2.71 | 34.1 | Carpinus betulus L. | 0.50 | 24.2 | 15.4 | 19.7 | 62.6 |
| Alnus glutinosa | 0.27 | 34.2 | 1.84 | 3.51 | 101 | Crataegus oxyacantha L. | 0.19 | 9.49 | 5.21 | 3.88 | 52.1 | |
| 4 | Acer platanoides | 0.74 | 16.9 | 3.78 | <0.1 | 79.1 | Sambucus nigra L. | 0.20 | 16.0 | 6.80 | 1.99 | 54.6 |
| Salix fragilis | 2.02 | 21.9 | 4.15 | <0.1 | 64.8 | Fagus sylvatica | 0.24 | 18.1 | 13.2 | 1.15 | 75.7 | |
| 5 | Salix fragilis | 1.07 | 16.7 | 4.54 | <0.1 | 106 | Alnus glutinosa | 0.18 | 16.5 | 9.31 | 9.80 | 57.1 |
| Acer negundo L. | 0.13 | 20.7 | 3.24 | <0.1 | 40.9 | Alnus glutinosa | 0.64 | 29.8 | 28.1 | 16.4 | 66.7 | |
| 6 | Alnus glutinosa | 0.12 | 30.8 | 4.73 | 5.73 | 79.5 | Sorbus aucuparia L. | 0.23 | 13.4 | 6.13 | 9.12 | 34.4 |
| Salix fragilis | 2.04 | 13.9 | 2.87 | <0.1 | 70.6 | Quercus robur L. | 0.24 | 20.4 | 3.84 | 10.9 | 54.0 | |
| 7 | Alnus glutinosa | 0.19 | 23.2 | 6.63 | <0.1 | 103 | Acer platanoides | 0.71 | 27.1 | 3.25 | 3.15 | 111 |
| Acer platanoides | 0.53 | 11.6 | 3.52 | <0.1 | 81.9 | Acer platanoides | 0.49 | 69.6 | 4.12 | 0.42 | 58.3 | |
| 8 | Alnus glutinosa | 0.12 | 19.0 | 2.12 | <0.1 | 68.0 | Fraxinus excelsior L. | 0.23 | 36.6 | 6.68 | 0.24 | 75.1 |
| Salix fragilis | 2.14 | 23.0 | 3.24 | 2.71 | 109 | Corylus avellana | 0.88 | 22.1 | 9.47 | 1.66 | 144 | |
| 9 | Acer pseudoplatanus L. | 0.16 | 24.8 | 3.57 | 7.36 | 48.2 | Corylus avellana | 0.25 | 32.9 | 15.1 | 0.38 | 67.4 |
| Sambucus nigra | 0.22 | 14.1 | 3.32 | <0.1 | 62.5 | Carpinus betulus | 0.21 | 20.3 | 5.89 | 1.16 | 35.7 | |
| 10 | Corylus avellana | 0.16 | 17.7 | 4.53 | 1.50 | 35.3 | Alnus glutinosa | 0.45 | 27.6 | 9.72 | 2.81 | 110 |
| Alnus glutinosa | 0.24 | 17.9 | 5.17 | 4.82 | 60.8 | Prunus avium L. | 0.23 | 30.8 | 8.56 | 2.82 | 49.7 | |
| Plot | Nedvědička (Site 1) | Hadůvka (Site 2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Cu | Pb | U | Zn | Cd | Cu | Pb | U | Zn | |
| 2 | 0.92 | 0.54 | 0.27 | 0.46 | 0.78 | 0.05 | 0.25 | 0.05 | 0.03 * | 0.85 |
| 3 | 0.82 | 1.0 | 0.002 ** | 0.64 | 0.53 | 0.08 | 0.046 * | 0.048 * | 0.09 | 0.86 |
| 4 | 0.20 | 0.69 | 0.03 * | 0.46 | 0.63 | 0.03 * | 0.046 * | 0.048 * | 0.02 * | 0.93 |
| 5 | 0.77 | 0.63 | 0.03 * | 0.46 | 0.66 | 0.13 | 0.07 | 0.06 | 0.11 | 0.98 |
| 6 | 0.36 | 0.98 | 0.03 * | 0.69 | 0.70 | 0.03 * | 0.046 * | 0.04 * | 0.07 | 0.53 |
| 7 | 0.98 | 0.52 | 0.18 | 0.46 | 0.82 | 0.47 | 0.36 | 0.04 * | 0.02 * | 0.46 |
| 8 | 0.33 | 0.84 | 0.004 ** | 0.82 | 0.93 | 0.36 | 0.11 | 0.046 * | 0.01 * | 0.13 |
| 9 | 0.80 | 0.69 | 0.02 * | 0.47 | 0.30 | 0.03 * | 0.09 | 0.049 * | 0.01 * | 0.70 |
| 10 | 0.81 | 0.56 | 0.13 | 0.62 | 0.20 | 0.07 | 0.11 | 0.047 * | 0.02 * | 0.56 |
| Cd | 0.042 | 0.067 | 0.018 | 0.32 * |
| Cu | 0.015 | 0.12 | 0.12 | |
| Pb | 0.88 *** | −0.20 | ||
| U | −0.28 | |||
| Zn |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecina, V.; Juřička, D.; Kynický, J.; Baltazár, T.; Komendová, R.; Brtnický, M. The Need to Improve Riparian Forests Management in Uranium Mining Areas Based on Assessment of Heavy Metal and Uranium Contamination. Forests 2020, 11, 952. https://doi.org/10.3390/f11090952
Pecina V, Juřička D, Kynický J, Baltazár T, Komendová R, Brtnický M. The Need to Improve Riparian Forests Management in Uranium Mining Areas Based on Assessment of Heavy Metal and Uranium Contamination. Forests. 2020; 11(9):952. https://doi.org/10.3390/f11090952
Chicago/Turabian StylePecina, Václav, David Juřička, Jindřich Kynický, Tivadar Baltazár, Renata Komendová, and Martin Brtnický. 2020. "The Need to Improve Riparian Forests Management in Uranium Mining Areas Based on Assessment of Heavy Metal and Uranium Contamination" Forests 11, no. 9: 952. https://doi.org/10.3390/f11090952
APA StylePecina, V., Juřička, D., Kynický, J., Baltazár, T., Komendová, R., & Brtnický, M. (2020). The Need to Improve Riparian Forests Management in Uranium Mining Areas Based on Assessment of Heavy Metal and Uranium Contamination. Forests, 11(9), 952. https://doi.org/10.3390/f11090952






